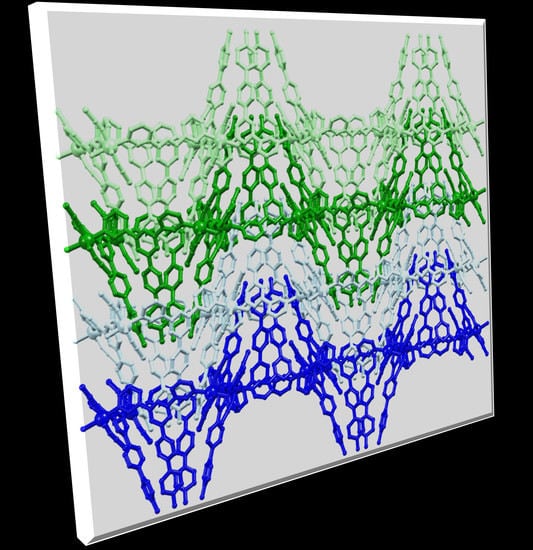
Adapting (4,4) Networks through Substituent Effects and Conformationally Flexible 3,2’:6’,3”-Terpyridines
Abstract
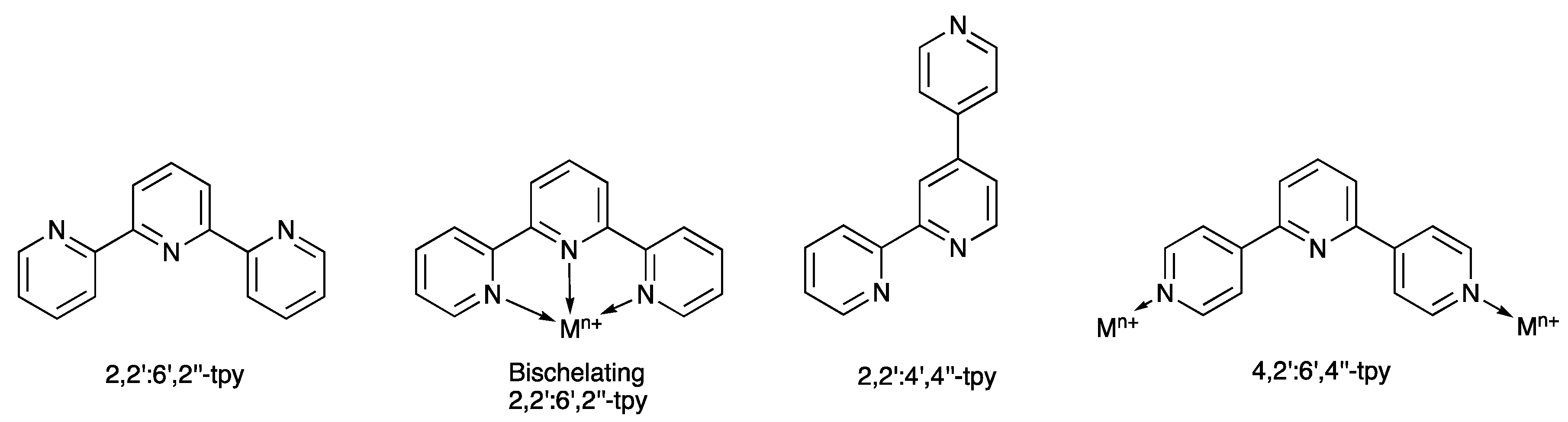
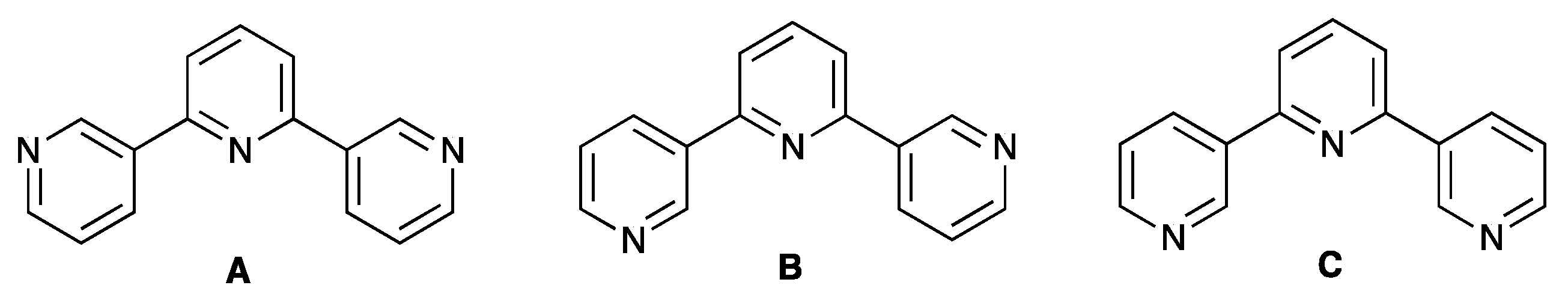
:1. Introduction
2. Results and Discussion
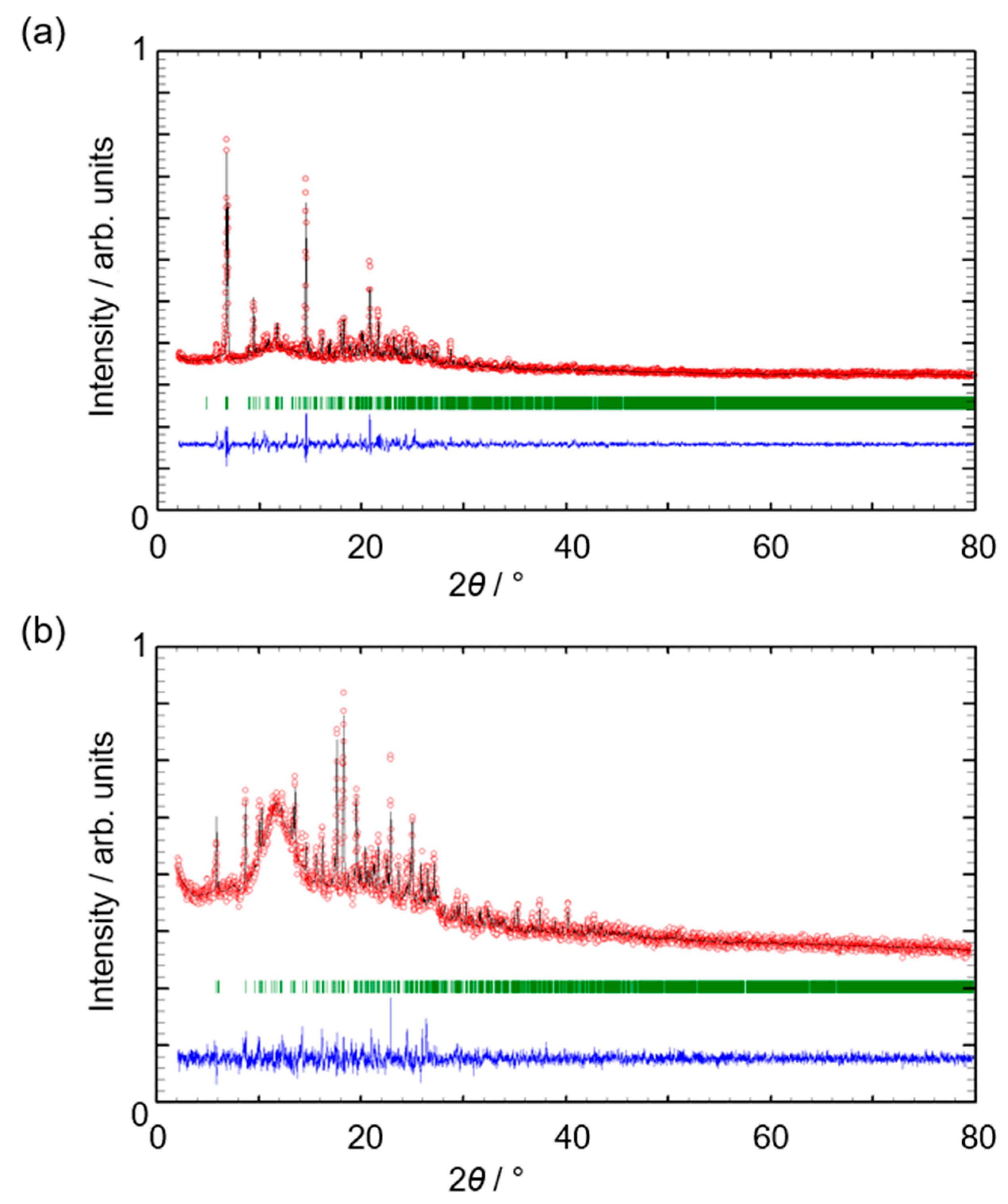
2.1. Crystal Growth and Bulk Material Characterization
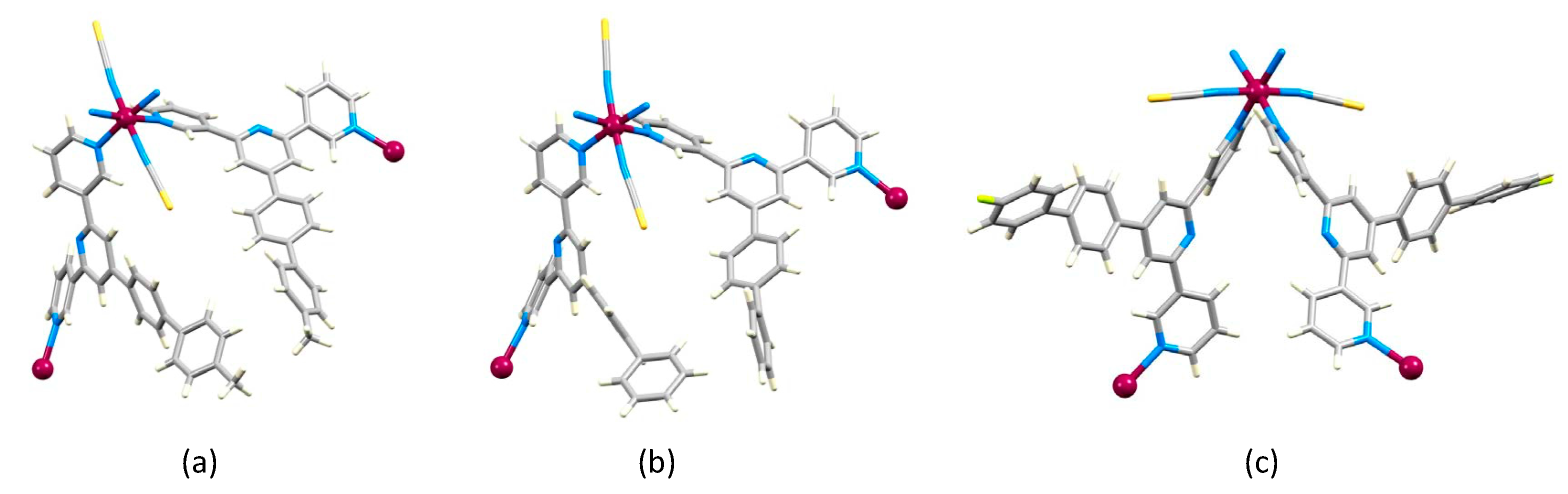
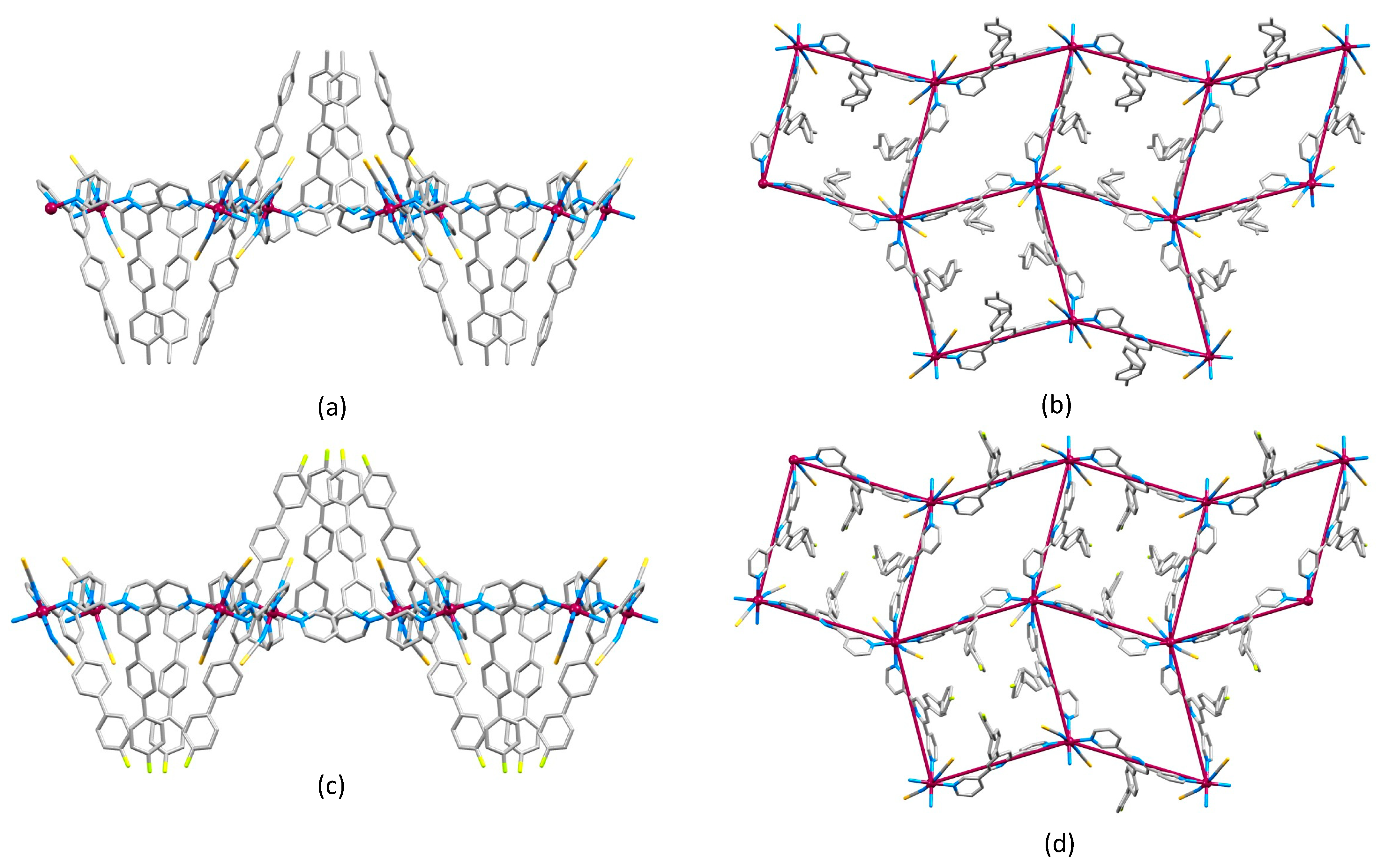
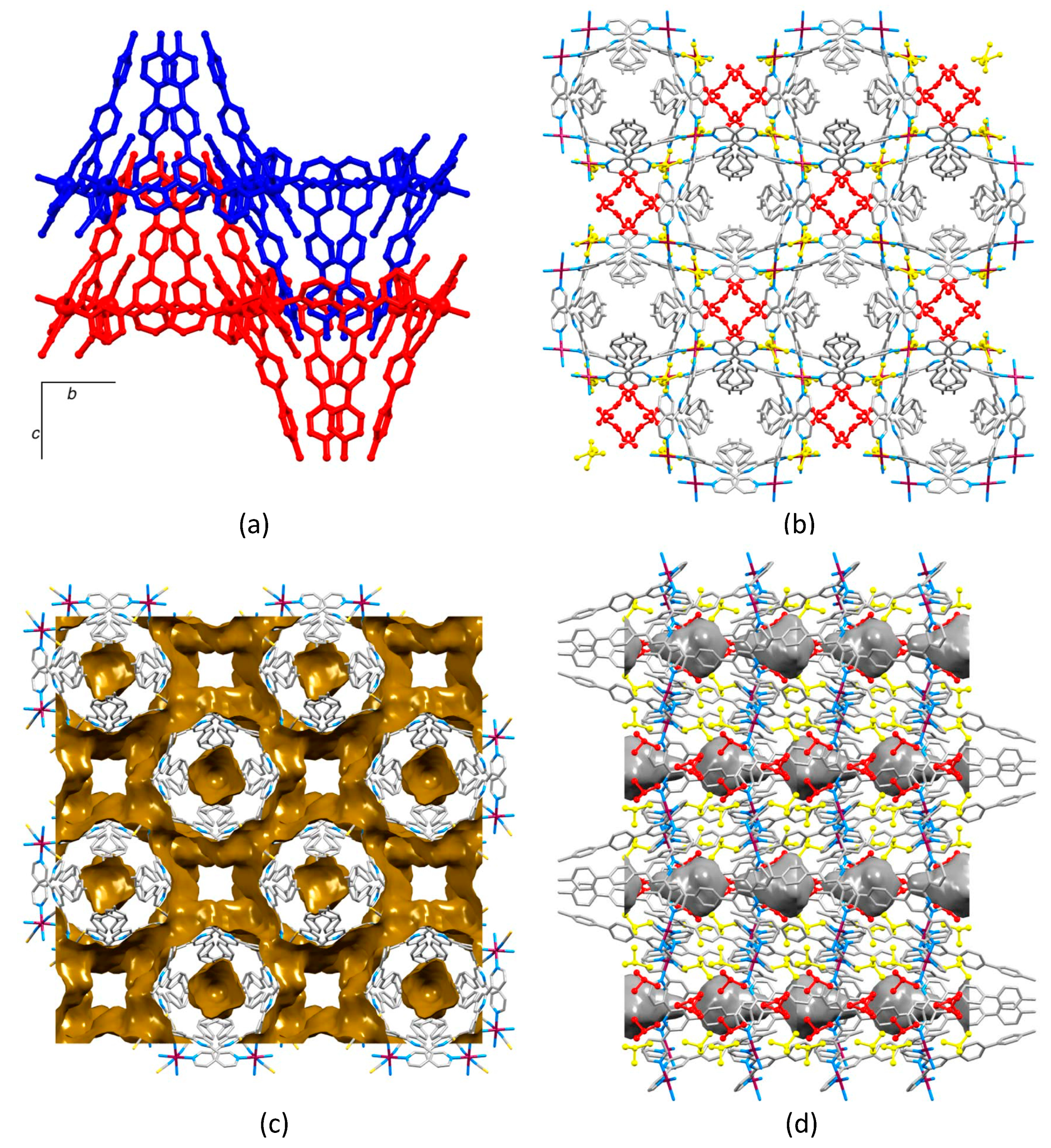
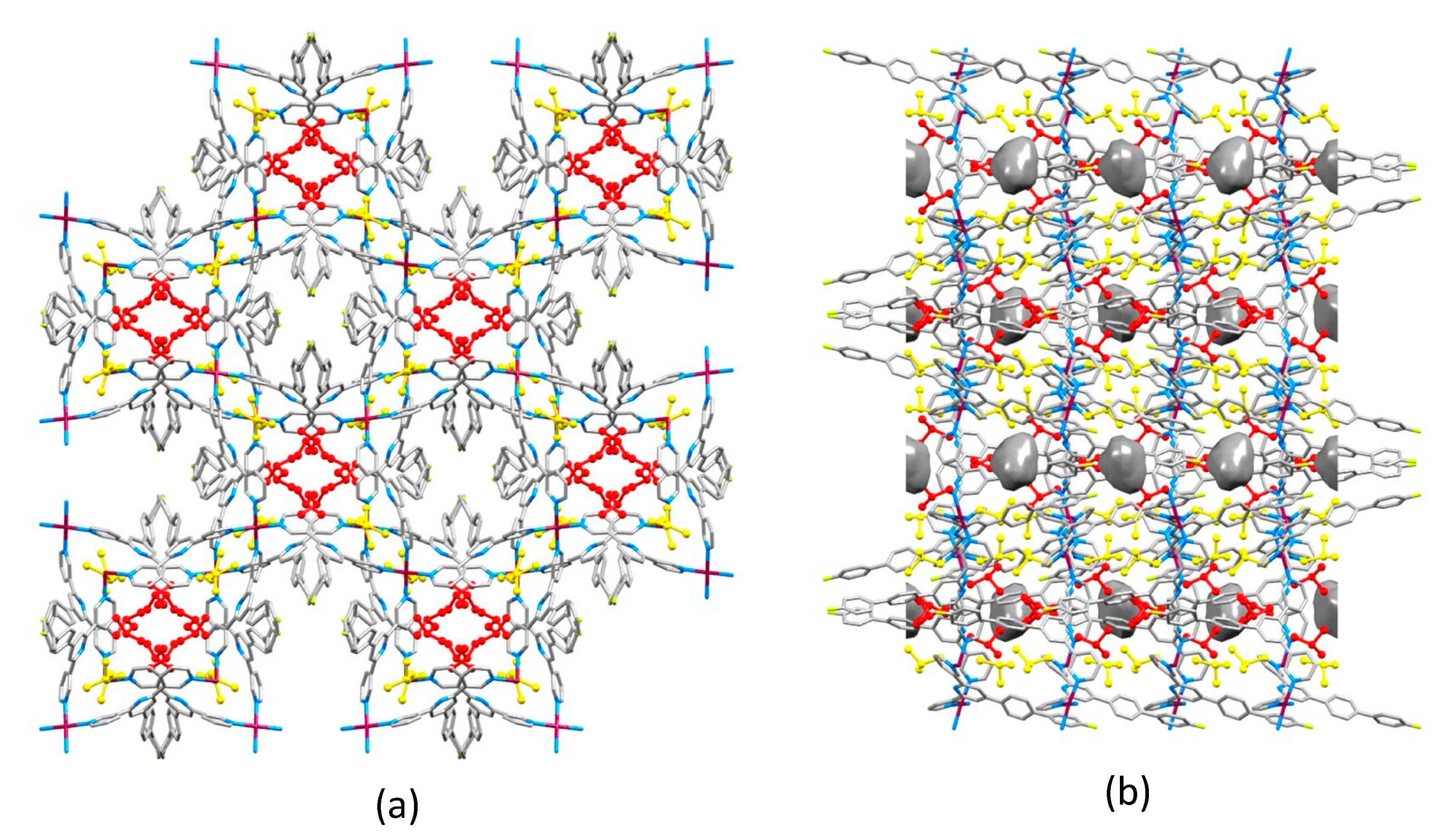
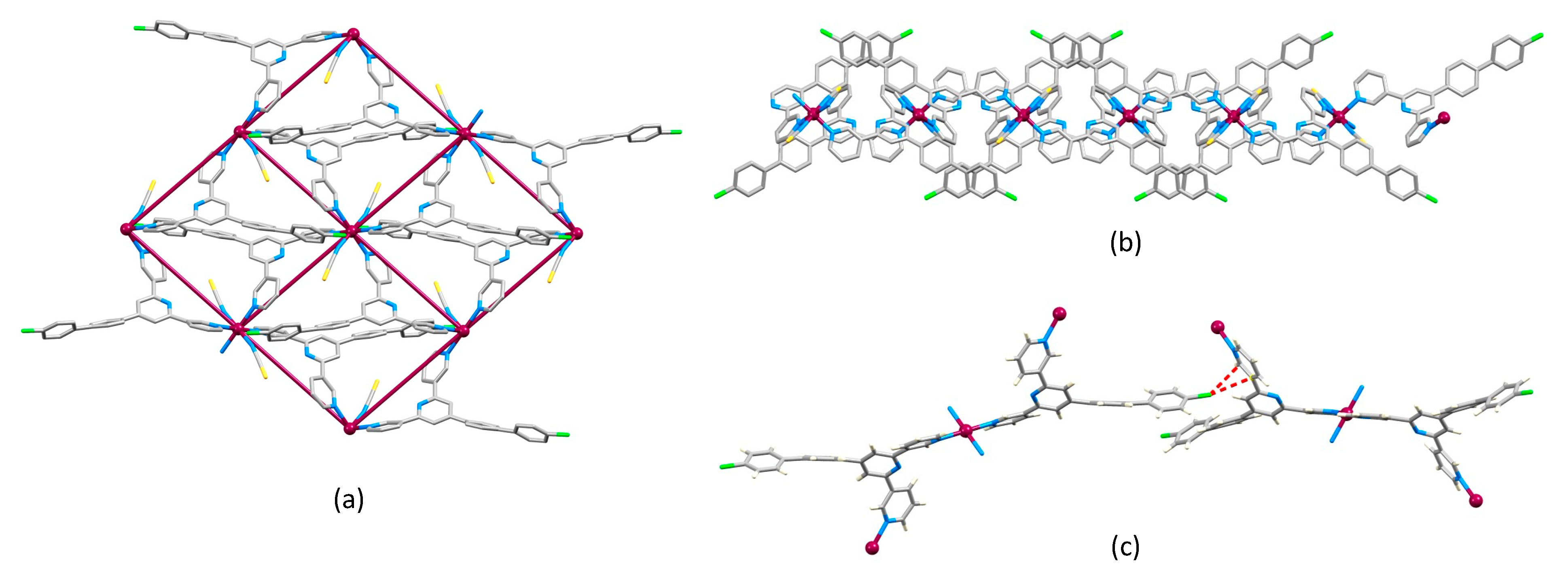
2.2. Crystal Structures of [Co(1)2(NCS)2]n·4.5nCHCl3, [Co(2)2(NCS)2]n·4.3nCHCl3 and [Co(3)2(NCS)2]n·4nCHCl3
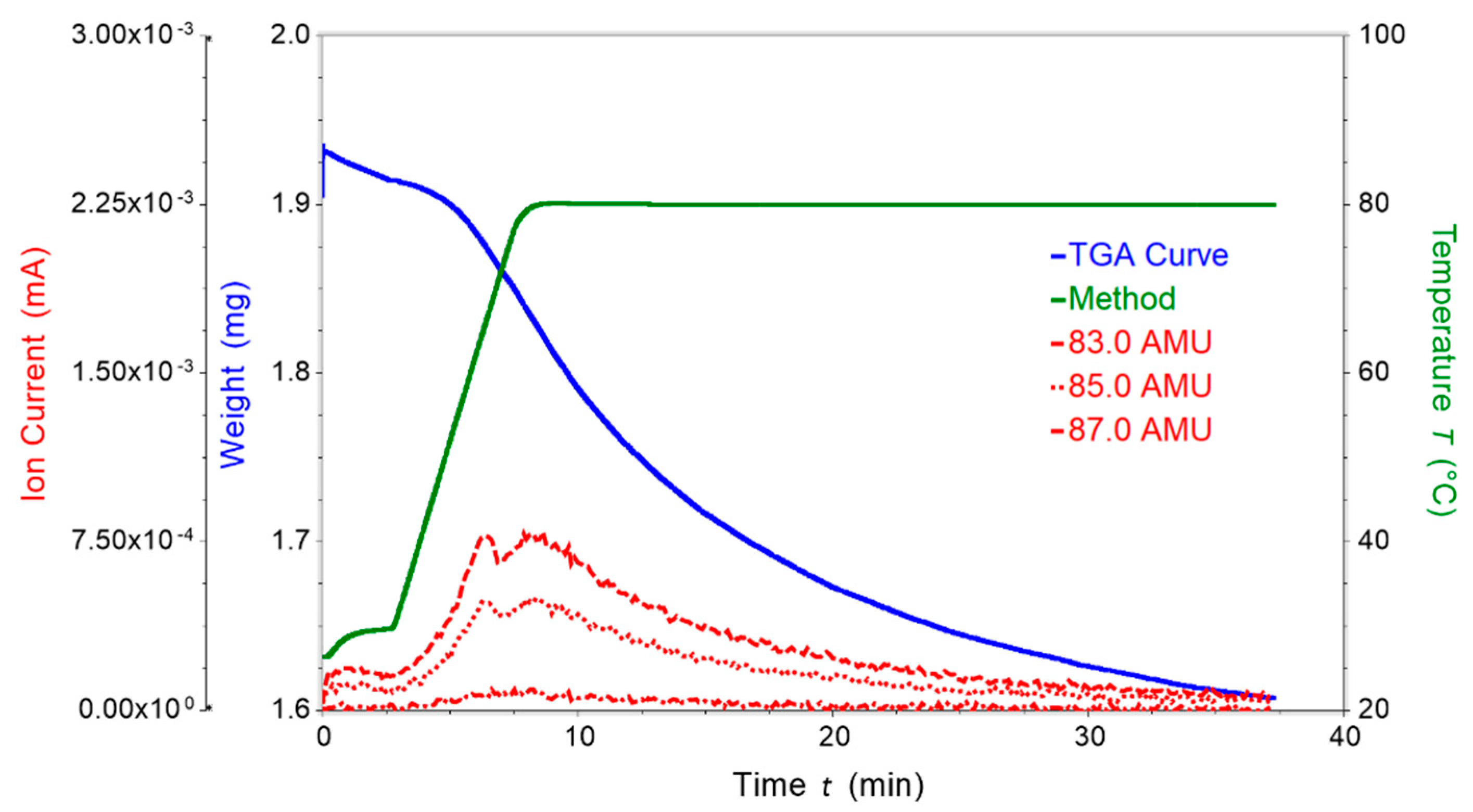
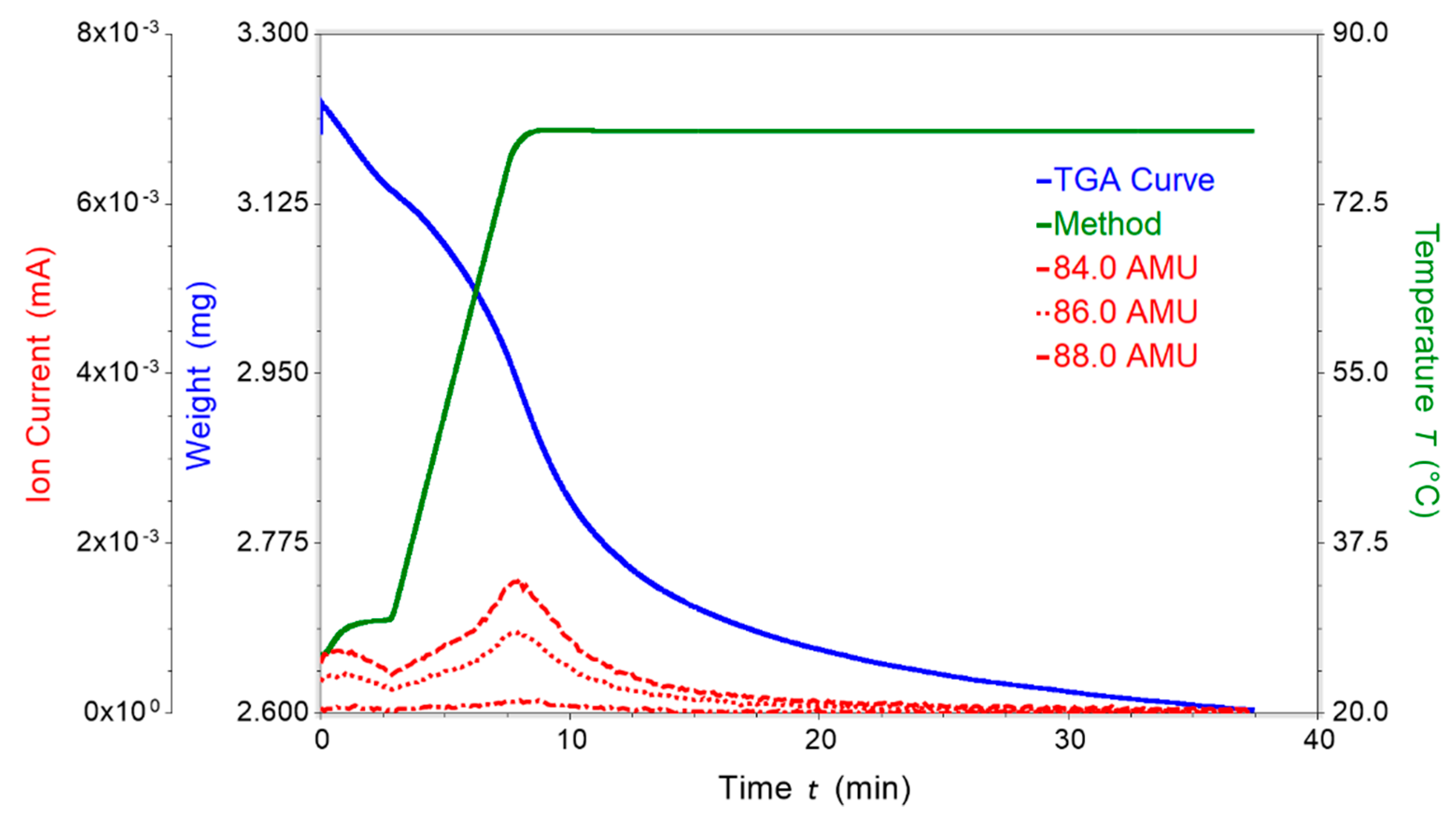
2.3. Thermogravimetric Analysis (TGA) of [Co(3)2(NCS)2]n·4nCHCl3
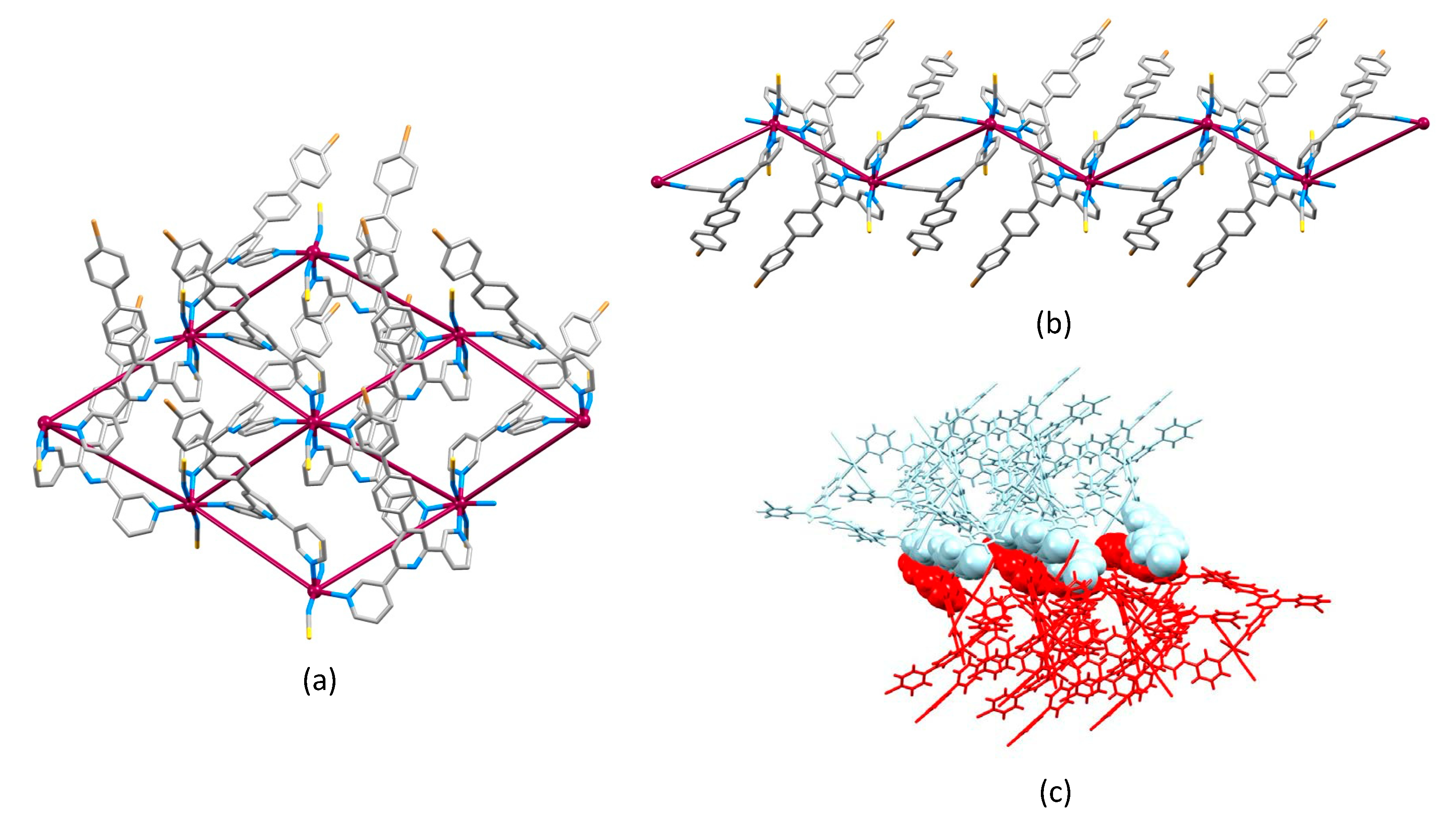
2.4. Crystal Structures of [Co(4)2(NCS)2]n and [Co(5)2(NCS)2]n·nCHCl3
3. Materials and Methods
3.1. General
3.2. [Co(1)2(NCS)2]n·4.5nCHCl3
3.3. [Co(2)2(NCS)2]n·4.3nCHCl3
3.4. [Co(3)2(NCS)2]n·4nCHCl3
3.5. [Co(4)2(NCS)2]n
3.6. [Co(5)2(NCS)2]n·nCHCl3
3.7. Crystallography
3.8. [Co(1)2(NCS)2]n·4.5nCHCl3
3.9. [Co(2)2(NCS)2]n·4.3nCHCl3
3.10. [Co(3)2(NCS)2]n·4nCHCl3
3.11. [Co(4)2(NCS)2]n
3.12. [Co(5)2(NCS)2]n·nCHCl3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Constable, E.C. The Coordination Chemistry of 2,2′:6′,2″-Terpyridine and Higher Oligopyridines. Adv. Inorg. Chem. 1986, 30, 69–121. [Google Scholar] [CrossRef]
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH Verlag & Co.: Weinheim, Germany, 2006. [Google Scholar]
- Constable, E.C. 2,2′:6′,2″-Terpyridines: From chemical obscurity to common supramolecular motifs. Chem. Soc. Rev. 2007, 36, 246–253. [Google Scholar] [CrossRef]
- Wei, C.; He, Y.; Shi, X.; Song, Z. Terpyridine-metal complexes: Applications in catalysis and supramolecular chemistry. Coord. Chem. Rev. 2019, 385, 1–19. [Google Scholar] [CrossRef]
- Chakraborty, S.; Newkome, G.R. Terpyridine-based metallosupramolecular constructs: Tailored monomers to precise 2D-motifs and 3D-metallocages. Chem. Soc. Rev. 2018, 47, 3991–4016. [Google Scholar] [CrossRef]
- Taniya, O.S.; Kopchuk, D.S.; Khasanov, A.F.; Kovalev, I.S.; Santra, S.; Zyryanov, G.V.; Majee, A.; Charushin, V.N.; Chupakhin, O.N. Synthetic approaches and supramolecular properties of 2,2′:n′,m″-terpyridine domains (n = 3, 4, 5, 6; m = 2, 3, 4) based on the 2,2’-bipyridine core as ligands with k2N-bidentate coordination mode. Coord. Chem. Rev. 2021, 442, 213980. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. The Terpyridine Isomer Game: From Chelate to Coordination Network Building Block. Chem. Commun. 2020, 56, 10786–10794. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. Isomers of terpyridine as ligands in coordination polymers and networks containing zinc(II) and cadmium(II). Molecules 2021, 26, 3110. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-T.; Zhang, J.-L.; Hua, H.-M.; Cheng, Y.; Xue, L.-L.; Wanga, X.; Wang, B.-Z. Syntheses, structures and luminescent properties of Zn/Cd coordination polymers based on 4′-(2-carboxyphenyl)-3,2′:6′,3″-terpyridine. Polyhedron 2018, 151, 43–50. [Google Scholar] [CrossRef]
- Rocco, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Directing 2D-coordination networks: Combined effects of a conformationally flexible 3,2′:6′,3″-terpyridine and chain length variation in 4′-(4-n-alkyloxyphenyl) substituents. Molecules 2020, 25, 1663. [Google Scholar] [CrossRef] [Green Version]
- Rocco, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Switching the conformation of 3,2′:6′,3″-tpy domains in 4′-(4-n-alkyloxyphenyl)-3,2′:6′,3″-terpyridines. Molecules 2020, 25, 3162. [Google Scholar] [CrossRef] [PubMed]
- Rocco, D.; Novak, S.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Manipulating the conformation of 3,2′:6′,3″-terpyridine in [Cu2(μ-OAc)4(3,2′:6′,3″-tpy)]n 1D-polymers. Chemistry 2021, 3, 15. [Google Scholar] [CrossRef]
- Zhao, M.; Tan, J.; Su, J.; Zhang, J.; Zhang, S.; Wu, J.; Tian, J. Syntheses, crystal structures and third-order nonlinear optical properties of two series of Zn(II) complexes using the thiophene-based terpyridine ligands. Dye. Pigm. 2016, 130, 216–225. [Google Scholar] [CrossRef]
- Klein, Y.M.; Lanzilotto, A.; Prescimone, A.; Kramer, K.W.; Decurtins, S.; Liu, S.X.; Constable, E.C.; Housecroft, C.E. Coordination behaviour of 1-(3,2’:6’,3’’-terpyridin-4’-yl)ferrocene: Structure and magnetic and electrochemical properties of a tetracopper dimetallomacrocycle. Polyhedron 2017, 129, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Granifo, J.; Varga, M.; Garland, M.T.; Ibáñez, A.; Gaviño, R.; Baggio, R. The novel ligand 4’-phenyl-3,2’:6’,3’’-terpyridine (L) and the supramolecular structure of the dinuclear complex [Zn2(μ-L)(acac)4]·H2O (acac = acetylacetonato). Inorg. Chem. Comm. 2008, 11, 1388–1391. [Google Scholar] [CrossRef]
- Granifo, J.; Gaviño, R.; Freire, E.; Baggio, R. The new sulphur-containing ligand 4’-(4-methylthiophenyl)-3,2’:6’,3’’-terpyridine (L1) and the supramolecular structure of the dinuclear complex [Zn2(μ-L)(acac)4] (acac = acetylacetonato): The key role of non-covalent S⋯O contacts and C–H⋯S hydrogen bonds. J. Mol. Struct. 2011, 1006, 684–691. [Google Scholar] [CrossRef]
- Henling, L.M.; Marsh, R.E. Some more space-group corrections. Acta Cryst. 2014, 70, 834–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kröhnke, F. The Specific Synthesis of Pyridines and Oligopyridines. Synthesis 1976, 1976, 1–24. [Google Scholar] [CrossRef]
- Wang, J.; Hanan, G.S. A facile route to sterically hindered and non-hindered 4′-aryl-2,2′:6′,2′’-terpyridines. Synlett 2005, 1251–1254. [Google Scholar] [CrossRef]
- Klein, Y.M.; Constable, E.C.; Housecroft, C.E.; Zampese, J.A.; Crochet, A. Greasy tails switch 1D-coordination [Zn2(OAc)4(4′-(4-ROC6H4)-4,2′:6′,4″-tpy)]n polymers to discrete [Zn2(OAc)4(4′-(4-ROC6H4)-4,2′:6′,4″-tpy)2] complexes. CrystEngComm 2014, 16, 9915–9929. [Google Scholar] [CrossRef] [Green Version]
- Rocco, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Straight versus branched chain substituents in 4’-(butoxyphenyl)-3,2′:6′,3″-terpyridines: Effects on (4,4) coordination network assemblies. Polymers 2020, 12, 1823. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Housecroft, C.E.; Vujovic, S.; Zampese, J.A.; Crochet, A.; Batten, S.R. Do perfluoroarene…arene and C–H…F interactions make a difference to 4,2’:6’,4”-terpyridine-based coordination polymers? CrystEngComm 2013, 15, 10068–10078. [Google Scholar] [CrossRef] [Green Version]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NIST Chemistry WebBook. Available online: http://webbook.nist.gov/chemistry (accessed on 5 October 2021).
- Prasanna, M.D.; Row, T.N.G. C–halogen…π interactions and their influence on molecular conformation and crystal packing: A database study. Cryst. Eng. 2000, 3, 135–154. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Supramolecular architectures sustained by delocalised C–I…π(arene) interactions in molecular crystals and the propensity of their formation. CrystEngComm 2021, 23, 904–928. [Google Scholar] [CrossRef]
- Shishkin, O.V. Evaluation of true energy of halogen bonding in crystals of halogen derivatives of trityl alcohol. Chem. Phys. Lett. 2008, 458, 96–100. [Google Scholar] [CrossRef]
- Software for the Integration of CCD Detector System Bruker Analytical X-ray Systems, Bruker axs, Madison, WI (after 2013).
- Sheldrick, G.M. ShelXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with ShelXL. Acta Cryst. 2015, 27, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef] [Green Version]
- Palatinus, L.; Prathapa, S.J.; van Smaalen, S. EDMA: A Computer Program for Topological Analysis of Discrete Electron Densities. J. Appl. Cryst. 2012, 45, 575–580. [Google Scholar] [CrossRef]
- LeBail, A.; Duroy, H.; Fourquet, J.L. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mat. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Pawley, G.S. Unit-cell refinement from powder diffraction scans. J. Appl. Cryst. 1981, 14, 357–361. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Physical B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríguez-Carvajal, J. Materials Science Forum. WinPLOTR: A Windows tool for powder diffraction patterns analysis. In Proceedings of the Seventh European Powder Diffraction Conference (EPDIC 7), Barcelona, Spain, 20–23 May 2001; pp. 118–123. [Google Scholar]


| Co–NNCS/Å | Co–Ntpy/Å | Angle between Planes of Pyridine Rings/° | Angle between Planes of Pyridine and Arene Rings/° | Angle between Planes of Rings in Biphenyl Unit/° | |
|---|---|---|---|---|---|
| [Co(1)2(NCS)2]n·4.5nCHCl3 | 2.066(4) | 2.211(4), 2.189(4) | 27.7, 28.4 | 17.5 | 55.6 |
| [Co(2)2(NCS)2]n·4.3nCHCl3 | 2.067(9) | 2.166(11), 2.193(10) | 24.7, 35.7 | 35.6 a | 79.7 a |
| [Co(3)2(NCS)2]n·4nCHCl3 | 2.064(7), 2.065(6) | 2.164(6), 2.192(6), 2.189(6), 2.213(6) | 28.3, 33.3 b 24.8, 37.1 c | 40.2 b 15.9 c | 48.0 b 58.2 c |
| Co–NNCS/Å | Co–Ntpy/Å | Angle between Planes of Pyridine Rings/° | Angle between Planes of Pyridine and Arene Rings/° | Angle between Planes of Rings in Biphenyl Unit/° | |
|---|---|---|---|---|---|
| [Co(4)2(NCS)2]n | 2.0776(17) | 2.1781(17), 2.2295(18) | 34.1, 39.0 | 41.4 | 28.8 |
| [Co(5)2(NCS)2]n·nCHCl3 | 2.061(2), 2.084(2) | 2.170(2), 2.221(2), 2.175(2), 2.199(2) | 29.1, 21.1 a 38.8, 48.3 b | 38.9 a 22.1 b | 40.9 a 30.6 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocco, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Adapting (4,4) Networks through Substituent Effects and Conformationally Flexible 3,2’:6’,3”-Terpyridines. Molecules 2021, 26, 6337. https://doi.org/10.3390/molecules26216337
Rocco D, Prescimone A, Constable EC, Housecroft CE. Adapting (4,4) Networks through Substituent Effects and Conformationally Flexible 3,2’:6’,3”-Terpyridines. Molecules. 2021; 26(21):6337. https://doi.org/10.3390/molecules26216337
Chicago/Turabian StyleRocco, Dalila, Alessandro Prescimone, Edwin C. Constable, and Catherine E. Housecroft. 2021. "Adapting (4,4) Networks through Substituent Effects and Conformationally Flexible 3,2’:6’,3”-Terpyridines" Molecules 26, no. 21: 6337. https://doi.org/10.3390/molecules26216337
APA StyleRocco, D., Prescimone, A., Constable, E. C., & Housecroft, C. E. (2021). Adapting (4,4) Networks through Substituent Effects and Conformationally Flexible 3,2’:6’,3”-Terpyridines. Molecules, 26(21), 6337. https://doi.org/10.3390/molecules26216337