Curcumin Nanoparticles as Promising Therapeutic Agents for Drug Targets
Abstract
:1. Introduction
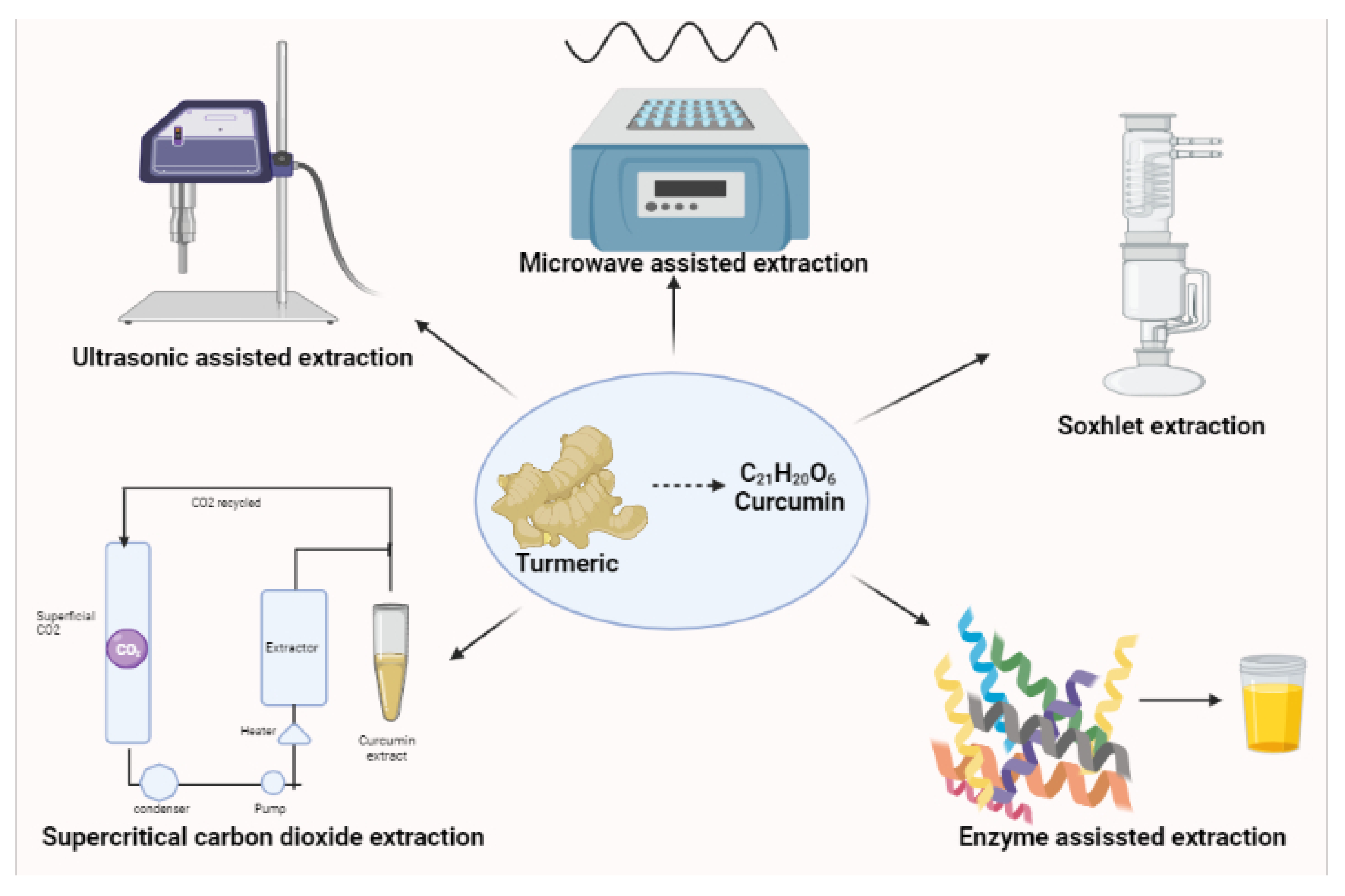
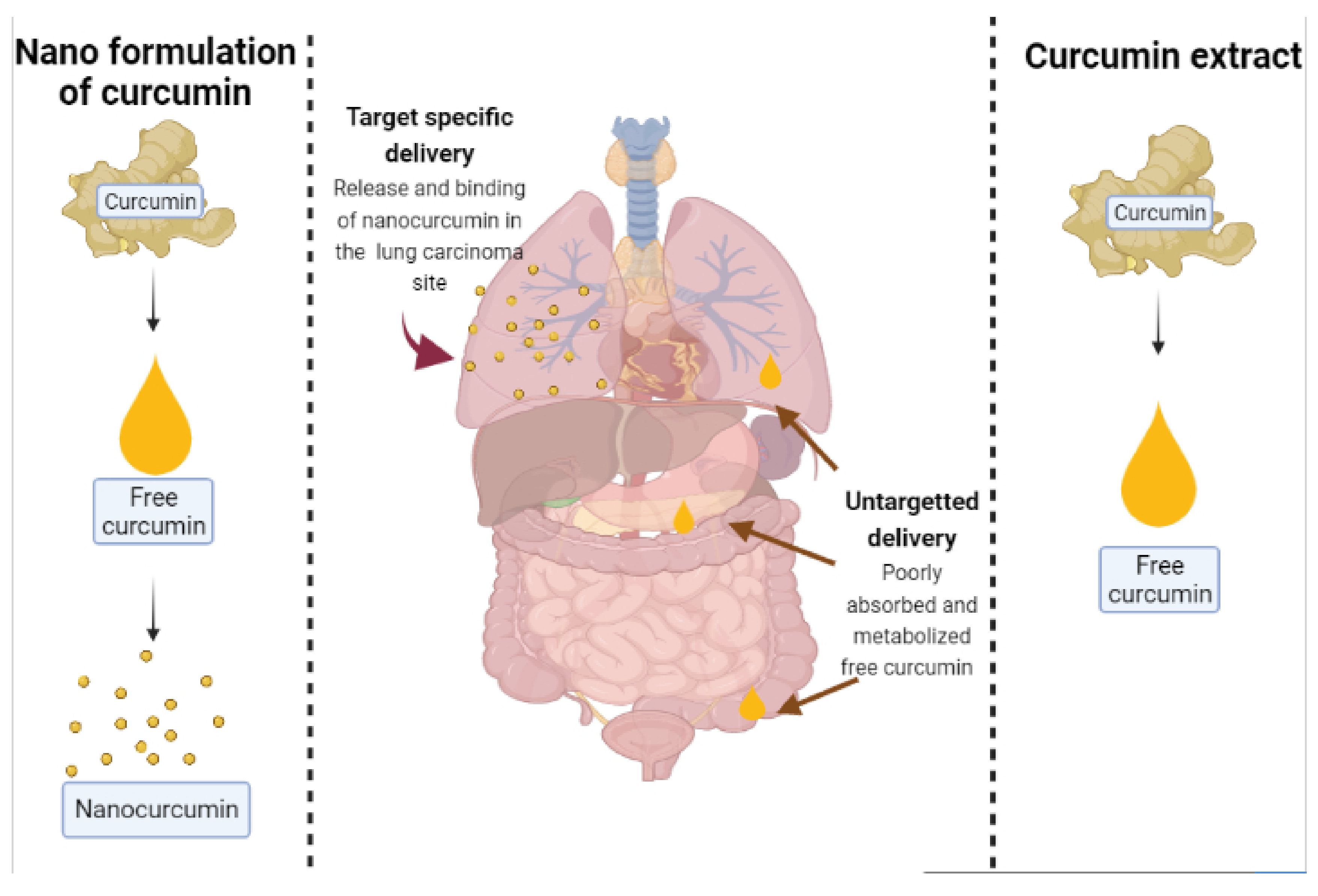
2. Recent Trends in Curcumin Formulations Techniques for Effective Drug Delivery
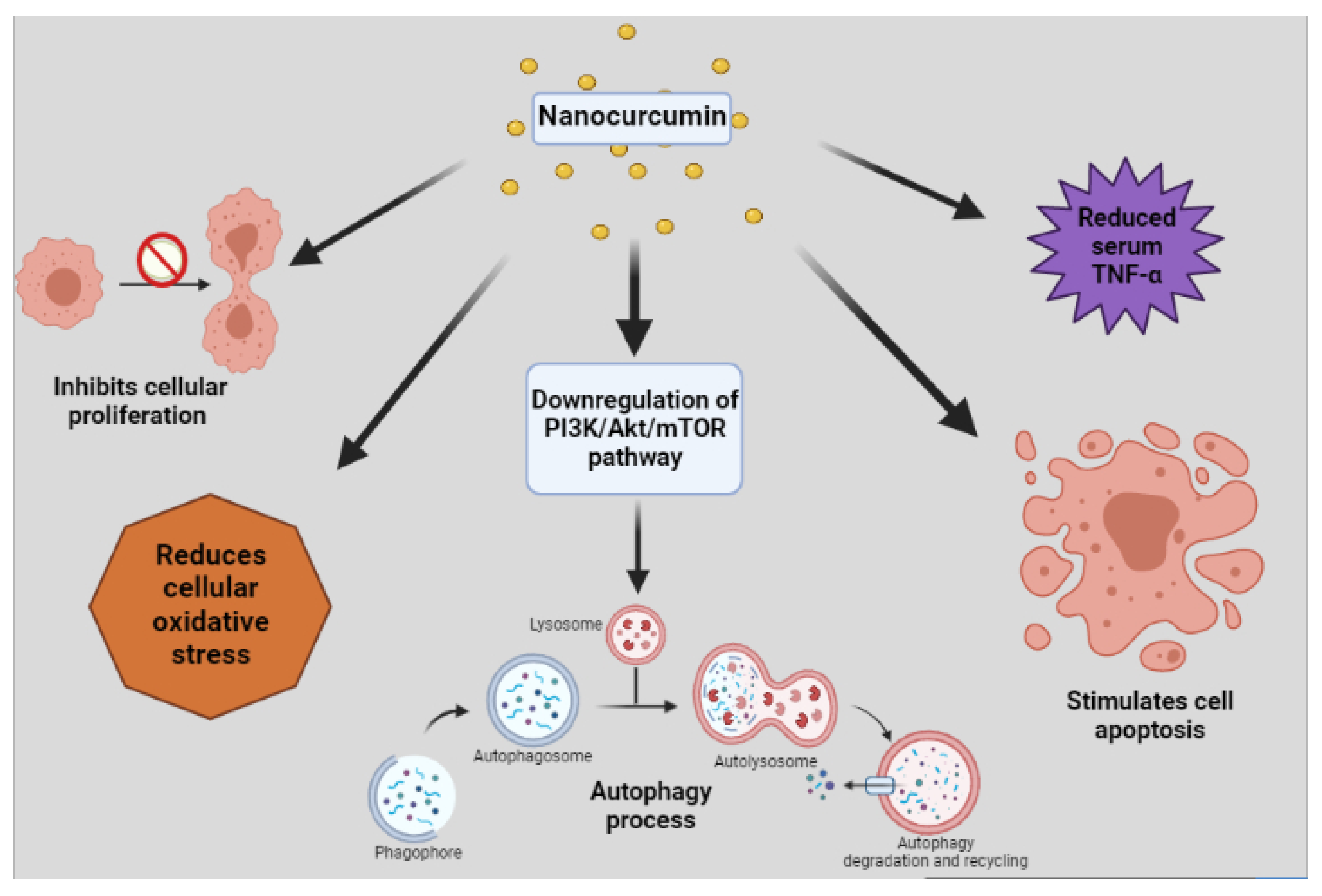
3. Uses of Nanocurcumin
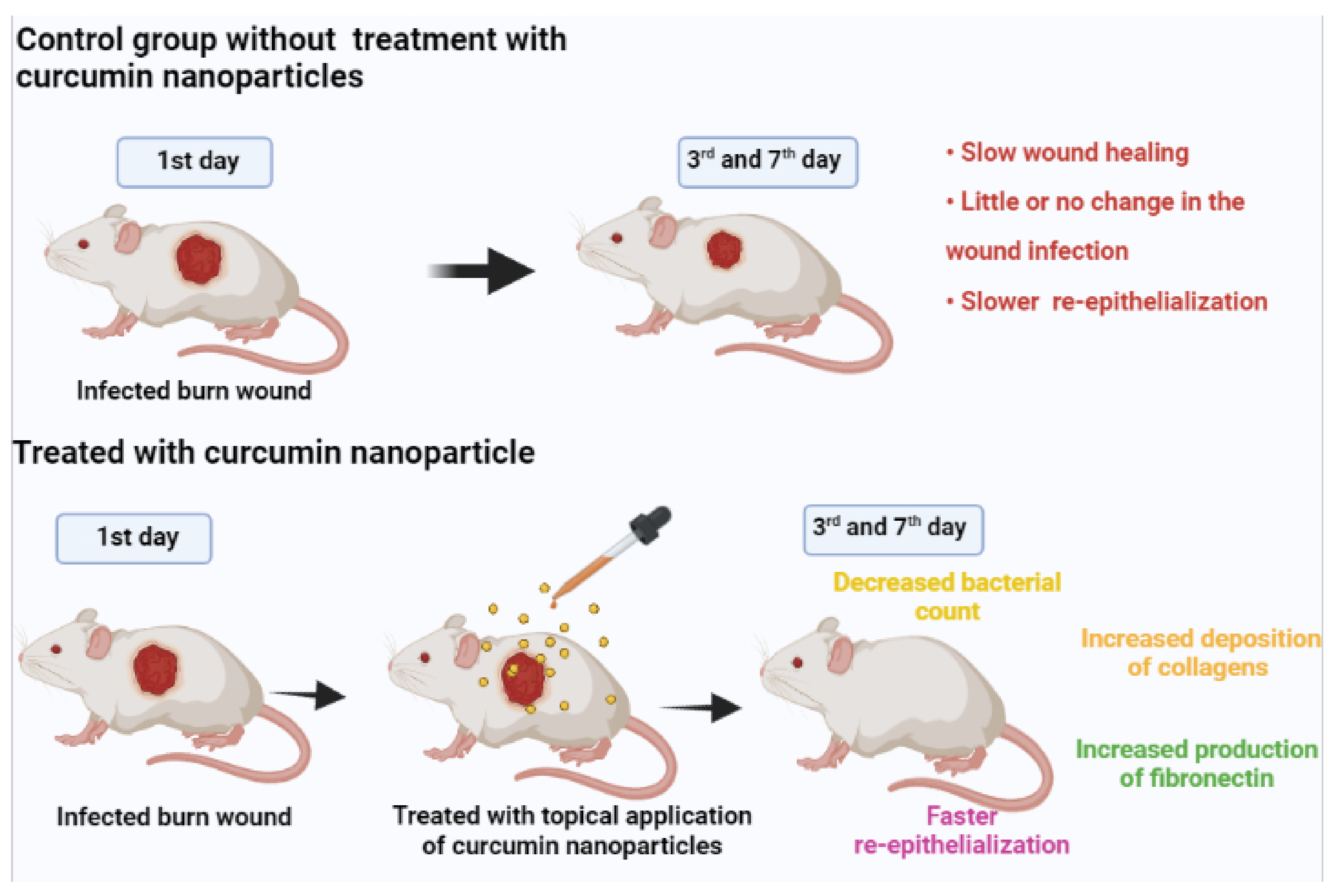
3.1. Wound Healing
3.2. Hepatoprotective
3.3. CVD
3.4. Nervous System
3.5. Lipid Profile
3.6. Antioxidant
3.7. Anti-Fibrinolytic Effect
3.8. Anti Protozoal Activity
3.9. Anti-Bacterial
3.10. Anti HIV
3.11. Anti-Inflammatory
3.12. Anti-Tumor
3.13. Anti-Diabetic
3.14. Anti-Coagulant Activity
4. Recent Patents for Nanocurcumin
5. Recent Findings with Use of Nanocurcumin
6. Research Gaps and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Tat | trans-activator of transcription |
| CCR5 | C-C chemokine receptor type 5 |
| VTE | Venous thromboembolism |
| TLR | Toll-like receptor |
| LPS | Lipopolysaccharide |
| GSK3b | Glycogen Synthase Kinase-3b |
| CoNS | Coagulase-Negative Staphylococci |
| HIV | Human Immunodeficiency Virus |
| ROS | Reactive Oxygen Species |
| DSPN | Diabetic Sensorimotor Polyneuropathies |
| LPS | Lipopolysaccharide |
| MAE | Mean Absolute Error |
| PGE2 | Prostaglandin E 2 |
| NO | Nitrous Oxide |
| NF-κB | Nuclear Factor kappa light chain enhancer of activated B cells |
| HEMA | 2-hydroxyethyl methacrylate |
| CCl4 | Carbon tetrachloride |
| ALT | Alanine Transaminase |
| AST | Aspartate aminotransferase |
| ALP | Alkaline phosphatase |
| CAT | Common Antioxidant Enzyme |
| CRP | c-reactive protein |
| SOD | Superoxide dismutase |
| GPX | Glutathione peroxidase |
| CuSO4 | Copper sulfate |
| LDH | Lactate dehydrogenase |
| CVS | Cardiovascular system |
| CYP3A4 | Cytochrome P4503A4 |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| ICAM-1 | Intracellular Adhesion Molecule 1 |
| Nrf2 | Nuclear factor erythroid 2 related factor |
| CNS | Central Nervous System |
| HDL-C | High Density Lipoprotein-Cholesterol |
| HLB | hydrophilic-lipophilic balance |
| LDL-C | Low Density Lipoprotein-Cholesterol |
| VLDL-C | Very low density Lipoprotein-Cholesterol |
| GSH | Glutathione |
| TAC | Total antioxidant capacity |
| uPA | urokinase plasminogen activator |
| JNK | Jun N-terminal kinase |
| p38MAPK | p38 mitogen activated protein kinase |
| PAI-I | Plasminogen Activator Inhibitor-I |
| PfGST | Glutathionetransferase |
| PLGA | poly (lactic-co-glycolic acid) |
| STZ toxin | Streptozotocin |
| Ftszprotein | filamenting temperature sensitive mutant |
| PT | Prothrombin Time |
| APTT | ActivatedPartialThromboplastin Time |
| FBS | Fasting Blood Sugar |
| HbA1c | GlycatedHaemoglobin |
| PVC | polyvinyl chloride |
| iNOS | inducible nitric oxide synthase |
References
- Hu, R.W.; Carey, E.J.; Lindor, K.D.; Tabibian, J.H. Curcumin in hepatobiliary disease: Pharmacotherapeutic properties and emerging potential clinical applications. Ann. Hepatol. 2018, 16, 835–841. [Google Scholar] [CrossRef]
- Bagheri, H.; Ghasemi, F.; Barreto, G.E.; Rafiee, R.; Sathyapalan, T.; Sahebkar, A. Effects of curcumin on mitochondria in neurodegenerative diseases. Biofactors 2020, 46, 5–20. [Google Scholar] [CrossRef]
- López-Malo, D.; Villarón-Casares, C.A.; Alarcón-Jiménez, J.; Miranda, M.; Díaz-Llopis, M.; Romero, F.J.; Villar, V.M. Curcumin as a Therapeutic Option in Retinal Diseases. Antioxidants 2020, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Ngadi, M.O.; Ma, Y. Optimisation of pulsed ultrasonic and microwave-assisted extraction for curcuminoids by response surface methodology and kinetic study. Food Chem. 2014, 165, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Hmar, B.Z.; Kalita, D.; Srivastava, B. Optimization of microwave power and curing time of turmeric rhizome (Curcuma longa L.) based on textural degradation. LWT 2017, 76, 48–56. [Google Scholar] [CrossRef]
- Najafpour, G. Formic acid and microwave assisted extraction of curcumin from turmeric (Curcuma longa L.). Int. J. Eng. 2016, 29, 145–151. [Google Scholar]
- Kapelle, I.B.D.; Manalu, W. Pengaruh Metode Proses Sintesis Analog Kurkumin Asimetris Terhadap Efek Hepatoprotektif Mencit (Mus musculus L.). J. Bioteknol. Biosains Indones. 2020, 7, 215–225. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Shirsath, S.; Sable, S.; Gaikwad, S.; Sonawane, S.; Saini, D.; Gogate, P. Intensification of extraction of curcumin from Curcuma amada using ultrasound assisted approach: Effect of different operating parameters. Ultrason. Sonochem. 2017, 38, 437–445. [Google Scholar] [CrossRef]
- Jatoi, S.A.; Kikuchi, A.; Gilani, S.A.; Watanabe, K.N. Phytochemical, pharmacological and ethnobotanical studies in mango ginger (Curcuma amada Roxb.; Zingiberaceae). Phytother. Res. 2007, 21, 507–516. [Google Scholar] [CrossRef]
- Mannaï, A.; Jableoui, C.; Hamrouni, L.; Allaf, K.; Jamoussi, B. DIC as a pretreatment prior to ultrasonic extraction for the improvement of rebaudioside A yield and preservation of vitamin B1 and B6. J. Food Meas. Charact. 2019, 13, 2764–2772. [Google Scholar] [CrossRef]
- Serpa Guerra, A.M.; Cock, J.A.; Vuerra, A.M. G yield and preservation of vitamin B1 and B6. as a pretreatment priEffect of ultra-fine friction grinding on the physical and chemical properties of curcuma (Curcuma longa L.) suspensions. J. Food Sci. 2020, 85, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Kiamahalleh, M.V.; Najafpour-Darzi, G.; Rahimnejad, M.; Moghadamnia, A.A.; Kiamahalleh, M.V. High performance curcumin subcritical water extraction from turmeric (Curcuma longa L.). J. Chromatogr. B 2016, 1022, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.-P.; Hu, D.-J.; Zhou, Y.-Q.; Zhang, Q.-W.; Zhao, J.; Li, S.-P. Preparation and application of standardized typical volatile components fraction from turmeric (Curcuma longa L.) by supercritical fluid extraction and step molecular distillation. Molecules 2018, 23, 1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurmudle, N.; Kagliwal, L.D.; Bankar, S.; Singhal, R.S. Enzyme-assisted extraction for enhanced yields of turmeric oleoresin and its constituents. Food Biosci. 2013, 3, 36–41. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Tortia, F.M.; Tortic, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Jordan, W.C.; Drew, C.R. Curcumin—A natural herb with anti-HIV activity. J. Natl. Med. Assoc. 1996, 88, 333. [Google Scholar] [PubMed]
- Taylor, D.L.; Nash, R.; Fellows, L.E.; Kang, M.S.; Tyms, A.S. Naturally occurring pyrrolizidines: Inhibition of α-glucosidase 1 and anti-HIV activity of one stereoisomer. Antivir. Chem. Chemother. 1992, 3, 273–277. [Google Scholar] [CrossRef] [Green Version]
- Mena, S.; Ortega, A.; Estrela, J.M. Oxidative stress in environmental-induced carcinogenesis. Mutat. Res. Toxicol. Environ. Mutagen. 2009, 674, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in cancer chemoprevention: Molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch. Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef]
- Kharat, M.; McClements, D.J. Recent advances in colloidal delivery systems for nutraceuticals: A case study—Delivery by Design of curcumin. J. Colloid Interface Sci. 2019, 557, 506–518. [Google Scholar] [CrossRef]
- Mohanty, C.; Das, M.; Sahoo, S.K. Emerging role of nanocarriers to increase the solubility and bioavailability of curcumin. Expert Opin. Drug Deliv. 2012, 9, 1347–1364. [Google Scholar] [CrossRef]
- Goldberg, M.; Langer, R.; Jia, X. Nanostructured materials for applications in drug delivery and tissue engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 241–268. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Hu, Q.; Ji, W.; Wright, G.; Gu, Z. Leveraging Physiology for Precision Drug Delivery. Physiol. Rev. 2017, 97, 189–225. [Google Scholar] [CrossRef]
- Maherani, B.; Arab-Tehrany, E.; Kheirolomoom, A.; Geny, D.; Linder, M. Calcein release behavior from liposomal bilayer; influence of physicochemical/mechanical/structural properties of lipids. Biochimie 2013, 95, 2018–2033. [Google Scholar] [CrossRef]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2019, 30, 336–365. [Google Scholar] [CrossRef]
- Jangle, R.D.; Thorat, B.N. Effect of Freeze-Thawing Study on Curcumin Liposome for Obtaining Better Freeze-Dried Product. Dry. Technol. 2013, 31, 966–974. [Google Scholar] [CrossRef]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, P.; Lalani, R.; Vhora, I.; Patil, S.; Amrutiya, J.; Misra, A.; Mashru, R. Liposomes encapsulating native and cyclodextrin enclosed paclitaxel: Enhanced loading efficiency and its pharmacokinetic evaluation. Int. J. Pharm. 2018, 536, 95–107. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Q.; Zhang, Z.; Yuan, L.; Liu, X.; Zhou, L. Preparation of Curcumin-Loaded Liposomes and Evaluation of Their Skin Permeation and Pharmacodynamics. Molecules 2012, 17, 5972–5987. [Google Scholar] [CrossRef]
- Wang, S.; Ha, Y.; Huang, X.; Chin, B.; Sim, W.; Chen, R. A New Strategy for Intestinal Drug Delivery via pH-Responsive and Membrane-Active Nanogels. ACS Appl. Mater. Interfaces 2018, 10, 36622–36627. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Edit. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Ganesh, G.N.; Singh, M.K.; Datri, S.; Karri, V.V. Design and development of curcumin nanogel for squamous cell carcinoma. J. Pharm. Sci. Res. 2019, 11, 1683. [Google Scholar]
- Khosropanah, M.H.; Dinarvand, A.; Nezhadhosseini, A.; Haghighi, A.; Hashemi, S.; Nirouzad, F.; Khatamsaz, S.; Entezari, M.; Hashemi, M.; Dehghani, H. Analysis of the antiproliferative effects of curcumin and nanocurcumin in MDA-MB231 as a breast cancer cell line. Iran. J. Pharm. Res. 2016, 15, 231. [Google Scholar] [PubMed]
- Gonçalves, C.; Schellenberg, P.M.; Gama, F.; Coutinho, P.J.; Pereira, P. Self-assembled dextrin nanogel as curcumin delivery system. J. Biomater. Nanobiotech. 2012, 3, 178–184. [Google Scholar] [CrossRef]
- Krausz, A.E.; Adler, B.L.; Cabral, V.; Navati, M.; Doerner, J.; Charafeddine, R.; Chandra, D.; Liang, H.; Gunther, L.; Clendaniel, A.; et al. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 195–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A promising candidate for therapeutic applications. Front. Pharm. 2020, 11, 487. [Google Scholar] [CrossRef]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin delivery mediated by bio-based nanoparticles: A review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Sun, L.; Wu, Q.; Guo, W.; Li, L.; Chen, Y.; Li, Y.; Gong, C.; Qian, Z.; Wei, Y. Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int. J. Pharm. 2013, 443, 175–182. [Google Scholar] [CrossRef]
- Sinjari, B.; Pizzicannella, J.; D’Aurora, M.; Zappacosta, R.; Gatta, V.; Fontana, A.; Trubiani, O.; Diomede, F. Curcumin/Liposome Nanotechnology as Delivery Platform for Anti-inflammatory Activities via NFkB/ERK/pERK Pathway in Human Dental Pulp Treated With 2-HydroxyEthyl MethAcrylate (HEMA). Front. Physiol. 2019, 10, 633. [Google Scholar] [CrossRef]
- Li, J.; Niu, R.; Dong, L.; Gao, L.; Zhang, J.; Zheng, Y.; Shi, M.; Liu, Z.; Li, K. Nanoencapsulation of Curcumin and Its Protective Effects against CCl4-Induced Hepatotoxicity in Mice. J. Nanomater. 2019, 2019, 7140132. [Google Scholar] [CrossRef]
- Alhusaini, A.; Hasan, I.; AlDowsari, N.; Alsaadan, N. Prophylactic Administration of Nanocurcumin Abates the Incidence of Liver Toxicity Induced by an Overdose of Copper Sulfate: Role of CYP4502E1, NF-κB and Bax Expressions. Dose-Response 2018, 16, 1559325818816284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maghsoumi, F.; Bidgoli, S.A. Hepatoprotective Effects of Curcumin Nanomicells in Alcohol-induced Liver Injury: Comparison with Curcumin and Silymarin in Mice Model. J. Med. Plants 2020, 4, 64–77. [Google Scholar] [CrossRef]
- Sayrafi, R.; Hosseini, S.M.; Ahmadi, M.A. The protective effects of nanocurcumin on liver toxicity induced by salinomycin in broiler chickens. Rev. Med. Vet. 2017, 168, 136–142. [Google Scholar]
- Sookoian, S.; Pirola, C.J. Alanine and aspartate aminotransferase and glutamine-cycling pathway: Their roles in pathogenesis of metabolic syndrome. World J. Gastroenterol. 2012, 8, 3775. [Google Scholar] [CrossRef]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.J. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2019, 16, 8. [Google Scholar] [CrossRef]
- Salehi, B.; Prado-Audelo, D.; María, L.; Cortés, H.; Leyva-Gómez, G.; Stojanović-Radić, Z.; Singh, Y.D.; Patra, J.K.; Das, G.; Martins, N.; et al. Therapeutic applications of curcumin nanomedicine formulations in cardiovascular diseases. J. Clin. Med. 2020, 9, 746. [Google Scholar] [CrossRef] [Green Version]
- Namdari, M.; Eatemadi, A. Cardioprotective effects of curcumin-loaded magnetic hydrogel nanocomposite (nanocurcumin) against doxorubicin-induced cardiac toxicity in rat cardiomyocyte cell lines. Artif. Cells Nanomed. Biotechnol. 2016, 45, 731–739. [Google Scholar] [CrossRef] [Green Version]
- Vafadar_afshar, G.; Khadem-Ansari, M.H.; Makhdomii, K.; Rasooli, J. The effects of nano-curcumin supplementation on serum level of hs-CRP, adhesion molecules, and lipid profiles in hemodialysis patients, a randomized controlled clinical trial. Iran. J. Kidney Dis. 2020, 14, 52. [Google Scholar]
- Helli, B.; Gerami, H.; Kavianpour, M.; Heybar, H.; Hosseini, S.K.; Haghighian, H.K. Curcumin Nanomicelle Improves Lipid Profile, Stress Oxidative Factors and Inflammatory Markers in Patients Undergoing Coronary Elective Angioplasty; A Randomized Clinical Trial. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 33397249. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Rana, S.; Banerjee, D.; Mitra, A.; Datta, R.; Naskar, S.; Sarkar, S. Improved bioavailability of targeted Curcumin delivery efficiently regressed cardiac hypertrophy by modulating apoptotic load within cardiac microenvironment. Toxicol. Appl. Pharm. 2016, 290, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, J.; Li, S.Y. Nano-Curcumin Simultaneously Protects the Blood-Brain Barrier and Reduces M1 Microglial Activation During Cerebral Ischemia-Reperfusion Injury. ACS Appl. Mater. Interfaces 2019, 11, 3763–3770. [Google Scholar] [CrossRef]
- Djiokeng Paka, G.; Doggui, S.; Zaghmi, A.; Safar, R.; Dao, L.; Reisch, A.; Klymchenko, A.; Roullin, V.G.; Joubert, O.; Ramassamy, C. Neuronal uptake and neuroprotective properties of curcumin-loaded nanoparticles on SK-N-SH cell line: Role of poly (lactide-co-glycolide) polymeric matrix composition. Mol. Pharm. 2016, 13, 391–403. [Google Scholar] [CrossRef]
- Panzarini, E.; Mariano, S.; Tacconi, S.; Carata, E.; Tata, A.M.; Dini, L. Novel Therapeutic Delivery of Nanocurcumin in Central Nervous System Related Disorders. Nanomaterials 2021, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Abdolahi, M.; Jafarieh, A.; Sarraf, P.; Sedighiyan, M.; Yousefi, A.; Tafakhori, A.; Abdollahi, H.; Salehinia, F.; Djalali, M. The Neuromodulatory Effects of ω-3 Fatty Acids and Nano-Curcumin on the COX-2/iNOS Network in Migraines: A Clinical Trial Study from Gene Expression to Clinical Symptoms. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 874–884. [Google Scholar] [CrossRef]
- Sahab-Negah, S.; Ariakia, F.; Jalili-Nik, M.; Afshari, A.R.; Salehi, S.; Samini, F.; Rajabzadeh, G.; Gorji, A. Curcumin Loaded in Niosomal Nanoparticles Improved the Anti-tumor Effects of Free Curcumin on Glioblastoma Stem-like Cells: An In Vitro Study. Mol. Neurobiol. 2020, 57, 3391–3411. [Google Scholar] [CrossRef]
- Murthy, K.C.; Monika, P.; Jayaprakasha, G.K.; Patil, B.S. Nanoencapsulation: An advanced nanotechnological approach to enhance the biological efficacy of curcumin. In Advances in Plant Phenolics: From Chemistry to Human Health; ACS: Washington, DC, USA, 2018; pp. 383–405. [Google Scholar]
- Shamsi-Goushki, A.; Mortazavi, Z.; Mirshekar, M.A.; Mohammadi, M.; Moradi-Kor, N.; Jafari-Maskouni, S.; Shahraki, M. Comparative Effects of Curcumin versus Nano-Curcumin on Insulin Resistance, Serum Levels of Apelin and Lipid Profile in Type 2 Diabetic Rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, F.; Fakhri, S.; Shakeryan, S.; Alizadeh, A. The Effect of Short–Term Nanocurcumin Supplementation on the Anthropometric Indices, Lipid Profile and C-Reactive Protein of Overweight Girls. Complement. Med. J. 2020, 10, 94–105. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Biswas, S.; Mukhopadhyay, S.; Chattopadhyay, B. Amelioration of Nicotine Induced Toxicity by Nanocurcumin in Protein Malnourished Condition. J. Biomed. Res. Environ. Sci. 2018, 4, 1–8. [Google Scholar]
- Carvalho, D.D.; Takeuchi, K.P.; Geraldine, R.M.; Moura, C.J.; Torres, M.C. Production, solubility and antioxidant activity of curcumin nanosuspension. Food Sci. Technol. 2015, 35, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Potphode, N.D.; Daunde, J.A.; Desai, S.S.; Walvekar, M.V. Nano-curcumin: A Potent Enhancer of Body Antioxidant System in Diabetic Mice. Int. J. Phytomed. 2018, 10, 162. [Google Scholar] [CrossRef]
- El-Desoky, G.E.; Wabaidur, S.M.; Alothman, Z.A.; Habila, M.A. Regulatory Role of Nano-Curcumin against Tartrazine-Induced Oxidative Stress, Apoptosis-Related Genes Expression, and Genotoxicity in Rats. Molecules 2020, 25, 5801. [Google Scholar]
- Ranjbar, A.; Gholami, L.; Ghasemi, H.; Kheiripour, N. Effects of nano-curcumin and curcumin on the oxidant and antioxidant system of the liver mitochondria in aluminum phosphide-induced experimental toxicity. Nanomed. J. 2020, 7, 58–64. [Google Scholar]
- Flora, G.; Gupta, D.; Tiwari, A. Preventive Efficacy of Bulk and Nanocurcumin Against Lead-Induced Oxidative Stress in Mice. Biol. Trace Element Res. 2013, 152, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Rasaie, D.; Asl, S.S.; Ahmadabadi, A.N.; Ranjbar, A. Evaluation of the protective effects of curcumin and nanocurcumin against lung injury induced by sub-acute exposure to paraquat in rats. Toxin Rev. 2019, 1–9. [Google Scholar] [CrossRef]
- Rajasekar, A.; Devasena, T. Facile Synthesis of Curcumin Nanocrystals and Validation of Its Antioxidant Activity Against Circulatory Toxicity in Wistar Rats. J. Nanosci. Nanotechnol. 2015, 15, 4119–4125. [Google Scholar] [CrossRef]
- Kazemi-Darabadi, S.; Nayebzadeh, R.; Shahbazfar, A.A.; Kazemi-Darabadi, F.; Fathi, E. Curcumin and Nanocurcumin Oral Supplementation Improve Muscle Healing in a Rat Model of Surgical Muscle Laceration. Bull. Emerg. Trauma 2019, 7, 292–299. [Google Scholar] [CrossRef]
- Madhyastha, R.; Nakajima, Y.; Omura, S.; Maruyama, M. Curcumin Facilitates Fibrinolysis and Cellular Migration during Wound Healing by Modulating Urokinase Plasminogen Activator Expression. Pathophysiol. Haemost. Thromb. 2009, 37, 59–66. [Google Scholar] [CrossRef]
- Malhotra, M.; Rai, A.; Malhotra, V. Curcumin in the Management of Oral Potentially Malignant Disorders. World J. Pharm. Res. 2019, 8, 1–21. [Google Scholar]
- Gouda, M.M.; Prabhu, A.; Varsha, S.V.; Jahan, R.; Bhandary, Y.P. Nano-Curcumin Regulates p53 Phosphorylation and PAI-1 Expression during Bleomycin Induced Injury in Alveolar Basal Epithelial Cells. Curr. Bioact. Compd. 2020, 16, 85–89. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zhu, R.; Liu, Q.; Fei, J.; Wang, S. Anti-inflammatory activity of curcumin-loaded solid lipid nanoparticles in IL-1β transgenic mice subjected to the lipopolysaccharide-induced sepsis. Biomaterials 2015, 53, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Yallapu, M.; Núñez, D.; Oyarzún, P.; López, M.; Jayaramudu, T.; Karthikeyan, C. Generation of engineered core–shell antibiotic nanoparticles. RSC Adv. 2019, 9, 8326–8332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Yao, Y.; Yu, Y.; Zeng, Y. Enhanced Antibacterial Activity of Curcumin by Combination with Metal Ions. Colloid Interface Sci. Commun. 2018, 25, 1–6. [Google Scholar] [CrossRef]
- Hosseini, S.; Chamani, J.; Rahimi, H.; Azmoodeh, N.; Ghasemi, F.; Abadi, P.H. An In vitro Study on Curcumin Delivery by Nano-Micelles for Esophageal Squamous Cell Carcinoma (KYSE-30). Rep. Biochem. Mol. Biol. 2018, 6, 137–143. [Google Scholar]
- Ganugula, R.; Arora, M.; Jaisamut, P.; Wiwattanapatapee, R.; Jørgensen, H.G.; Venkatpurwar, V.P.; Zhou, B.; Hoffmann, A.R.; Basu, R.; Guo, S.; et al. Nano-curcumin safely prevents streptozotocin-induced inflammation and apoptosis in pancreatic beta cells for effective management of Type 1 diabetes mellitus. Br. J. Pharm. 2017, 174, 2074–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldahoun, M.A.; Jaafar, M.S.; Al-Akhras, M.-A.; Bououdina, M. Enhanced nanocurcumin toxicity against (PC3) tumor and microbial by using magnetic field in vitro. Artif. Cells Nanomed. Biotechnol. 2016, 45, 843–853. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Abdelfatah, E.A.; Khalil, W.A. Antitumor Activity of Curcumin-Green Synthesized Gold Nanoparticles: In Vitro Study. BioNanoScience 2019, 9, 813–820. [Google Scholar] [CrossRef]
- Capela, R.; Moreira, R.; Lopes, F. An Overview of Drug Resistance in Protozoal Diseases. Int. J. Mol. Sci. 2019, 20, 5748. [Google Scholar] [CrossRef] [Green Version]
- Saheb, E.J. The prevalence of parasitic protozoan diseases in Iraq, 2016. Karbala Int. J. Mod. Sci. 2018, 4, 21–25. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Anasane, N.; Dos Santos, C.A. Curcumin and curcumin-loaded nanoparticles: Antipathogenic and antiparasitic activities. Expert Rev. Anti. Infect. Ther. 2020, 18, 367–379. [Google Scholar] [CrossRef]
- Subramani, P.A.; Panati, K.; Lebaka, V.R.; Reddy, D.D.; Narala, V.R. Nanostructures for Curcumin Delivery: Possibilities and Challenges; Nano-and Microscale Drug Delivery Systems Elsevier: Amsterdam, The Netherlands, 2017; pp. 393–418. [Google Scholar]
- Montanaro, L.; Campoccia, D.; Arciola, C.R. Advancements in molecular epidemiology of implant infections and future perspectives. Biomaterials 2007, 28, 5155–5168. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Kannan, I.; Jeyakumari, D.; Premavathi, R.K.; Sukumar, R.G.; Shantha, S. In vitro antibacterial activity of synthesized curcumin-silver nanoparticles. Int. J. Med. Clin. Appl. Micr. 2017, 1, 1–8. [Google Scholar]
- Soumya, K.R.; Jishma, P.; Dhivya, R.; Annaraj, J.; Sugathan, S.; Mathew, J.; Radhakrishnan, E.K. Role of Nanocurcumin as a Surface Modifying Agent with Excellent Preventive Effect on Device-Related CoNS Infections. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2019, 90, 29–35. [Google Scholar] [CrossRef]
- Perera, W.P.T.D.; Dissanayake, R.K.; Ranatunga, U.I.; Hettiarachchi, N.M.; Perera, K.D.C.; Unagolla, J.M.; De Silva, R.T.; Pahalagedara, L.R. Curcumin loaded zinc oxide nanoparticles for activity-enhanced antibacterial and anticancer applications. RSC Adv. 2020, 10, 30785–30795. [Google Scholar] [CrossRef]
- Reda, F.M.; El-Saadony, M.T.; ElNesr, S.S.; Alagawany, M.; Tufarelli, V. Effect of Dietary Supplementation of Biological Curcumin Nanoparticles on Growth and Carcass Traits, Antioxidant Status, Immunity and Caecal Microbiota of Japanese Quails. Animals 2020, 10, 754. [Google Scholar] [CrossRef]
- Adahoun, M.A.; Al-Akhras, M.-A.; Jaafar, M.S.; Bououdina, M. Enhanced anti-cancer and antimicrobial activities of curcumin nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 45, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Fathi, N.; Memar, M.Y.; Hosseiniyan Khatibi, S.M.; Khalilov, R.; Negahdari, R.; Zununi Vahed, S.; Maleki Dizaj, S. Anti-microbial activity of curcumin nanoformulations: New trends and future perspectives. Phytother. Res. 2020, 34, 1926–1946. [Google Scholar] [CrossRef] [PubMed]
- Praditya, D.; Kirchhoff, L.; Brüning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-infective Properties of the Golden Spice Curcumin. Front. Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef] [Green Version]
- Laila, U.; Akram, M.; Shariati, M.A.; Hashmi, A.M.; Akhtar, N.; Tahir, I.M.; Ghauri, A.O.; Munir, N.; Riaz, M.; Shaheen, G.; et al. Role of medicinal plants in HIV/AIDS therapy. Clin. Exp. Pharmacol. Physiol. 2019, 46, 1063–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.K.; Cwiklinski, K.; Aalinkeel, R.; Reynolds, J.L.; Sykes, D.E.; Quaye, E.; Oh, J.; Mahajan, S.D.; Schwartz, S.A. Immunomodulatory activities of curcumin-stabilized silver nanoparticles: Efficacy as an antiretroviral therapeutic. Immunol. Investig. 2017, 46, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Keikha, M.; Ghazvini, K. Can Curcumin be Used as an Anti-HIV Therapeutic Option? Iran. J. Virol. 2018, 12, 34–37. [Google Scholar]
- Schett, G.; Neurath, M.F. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef]
- Slam, M.A.; Pröll, M.; Hölker, M.; Tholen, E.; Tesfaye, D.; Looft, C.; Schellander, K.; Cinar, M.U. Alveolar macrophage phagocytic activity is enhanced with LPS priming, and combined stimulation of LPS and lipoteichoic acid synergistically induce pro-inflammatory cytokines in pigs. Innate Immun. 2013, 19, 631–643. [Google Scholar]
- Kim, S.A.; Choi, H.C. Metformin inhibits inflammatory response via AMPK–PTEN pathway in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2012, 425, 866–872. [Google Scholar] [CrossRef]
- Almarzany, Z.S. Anti-inflammatory and Anti-Arthritic Activity of Nanocurcumin in Albino Rats against Freund’s Complete Adjuvant Induced-Arthritis. Syst. Rev. Pharm. 2020, 11, 98–105. [Google Scholar]
- Hafezi, K.; Hemmati, A.A.; Abbaszadeh, H.; Valizadeh, A.; Makvandi, M. Anticancer activity and molecular mechanisms of α-conidendrin, a polyphenolic compound present in Taxus yunnanensis, on human breast cancer cell lines. Phytother. Res. 2020, 34, 1397–1408. [Google Scholar] [CrossRef]
- Liu, H.-T.; Ho, Y.-S. Anticancer effect of curcumin on breast cancer and stem cells. Food Sci. Hum. Wellness 2018, 7, 134–137. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Bagherian, M.; Azami, N.; Bejandi, A.K.; Hushmandi, K.; Ang, H.L.; et al. Polychemotherapy with Curcumin and Doxorubicin via Biological Nanoplatforms: Enhancing Antitumor Activity. Pharmaceutics 2020, 12, 1084. [Google Scholar] [CrossRef]
- Ma, C.; Zhuang, Z.; Su, Q.; He, J.; Li, H. Curcumin Has Anti-Proliferative and Pro-Apoptotic Effects on Tongue Cancer in vitro: A Study with Bioinformatics Analysis and in vitro Experiments. Drug Des. Dev. Ther. 2020, 14, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh, E.; Bhatt, M.L.; Tripathi, M. Role of nano-curcumin: A treatment for cancer. J. Med. Plants 2017, 5, 394–397. [Google Scholar]
- Abou Zaid, O.A.; Ahmed, F.A.; Badwi, A.F.; Ibrahim, N.M. Protective role of new nanocomposite natural product (basic nano-curcumin) against breast carcinoma. Benha Vet. Med. J. 2017, 33, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.; Tran, N.T.N.; Le, P.N.; Nguyen, T.B.T.; Bach, L.G.; Doan, V.N.; Tran, H.L.B.; Le, V.T.; Tran, N.Q. Synergic Activity Against MCF-7 Breast Cancer Cell Growth of Nanocurcumin-Encapsulated and Cisplatin-Complexed Nanogels. Molecules 2018, 23, 3347. [Google Scholar] [CrossRef] [Green Version]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabet. Metab. Synd. Obes. 2014, 7, 587. [Google Scholar] [CrossRef] [Green Version]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Khamoshian, K.; Sotoudeh, G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo- controlled clinical trial. Complement. Ther. Med. 2019, 43, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes-Franco, S.; Padilla-Tejeda, D.E.; Carrillo-Ibarra, S.; Miranda-Díaz, A.G. Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy. Int. J. Endocrinol. 2018, 2018, 1875870. [Google Scholar] [CrossRef]
- Chaichian, S.; Shafabakhsh, R.; Mirhashemi, S.M.; Moazzami, B.; Asemi, Z. Circular RNAs: A novel biomarker for cervical cancer. J. Cell. Physiol. 2019, 235, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Gouda, W.; Hafiz, N.A.; Mageed, L.; Alazzouni, A.S.; Khalil, W.K.B.; Afify, M.; Abdelmaksoud, M.D.E. Effects of nano-curcumin on gene expression of insulin and insulin receptor. Bull. Natl. Res. Cent. 2019, 43, 128. [Google Scholar] [CrossRef] [Green Version]
- Mohiti-Ardekani, J.; Asadi, S.; Ardakani, A.M.; Rahimifard, M.; Baeeri, M.; Momtaz, S. Curcumin increases insulin sensitivity in C2C12 muscle cells via AKT and AMPK signaling pathways. Cogent Food Agric. 2019, 5, 1577532. [Google Scholar] [CrossRef]
- Abbaszadeh, F.; Fakhri, S.; Khan, H. Targeting apoptosis and autophagy following spinal cord injury: Therapeutic approaches to polyphenols and candidate phytochemicals. Pharm. Res. 2020, 160, 105069. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Hashemzaei, M.; Sahebkar, A. The regulatory role of curcumin on platelet functions. J. Cell. Biochem. 2018, 119, 8713–8722. [Google Scholar] [CrossRef]
- Falanga, A.; Russo, L.; Milesi, V.; Vignoli, A. Mechanisms and risk factors of thrombosis in cancer. Crit. Rev. Oncol. 2017, 118, 79–83. [Google Scholar] [CrossRef]
- Huang, M.-J.; Wei, R.-B.; Wang, Y.; Su, T.-Y.; Di, P.; Li, Q.-P.; Yang, X.; Li, P.; Chen, X.-M. Blood coagulation system in patients with chronic kidney disease: A prospective observational study. BMJ Open 2017, 7, e014294. [Google Scholar] [CrossRef] [Green Version]
- Keihanian, F.; Saeidinia, A.; Bagheri, R.K.; Johnston, T.P.; Sahebkar, A. Curcumin, hemostasis, thrombosis, and coagulation. J. Cell Physiol. 2018, 233, 4497–4511. [Google Scholar] [CrossRef]
- Kar, S.K.; Akhtar, F.; Ray, G.; Pandey, A.K. Curcumin Nanoparticles and Methods of Producing the Same. U.S. Patent Application No. 13/056,515, 4 August 2011. [Google Scholar]
- Bansal, A.K.; Munjal, B.; Patel, S.B. Self-Nano-Emulsifying Curcuminoids Composition with Enhanced Bioavailability. WIPO Patent WO2010010431A1, 28 January 2010. [Google Scholar]
- Ranjan, A.P.; Mukerjee, A.; Vishwanatha, J.K.; Helson, L. Curcumin-Er, a Liposomal-PLGA Sustained Release Nanocurcumin for Minimizing QT Prolongation for Cancer Therapy. U.S. Patent No. 9,138,411, 22 September 2015. [Google Scholar]
- Kar, S.K.; Das, G.; Suar, M. A Pharmaceutical Combination for Treating Tuberculosis. WIPO Patent WO2014170820A2, 23 October 2014. [Google Scholar]
- Helson, L. Intravenous Infusion of Curcumin and a Calcium Channel Blocker. U.S. Patent No. 8,747,890, 10 June 2014. [Google Scholar]
- Chaniyiyilparampu, R.N.; Mungala, M.; Kapoor, A.; Gokaraju, G.R.; Gokaraju, R.R.; Bhupathiraju, K.; Golakati, T.; Nair, A.K.; Murali, M.R.; Parthasarathy, K. Topical Formulation(s) for the Treatment of Inflammation, Skin and Mucosal Disorders and Other Diseases Thereof. U.S. Patent No. 8,535,693, 17 September 2013. [Google Scholar]
- Sezgin, V.C.; Bayraktar, O. Development of Curcumin and Piperine Loaded Double-Layered Biopolymer Based Nano Delivery Systems by Using Electrospray/Coating Method. U.S. Patent 10,398,650, 3 September 2019. [Google Scholar]
- Braden, A.R.C.; Vishwanatha, J.K. Formulation of Active Agent Loaded Activated PLGA Nanoparticles for Targeted Cancer Nanotherapeutics. Canada Patent CA2683777C, 23 August 2016. [Google Scholar]
- Ranjan, A.P.; Mukerjee, A.; Vishwanatha, J.K. University of North Texas Health Science Center, Assignee. Solid in Oil/Water Emulsion-Diffusion-Evaporation Formulation for Preparing Curcumin-Loaded PLGA Nanoparticles. U.S. Patent Application No. 12/766,068, 18 November 2010. [Google Scholar]
- Frautschy, S.A.; Cole, G.M. Bioavailable Curcuminoid Formulations for Treating Alzheimer’s Disease and Other Age-Related Disorders. U.S. Patent No. 9,192,644, 24 November 2015. [Google Scholar]
- Valizadeh, H.; Abdolmohammadi-Vahid, S.; Danshina, S.; Gencer, M.Z.; Ammari, A.; Sadeghi, A.; Roshangar, L.; Aslani, S.; Esmaeilzadeh, A.; Ghaebi, M.; et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharmacol. 2020, 89, 107088. [Google Scholar] [CrossRef] [PubMed]
- Saber-Moghaddam, N.; Salari, S.; Hejazi, S.; Amini, M.; Taherzadeh, Z.; Eslami, S.; Rezayat, S.M.; Jaafari, M.R.; Elyasi, S. Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease -19 patients: An open label nonrandomized clinical trial. Phytother. Res. 2021, 35, 2616–2623. [Google Scholar] [CrossRef] [PubMed]
- Osali, A. Aerobic exercise and nano-curcumin supplementation improve inflammation in elderly females with metabolic syndrome. Diabetol. Metab. Syndr. 2020, 12, 26–27. [Google Scholar] [CrossRef]
- Bakhshi, M.; Gholami, S.; Mahboubi, A.; Jaafari, M.R.; Namdari, M. Combination Therapy with 1% Nanocurcumin Gel and 0.1% Triamcinolone Acetonide Mouth Rinse for Oral Lichen Planus: A Randomized Double-Blind Placebo Controlled Clinical Trial. Dermatol. Res. Pract. 2020, 2020, 4298193. [Google Scholar] [CrossRef]
- Cheragh-Birjandi, S.; Moghbeli, M.; Haghighi, F.; Safdari, M.R.; Baghernezhad, M.; Akhavan, A.; Ganji, R. Impact of resistance exercises and nano-curcumin on synovial levels of collagenase and nitric oxide in women with knee osteoarthritis. Transl. Med. Commun. 2020, 5, 3. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, Y.; Wei, Y.; Gao, Y.; Jiang, W.; Wang, G.; Wang, D. Facile synthetic nano-curcumin encapsulated Bio-fabricated nanoparticles induces ROS-mediated apoptosis and migration blocking of human lung cancer cells. Process. Biochem. 2020, 95, 91–98. [Google Scholar] [CrossRef]
- Djalali, M.; Abdolahi, M.; Hosseini, R.; Miraghajani, M.; Mohammadi, H.; Djalali, M. The effects of nano-curcumin supplementation on Th1/Th17 balance in migraine patients: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2020, 41, 101256. [Google Scholar] [CrossRef]
- Djalali, M.; Djalali, M.; Abdolahi, M.; Mohammadi, H.; Heidari, H.; Hosseini, S.; Sadeghizadeh, M. The Effect of Nano-Curcumin Supplementation on Pentraxin 3 Gene Expression and Serum Level in Migraine Patients. Rep. Biochem. Mol. Biol. 2020, 9, 32821745. [Google Scholar]
- Arzani, H.; Adabi, M.; Mosafer, J.; Dorkoosh, F.; Khosravani, M.; Maleki, H.; Nekounam, H.; Kamali, M. Preparation of curcumin-loaded PLGA nanoparticles and investigation of its cytotoxicity effects on human glioblastoma U87MG cells. Biointerface Res. Appl. Chem. 2019, 9, 4225–4231. [Google Scholar]
| Size | Activity | Study Type | Reference | |
|---|---|---|---|---|
| Curcumin loaded in Solid-liquid nanoparticles | 60 nm | Prevents LPS induced sepsis in | Pre-clinical study | [78] |
| (Cur-SLNs) | Animal used: mice | |||
| Zinc oxide–curcumin core–shell nanoparticles | ~45 nm ZnO core and ~12 nm curcumin shell | Antibacterial activity | In vitro study | [79] |
| (ZnO–Cum) | (including the antibiotic resistant bacteria) | bacterial strains using the diffusion method | ||
| Curcumin-TA-metal complex |
| Antibacterial activity | In vitro study | [80] |
| Microbiological study on agar plates | |||
| Nano-micelle containing curcumin (Sina Curcumin ®) | 10 nm |
| Pre-clinical study | [81] |
| (study performed on diabetic rats) | ||||
| Nanocurcumin | 300 nm |
| Pre-clinical study | [82] |
| Animal used—rats | ||||
| NANOCUR-MF | 34–359 nm |
| In vitro study | [83] |
| Nanocurcumin combined with magnetic field | 8 MT magnetic field | (on mammalian cell line) | ||
| Curcumin-reduced gold nanoparticles | 26 nm |
| In vitro study | [84] |
| (AuNP’s-Cur) | (on human cell line) |
| Disease Targeted | Outcome of Study | Reference |
|---|---|---|
| Coronavirus disease 2019 | Nanocurcumin modulated increase in rate of inflammatory cytokines in COVID-2019 patients. | [133] |
| Coronavirus disease 2019 | Symptoms of COVID-2019 resolved faster in group administered with Nanocurcumin and improved recovery rate. | [134] |
| Metabolic syndrome | Levels of Brain-derived neurotrophic factor, IL-10, serum concentrations of malondialdehyde decreased. | [135] |
| Oral lichen planus | Decrease in reticular-erosive-ulcerative (REU) score observed. | [136] |
| Knee Osteoarthritis | Reduced levels of Collagenase-2 and NO | [137] |
| Human lung cancer | Significantly inhibited the migration ability of A549 cells; promote intracellular ROS overproduction and induced apoptosis. | [138] |
| Migraine | Significant reduction in serum levels and expression of IL-17 mRNA | [139] |
| Migraine | PTX3 gene expression and serum levels were both significantly less | [140] |
| Human Glioblastoma | Enhancement in cytotoxicity against U87MG cell lines | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chopra, H.; Dey, P.S.; Das, D.; Bhattacharya, T.; Shah, M.; Mubin, S.; Maishu, S.P.; Akter, R.; Rahman, M.H.; Karthika, C.; et al. Curcumin Nanoparticles as Promising Therapeutic Agents for Drug Targets. Molecules 2021, 26, 4998. https://doi.org/10.3390/molecules26164998
Chopra H, Dey PS, Das D, Bhattacharya T, Shah M, Mubin S, Maishu SP, Akter R, Rahman MH, Karthika C, et al. Curcumin Nanoparticles as Promising Therapeutic Agents for Drug Targets. Molecules. 2021; 26(16):4998. https://doi.org/10.3390/molecules26164998
Chicago/Turabian StyleChopra, Hitesh, Protity Shuvra Dey, Debashrita Das, Tanima Bhattacharya, Muddaser Shah, Sidra Mubin, Samka Peregrine Maishu, Rokeya Akter, Md. Habibur Rahman, Chenmala Karthika, and et al. 2021. "Curcumin Nanoparticles as Promising Therapeutic Agents for Drug Targets" Molecules 26, no. 16: 4998. https://doi.org/10.3390/molecules26164998
APA StyleChopra, H., Dey, P. S., Das, D., Bhattacharya, T., Shah, M., Mubin, S., Maishu, S. P., Akter, R., Rahman, M. H., Karthika, C., Murad, W., Qusty, N., Qusti, S., Alshammari, E. M., Batiha, G. E.-S., Altalbawy, F. M. A., Albooq, M. I. M., & Alamri, B. M. (2021). Curcumin Nanoparticles as Promising Therapeutic Agents for Drug Targets. Molecules, 26(16), 4998. https://doi.org/10.3390/molecules26164998










