Photoluminescence of Ni(II), Pd(II), and Pt(II) Complexes [M(Me2dpb)Cl] Obtained from C‒H Activation of 1,5-Di(2-pyridyl)-2,4-dimethylbenzene (Me2dpbH)
Abstract
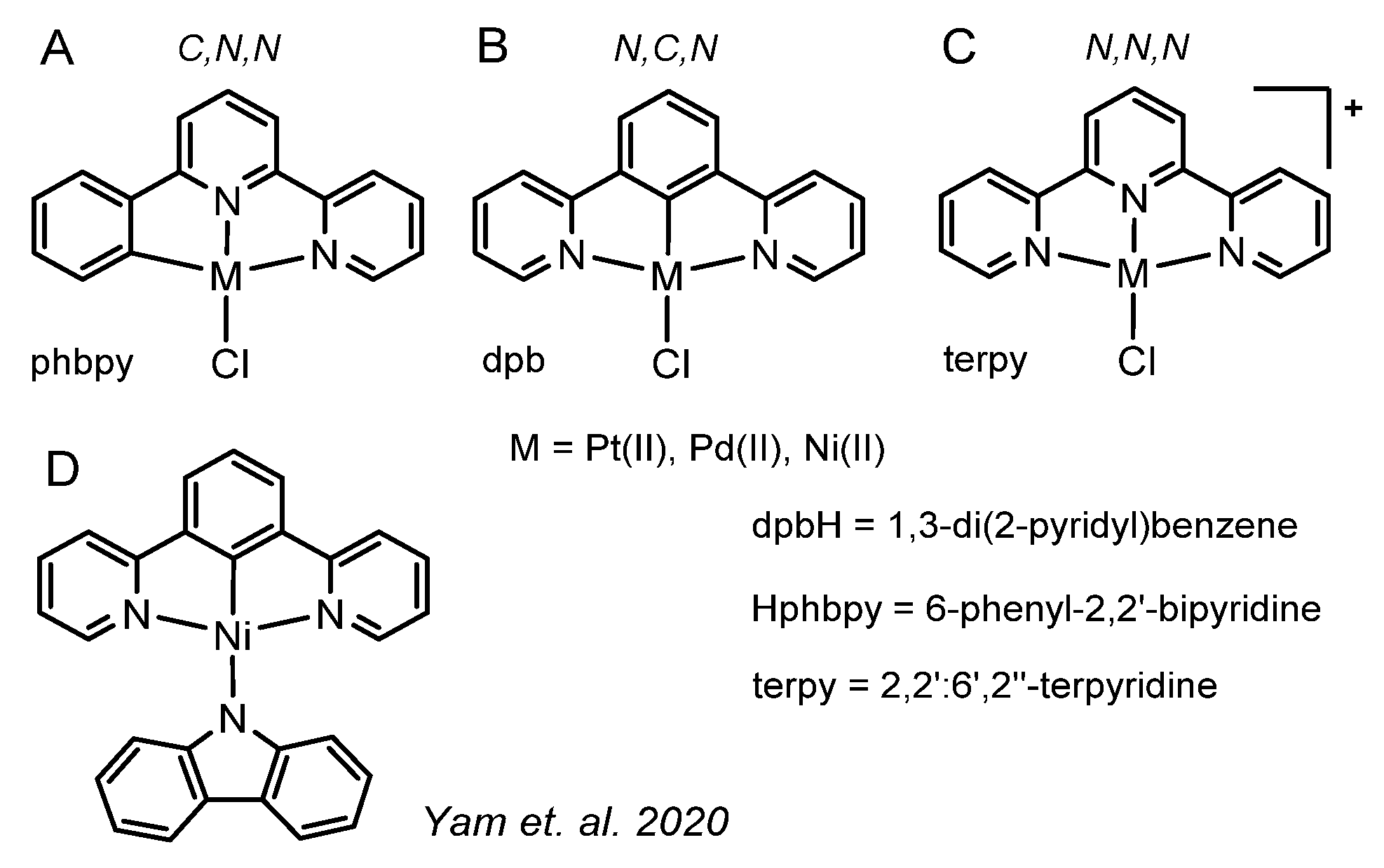
:1. Introduction
2. Results and Discussion
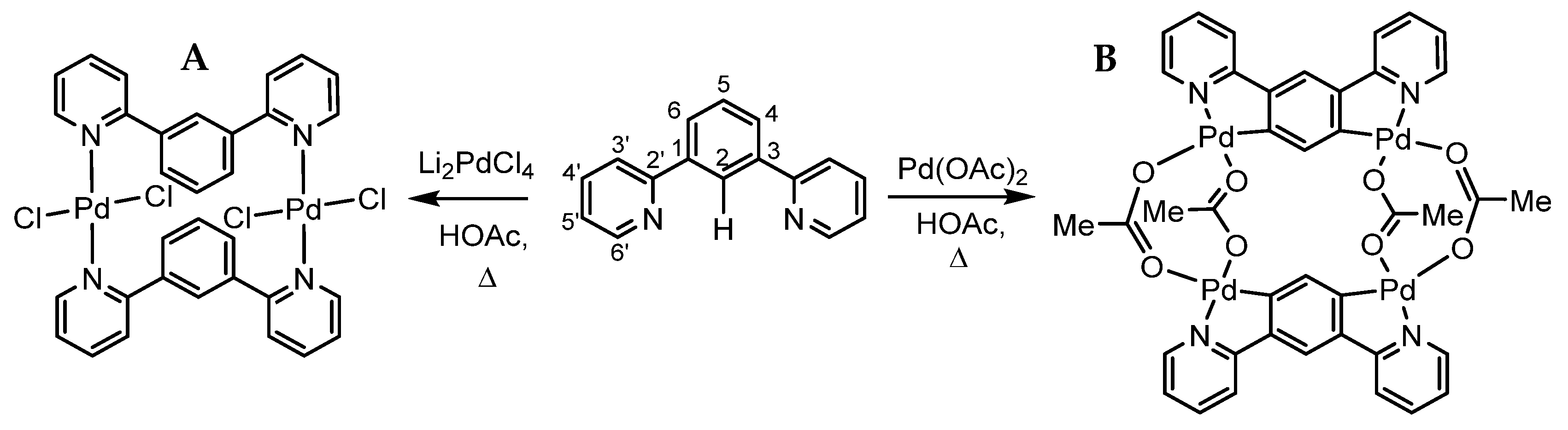
2.1. Preparation and Analytical Characterisation
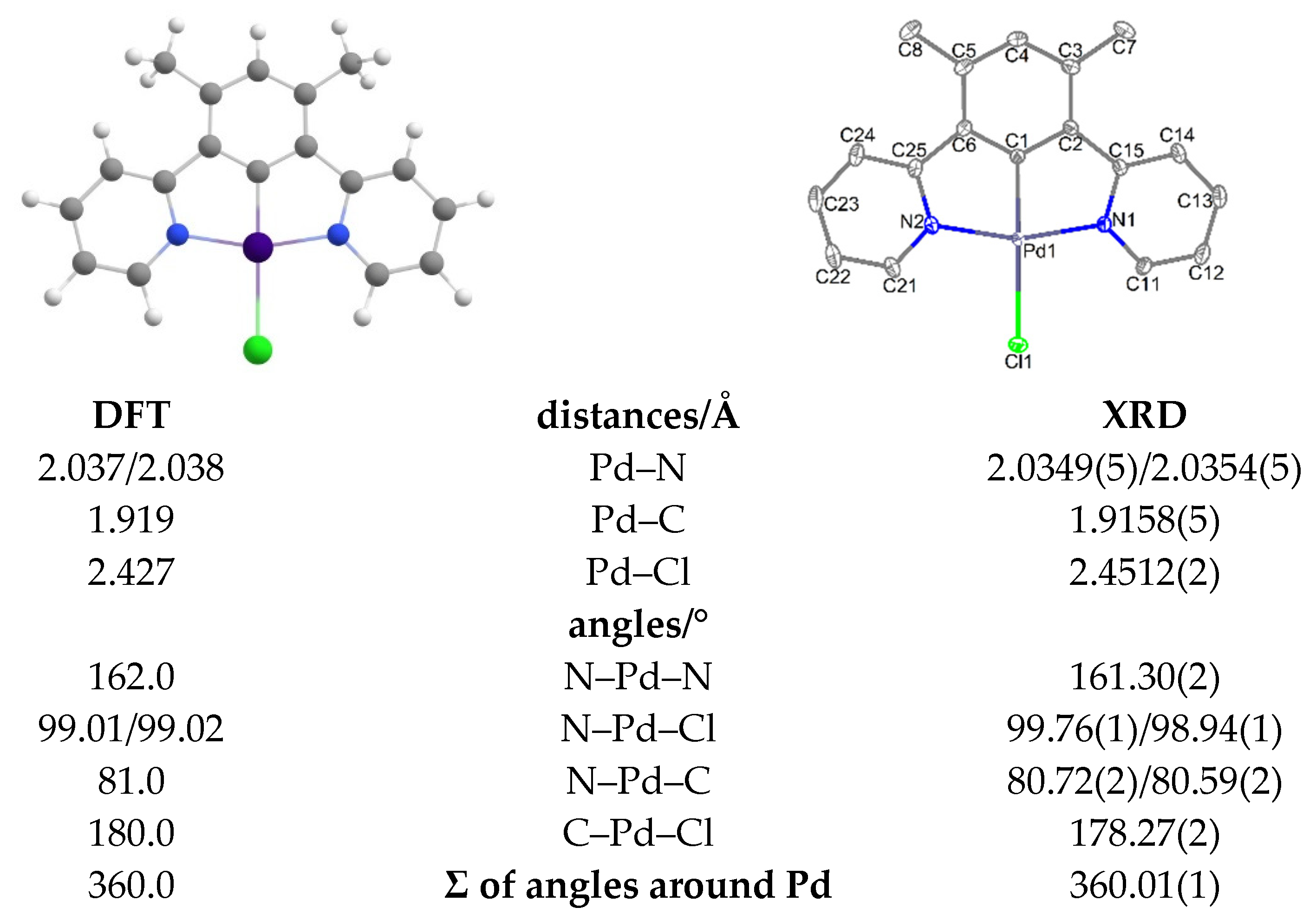
2.2. X-ray Diffractometric Analysis of Single Crystals and DFT-Calculated Structures
2.3. Electrochemistry and DFT Calculations of Frontier Orbitals
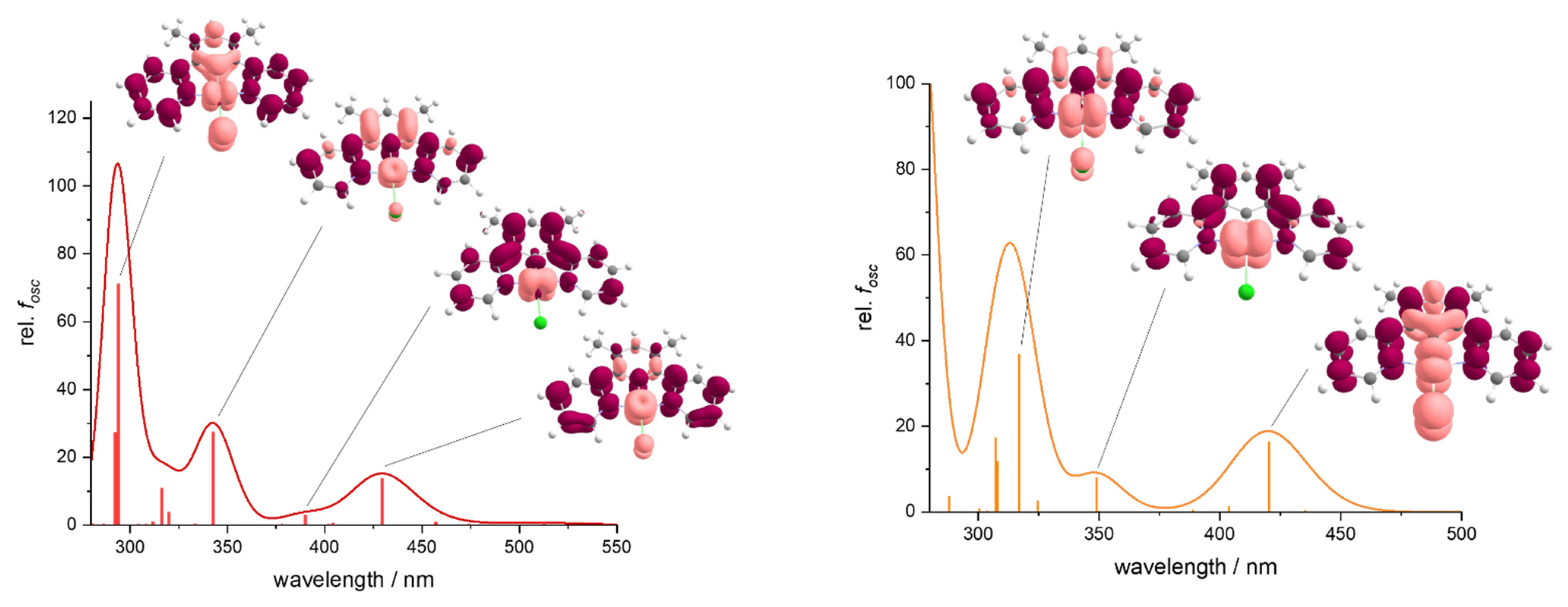
2.4. UV-Vis Absorption Spectroscopy and TD-DFT Calculated Transitions
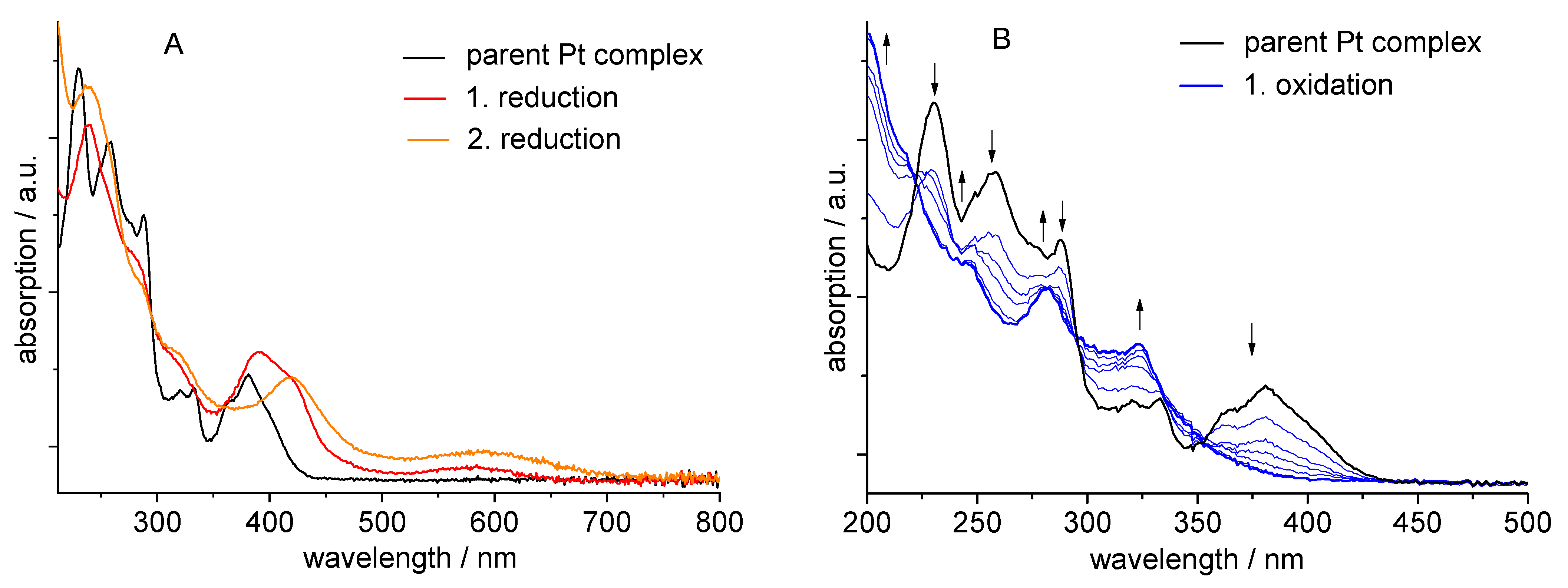
2.5. Spectroelectrochemistry
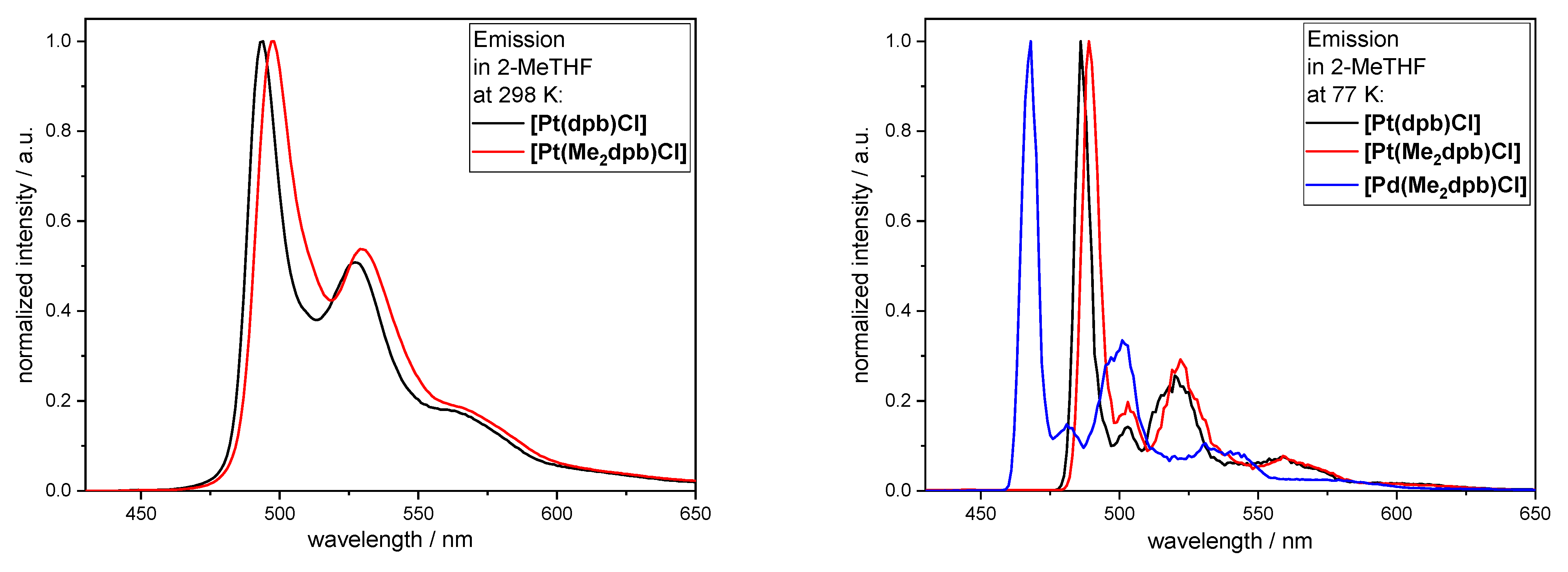
2.6. Photoluminescence Spectroscopy
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mori, K.; Yamashita, H. Metal Complexes Supported on Solid Matrices for Visible-Light-Driven Molecular Transformations. Chem. Eur. J. 2016, 22, 11122–11137. [Google Scholar] [CrossRef]
- Parasram, M.; Gevorgyan, V. Visible light-induced transition metal-catalyzed transformations: Beyond conventional photosensitizers. Chem. Soc. Rev. 2017, 46, 6227–6240. [Google Scholar] [CrossRef]
- Guerchais, V.; Fillaut, J.-L. Sensory luminescent iridium(III) and platinum(II) complexes for cation recognition. Coord. Chem. Rev. 2011, 255, 2448–2457. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, F.; Huang, C. Phosphorescent chemosensors based on heavy-metal complexes. Chem. Soc. Rev. 2010, 39, 3007–3030. [Google Scholar] [CrossRef]
- Kalinowski, J.; Fattori, V.; Cocchi, M.; Gareth Williams, J.A. Light-emitting devices based on organometallic platinum complexes as emitters. Coord. Chem. Rev. 2011, 255, 2401–2425. [Google Scholar] [CrossRef]
- Li, K.; Tong, G.S.M.; Wan, Q.; Cheng, G.; Tong, W.-Y.; Ang, W.-H.; Kwong, W.-L.; Che, C.-M. Highly phosphorescent platinum(II) emitters: Photophysics, materials and biological application. Chem. Sci. 2016, 7, 1653–1673. [Google Scholar] [CrossRef] [Green Version]
- Yam, V.W.-W.; Law, A.S.-Y. Luminescent d8 metal complexes of platinum(II) and gold(III): From photophysics to photofunctional materials and probes. Coord. Chem. Rev. 2020, 414, 213298. [Google Scholar] [CrossRef]
- Fantacci, S.; De Angelis, F. A computational approach to the electronic and optical properties of Ru(II) and Ir(III) polypyridyl complexes: Applications to DSC, OLED and NLO. Coord. Chem. Rev. 2011, 255, 2704–2726. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. Over the LEC rainbow: Colour and stability tuning of cyclometallated iridium(III) complexes in light-emitting electrochemical cells. Coord. Chem. Rev. 2017, 350, 155–177. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, Y.; Song, J.; Qu, J.; Li, B.; Zhu, W.; Wong, W.-Y. Near-Infrared Emitting Materials via Harvesting Triplet Excitons: Molecular Design, Properties, and Application in Organic Light Emitting Diodes. Adv. Opt. Mater. 2018, 6, 1800466. [Google Scholar] [CrossRef]
- Yersin, H.; Rausch, A.F.; Czerwieniec, R.; Hofbeck, T.; Fischer, T. The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs. Coord. Chem. Rev. 2011, 255, 2622–2652. [Google Scholar] [CrossRef]
- Bizzarri, C.; Spuling, E.; Knoll, D.M.; Volz, D.; Bräse, S. Sustainable metal complexes for organic light-emitting diodes (OLEDs). Coord. Chem. Rev. 2018, 373, 49–82. [Google Scholar] [CrossRef]
- Fleetham, T.; Li, G.; Li, J. Phosphorescent Pt(II) and Pd(II) Complexes for Efficient, High-Color-Quality, and Stable OLEDs. Adv. Mater. 2017, 29, 1601861. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.; Weinstein, J.A. Charge-separated excited states in platinum(II) chromophores: Photophysics, formation, stabilization and utilization in solar energy conversion. Coord. Chem. Rev. 2012, 256, 2530–2561. [Google Scholar] [CrossRef]
- Strassner, T. Phosphorescent Platinum(II) Complexes with C^C* Cyclometalated NHC Ligands. Acc. Chem. Res. 2016, 49, 2680–2689. [Google Scholar] [CrossRef]
- Cebrián, C.; Mauro, M. Recent advances in phosphorescent platinum complexes for organic light-emitting diodes. Beilstein J. Org. Chem. 2018, 14, 1459–1481. [Google Scholar] [CrossRef]
- Koshevoy, I.O.; Krause, M.; Klein, A. Non-Covalent Intramolecular Interactions through Ligand-Design Promoting Efficient Luminescence from Transition Metal Complexes. Coord. Chem. Rev. 2020, 405, 213094. [Google Scholar] [CrossRef]
- Sivchik, V.; Kochetov, A.; Eskelinen, T.; Kisel, K.S.; Solomatina, A.I.; Grachova, E.V.; Tunik, S.P.; Hirva, P.; Koshevoy, I.O. Modulation of Metallophilic and p–p Interactions in Platinum Cyclometalated Luminophores with Halogen Bonding. Chem. Eur. J. 2021, 27, 1787–1794. [Google Scholar] [CrossRef]
- Ravotto, L.; Ceroni, P. Aggregation induced phosphorescence of metal complexes: From principles to applications. Coord. Chem. Rev. 2017, 346, 62–76. [Google Scholar] [CrossRef]
- Gray, H.B.; Záliš, S.; Vlček, A. Electronic structures and photophysics of d8-d8 complexes. Coord. Chem. Rev. 2017, 345, 297–317. [Google Scholar] [CrossRef]
- Ganesan, P.; Hung, W.; Tso, J.; Ko, C.; Wang, T.; Chen, P.; Hsu, H.; Liu, S.; Lee, G.; Chou, P.; et al. Functional Pyrimidinyl Pyrazolate Pt(II) Complexes: Role of Nitrogen Atom in Tuning the Solid-State Stacking and Photophysics. Adv. Funct. Mater. 2019, 29, 1900923. [Google Scholar] [CrossRef]
- Cinninger, L.M.; Bastatas, L.D.; Shen, Y.; Holliday, B.J.; Slinker, J.D. Luminescent properties of a 3,5-diphenylpyrazole-bridged Pt(II) dimer. Dalton Trans. 2019, 48, 9684–9691. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Au, V.K.-M.; Leung, S.Y.-L. Light-Emitting Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef]
- Zou, C.; Lin, J.; Suo, S.; Xie, M.; Chang, X.; Lu, W. Palladium(II) N-heterocyclic allenylidene complexes with extended intercationic Pd···Pd interactions and MMLCT phosphorescence. Chem. Commun. 2018, 54, 5319–5322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana, M.D.; López-Banet, L.; Sánchez, G.; Pérez, J.; Pérez, E.; García, L.; Serrano, J.L.; Espinosa, A. Non-covalent stacking interactions directing the structural and photophysical features of mono- and dinuclear cyclometalated palladium(II) complexes. Dalton Trans. 2016, 45, 8601–8613. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Hussain, F.; Zeng, C.; Wang, B.; Li, Z.; Kozin, I.; Wang, S. Multiresponsive Tetradentate Phosphorescent Metal Complexes as Highly Sensitive and Robust Luminescent Oxygen Sensors: Pd(II) Versus Pt(II) and 1,2,3-Triazolyl Versus 1,2,4-Triazolyl. ACS Appl. Mater. Interfaces 2019, 11, 12666–12674. [Google Scholar] [CrossRef]
- Chow, P.-K.; Cheng, G.; Tong, G.S.-M.; Ma, C.; Kwok, W.-M.; Ang, W.-H.; Chung, C.Y.-S.; Yang, C.; Wang, F.; Che, C.-M. Highly luminescent palladium(II) complexes with sub-millisecond blue to green phosphorescent excited states. Photocatalysis and highly efficient PSF-OLEDs. Chem. Sci. 2016, 7, 6083–6098. [Google Scholar] [CrossRef] [Green Version]
- Fleetham, T.; Ji, Y.; Huang, L.; Fleetham, T.S.; Li, J. Efficient and stable single-doped white OLEDs using a palladium-based phosphorescent excimer. Chem. Sci. 2017, 8, 7983–7990. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Zou, C.; Zhang, X.; Gao, Q.; Suo, S.; Zhuo, Q.; Chang, X.; Xie, M.; Lu, W. Highly phosphorescent organopalladium(II) complexes with metal–metal-to-ligand charge-transfer excited states in fluid solutions. Dalton Trans. 2019, 48, 10417–10421. [Google Scholar] [CrossRef]
- Krause, M.; Von der Stück, R.; Brünkink, D.; Buss, S.; Doltsinis, N.L.; Strassert, C.A.; Klein, A. Platinum and palladium complexes of tridentate −C^N^N (phen-ide)-pyridine-thiazol ligands—A case study involving spectroelectrochemistry, photoluminescence spectroscopy and TD-DFT calculations. Inorg. Chim. Acta 2021, 518, 120093. [Google Scholar] [CrossRef]
- Föller, J.; Friese, D.H.; Riese, S.; Kaminski, J.M.; Metz, S.; Schmidt, D.; Würthner, F.; Lambert, C.; Marian, C.M. On the photophysical properties of IrIII, PtII, and PdII (phenylpyrazole) (phenyldipyrrin) complexes. Phys. Chem. Chem. Phys. 2020, 22, 3217–3233. [Google Scholar] [CrossRef]
- Eskelinen, T.; Buss, S.; Petrovskii, S.K.; Grachova, E.V.; Krause, M.; Klein, A.; Strassert, C.A.; Koshevoy, I.O.; Hirva, P. Photophysics and Excited State Dynamics of Cyclometalated [M(C^N^N)(CN)] (M = Ni, Pd, Pt) Complexes: A Theoretical and Experimental Study. Inorg. Chem. 2021, 60, 8777–8789. [Google Scholar] [CrossRef]
- Wong, Y.-S.; Tang, M.-C.; Ng, M.; Yam, V.W.-W. Toward the Design of Phosphorescent Emitters of Cyclometalated Earth-Abundant Nickel(II) and Their Supramolecular Study. J. Am. Chem. Soc. 2020, 142, 7638–7646. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.T.; Chi, Y.; Chung, M.-W.; Lin, C.-C. Harvesting luminescence via harnessing the photophysical properties of transition metal complexes. Coord. Chem. Rev. 2011, 255, 2653–2665. [Google Scholar] [CrossRef]
- Tong, G.S.-M.; Che, C.-M. Emissive or Nonemissive? A Theoretical Analysis of the Phosphorescence Efficiencies of Cyclometalated Platinum(II) Complexes. Chem. Eur. J. 2009, 15, 7225–7237. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Li, Y.-R.; Wang, X.; Bai, F.-Q. Theoretical investigation of N^C^N-coordinated Pt(II) and Pd(II) complexes for long-lived two-photon photodynamic therapy. Dyes Pigm. 2017, 142, 55–61. [Google Scholar] [CrossRef]
- Haque, A.; Xu, L.; Al-Balushi, R.A.; Al-Suti, M.K.; Ilmi, R.; Guo, Z.; Khan, M.S.; Wong, W.-Y.; Raithby, P.R. Cyclometallated tridentate platinum(II) arylacetylide complexes: Old wine in new bottles. Chem. Soc. Rev. 2019, 48, 5547–5563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gareth Williams, J.A. The coordination chemistry of dipyridylbenzene: N-deficient terpyridine or panacea for brightly luminescent metal complexes? Chem. Soc. Rev. 2009, 38, 1783–1801. [Google Scholar] [CrossRef]
- Garbe, S.; Krause, M.; Klimpel, A.; Neundorf, I.; Lippmann, P.; Ott, I.; Brünkink, D.; Strassert, C.A.; Doltsinis, N.L.; Klein, A. Cyclometalated Pt Complexes of CNC Pincer Ligands: Luminescence and Cytotoxic Evaluation. Organometallics 2020, 39, 746–756. [Google Scholar] [CrossRef]
- Hebenbrock, M.; Stegemann, L.; Kösters, N.L.; Doltsinis, N.L.; Müller, J.; Strassert, C.A. Phosphorescent Pt(II) complexes bearing a monoanionic C^N^N luminophore and tunable ancillary ligands. Dalton Trans. 2017, 46, 3160–3169. [Google Scholar] [CrossRef]
- Cnudde, M.; Brünkink, D.; Doltsinis, N.L.; Strassert, C.A. Tetradentate N^N°N^N-type luminophores for Pt(II) complexes: Synthesis, photophysical and quantum-chemical investigation. Inorg. Chim. Acta 2021, 518, 120090. [Google Scholar] [CrossRef]
- Lai, S.-W.; Cheung, T.-C.; Chan, M.C.W.; Cheung, K.-K.; Peng, S.-M.; Che, C.-M. Luminescent Mononuclear and Binuclear Cyclometalated Palladium(II) Complexes of 6-Phenyl-2,2′-bipyridines: Spectroscopic and Structural Comparisons with Platinum(II) Analogues. Inorg. Chem. 2000, 39, 255–262. [Google Scholar] [CrossRef]
- Cheung, T.-C.; Cheung, K.-K.; Peng, S.-M.; Che, C.-M. Photoluminescent cyclometallated diplatinum(II,II) complexes: Photophysical properties and crystal structures of [PtL(PPh3)]ClO4, and [Pt2L2(µ-dppm)][ClO4] (HL = 6-phenyl-2,2′-bipyridine, dppm = Ph2PCH2PPh2). J. Chem. Soc. Dalton Trans. 1996, 8, 1645–1651. [Google Scholar] [CrossRef]
- Karlen, T.; Ludi, A.; Güdel, H.U. One-Dimensional Migration of 3MLCT Excitation Energy in PdII(phbpy)Cl (phbpy = 6-Phenyl-2,2′-bipyridine). Inorg. Chem. 1991, 10, 2249–2250. [Google Scholar] [CrossRef]
- Niazi, M.; Klein, A. DFT Investigation of the Molecular Properties of the Dimethylglyoximato Complexes [M(Hdmg)2] (M = Ni, Pd, Pt). Inorganics 2021, 9, 47. [Google Scholar] [CrossRef]
- Haseloer, A.; Jordan, R.; Denkler, L.M.; Reimer, M.; Olthof, S.; Schmidt, I.; Meerholz, K.; Hörner, G.; Klein, A. Ni, Pd, and Pt complexes of a tetradentate dianionic thiosemicarbazone-based O^N^N^S ligand. Dalton Trans. 2021, 50, 4311–4322. [Google Scholar] [CrossRef] [PubMed]
- Kletsch, L.; Hörner, G.; Klein, A. Cyclometalated Ni(II) complexes [Ni(N^C^N)X] of the tridentate 2,6-di(2-pyridyl)-phen-ide ligand. Organometallics 2020, 39, 2820–2829. [Google Scholar] [CrossRef]
- Vogt, N.; Sivchik, V.; Sandleben, A.; Hörner, G.; Klein, A. Direct Base-Assisted C‒H Cyclonickelation of 6-Phenyl-2,2′-bipyridine. Molecules 2020, 25, 997. [Google Scholar] [CrossRef] [Green Version]
- Cardenas, D.J.; Echavarren, A.M.; Ramirez de Arellano, M.C. Divergent Behavior of Palladium(II) and Platinum(II) in the Metalation of 1,3-Di(2-pyridyl)benzene. Organometallics 1999, 18, 3337–3341. [Google Scholar] [CrossRef]
- Gareth Williams, J.A.; Beeby, A.; Davies, E.S.; Weinstein, J.A.; Wilson, C. An Alternative Route to Highly Luminescent Platinum(II) Complexes: Cyclometalation with N^C^N-Coordinating Dipyridylbenzene Ligands. Inorg. Chem. 2003, 42, 8609–8611. [Google Scholar] [CrossRef]
- Hofmann, A.; Dahlenburg, L.; Van Eldik, R. Cyclometalated Analogues of Platinum Terpyridine Complexes: Kinetic Study of the Strong σ-Donor Cis and Trans Effects of Carbon in the Presence of a π-Acceptor Ligand Backbone. Inorg. Chem. 2003, 42, 6528–6538. [Google Scholar] [CrossRef]
- Soro, B.; Stoccoro, S.; Minghetti, G.; Zucca, A.; Cinellu, M.A.; Manassero, M.; Gladiali, S. The first pincer-aryl [M–(NCN)] complexes {M = Pd(II); Pt(II)} with chiral pyridine donors: Synthesis and catalytic activity in C–C bond formation. Inorg. Chim. Acta 2006, 359, 1879–1888. [Google Scholar] [CrossRef]
- Abe, T.; Shinozaki, K.; Ikeda, N.; Suzuki, T. [2,6-Bis(5-methyl-2-pyridyl)phenyl-κ3N,C1,N′]chloridoplatinum(II). Acta Cryst. C Cryst. Struct. Commun. 2007, C63, m456–m458. [Google Scholar] [CrossRef]
- Wang, Z.; Turner, E.; Mahoney, V.; Madakuni, S.; Groy, T.; Li, J. Facile Synthesis and Characterization of Phosphorescent Pt(N∧C∧N)X Complexes. Inorg. Chem. 2010, 49, 11276–11286. [Google Scholar] [CrossRef]
- Soro, B.; Stoccoro, S.; Minghetti, G.; Zucca, A.; Cinellu, M.A.; Gladiali, S.; Manassero, M.; Sansoni, M. Synthesis of the First C-2 Cyclopalladated Derivatives of 1,3-Bis(2-pyridyl)benzene. Crystal Structures of [Hg(N-C-N)Cl], [Pd(N-C-N)Cl], and [Pd2(N-C-N)2(µ-OAc)]2[Hg2Cl6]. Catalytic Activity in the Heck Reaction. Organometallics 2005, 24, 53–61. [Google Scholar] [CrossRef]
- Constable, E.C.; Henney, R.P.G.; Leese, T.A. Cyclometallation Reactions of 6-Phenyl-2,2′-bipyridine; a Potential C,N,N-Donor Analogue of 2,2′:6′, 2″-Terpyridine. Crystal and Molecular Structure of Dichlorobis(6-phenyl-2,2′-bipyridine)ruthenium(II). J. Chem. Soc. Dalton Trans. 1990, 443–449. [Google Scholar] [CrossRef]
- Yamamoto, K.; Higuchi, K.; Ogawa, M.; Sogawa, H.; Kuwata, S.; Hayashi, Y.; Kawauchi, S.; Takata, T. Macrocyclic Metal Complexes Bearing Rigid Polyaromatic Ligands: Synthesis and Catalytic Activity. Chem. Asian J. 2020, 15, 356–359. [Google Scholar] [CrossRef]
- Iwakiri, A.; Konno, Y.; Shinozaki, K. Determination of excimer emission quantum yield of Pt(dpb)Cl (dpbH = 1,3-di(2-pyridyl)benzene and its analogues in solution. J. Lumin. 2019, 207, 482–490. [Google Scholar] [CrossRef]
- Garoni, E.; Boixel, J.; Dorcet, V.; Roisnel, T.; Roberto, D.; Jacquemin, D.; Guerchais, V. Controlling the emission in flexibly-linked (N^C^N)platinum dyads. Dalton Trans. 2018, 47, 224–232. [Google Scholar] [CrossRef]
- Schulze, B.; Friebe, C.; Jäger, M.; Görls, H.; Birckner, E.; Winter, A.; Schubert, U.S. PtII Phosphors with Click-Derived 1,2,3-Triazole-Containing Tridentate Chelates. Organometallics 2018, 37, 145–155. [Google Scholar] [CrossRef]
- Rodrigue-Witchel, A.; Rochester, D.L.; Zhao, S.-B.; Lavelle, K.B.; Gareth Williams, J.A.; Wang, S.; Connick, W.B.; Reber, C. Pressure-induced variations of MLCT and ligand-centered luminescence spectra in square-planar platinum(II) complexes. Polyhedron 2016, 108, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Rausch, A.F.; Murphy, L.; Gareth Williams, J.A.; Yersin, H. Improving the Performance of Pt(II) Complexes for Blue Light Emission by Enhancing the Molecular Rigidity. Inorg. Chem. 2012, 51, 312–319. [Google Scholar] [CrossRef]
- Rausch, A.F.; Murphy, L.; Gareth Williams, J.A.; Yersin, H. Probing the Excited State Properties of the Highly Phosphorescent Pt(dpyb)Cl Compound by High-Resolution Optical Spectroscopy. Inorg. Chem. 2009, 48, 11407–11414. [Google Scholar] [CrossRef]
- Abe, T.; Itakura, T.; Ikeda, N.; Shinozaki, K. Luminescence color change of a platinum(II) complex solid upon mechanical grinding. Dalton Trans. 2009, 1, 711–715. [Google Scholar] [CrossRef]
- Farley, S.J.; Rochester, D.L.; Thompson, A.L.; Howard, J.A.K.; Gareth Williams, J.A. Controlling Emission Energy, Self-Quenching, and Excimer Formation in Highly Luminescent N^C^N-Coordinated Platinum(II) Complexes. Inorg. Chem. 2005, 44, 9690–9703. [Google Scholar] [CrossRef]
- Klein, A.; Rausch, B.; Kaiser, A.; Vogt, N.; Krest, A. The cyclometalated nickel complex [(Phbpy)NiBr] (Phbpy− = 2,2′-bipyridine-6-phen-2-yl)—Synthesis, spectroscopic and electrochemical studies. J. Organomet. Chem. 2014, 774, 86–93. [Google Scholar] [CrossRef]
- Sandleben, A.; Vogt, V.; Hörner, G.; Klein, A. Redox Series of Cyclometalated Nickel Complexes [Ni((R)Ph(R′)bpy)Br]+/0/−/2− (H−(R)Ph(R′)bpy = Substituted 6-Phenyl-2,2′-bipyridine). Organometallics 2018, 37, 3332–3341. [Google Scholar] [CrossRef]
- Jensen, K.P. Bioinorganic Chemistry Modeled with the TpSSh Density Functional. Inorg. Chem. 2008, 47, 10357–10365. [Google Scholar] [CrossRef] [PubMed]
- Alrefai, R.; Hörner, G.; Schubert, H.; Berkefeld, A. Broadly versus Barely Variable Complex Chromophores of Planar Nickel(II) from κ3-N,N′,C and κ3-N,N′,O Donor Platforms. Organometallics 2021, 40, 1163–1177. [Google Scholar] [CrossRef]
- Vogt, N.; Sandleben, A.; Kletsch, L.; Schäfer, S.; Chin, M.T.; Vicic, D.A.; Hörner, G.; Klein, A. On the Role of the X Coligands in Cyclometalated [Ni(Phbpy)X] Complexes (HPhbpy = 6-phenyl-2,2′-bipyridine). Organometallics 2021, 40, 1776–1785. [Google Scholar] [CrossRef]
- Von der Stück, R.; Schmitz, S.; Klein, A. C‒X vs. C‒H activation for the synthesis of the cyclometalated complexes [Pd(YPhbpy)X] (HPhbpy = 6-phenyl-2,2′-bipyridine; X/Y = (pseudo)halides). Inorg. Chem. Res. 2021, 5, 173–192. [Google Scholar]
- Lai, S.-W.; Chan, M.C.-W.; Cheung, T.-C.; Peng, S.-M.; Che, C.-M. Probing d8-d8 Interactions in Luminescent Mono- and Binuclear Cyclometalated Platinum(II) Complexes of 6-Phenyl-2,2′-bipyridines. Inorg. Chem. 1999, 38, 4046–4055. [Google Scholar] [CrossRef]
- Kaim, W.; Fiedler, J. Spectroelectrochemistry: The best of two worlds. Chem. Soc. Rev. 2009, 38, 3373–3382. [Google Scholar] [CrossRef]
- APEX3—Software Suite for Crystallographic Programs; Bruker AXS, Inc.: Madison, WI, USA, 2015.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, 1327. [Google Scholar] [CrossRef]
- Chemcraft—Graphical Software for Visualization of Quantum Chemistry Computations. Available online: https://www.chemcraftprog.com (accessed on 19 August 2021).
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P.; Yue, W. Accurate and simple density functional for the electronic exchange energy: Generalized gradient approximation. Phys. Rev. B 1986, 33, 8800–8802. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2 1993, 799–805. [Google Scholar] [CrossRef]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Petrenko, T.; Kossmann, S.; Neese, F. Efficient time-dependent density functional theory approximations for hybrid density functionals: Analytical gradients and parallelization. J. Chem. Phys. 2011, 134, 054116. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Head-Gordon, M. Time-dependent density functional theory within the Tamm-Dancoff approximation. Chem. Phys. Lett. 1999, 314, 291–299. [Google Scholar] [CrossRef]
- Tao, J.; Perdew, J.P.; Stavoverov, V.N.; Scuseria, G.E. Climbing the density functional ladder: Nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys. Rev. Lett. 2003, 91, 146401. [Google Scholar] [CrossRef] [PubMed] [Green Version]

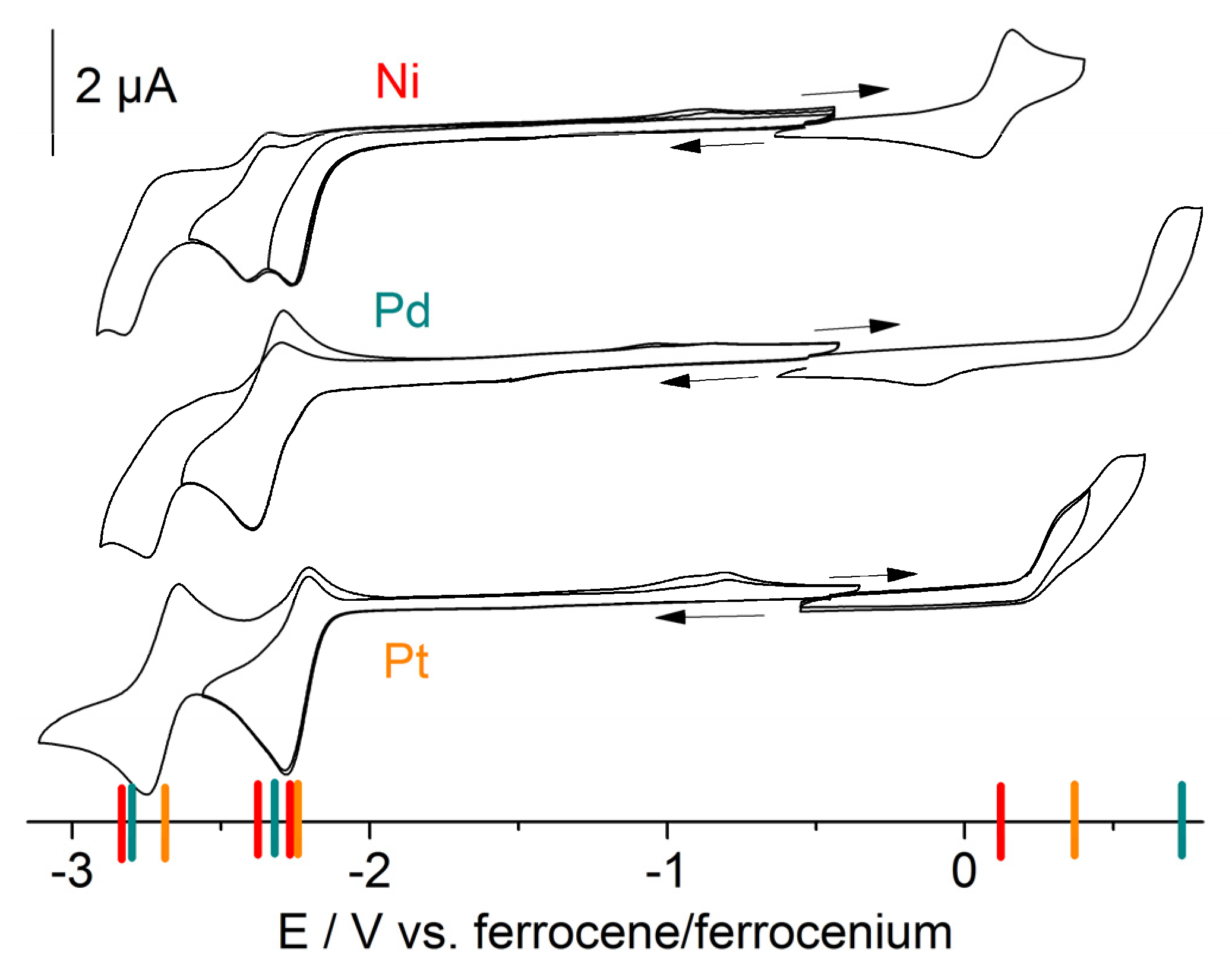

| Epc (Red2) | E1/2 (Red1) | Epa (ox1) | ΔE (Red1‒Red2) | ΔE (ox1‒Red1) | Solvent | DFTk | |
|---|---|---|---|---|---|---|---|
| Me2dpbH | −2.52 | −1.74 irr | - | 0.70 | - | THF | |
| [Ni(Me2dpb)Cl] | −2.37 rev b | −2.26 irr | 0.10 rev | 0.11 (0.45) b | 2.36 | THF | 3.07 |
| [Pd(Me2dpb)Cl] | −2.75 | −2.34 | 0.74 | 0.41 | 3.07 | THF | 3.39 |
| [Pt(Me2dpb)Cl] | −2.69 | −2.24 | 0.35 c | 0.45 | 2.59 | THF | 3.15 |
| [Pt(dpb)Cl] d | - | −2.14 | 0.35 | - | 2.49 | MeCN | |
| [Pt(4-Medpb)Cl] d | - | −2.15 | 0.29 | - | 2.44 | MeCN | |
| [Pt(4-MeOOCdpb)Cl] d | - | −2.04 | 0.39 | - | 2.43 | MeCN | |
| [Pt(dpb)Cl] e | - | −2.18 | 0.41 | - | 2.59 | DMF | |
| [Pt(3,5-(CF3)2dpb)Cl] e | - | −1.92 | 0.50 | - | 2.42 | DMF | |
| [Pt(3,4,5-F3dpb)Cl] e | - | −2.07 | 0.51 | - | 2.58 | DMF | |
| [Pt(Me2dpb)Cl] f | - | −2.03 | 0.43 | - | 2.46 | CH2Cl2 | |
| [Ni(dpb)Cl] g | −2.57 | −2.33 irr | 0.06 rev | 0.24 | 2.39 | THF | |
| [Ni(phbpy)Cl] h,i | −2.60 rev | −1.93 | 0.06 rev | 0.67 | 1.99 | THF | |
| [Pd(phbpy)Cl)] i | −2.60 rev | −1.92 | 0.80 b | 0.68 | 2.72 | THF | 3.58 |
| [Pt(phbpy)Cl] i | −2.48 rev | −1.78 | 0.41 | 0.70 | 2.19 | THF |
| λ1 (ε) | λ2 (ε) | λ3 (ε) | λ4 (ε) | λ5 (ε) | λ6 (ε) | λ7 (ε) | |
|---|---|---|---|---|---|---|---|
| Me2dpbH—this work | 227 (28.2) | 260 (19.7) | 340 (0.4) | 636 (0.3) | - | - | - |
| [Pt(Me2dpb)Cl]—this work | 231 (33.7) | 256 (30.0) | 287 (22.9) | 331 (9.2) | 380 (8.3) | 413 (1.5) | 480 (0.1) |
| [Pd(Me2dpb)Cl]—this work | 239 (26.7) | 275 (23.2) | 283 (22.0) | 327 (8.3) | 360 (7.4) | 375 (1.2) | - |
| [NiMe2dpb)Cl]—this work | 230 (33.0) | 274 (31.2) | 297 (14.7) | 333 (9.0) | 395 (6.1) b | - | 465 (1.1) |
| [Pt(dpb)Cl] c,e,e,f | - | 255 (25.2) | 289 (21.1) | 332 (6.3) | 379 (8.6) | 402 (7.0) | 485 (0.1) |
| [Pt(4-Medpb)Cl] d | - | - | - | 335 (5.7) | 381 (6.9) | 412 (6.8) | 495 (0.1) |
| [Pt(4-MeOOCdpb)Cl] d | - | - | - | 329 (7.5) | 380 (9.9) | 397 (7.9) | 478 (0.2) |
| [Pt(3-F-dpb)Cl] e | - | - | - | - | 379 (9.1) | 401 (5.2) | 477 (0.1) |
| [Pt(3,5-F2-dpb)Cl] e | - | - | - | - | 375 (7.6) | - | 467 (0.1) |
| [Pt(3,4,5-F3-dpb)Cl] c | - | - | - | - | 380 (10.4) | 405 (4.6) | 480 (0.1) |
| [Pt(3,5-(CF3)2-dpb)Cl] c | - | - | - | - | 382 (10.4) | 408 (8.6) | 479 (0.2) |
| [Ni(dpb)Cl] g | 236 (35.5) | 279 (26.8) | - | 332 (5.7) | 412 (6.3) | 437 (6.7) | |
| [Ni(phbpy)Cl] g,h | 281 | 321 | 354 | 391 | 596 | ||
| [Pd(phbpy)Cl] h,i,k | 266 (20) | 278 (21) | 311 (12) | 325 (13) | 342 (8) | 402 (1) | |
| [Pt(phbpy)Cl] h,i | 278 | 302 | 330 | 363 | 410 | 430 |
| [Pt(dpb)Cl] | [Pt(Me2dpb)Cl] | [Pd(Me2dpb)Cl] | ||||||
|---|---|---|---|---|---|---|---|---|
| Solvent | DCM | 2-MeTHF | DCM | 2-MeTHF | 2-MeTHF | |||
| Temperature | 298 K | 298 K | 77 K | 298 K | 298 K | 77 K | 77 K | |
| λEm/nm | 490, 523, 560sh | 494, 526, 565sh | 486, 503, 520, 560sh | 495, 527, 565 | 498, 530, 567sh | 489, 503, 522, 560sh | 468, 481, 501, 531sh | |
| λExc/nm | 289, 330sh, 381, 403sh | 298, 330sh, 388, 415 | 295, 335, 380, 410 | 300, 342, 390 | 302, 343, 394 | 303, 343, 373, 390 | 300, 347, 373 | |
| τav/µs b | Air-equilibrated | 0.4464 ± 0.0015 | 0.1659 ± 0.0006 | 5.717 ± 0.016 | 0.3428 ± 0.0015 | 0.1185 ± 0.0006 | 5.794 ± 0.017 c | 152.8 ± 0.6 [157 ± 3 (92); 102 ± 12 (8)] d |
| Deaerated | 6.050 ± 0.019 | 3.842 ± 0.009 | 6.420 ± 0.016 | 4.608 ± 0.010 | ||||
| ΦL ± 0.02 | Air-equilibrated | 0.04 | <0.02 | 0.98 | 0.04 | < 0.02 | 0.98 | 0.98 |
| Deaerated | 0.64 | 0.52 | 0.75 | 0.64 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kletsch, L.; Jordan, R.; Köcher, A.S.; Buss, S.; Strassert, C.A.; Klein, A. Photoluminescence of Ni(II), Pd(II), and Pt(II) Complexes [M(Me2dpb)Cl] Obtained from C‒H Activation of 1,5-Di(2-pyridyl)-2,4-dimethylbenzene (Me2dpbH). Molecules 2021, 26, 5051. https://doi.org/10.3390/molecules26165051
Kletsch L, Jordan R, Köcher AS, Buss S, Strassert CA, Klein A. Photoluminescence of Ni(II), Pd(II), and Pt(II) Complexes [M(Me2dpb)Cl] Obtained from C‒H Activation of 1,5-Di(2-pyridyl)-2,4-dimethylbenzene (Me2dpbH). Molecules. 2021; 26(16):5051. https://doi.org/10.3390/molecules26165051
Chicago/Turabian StyleKletsch, Lukas, Rose Jordan, Alicia S. Köcher, Stefan Buss, Cristian A. Strassert, and Axel Klein. 2021. "Photoluminescence of Ni(II), Pd(II), and Pt(II) Complexes [M(Me2dpb)Cl] Obtained from C‒H Activation of 1,5-Di(2-pyridyl)-2,4-dimethylbenzene (Me2dpbH)" Molecules 26, no. 16: 5051. https://doi.org/10.3390/molecules26165051
APA StyleKletsch, L., Jordan, R., Köcher, A. S., Buss, S., Strassert, C. A., & Klein, A. (2021). Photoluminescence of Ni(II), Pd(II), and Pt(II) Complexes [M(Me2dpb)Cl] Obtained from C‒H Activation of 1,5-Di(2-pyridyl)-2,4-dimethylbenzene (Me2dpbH). Molecules, 26(16), 5051. https://doi.org/10.3390/molecules26165051








