Subcritical Extracts from Major Species of Oil-Bearing Roses—A Comparative Chemical Profiling
Abstract
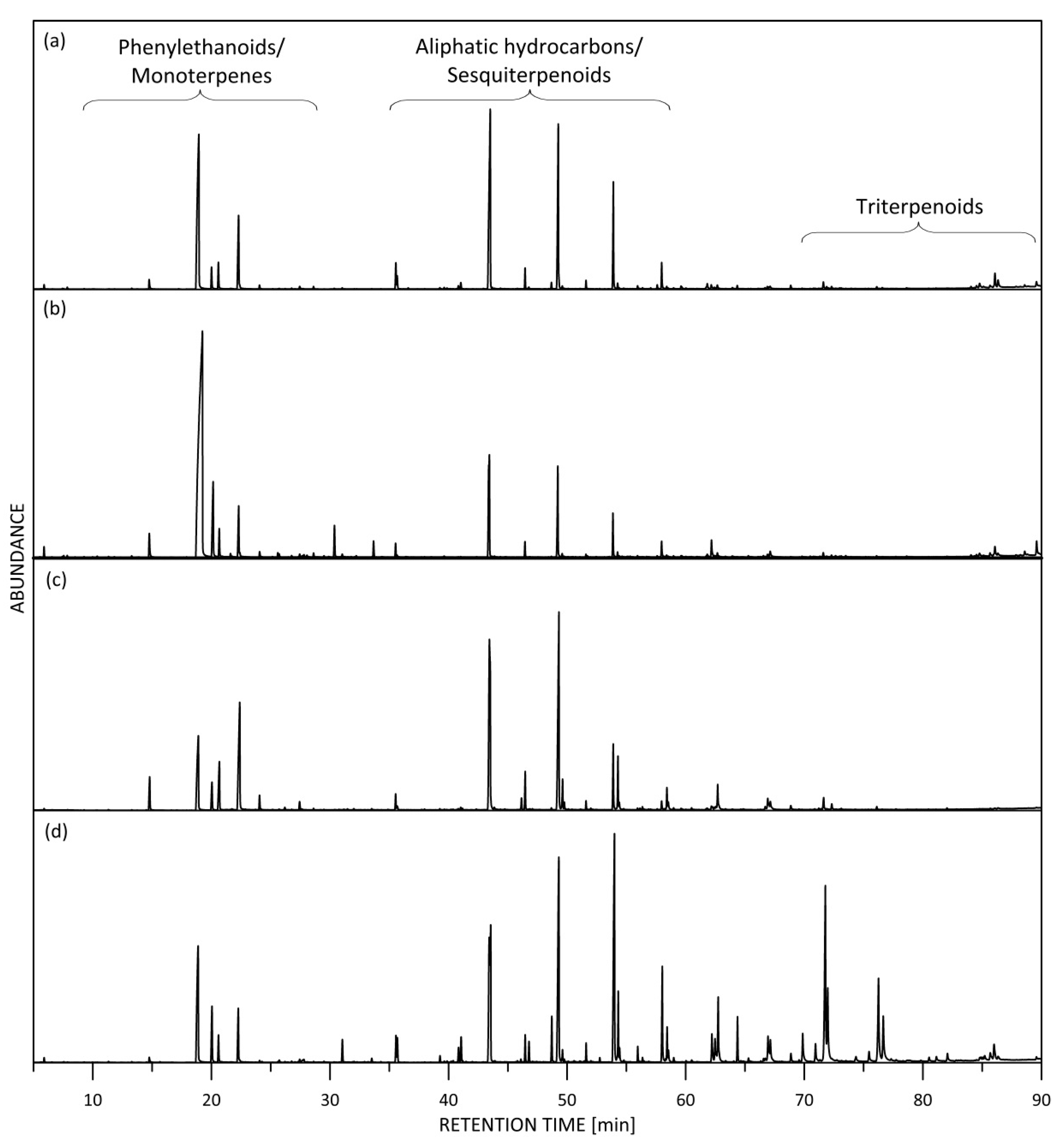
:1. Introduction
- -
- -
- Very high chemical resistance—no interaction with the processed plant, its compounds and equipment walls (high quality and purity of final product) [37];
- -
- Low viscosity and surface tension of liquid solvent [37]—intensive extraction process for short extraction times, even at low temperatures;
- -
- -
- Low boiling points [37]—easy and effective separation of final product from solvent;
- -
- Low values of vaporization heat [37]—high level of energy efficiency and low energy input for micellar separation;
- -
- Absence of own smells and taste [38]—high purity of final extracts;
- -
- Safe for the human health [38] and suitable for food-grade aroma preparations;
- -
- Fire and explosion safe properties [38];
- -
2. Results and Discussion
2.1. Phenylethanoids and Phenylpropanoids
2.2. Terpenoids
2.2.1. Monoterpenes and Their Oxygenated Derivatives
2.2.2. Sesquiterpenes
2.2.3. Triterpenoids
2.3. Aliphatic Hydrocarbons (Stearopten)
2.4. Others
3. Materials and Methods
3.1. Raw Plant Material
3.2. Extraction
3.3. Analysis
3.3.1. Gas Chromatography-Mass Spectrometry (GC/MS)
3.3.2. Gas Chromatography with Flame-Ionization Detector (GC-FID)
3.3.3. Identification, Quantitative Analysis and Chemometrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Panda, H. Cultivation and Utilization of Aromatic Plants; Asia Pacific Business Press: Delhi, India, 2006; ISBN 978-81-7833-027-3. [Google Scholar]
- Kovacheva, N.; Zheljazkov, V.D.; Astatkie, T. Productivity, Oil Content, Composition, and Bioactivity of Oil-Bearing Rose Accessions. HortScience 2011, 46, 710–714. [Google Scholar] [CrossRef] [Green Version]
- Pal, P.K. Evaluation, Genetic Diversity, Recent Development of Distillation Method, Challenges and Opportunities of Rosa damascena: A Review. J. Essent. Oil Bear. Plants 2013, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mileva, M.; Ilieva, Y.; Jovtchev, G.; Gateva, S.; Zaharieva, M.M.; Georgieva, A.; Dimitrova, L.; Dobreva, A.; Angelova, T.; Vilhelmova-Ilieva, N.; et al. Rose Flowers—A Delicate Perfume or a Natural Healer? Biomolecules 2021, 11, 127. [Google Scholar] [CrossRef]
- Mahboubi, M. Rosa damascena as Holy Ancient Herb with Novel Applications. J. Tradit. Complement. Med. 2016, 6, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulusoy, S.; Boşgelmez-Tınaz, G.; Seçilmiş-Canbay, H. Tocopherol, Carotene, Phenolic Contents and Antibacterial Properties of Rose Essential Oil, Hydrosol and Absolute. Curr. Microbiol. 2009, 59, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Aromatherapie: Grundlagen-Wirkprinzipien-Praxis; Wabner, D.; Beier, C. (Eds.) 2. Aufl.; Elsevier, Urban & Fischer: München, Germany, 2012; ISBN 978-3-437-56991-3. [Google Scholar]
- Ayati, Z.; Ramezani, M.; Amiri, M.S.; Sahebkar, A.; Emami, S.A. Genus Rosa: A Review of Ethnobotany, Phytochemistry and Traditional Aspects According to Islamic Traditional Medicine (ITM). In Pharmacological Properties of Plant-Derived Natural Products and Implications for Human Health; Barreto, G.E., Sahebkar, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 1308, pp. 353–401. ISBN 978-3-030-64871-8. [Google Scholar]
- Kovacheva, N.; Rusanov, K.; Atanassov, I. Industrial Cultivation of Oil Bearing Rose and Rose Oil Production in Bulgaria During 21st Century, Directions and Challenges. Biotechnol. Biotechnol. Equip. 2010, 24, 1793–1798. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization. ISO Standard 9842:2003 Oil of Rose (Rosa x damascena Miller); International Organization for Standardization: London, UK, 2003. [Google Scholar]
- Babu, K.G.D.; Singh, B.; Joshi, V.P.; Singh, V. Essential Oil Composition of Damask Rose (Rosa damascena Mill.) Distilled under Different Pressures and Temperatures. Flavour Fragr. J. 2002, 17, 136–140. [Google Scholar] [CrossRef]
- Buccellato, F. An Anatomy of Rose. Perfum. Flavorist 1980, 5, 29–32. [Google Scholar]
- Dobreva, A.; Getchovska, K.; Nedeltcheva-Antonova, D. A Comparative Study of Saudi Arabia and Bulgarian Rose Oil Chemical Profile: The Effect of the Technology and Geographic Origin. Flavour Fragr. J. 2020, 35, 584–596. [Google Scholar] [CrossRef]
- Dobreva, A.; Kovacheva, N. Daily Dynamics of Essential Oils of Rosa damascena Mill. and Rosa alba L. Agric. Sci. Technol. 2010, 2, 71–74. [Google Scholar]
- Garnero, J.; Guichard, G.; Buil, P. L’huile Essentielle et La Concrete de Rose de Turquie. Riv. Ital. Essenze Profumi Piante Off. Aromi Saponi Cosmet. Aerosol 1976, 58, 160–179. [Google Scholar]
- Koksall, N.; Aslancan, H.; Sadighazadi, S.; Kafkas, E. Chemical Investigation on Rosa damascena Mill. Volatiles: Effects of Storage and Drying Conditions. Acta Sci. Pol. Hortorum Cultus 2015, 14, 105–114. [Google Scholar]
- Kováts, E. Composition of Essential Oils. J. Chromatogr. A 1987, 406, 185–222. [Google Scholar] [CrossRef]
- Nedkov, N.; Dobreva, A.; Kovacheva, N.; Bardarov, V.; Velcheva, A. Bulgarian Rose Oil of White Oil-Bearing Rose. Bulg. J. Agric. Sci. 2009, 15, 318–322. [Google Scholar]
- Ohloff, G.; Demole, E. Importance of the Odoriferous Principle of Bulgarian Rose Oil in Flavour and Fragrance Chemistry. J. Chromatogr. A 1987, 406, 181–183. [Google Scholar] [CrossRef]
- Pellati, F.; Orlandini, G.; van Leeuwen, K.A.; Anesin, G.; Bertelli, D.; Paolini, M.; Benvenuti, S.; Camin, F. Gas Chromatography Combined with Mass Spectrometry, Flame Ionization Detection and Elemental Analyzer/Isotope Ratio Mass Spectrometry for Characterizing and Detecting the Authenticity of Commercial Essential Oils of Rosa damascena Mill.: GC/MS, GC/FID and GC/C/IRMS Analysis of Rosa damascena Essential Oil. Rapid Commun. Mass Spectrom. 2013, 27, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Luo, J.; Niu, Y.; Wu, M. Characterization of Key Aroma Compounds from Different Rose Essential Oils Using Gas Chromatography-Mass Spectrometry, Gas Chromatography–Olfactometry and Partial Least Squares Regression. Nat. Prod. Res. 2018, 32, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Cebi, N.; Arici, M.; Sagdic, O. The Famous Turkish Rose Essential Oil: Characterization and Authenticity Monitoring by FTIR, Raman and GC–MS Techniques Combined with Chemometrics. Food Chem. 2021, 354, 129495. [Google Scholar] [CrossRef]
- Aydinli, M.; Tutaș, M. Production of Rose Absolute from Rose Concrete. Flavour Fragr. J. 2003, 18, 26–31. [Google Scholar] [CrossRef]
- Nedeltcheva-Antonova, D.; Stoicheva, P.; Antonov, L. Chemical Profiling of Bulgarian Rose Absolute (Rosa damascenaMill.) Using Gas Chromatography–Mass Spectrometry and Trimethylsilyl Derivatives. Ind. Crops Prod. 2017, 108, 36–43. [Google Scholar] [CrossRef]
- Ohashi, T.; Miyazawa, Y.; Ishizaki, S.; Kurobayashi, Y.; Saito, T. Identification of Odor-Active Trace Compounds in Blooming Flower of Damask Rose (Rosa damascena). J. Agric. Food Chem. 2019, 67, 7410–7415. [Google Scholar] [CrossRef]
- Aycı, F.; Aydınlı, M.; Bozdemir, Ö.A.; Tutaş, M. Gas Chromatographic Investigation of Rose Concrete, Absolute and Solid Residue. Flavour Fragr. J. 2005, 20, 481–486. [Google Scholar] [CrossRef]
- Krupčík, J.; Gorovenko, R.; Špánik, I.; Sandra, P.; Armstrong, D.W. Enantioselective Comprehensive Two-Dimensional Gas Chromatography. A Route to Elucidate the Authenticity and Origin of Rosa damascena Miller Essential Oils: Gas Chromatography. J. Sep. Sci. 2015, 38, 3397–3403. [Google Scholar] [CrossRef] [PubMed]
- Kurkcuoglu, M.; Baser, K.H.C. Studies on Turkish Rose Concrete, Absolute, and Hydrosol. Chem. Nat. Compd. 2003, 39, 457–464. [Google Scholar] [CrossRef]
- Özkan, G.; Sagdiç, O.; Baydar, N.G.; Baydar, H. Antioxidant and Antibacterial Activities of Rosa damascena Flower Extracts. Food Sci. Technol. Int. 2004, 10, 277–281. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Fabiano-Tixier, A.-S.; Pierson, J.T.; Bily, A. Green Extraction: From Concepts to Research, Education, and Economical Opportunities. In Green Extraction of Natural Products; Chemat, F., Strube, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 1–36. ISBN 978-3-527-67682-8. [Google Scholar]
- Gochev, V.; Girova, T.; Stoilova, I.; Atanasova, T.; Nenov, N.; Stanchev, V.; Stoyanova, A. Low Temperature Extraction of Essential Oil Bearing Plants by Liquefied Gases. 7. Seeds from Cardamom (Elettaria Cardamomum (L.) Maton). J. Biosci. Biotechnol. 2012, 1, 135–139. [Google Scholar]
- Khambay, B.P.S. Extraction and Isolation of Artemisin with HFC-134a. In Proceedings of the Artemisinin Forum 2008, Guilin, China, 26 November 2008. [Google Scholar]
- Mihov, R.; Nikovska, K.; Nenov, N.; Slavchev, A. Evaluation of Mayonnaise-like Food Emulsions with Extracts of Herbs and Spices. Emir. J. Food Agric. 2012, 24, 191–199. [Google Scholar]
- Nenov, N.; Gochev, V.; Girova, T.; Stoilova, I.; Atanasova, T.; Stanchev, V.; Stoyanova, A. Low Temperature Extraction of Essential Oil Bearing Plants by Liquefied Gases. 6. Barks from Cinnamon (Cinnamomum Zeylanicum Nees). J. Essent. Oil Bear. Plants 2011, 14, 67–75. [Google Scholar] [CrossRef]
- Stoyanova, A.; Nenov, N.; Slavchev, A.; Jirovetz, L.; Buchbauer, G.; Lien, H.; Schmidt, E.; Geissler, M. C2H2F4-Oleoresins of Black Pepper(Piper NigrumL.)and Ginger (Zingiber Officinale(L.)Rosc.) from Vietnam: Antimicrobial Testings, Gas Chromatographic Analysis and Olfactoric Evaluation. Electron. J. Environ. Agric. Food Chem. 2006, 5, 1615–1623. [Google Scholar]
- Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 on the Approximation of the Laws of the Member States on Extraction Solvents Used in the Production of Foodstuffs and Food Ingredients. Off. J. Eur. Union 2009, 52.
- Solkane®134a Thermodynamics; SOLVAY FLUOR GMBH: Hannover, Germany, 2018; Release 1.05.
- SOLKANE® 227 Pharma SOLKANE® 134a Pharma; SOLVAY FLUOR: Hannover, Germany, 2018.
- Haghighi, A.; Khajenoori, M. Subcritical Water Extraction. In Mass Transfer-Advances in Sustainable Energy and Environment Oriented Numerical Modeling; Nakajima, H., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1170-2. [Google Scholar]
- Özel, M.Z.; Göğüş, F.; Lewis, A.C. Comparison of Direct Thermal Desorption with Water Distillation and Superheated Water Extraction for the Analysis of Volatile Components of Rosa damascena Mill. Using GCxGC-TOF/MS. Anal. Chim. Acta 2006, 566, 172–177. [Google Scholar] [CrossRef]
- Manouchehri, R.; Saharkhiz, M.J.; Karami, A.; Niakousari, M. Extraction of Essential Oils from Damask Rose Using Green and Conventional Techniques: Microwave and Ohmic Assisted Hydrodistillation versus Hydrodistillation. Sustain. Chem. Pharm. 2018, 8, 76–81. [Google Scholar] [CrossRef]
- Reverchon, E.; Delta Porta, G. Rose Concrete Fractionation by Supercritical CO2. J. Supercrit. Fluids 1996, 9, 199–204. [Google Scholar] [CrossRef]
- Boelens, M.H.; Boelens, H. Differences in Chemical and Sensory Properties of Orange Flower and Rose Oils Obtained from Hydrodistillation and from Supercritical C02 Extraction. Perfum. Flavorist 1997, 22, 31–35. [Google Scholar]
- Reverchon, E.; Della Porta, G.; Gorgoglione, D. Supercritical CO2 Extraction of Volatile Oil from Rose Concrete. Flavour Fragr. J. 1997, 12, 37–41. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Hedayati, A.; Mousavi, S.O. Quercetin Extraction from Rosa damascena Mill via Supercritical CO2: Neural Network and Adaptive Neuro Fuzzy Interface System Modeling and Response Surface Optimization. J. Supercrit. Fluids 2016, 112, 57–66. [Google Scholar] [CrossRef]
- Wilde, P.F.; McClory, P.J. New Solvents for Extraction. Perfum. Flavorist 1994, 19, 25–28. [Google Scholar]
- Baser, K.H.C.; Kurkcuoglu, M.; Özek, T. Turkish Rose Oil Research: Recent Results. Perfum. Flavorist 2003, 28, 34–42. [Google Scholar]
- Antonova, D.V.; Medarska, Y.N.; Stoyanova, A.S.; Nenov, N.S.; Slavov, A.M.; Antonov, L.M. Chemical Profile and Sensory Evaluation of Bulgarian Rose (Rosa damascena Mill.) Aroma Products, Isolated by Different Techniques. J. Essent. Oil Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Nenov, N.; Atanasova, T.; Gochev, V.; Merdzhanov, P.; Girova, T.; Djurkov, T.; Stoyanova, A. New Product from Bulgarian Rose. Int. Sci. Pract. Conf. World Sci. 2016, 1, 17–22. [Google Scholar]
- Roth, M. Thermodynamic Prospects of Alternative Refrigerants as Solvents for Supercritical Fluid Extraction. Anal. Chem. 1996, 68, 4474–4480. [Google Scholar] [CrossRef]
- Joichi, A.; Yomogida, K.; Awano, K.; Ueda, Y. Volatile Components of Tea-Scented Modern Roses and Ancient Chinese Roses. Flavour Fragr. J. 2005, 20, 152–157. [Google Scholar] [CrossRef]
- Hirata, H.; Ohnishi, T.; Watanabe, N. Biosynthesis of Floral Scent 2-Phenylethanol in Rose Flowers. Biosci. Biotechnol. Biochem. 2016, 80, 1865–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusanov, K.; Kovacheva, N.; Rusanova, M.; Atanassov, I. Reducing Methyl Eugenol Content in Rosa damascena Mill Rose Oil by Changing the Traditional Rose Flower Harvesting Practices. Eur. Food Res. Technol. 2012, 234, 921–926. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Methyl Eugenol: Its Occurrence, Distribution, and Role in Nature, Especially in Relation to Insect Behavior and Pollination. J. Insect Sci. 2012, 12, 1–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobreva, A.; Velcheva, A.; Bardarov, V.; Bardarov, K. Chemical Composition of Different Genotypes Oil - Bearing Roses. Bulg. J. Agric. Sci. 2013, 19, 1213–1218. [Google Scholar]
- Rusanov, K.; Kovacheva, N.; Atanassov, I. Comparative GC/MS Analysis of Rose Flower and Distilled Oil Volatiles of The Oil Bearing Rose Rosa damascena. Biotechnol. Biotechnol. Equip. 2011, 25, 2210–2216. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S. Triterpenoids: Structural diversity, biosynthetic pathway, and bioactivity. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 67, pp. 411–461. ISBN 978-0-12-819483-6. [Google Scholar]
- Riffault-Valois, L.; Destandau, E.; Pasquier, L.; André, P.; Elfakir, C. Complementary Analytical Methods for the Phytochemical Investigation of ‘Jardin de Granville’, a Rose Dedicated to Cosmetics. Comptes Rendus Chim. 2016, 19, 1101–1112. [Google Scholar] [CrossRef] [Green Version]
- Da Porto, C.; Decorti, D.; Natolino, A. Application of a Supercritical CO 2 Extraction Procedure to Recover Volatile Compounds and Polyphenols from Rosa damascena. Sep. Sci. Technol. 2015, 50, 1175–1180. [Google Scholar] [CrossRef]
- Staikov, V.; Decheva, R.; Balinova-Tsvetkova, A. Studies on the Composition of Rose Oil Obtained from the Flowers in Different Stages of Their Development. Riv. Ital. 1975, 57, 192–195. [Google Scholar]
- Seify, Z.; Yadegari, M.; Pirbalouti, A. Essential Oil Composition of Rosa damascena Mill. Produced With Different Storage Temperatures and Durations. Korean J. Hortic. Sci. Technol. 2018, 36. [Google Scholar] [CrossRef]
- Barao, T.; de Castro, C.A.N.; Mardolcar, U.V.; Okambawa, R.; St-Arnaud, J.M. Dielectric Constant, Dielectric Virial Coefficients, and Dipole Moments of 1,1,1,2-Tetrafluoroethane. J. Chem. Eng. Data 1995, 40, 1242–1248. [Google Scholar] [CrossRef]

| No | Compound | LRIexp DB-17HT | Rel.%, as Determined Using GC-FID | |||
|---|---|---|---|---|---|---|
| R. galica | R. damascena | R. alba | R. centifolia | |||
| 1. | α-Pinene | 845 | 0.24 | 0.42 | 0.12 | 0.16 |
| 2. | β-Pinene | 944 | n.d. | 0.08 | 0.01 | 0.02 |
| 3. | β-Myrcene | 968 | 0.17 | 0.09 | 0.03 | 0.02 |
| 4. | Limonene | 1003 | n.d. | n.d. | 0.08 | 0.01 |
| 5. | p-Cymene | 1026 | n.d. | n.d. | 0.01 | n.d. |
| 6. | Benzaldehyde | 1051 | n.d. | n.d. | n.d. | n.d. |
| 7. | Linalool | 1201 | 0.07 | 0.07 | 0.10 | n.d. |
| 8. | Rose oxide | 1205 | n.d. | 0.01 | n.d. | n.d. |
| 9. | Benzyl alcohol | 1223 | 0.84 | 1.48 | 4.43 | 0.27 |
| 10. | Octanoic acid | 1298 | 0.14 | n.d. | 0.02 | n.d. |
| 11. | 2-Phenyl ethyl alcohol | 1317 | 27.22 | 59.15 | 14.07 | 8.99 |
| 12. | β-Citronellol | 1366 | 1.72 | 5.27 | 2.84 | 2.85 |
| 13. | Nerol | 1374 | 2.24 | 1.49 | 6.13 | 1.26 |
| 14. | Phenyl ethyl formate | 1381 | 0.12 | 0.12 | 0.08 | n.d. |
| 15. | Geraniol | 1414 | 8.46 | 3.01 | 14.41 | 2.87 |
| 16. | Neral | 1392 | n.d. | 0.21 | 0.07 | 0.10 |
| 17. | Geranial | 1440 | 0.34 | 0.24 | 0.95 | 0.09 |
| 18. | Phenyl ethyl acetate | 1469 | n.d. | 0.15 | 0.04 | 0.11 |
| 19. | β-Elemene | 1472 | n.d. | 0.12 | 0.01 | n.d. |
| 20. | Cytronellyl acetate | 1475 | n.d. | n.d. | 0.08 | n.d. |
| 21. | Anethole (Benzene,1-methoxy-4(1-propenyl)) | 1482 | n.d. | n.d. | 0.25 | n.d. |
| 22. | Pentadecane(C15) | 1500 | 0.07 | 0.06 | 0.09 | n.d. |
| 23. | β-Caryophyllene | 1506 | 0.25 | 0.17 | 0.98 | 0.16 |
| 24. | Geranic acid | 1522 | 0.11 | 0.48 | 0.26 | 0.37 |
| 25. | β-Copaene | 1520 | n.d. | n.d. | n.d. | 0.26 |
| 26. | α-Guaiene | 1518 | n.d. | 0.06 | n.d. | n.d. |
| 27. | Geranyl acetate | 1540 | 0.17 | 0.16 | 0.03 | n.d. |
| 28. | α-Caryophyllene | 1552 | n.d. | n.d. | n.d. | n.d. |
| 29. | Hydroxy linalool | 1568 | n.d. | n.d. | 0.03 | n.d. |
| 30. | Eugenol | 1574 | 0.07 | 1.26 | 0.08 | n.d. |
| 31. | Germacrene D | 1593 | 0.09 | 0.11 | n.d. | 0.74 |
| 32. | β-Cubebene | 1598 | n.d. | n.d. | n.d. | n.d. |
| 33. | α-Muurolene | 1607 | n.d. | n.d. | n.d. | n.d. |
| 34. | β-Guaiene | 1611 | n.d. | 0.06 | n.d. | n.d. |
| 35. | β-Copaene | 1616 | n.d. | n.d. | n.d. | n.d. |
| 36 | β-Cadinene | 1646 | n.d. | n.d. | n.d. | 0.12 |
| 37. | Methyl eugenol | 1654 | n.d. | 0.49 | n.d. | n.d. |
| 38. | Heptadecane (C17) | 1700 | 1.48 | 0.46 | 1.65 | 0.88 |
| 39. | Bulnesol | 1703 | 0.81 | 0.09 | 0.25 | 1.10 |
| 40. | Tetradecanal(Myristyl aldehyde) | 1706 | 0.10 | n.d. | n.d. | n.d. |
| 41. | Heptadecene (C17:1) | 1710 | n.d. | n.d. | 0.01 | n.d. |
| 42. | Benzyl tiglate + Heptadecadiene (C17:2) | 1714 | 0.14 | n.d. | 0.07 | 0.27 |
| 43. | Octadecane(C18) | 1744 | 0.14 | 0.06 | n.d. | 0.07 |
| 44. | γ-Eudesmol | 1796 | n.d. | n.d. | 0.02 | 0.05 |
| 45. | τ-Cadinol | 1805 | n.d. | n.d. | n.d. | n.d. |
| 46. | α-Eudesmol | 1819 | 0.24 | n.d. | 0.02 | 0.49 |
| 47. | β-Eudesmol | 1826 | 0.36 | n.d. | 0.14 | 0.81 |
| 48. | α-Cadinol | 1833 | n.d. | n.d. | 0.09 | n.d. |
| 49. | Nonadecane + Nonadecene (C19 + C19:1) | 1900 | 17.97 | 5.09 | 15.66 | 12.75 |
| 50. | Hexadecanal | 1936 | 0.07 | n.d | 0.04 | n.d |
| 51. | Eicosane(C20) | 2000 | 0.98 | 0.46 | 1.50 | 0.65 |
| 52. | Selina-4.7-diol | 2046 | 0.08 | n.d | n.d | 0.43 |
| 53. | Unknown sesquiterpene | 2054 | 0.31 | n.d | n.d | 0.92 |
| 54. | Heneicosane(C21) | 2100 | 9.67 | 3.09 | 11.83 | 8.34 |
| 55. | Heneicosene(C21:1) | 2105 | 0.24 | 0.17 | 1.31 | 0.34 |
| 56. | Heneicosene(C21:1), isomer | 2121 | n.d | 0.03 | 0.25 | 0.07 |
| 57. | Docosane(C22) | 2200 | 0.47 | 0.12 | n.d | 0.44 |
| 58. | Docosene(C22:1) | 2211 | n.d | n.d | n.d | 0.06 |
| 59. | Eudesm-4-en-3-one,11-hydroxy | 2243 | 4.67 | n.d | n.d | 0.11 |
| 60. | Tricosane(C23) | 2300 | 0.07 | 1.27 | 2.46 | 8.63 |
| 61. | Tricosene(C23:1) | 2318 | 0.30 | 0.18 | 1.86 | 1.65 |
| 62. | Tricosene(C23:1), isomer | 2334 | 0.10 | 0.21 | 0.33 | 0.35 |
| 63. | 1,1,9-Eicosadiene | 2348 | 0.09 | 0.04 | 0.12 | 0.08 |
| 64. | Tetracosane (C24) | 2400 | 0.25 | 0.09 | n.d | 0.44 |
| 65. | Farnesol acetate | 2423 | 0.14 | n.d | n.d | n.d |
| 66. | Hexanoic acid,2-ethyl,tetradecyl ester | 2449 | 0.33 | 0.09 | n.d | n.d |
| 67. | Pentacosene(C25:1) | 2511 | 0.22 | 0.07 | 0.21 | 0.17 |
| 68. | Pentacosene(C25:1) | 2524 | 0.07 | 0.05 | 0.97 | 0.88 |
| 69. | Pentacosene(C25:1) | 2532 | n.d | n.d | 0.32 | 0.30 |
| 70. | Octanoic acid, tetradecyl ester | 2548 | 0.10 | 0.07 | 0.04 | n.d |
| 71. | Hexacosane(C26) | 2600 | 0.10 | 0.06 | 0.05 | 0.11 |
| 72. | Hexacosene(C26:1) | 2612 | n.d | 0.05 | 0.20 | 0.13 |
| 73. | Octanoic acid, hexadecyl ester | 2668 | n.d | n.d | n.d | 0.22 |
| 74. | Heptacosane +Heptacosene (C27 + C27:1) | 2700 | 0.10 | 0.08 | 0.21 | 0.80 |
| 75. | Heptacosene(C27:1), isomer | 2708 | 0.42 | 0.32 | 0.27 | 0.33 |
| 76. | Heptacosene(C27:1), isomer | 2721 | n.d | n.d | 1.19 | 1.93 |
| 77. | Heptacosanol | 2789 | n.d | n.d | n.d | 0.10 |
| 78. | Citronellyl ester (stearate) | 2843 | 0.08 | 0.18 | n.d | 0.25 |
| 79. | Geranyl ester | 2876 | n.d | n.d | n.d | 0.10 |
| 80. | Nonacosene(C29:1) | 2911 | 1.03 | 0.81 | 0.49 | 2.13 |
| 81. | Phenyl ethyl ester | 2925 | 0.33 | 0.52 | 0.52 | 0.71 |
| 82. | Squalene | 2931 | 0.62 | 0.08 | n.d | 0.55 |
| 83. | Dodecanoic acid, phenyl methyl ester | 2939 | n.d | n.d | n.d | 0.16 |
| 84. | Citronellyl ester (phenyl acetate) | 2942 | n.d | n.d | n.d | 1.28 |
| 85. | Neryl ester (phenyl acetate) | 2956 | n.d | 0.06 | n.d | 1.15 |
| 86. | Neryl ester | 2961 | n.d | n.d | n.d | 7.35 |
| 87. | Phenyl ethyl ester | 2964 | n.d | n.d | n.d | 1.95 |
| 88. | Phenyl ethyl ester (linoleate) | 2984 | 0.08 | 0.29 | n.d | 0.10 |
| 89. | Citronellyl ester | 3016 | n.d | n.d | n.d | 0.55 |
| 90. | Geranyl ester (stearate) | 3058 | n.d | n.d | n.d | 0.87 |
| 91. | Neryl ester (stearate) | 3076 | 0.07 | 0.06 | n.d | 4.82 |
| 92. | Phenyl ethyl ester (stearate) | 3112 | n.d | n.d | n.d | 1.83 |
| 93. | Citronellyl ester | 3224 | n.d | n.d | n.d | 0.54 |
| 94. | Phenyl ethyl ester | 3421 | 0.13 | n.d | n.d | 0.45 |
| 95. | Neryl ester | 3432 | n.d | 0.06 | 0.29 | 0.86 |
| 96. | β-Amyrin | 3442 | 0.93 | 0.45 | n.d | 0.14 |
| 97. | Phenyl ethyl ester | 3456 | 0.31 | 0.12 | n.d | 0.35 |
| 98. | 9,19-Cyclolanost-24-en-3-ol acetate | 3462 | n.d | n.d | n.d | 0.27 |
| 99. | Olean-12-en-3-one (Amirenone) | 3468 | 0.27 | 0.28 | 0.15 | 0.65 |
| 100. | α-Amyrin + Unindentified triterpene | 3476 | 0.14 | 0.17 | 0.09 | 0.78 |
| 101. | Unindentified triterpene | 3519 | 0.79 | 0.23 | n.d | 0.25 |
| 102. | Lup20(29)-en-3-one | 3546 | 0.20 | 0.35 | n.d | n.d |
| 103. | Lupeol | 3616 | 0.74 | 1.17 | 0.15 | 0.19 |
| Phenylethanoids and phenylpropanoids | 27.29 | 60.90 | 14.40 | 8.99 | ||
| Monoterpenes | 13.67 | 11.89 | 25.44 | 25.52 | ||
| -Monoterpene hydrocarbons | 0.41 | 0.59 | 0.25 | 0.21 | ||
| -Oxygeneted monoterpenes | 13.26 | 11.30 | 25.19 | 25.31 | ||
| Aliphatic hydrocarbons | 33.92 | 12.77 | 40.98 | 41.70 | ||
| Sesquiterpenes | 6.95 | 0.61 | 2.05 | 5.19 | ||
| Triterpenes | 3.69 | 2.73 | 0.38 | 2.83 | ||
| Others | 1.15 | 1.48 | 4.96 | 0.38 | ||
| TOTAL identified | 89.81 | 92.54 | 89.73 | 93.68 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobreva, A.; Nedeltcheva-Antonova, D.; Nenov, N.; Getchovska, K.; Antonov, L. Subcritical Extracts from Major Species of Oil-Bearing Roses—A Comparative Chemical Profiling. Molecules 2021, 26, 4991. https://doi.org/10.3390/molecules26164991
Dobreva A, Nedeltcheva-Antonova D, Nenov N, Getchovska K, Antonov L. Subcritical Extracts from Major Species of Oil-Bearing Roses—A Comparative Chemical Profiling. Molecules. 2021; 26(16):4991. https://doi.org/10.3390/molecules26164991
Chicago/Turabian StyleDobreva, Ana, Daniela Nedeltcheva-Antonova, Nenko Nenov, Kamelia Getchovska, and Liudmil Antonov. 2021. "Subcritical Extracts from Major Species of Oil-Bearing Roses—A Comparative Chemical Profiling" Molecules 26, no. 16: 4991. https://doi.org/10.3390/molecules26164991
APA StyleDobreva, A., Nedeltcheva-Antonova, D., Nenov, N., Getchovska, K., & Antonov, L. (2021). Subcritical Extracts from Major Species of Oil-Bearing Roses—A Comparative Chemical Profiling. Molecules, 26(16), 4991. https://doi.org/10.3390/molecules26164991








