Low-Frequency Ultrasound Coupled with High-Pressure Technologies: Impact of Hybridized Techniques on the Recovery of Phytochemical Compounds
Abstract
:1. Introduction
2. Fundamentals and Mechanisms
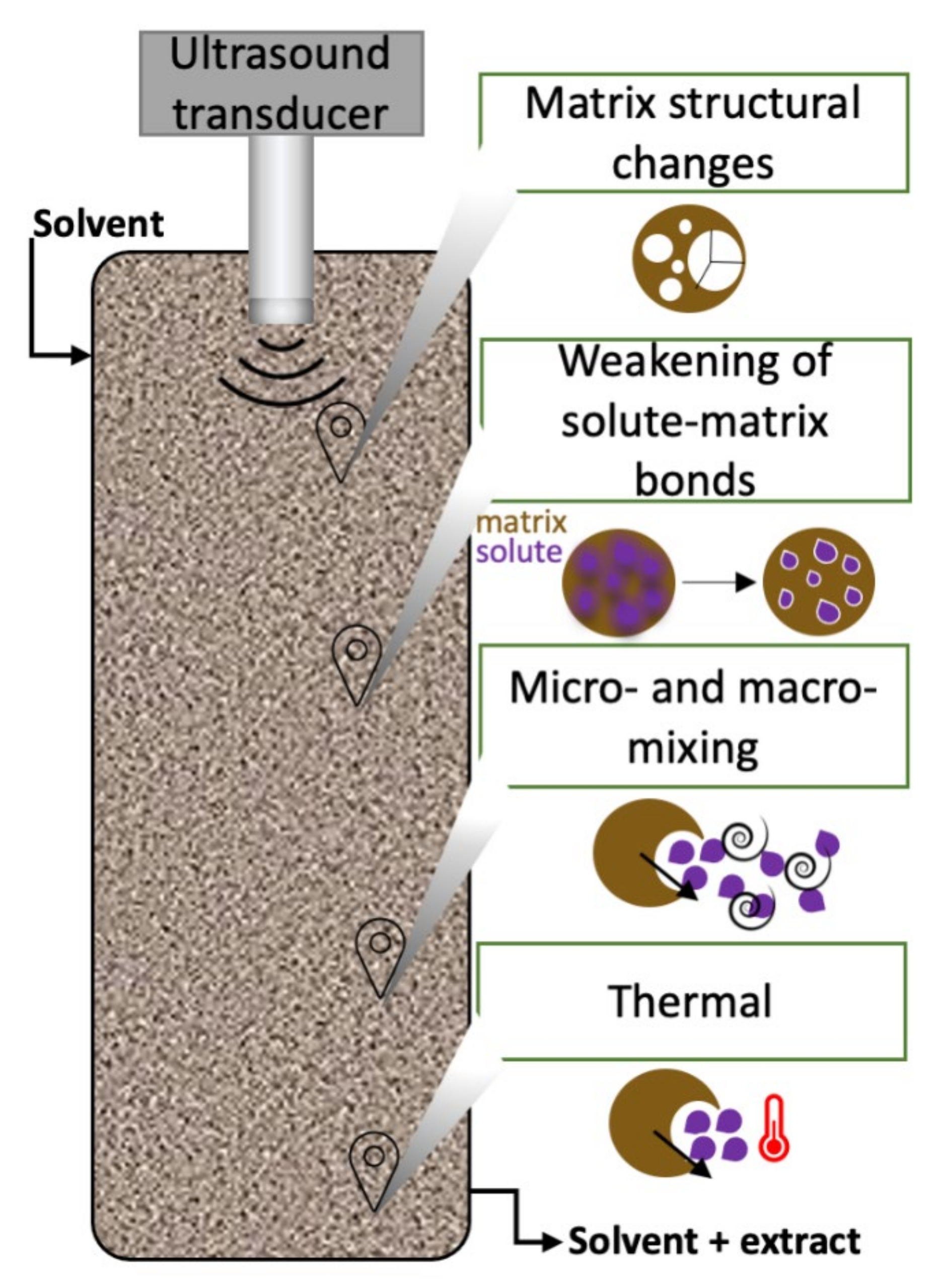
2.1. Low-Frequency Ultrasound Technology
2.2. High-Pressure Technologies
2.2.1. Supercritical Fluid Extraction (SFE)
2.2.2. Pressurized Liquid Extraction (PLE)
2.2.3. Gas-Expanded Liquids (GXLs) Extraction
2.3. Low-Frequency Ultrasound Coupled with High-Pressure Technologies
2.3.1. Low-Frequency Ultrasound Coupled with SFE
2.3.2. Low-Frequency Ultrasound Coupled with PLE
3. Innovative Processes for the Extraction of Phytochemical Compounds
3.1. Low-Frequency Ultrasound as a Single Operation
3.2. Low-Frequency Ultrasound Coupled with High-Pressure Technologies
4. Low-Frequency Ultrasound in the Industrial Scale
5. Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sharifzadeh, Z.; Berijani, K.; Morsali, A. High performance of ultrasonic-assisted synthesis of two spherical polymers for enantioselective catalysis. Ultrason. Sonochem. 2021, 73, 105499. [Google Scholar] [CrossRef] [PubMed]
- Cherifi, Z.; Boukoussa, B.; Zaoui, A.; Belbachir, M.; Meghabar, R. Structural, morphological and thermal properties of nanocomposites poly(GMA)/clay prepared by ultrasound and in-situ polymerization. Ultrason. Sonochem. 2018, 48, 188–198. [Google Scholar] [CrossRef]
- Leães, Y.S.V.; Pinton, M.B.; Rosa, C.T.D.A.; Robalo, S.S.; Wagner, R.; de Menezes, C.R.; Barin, J.S.; Campagnol, P.C.B.; Cichoski, A.J. Ultrasound and basic electrolyzed water: A green approach to reduce the technological defects caused by NaCl reduction in meat emulsions. Ultrason. Sonochem. 2019, 61, 104830. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Lopez, L.M.; Garcia-Galicia, I.A.; Tirado-Gallegos, J.M.; Sanchez-Vega, R.; Huerta-Jimenez, M.; Ashokkumar, M.; Alarcon-Rojo, A.D. Recent advances in the application of ultrasound in dairy products: Effect on functional, physical, chemical, microbiological and sensory properties. Ultrason. Sonochem. 2021, 73, 105467. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Costa, A.L.R.; Rodrigues, P.D.; Castro, R.J.S.; Silva, E.K. Sonoprocessing of freshly squeezed orange juice: Ascorbic acid content, pectin methylesterase activity, rheological properties and cloud stability. Food Control. 2021, 131, 108391. [Google Scholar] [CrossRef]
- Costa, J.M.; Neto, A.F.D.A. Ultrasound-assisted electrodeposition and synthesis of alloys and composite materials: A review. Ultrason. Sonochem. 2020, 68, 105193. [Google Scholar] [CrossRef]
- Yuan, D.; Shao, S.; Guo, C.; Jiang, F.; Wang, J. Grain refining of Ti-6Al-4V alloy fabricated by laser and wire additive manufacturing assisted with ultrasonic vibration. Ultrason. Sonochem. 2021, 73, 105472. [Google Scholar] [CrossRef]
- Huerta, R.R.; Silva, E.K.; Ekaette, I.; El-Bialy, T.; Saldaña, M.D.A. High-intensity ultrasound-assisted formation of cellulose nanofiber scaffold with low and high lignin content and their cytocompatibility with gingival fibroblast cells. Ultrason. Sonochem. 2019, 64, 104759. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V.B.; Gedanken, A. Sonochemical synthesis of carbon dots, mechanism, effect of parameters, and catalytic, energy, biomedical and tissue engineering applications. Ultrason. Sonochem. 2020, 64, 105009. [Google Scholar] [CrossRef]
- Yan, Y.; Manickam, S.; Lester, E.; Wu, T.; Pang, C.H. Synthesis of graphene oxide and graphene quantum dots from miscanthus via ultrasound-assisted mechano-chemical cracking method. Ultrason. Sonochem. 2021, 73, 105519. [Google Scholar] [CrossRef]
- Silva, E.K.; Azevedo, V.M.; Cunha, R.L.; Hubinger, M.D.; Meireles, M.A.A. Ultrasound-assisted encapsulation of annatto seed oil: Whey protein isolate versus modified starch. Food Hydrocoll. 2016, 56, 71–83. [Google Scholar] [CrossRef]
- Huerta, R.R.; Silva, E.K.; El-Bialy, T.; Saldaña, M.D.A. Clove essential oil emulsion-filled cellulose nanofiber hydrogel produced by high-intensity ultrasound technology for tissue engineering applications. Ultrason. Sonochem. 2019, 64, 104845. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT 2018, 101, 342–350. [Google Scholar] [CrossRef]
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Food Res. Int. 2018, 119, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Picó, Y. Ultrasound-assisted extraction for food and environmental samples. TrAC Trends Anal. Chem. 2013, 43, 84–99. [Google Scholar] [CrossRef]
- Martínez, J.; de Aguiar, A.C.; Machado, A.P.d.F.; Barrales, F.M.; Viganó, J.; dos Santos, P. 2.51—Process Integration and Intensification. In Comprehensive Foodomics; Cifuentes, A., Ed.; Elsevier: Oxford, UK, 2021; pp. 786–807. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2020, 70, 105325. [Google Scholar] [CrossRef]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-Assisted Extraction of Natural Products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Silva, E.K.; Saldaña, M.D.A. High-intensity ultrasound-assisted recovery of cinnamyl alcohol glycosides from Rhodiola rosea roots: Effect of probe diameter on the ultrasound energy performance for the extraction of bioactive compounds. Food Bioprod. Process. 2020, 122, 245–253. [Google Scholar] [CrossRef]
- Neves, M.I.L.; Strieder, M.M.; Vardanega, R.; Silva, E.K.; Meireles, M.A.A. Biorefinery of turmeric (Curcuma longa L.) using non-thermal and clean emerging technologies: An update on the curcumin recovery step. RSC Adv. 2019, 10, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, D.; B.N., R.; Gogate, P.R.; Rathod, V.K. Extraction of vanillin from vanilla pods: A comparison study of conventional soxhlet and ultrasound assisted extraction. J. Food Eng. 2009, 93, 421–426. [Google Scholar] [CrossRef]
- Tarone, A.G.; Silva, E.K.; Barros, H.D.D.F.Q.; Cazarin, C.B.B.; Junior, M.R.M. High-intensity ultrasound-assisted recovery of anthocyanins from jabuticaba by-products using green solvents: Effects of ultrasound intensity and solvent composition on the extraction of phenolic compounds. Food Res. Int. 2021, 140, 110048. [Google Scholar] [CrossRef] [PubMed]
- Gómez-López, V.M.; Pataro, G.; Tiwari, B.; Gozzi, M.; Meireles, M.A.; Wang, S.; Guamis, B.; Pan, Z.; Ramaswamy, H.; Sastry, S.; et al. Guidelines on reporting treatment conditions for emerging technologies in food processing. Crit. Rev. Food Sci. Nutr. 2021, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Dassoff, E.S.; Li, Y.O. Mechanisms and effects of ultrasound-assisted supercritical CO2 extraction. Trends Food Sci. Technol. 2019, 86, 492–501. [Google Scholar] [CrossRef]
- Brunner, G. Applications of Supercritical Fluids. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 321–342. [Google Scholar] [CrossRef]
- Mezzomo, N.; Mileo, B.R.; Friedrich, M.T.; Martínez, J.; Ferreira, S.R.S. Supercritical fluid extraction of peach (Prunus persica) almond oil: Process yield and extract composition. Bioresour. Technol. 2010, 101, 5622–5632. [Google Scholar] [CrossRef]
- Brunner, G. Supercritical fluids: Technology and application to food processing. J. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- Almeida, A.P.; Rodríguez-Rojo, S.; Serra, A.T.; Vila-Real, H.; Simplício, A.L.; Delgadilho, I.; da Costa, S.B.; da Costa, L.B.; Nogueira, I.D.; Duarte, C.M.M. Microencapsulation of oregano essential oil in starch-based materials using supercritical fluid technology. Innov. Food Sci. Emerg. Technol. 2013, 20, 140–145. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Zabot, G.L.; Moraes, M.N.; Meireles, M.A.A. Influence of the bed geometry on the kinetics of rosemary compounds extraction with supercritical CO2. J. Supercrit. Fluids 2014, 94, 234–244. [Google Scholar] [CrossRef]
- Aguiar, A.C.; Visentainer, J.V.; Martínez, J. Extraction from striped weakfish (Cynoscion striatus) wastes with pressurized CO2: Global yield, composition, kinetics and cost estimation. J. Supercrit. Fluids 2012, 71, 1–10. [Google Scholar] [CrossRef]
- Viganó, J.; Machado, A.P.D.F.; Martínez, J. Sub- and supercritical fluid technology applied to food waste processing. J. Supercrit. Fluids 2015, 96, 272–286. [Google Scholar] [CrossRef]
- Viganó, J.; Coutinho, J.P.; Souza, D.S.; Baroni, N.A.F.; Godoy, H.T.; Macedo, J.A.; Martínez, J. Exploring the selectivity of supercritical CO2 to obtain nonpolar fractions of passion fruit bagasse extracts. J. Supercrit. Fluids 2016, 110, 1–10. [Google Scholar] [CrossRef]
- Santos, L.C.; Johner, J.C.F.; Scopel, E.; Pontes, P.V.A.; Ribeiro, A.F.B.; Zabot, G.L.; Batista, E.A.C.; Meireles, M.A.A.; Martínez, J. Integrated supercritical CO2 extraction and fractionation of passion fruit (Passiflora edulis Sims) by-products. J. Supercrit. Fluids 2020, 168, 105093. [Google Scholar] [CrossRef]
- Meireles, M.A.A. Extraction of Bioactive Compounds from Latin American Plants. In Supercritical Fluid Extraction of Nutraceuti-cals and Bioactive Compounds; CRC Press: Boca Raton, FL, USA, 2007; pp. 243–274. [Google Scholar] [CrossRef]
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Revilla-Ruiz, P.; Hernández-Méndez, J. Pressurized liquid extraction in the analysis of food and biological samples. J. Chromatogr. A 2005, 1089, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ponce, M.T.; Parjikolaei, B.R.; Lari, H.N.; Casas, L.; Mantell, C.; de la Ossa, E.J.M. Pilot-plant scale extraction of phenolic compounds from mango leaves using different green techniques: Kinetic and scale up study. Chem. Eng. J. 2016, 299, 420–430. [Google Scholar] [CrossRef]
- Ferro, D.M.; Mayer, D.A.; Müller, C.M.O.; Ferreira, S.R.S. Scale-up simulation of PLE process applied to recover bio-based materials from Sida rhombifolia leaves. J. Supercrit. Fluids 2020, 166, 105033. [Google Scholar] [CrossRef]
- Henry, M.C.; Yonker, C.R. Supercritical Fluid Chromatography, Pressurized Liquid Extraction, and Supercritical Fluid Extraction. Anal. Chem. 2006, 78, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mendoza, M.d.P.; Espinosa-Pardo, F.A.; Baseggio, A.M.; Barbero, G.F.; Maróstica Junior, M.R.; Rostagno, M.A.; Martínez, J. Extraction of phenolic compounds and anthocyanins from juçara (Euterpe edulis Mart.) residues using pressurized liquids and supercritical fluids. J. Supercrit. Fluids 2017, 119, 9–16. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Tarone, A.G.; Cazarin, C.B.B.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from grape marc. J. Food Eng. 2018, 240, 105–113. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. TrAC Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Souza, M.C.; Silva, L.C.; Chaves, J.O.; Salvador, M.P.; Sanches, V.L.; da Cunha, D.T.; Carneiro, T.F.; Rostagno, M.A. Simultaneous extraction and separation of compounds from mate (Ilex paraguariensis) leaves by pressurized liquid extraction coupled with solid-phase extraction and in-line UV detection. Food Chem. Mol. Sci. 2021, 2, 100008. [Google Scholar] [CrossRef]
- Silva, L.C.; Souza, M.C.; Sumere, B.R.; Silva, L.G.S.; da Cunha, D.T.; Barbero, G.F.; Bezerra, R.M.N.; Rostagno, M.A. Simultaneous extraction and separation of bioactive compounds from apple pomace using pressurized liquids coupled on-line with solid-phase extraction. Food Chem. 2020, 318, 126450. [Google Scholar] [CrossRef]
- Strieder, M.M.; Neves, M.I.L.; Silva, E.K.; Meireles, M.A.A. Low-frequency and high-power ultrasound-assisted production of natural blue colorant from the milk and unripe Genipa americana L. Ultrason. Sonochem. 2020, 66, 105068. [Google Scholar] [CrossRef] [PubMed]
- Turner, C. From supercritical carbon dioxide to gas expanded liquids in extraction and chromatography of lipids. Lipid Technol. 2015, 27, 275–277. [Google Scholar] [CrossRef]
- Herrero, M.; Castro-Puyana, M.; Mendiola, J.A.; Ibañez, E. Compressed fluids for the extraction of bioactive compounds. TrAC Trends Anal. Chem. 2013, 43, 67–83. [Google Scholar] [CrossRef]
- Herrero, M.; Ibañez, E. Green extraction processes, biorefineries and sustainability: Recovery of high added-value products from natural sources. J. Supercrit. Fluids 2018, 134, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Camargo, A.D.P.; Mendiola, J.A.; Ibáñez, E. CHAPTER 17. Gas Expanded-Liquids. In Supercritical and Other High-Pressure Solvent Systems; Royal Society of Chemistry: London, UK, 2018; pp. 512–531. [Google Scholar]
- Jessop, P.G.; Subramaniam, B. Gas-Expanded Liquids. Chem. Rev. 2007, 107, 2666–2694. [Google Scholar] [CrossRef]
- Sakai, H.; Ono, K.; Tokunaga, S.; Sharmin, T.; Aida, T.M.; Mishima, K. Extraction of Natural Pigments from Gardenia Jasminoides J. Ellis Fruit Pulp Using CO2-Expanded Liquids and Direct Sonication. Separations 2020, 8, 1. [Google Scholar] [CrossRef]
- Golmakani, M.-T.; Mendiola, J.A.; Rezaei, K.; Ibáñez, E. Expanded ethanol with CO2 and pressurized ethyl lactate to obtain fractions enriched in γ-Linolenic Acid from Arthrospira platensis (Spirulina). J. Supercrit. Fluids 2012, 62, 109–115. [Google Scholar] [CrossRef]
- Reyes, F.A.; Mendiola, J.A.; Ibañez, E.; del Valle, J.M. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Viganó, J.; Assis, B.F.D.P.; Náthia-Neves, G.; dos Santos, P.; Meireles, M.A.A.; Veggi, P.C.; Martínez, J. Extraction of bioactive compounds from defatted passion fruit bagasse (Passiflora edulis sp.) applying pressurized liquids assisted by ultrasound. Ultrason. Sonochem. 2020, 64, 104999. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.T.V.; Zabot, G.L.; Reyes, F.G.R.; Iglesias, A.H.; Martínez, J. Integration of pressurized liquids and ultrasound in the extraction of bioactive compounds from passion fruit rinds: Impact on phenolic yield, extraction kinetics and technical-economic evaluation. Innov. Food Sci. Emerg. Technol. 2020, 67, 102549. [Google Scholar] [CrossRef]
- Sumere, B.R.; Souza, M.C.d.; Santos, M.P.d.; Bezerra, R.M.N.; Cunha, D.T.d.; Martinez, J.; Rostagno, M.A. Combining pres-surized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.). Ultrason. Sonochem. 2018, 48, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.P.; Souza, M.C.; Sumere, B.R.; da Silva, L.C.; Cunha, D.T.; Bezerra, R.M.N.; Rostagno, M.A. Extraction of bioactive compounds from pomegranate peel (Punica granatum L.) with pressurized liquids assisted by ultrasound combined with an expansion gas. Ultrason. Sonochem. 2019, 54, 11–17. [Google Scholar] [CrossRef]
- Arruda, H.S.; Silva, E.K.; Pereira, G.A.; Angolini, C.F.F.; Eberlin, M.N.; Meireles, M.A.A.; Pastore, G.M. Effects of high-intensity ultrasound process parameters on the phenolic compounds recovery from araticum peel. Ultrason. Sonochem. 2018, 50, 82–95. [Google Scholar] [CrossRef]
- Araujo, N.M.P.; Silva, E.K.; Arruda, H.S.; de Morais, D.R.; Meireles, M.A.A.; Pereira, G.A.; Pastore, G.M. Recovering phenolic compounds from Eugenia calycina Cambess employing high-intensity ultrasound treatments: A comparison among its leaves, fruit pulp, and seed as promising sources of bioactive compounds. Sep. Purif. Technol. 2021, 272, 118920. [Google Scholar] [CrossRef]
- Varaee, M.; Honarvar, M.; Eikani, M.H.; Omidkhah, M.R.; Moraki, N. Supercritical fluid extraction of free amino acids from sugar beet and sugar cane molasses. J. Supercrit. Fluids 2018, 144, 48–55. [Google Scholar] [CrossRef]
- Saldaña, M.D.A.; Martinez, E.R.; Sekhon, J.K.; Vo, H. The effect of different pressurized fluids on the extraction of anthocyanins and total phenolics from cranberry pomace. J. Supercrit. Fluids 2021, 175, 105279. [Google Scholar] [CrossRef]
- Limsangouan, N.; Charunuch, C.; Sastry, S.K.; Srichamnong, W.; Jittanit, W. High pressure processing of tamarind (Tamarindus indica) seed for xyloglucan extraction. LWT 2020, 134, 110112. [Google Scholar] [CrossRef]
- Jesus, A.L.T.; Cristianini, M.; dos Santos, N.M.; Júnior, M.R.M. Effects of high hydrostatic pressure on the microbial inactivation and extraction of bioactive compounds from açaí (Euterpe oleracea Martius) pulp. Food Res. Int. 2019, 130, 108856. [Google Scholar] [CrossRef] [PubMed]
- Gagneten, M.; Leiva, G.; Salvatori, D.; Schebor, C.; Olaiz, N. Optimization of Pulsed Electric Field Treatment for the Extraction of Bioactive Compounds from Blackcurrant. Food Bioprocess Technol. 2019, 12, 1102–1109. [Google Scholar] [CrossRef]
- Redondo, D.; Venturini, M.E.; Luengo, E.; Raso, J.; Arias, E. Pulsed electric fields as a green technology for the extraction of bioactive compounds from thinned peach by-products. Innov. Food Sci. Emerg. Technol. 2018, 45, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, H.; Du, W.; Qian, L.; Xu, Y.; Huang, Y.; Xiong, Q.; Li, H.; Yuan, J. Comparison of different extraction methods for polysaccharides from Crataegus pinnatifida Bunge. Int. J. Biol. Macromol. 2019, 150, 1011–1019. [Google Scholar] [CrossRef]
- Ismail, B.; Guo, M.; Pu, Y.; Wang, W.; Ye, X.; Liu, D. Valorisation of baobab (Adansonia digitata) seeds by ultrasound assisted extraction of polyphenolics. Optimisation and comparison with conventional methods. Ultrason. Sonochem. 2018, 52, 257–267. [Google Scholar] [CrossRef]
- Muhammad, D.R.A.; Tuenter, E.; Patria, G.D.; Foubert, K.; Pieters, L.; Dewettinck, K. Phytochemical composition and anti-oxidant activity of Cinnamomum burmannii Blume extracts and their potential application in white chocolate. Food Chem. 2021, 340, 127983. [Google Scholar] [CrossRef]
- Vázquez-González, Y.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M. Characterization and antifungal activity of jackfruit (Artocarpus heterophyllus Lam.) leaf extract obtained using conventional and emerging technologies. Food Chem. 2020, 330, 127211. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-F.; Zhu, L.-F.; Wu, Z.; Wang, G.; Ahmad, Z.; Chang, M.-W. Production of triterpenoid compounds from Ganoderma lucidum spore powder using ultrasound-assisted extraction. Prep. Biochem. Biotechnol. 2020, 50, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Bao, P.; Zhang, S.; Liu, A.; Shen, L.; Yu, R.; Liu, Y.; Chen, M.; Li, J.; Wu, X.; et al. Extraction and characterization of extracellular polymeric substances from a mixed fungal culture during the adaptation process with waste printed circuit boards. Environ. Sci. Pollut. Res. 2019, 26, 22137–22146. [Google Scholar] [CrossRef] [PubMed]
- Sallet, D.; Souza, P.O.; Fischer, L.T.; Ugalde, G.A.; Zabot, G.L.; Mazutti, M.A.; Kuhn, R.C. Ultrasound-assisted extraction of lipids from Mortierella isabellina. J. Food Eng. 2018, 242, 1–7. [Google Scholar] [CrossRef]
- Hernández-Santos, B.; Rodriguez-Miranda, J.; Herman-Lara, E.; Torruco-Uco, J.G.; Carmona-García, R.; Juárez-Barrientos, J.M.; Chávez-Zamudio, R.; Martínez-Sánchez, C.E. Effect of oil extraction assisted by ultrasound on the physicochemical properties and fatty acid profile of pumpkin seed oil (Cucurbita pepo). Ultrason. Sonochem. 2016, 31, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Pavlić, B.; Teslić, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.K.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef]
- Fabrowska, J.; Messyasz, B.; Szyling, J.; Walkowiak, J.; Leska, B. Isolation of chlorophylls and carotenoids from freshwater algae using different extraction methods. Phycol. Res. 2017, 66, 52–57. [Google Scholar] [CrossRef]
- Madhavamenon, K.I.; Maliakel, B.P. Ultrasound-Assisted High Throughput Continuous Extraction for Complete Fragmentation of Cocoabeans into Valuable Fractions and Their Formulations Thereof. U.S. Patent 2015/0196610, A1, 20 June 2017. [Google Scholar]
- Cares-Pacheco, M.G.; Gaete-Garreton, L.F.J.; Vargas-Hernández, Y.D.P.S.; Alarcon-Camacho, J.G.; Sainz-Lobo, J.I. Device and Method for Extracting Active Principles from Natural Sources, Using a Counter-Flow Extractor Assisted by a Sound Trans-duction System. WO Patent 2011/079404, 7 July 2011. [Google Scholar]
- Santos, P.; Aguiar, A.C.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Supercritical carbon dioxide extraction of capsaicinoids from malaguetapepper (Capsicum frutescens L.) assisted by ultrasound. Ultrason. Sonochem. 2015, 22, 78–88. [Google Scholar] [CrossRef]
- Reyes-Escogido, M.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and pharmacological aspects of capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef] [Green Version]
- Santos-Zea, L.; Antunes-Ricardo, M.; Gutierrez-Uribe, J.A.; García-Pérez, J.V.; Benedito, J. Effect of ultrasound transducer design on the acoustically-assisted supercritical fluid extraction of antioxidants from oregano. Ultrason. Sonochem. 2018, 47, 47–56. [Google Scholar] [CrossRef]
- Dias, A.L.B.; Sergio, C.S.A.; Santos, P.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Effect of ultrasound on the supercritical CO2 extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L. var. pendulum). Ultrason. Sonochem. 2016, 31, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, H.; Mishima, K.; Sharmin, T.; Ito, S.; Kawakami, R.; Kato, T.; Misumi, M.; Suetsugu, T.; Orii, H.; Kawano, H.; et al. Ultrasonically enhanced extraction of luteolin and apigenin from the leaves of Perilla frutescens (L.) Britt. Using liquid carbon dioxide and ethanol. Ultrason. Sonochem. 2015, 29, 19–26. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Ou, H.; Gregersen, H. Ultrasound-assisted supercritical CO2 extraction of cucurbitacin E from Iberis amara seeds. Ind. Crop. Prod. 2020, 145, 112093. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Barrales, F.M.; Martinez, J.; Filho, R.M. Supercritical CO2 Extraction of Lipophilic Molecules from Sugar-cane Straw. Chem. Eng. Trans. 2020, 80, 313–318. [Google Scholar]
- Tonato, D.; Luft, L.; Confortin, T.C.; Zabot, G.L.; Mazutti, M.A. Enhancement of fatty acids in the oil extracted from the fungus Nigrospora sp. by supercritical CO2 with ethanol as a cosolvent. J. Supercrit. Fluids 2019, 146, 180–188. [Google Scholar] [CrossRef]
- Fan, X.-D.; Hou, Y.; Huang, X.-X.; Qiu, T.-Q.; Jiang, J.-G. Ultrasound-Enhanced Subcritical CO2 Extraction of Lutein from Chlorella pyrenoidosa. J. Agric. Food Chem. 2015, 63, 4597–4605. [Google Scholar] [CrossRef]
- Donadone, D.B.D.S.; Giombelli, C.; Silva, D.L.G.; Stevanato, N.; Da Silva, C.; Barros, B.C.B. Ultrasound-assisted extraction of phenolic compounds and soluble sugars from the stem portion of peach palm. J. Food Process. Preserv. 2020, 44, e14636. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar] [CrossRef]
- Bates, D.M.; McLoughlin, A.R.; Yap, A.S.J. High Energy Ultrasound Extraction Method and Apparatus. U.S. Patent 008343562B2; Application granted: 2013-01-01, 21 January 2010. [Google Scholar]
- Mauricio, A.R. Integrated System of Analysis for the Determination of Chemicals and Use. BR Patent 102015030939A2, 10 December 2015. [Google Scholar]
- Hielscher. Hielscher Ultrasonics GmbH. Available online: https://www.hielscher.com/ (accessed on 20 September 2020).
- Reus. Ultrasounds Serving Nature. Available online: http://www.etsreus.com/ (accessed on 19 September 2020).
- Sonics. Ultrasonic Processor for Large Volume and Industrial Applications. Available online: https://www.sonics.com/ (accessed on 15 September 2020).
- Duas-Rodas. Flavors & Botanicals. Available online: https://www.duasrodas.com/ (accessed on 20 September 2020).
- GMC. Herb Extraction Ultrasound. Available online: https://www.gmariani.it/ (accessed on 14 September 2020).
- Khadhraoui, B.; Ummat, V.; Tiwari, B.K.; Fabiano-Tixier, A.S.; Chemat, F. Review of ultrasound combinations with hybrid and innovative techniques for extraction and processing of food and natural products. Ultrason. Sonochem. 2021, 76, 105625. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.L.B.; de Aguiar, A.C.; Rostagno, M.A. Extraction of natural products using supercritical fluids and pressurized liquids assisted by ultrasound: Current status and trends. Ultrason. Sonochem. 2021, 74, 105584. [Google Scholar] [CrossRef] [PubMed]
| Target Compounds | Yield | Biomass | Temperature (°C) | Frequency (kHz) | Solvent | S/F (mL/g) | Processing Time (min) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Flavonoids | 14.4 mg/g oleoresin | Cinnamomum burmannii | 30 | 80 | Water | 10 | 60 | [74] |
| Flavonoids | 29.8 mg/g oleoresin | Cinnamomum burmannii | 30 | 80 | Ethyl alcohol | 10 | 60 | [74] |
| Total soluble phenols | 155 mg/g leaves | Artocarpus heterophyllus Lam. | 10 | 42 | Ethyl alcohol: water (80:20, v/v) | 10 | 30 | [75] |
| Triterpenoids | 14.3 mg/g powder | Ganoderma lucidum spore powder | 60 | - | Ethyl alcohol: water (95:5, v/v) | 50 | 10 | [76] |
| Exopoly-saccharides | 46.7 mg/g fungal mycelium | Purpureocillium lilacinum and Aspergillus niger | - | - | Water | - | 10 | [77] |
| Fatty acids | 195 mg/g fungal biomass | Mortierella isabellina | 10 | 24 | Chloroform: methanol: water (2:1:0.8, v/v/v) | 40 | 30 | [78] |
| Fatty acids | 625 mg/g seeds | Cucurbita pepo | - | 20 | Hexane | 10 | 5 | [79] |
| Terpenoids | 210 mg/g extract | Mentha piperita L. | 50 | 40 | Methylene chloride | 10 | 40 | [80] |
| Carotenoids | 2.2 ± 0.1 mg/L algae extract | Ulva flexuosa | 40 | - | Ethyl alcohol: water (7:3, v/v) | 25 | 60 | [81] |
| Target Compounds | Yield | Biomass | Integrated Technology | Temperature (°C) | Frequency (kHz) | Nominal Power (W) | Solvent | S/F (g/g) | Processing Time (min) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic compounds | 43.3 ± 0.8 mg GAE/g dry peels | Punica granatum L. | Pressurized liquid extraction—10 MPa | 70 | 19 | 400 | Water | - | 10 | [62] |
| Phenolic compounds | 6.5 mg GAE/g dry leaves | Origanum vulgare L. | Supercritical fluid extraction—35 MPa | 35 | 30 | 60 | CO2: ethyl alcohol (97.7:2.3, w/w) | 5.5 | 60 | [86] |
| Capsaicinoids | 1.5 ± 0.3 mg/g dry fruits | Capsicum baccatum L. var. pendulum | Supercritical fluid extraction—25 MPa | 40 | - | 200 | CO2 | 484 | 40 | [87] |
| Luteolin | 0.06 mg/g dry leaves | Perilla frutescens L. | Liquid CO2 extraction—10 MPa | 25 | 20 | 188 | CO2: ethyl alcohol (84.2:15.8, w/w) | - | 2.1 | [88] |
| Cucurbitacin | 7.34 ± 0.05 mg/g dry seeds | Iberis amara | Supercritical fluid extraction—25 MPa | 55 | 40 | 200 | CO2: ethyl alcohol (88:12, w/w) | 30 | 60 | [89] |
| Lipophilic molecules | 9 ± 1 mg/g dry straw | Saccharum officinarum L. | Supercritical fluid extraction—25 MPa | 60 | - | 800 | CO2 | 126 | 120 | [90] |
| Fatty acids | 455 mg/g oil (39.4 mg oil/g fungal biomass) | Nigrospora sp. | Supercritical fluid extraction—25 MPa | 80 | 40 | 132 | CO2: ethyl alcohol (50:50, w/w) | 113 | 85 | [91] |
| Lutein | 1.2 mg/g algae biomass | Chlorella pyrenoidosa | Pressurized fluid extraction—25 MPa | 24 | 20 | 1000 | CO2: ethyl alcohol | - | 240 | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabot, G.L.; Viganó, J.; Silva, E.K. Low-Frequency Ultrasound Coupled with High-Pressure Technologies: Impact of Hybridized Techniques on the Recovery of Phytochemical Compounds. Molecules 2021, 26, 5117. https://doi.org/10.3390/molecules26175117
Zabot GL, Viganó J, Silva EK. Low-Frequency Ultrasound Coupled with High-Pressure Technologies: Impact of Hybridized Techniques on the Recovery of Phytochemical Compounds. Molecules. 2021; 26(17):5117. https://doi.org/10.3390/molecules26175117
Chicago/Turabian StyleZabot, Giovani Leone, Juliane Viganó, and Eric Keven Silva. 2021. "Low-Frequency Ultrasound Coupled with High-Pressure Technologies: Impact of Hybridized Techniques on the Recovery of Phytochemical Compounds" Molecules 26, no. 17: 5117. https://doi.org/10.3390/molecules26175117
APA StyleZabot, G. L., Viganó, J., & Silva, E. K. (2021). Low-Frequency Ultrasound Coupled with High-Pressure Technologies: Impact of Hybridized Techniques on the Recovery of Phytochemical Compounds. Molecules, 26(17), 5117. https://doi.org/10.3390/molecules26175117








