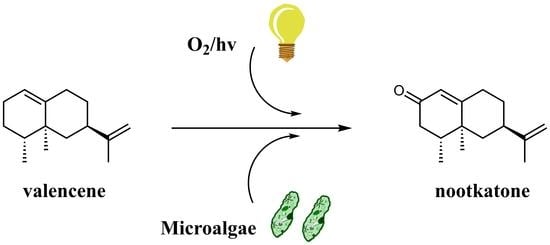
Oxidation of Terpenoids to Achieve High-Value Flavor and Fragrances—Questioning Microalgae Oxidative Capabilities in the Biotransformation of the Sesquiterpene Valencene and of Selected Natural Apocarotenoids
Abstract
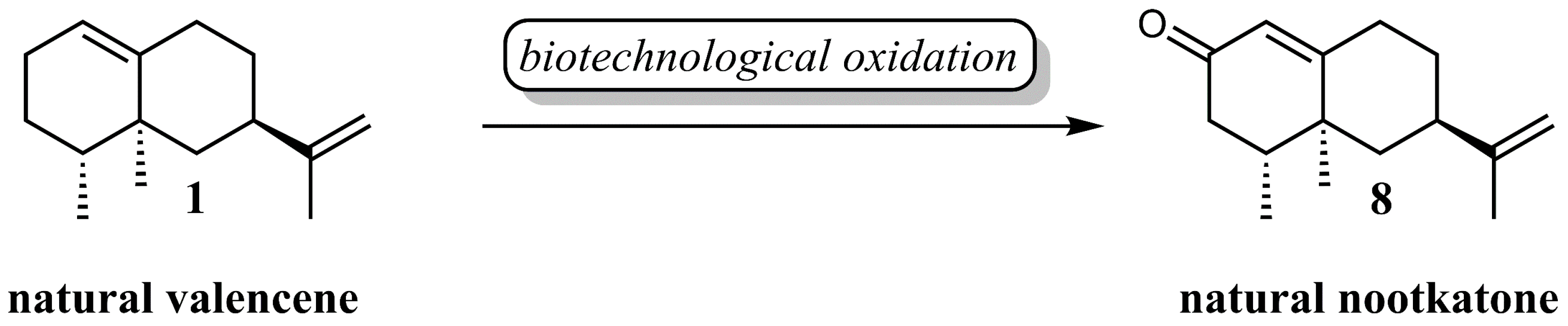
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microalgae Culture
2.3. Microalgal Vitality Evaluation
2.4. Nootkatone Toxicity
2.5. Biotransformation Experiments and Analysis
3. Results
3.1. Microalgal Vitality Evaluation and Nootkatone Toxicity
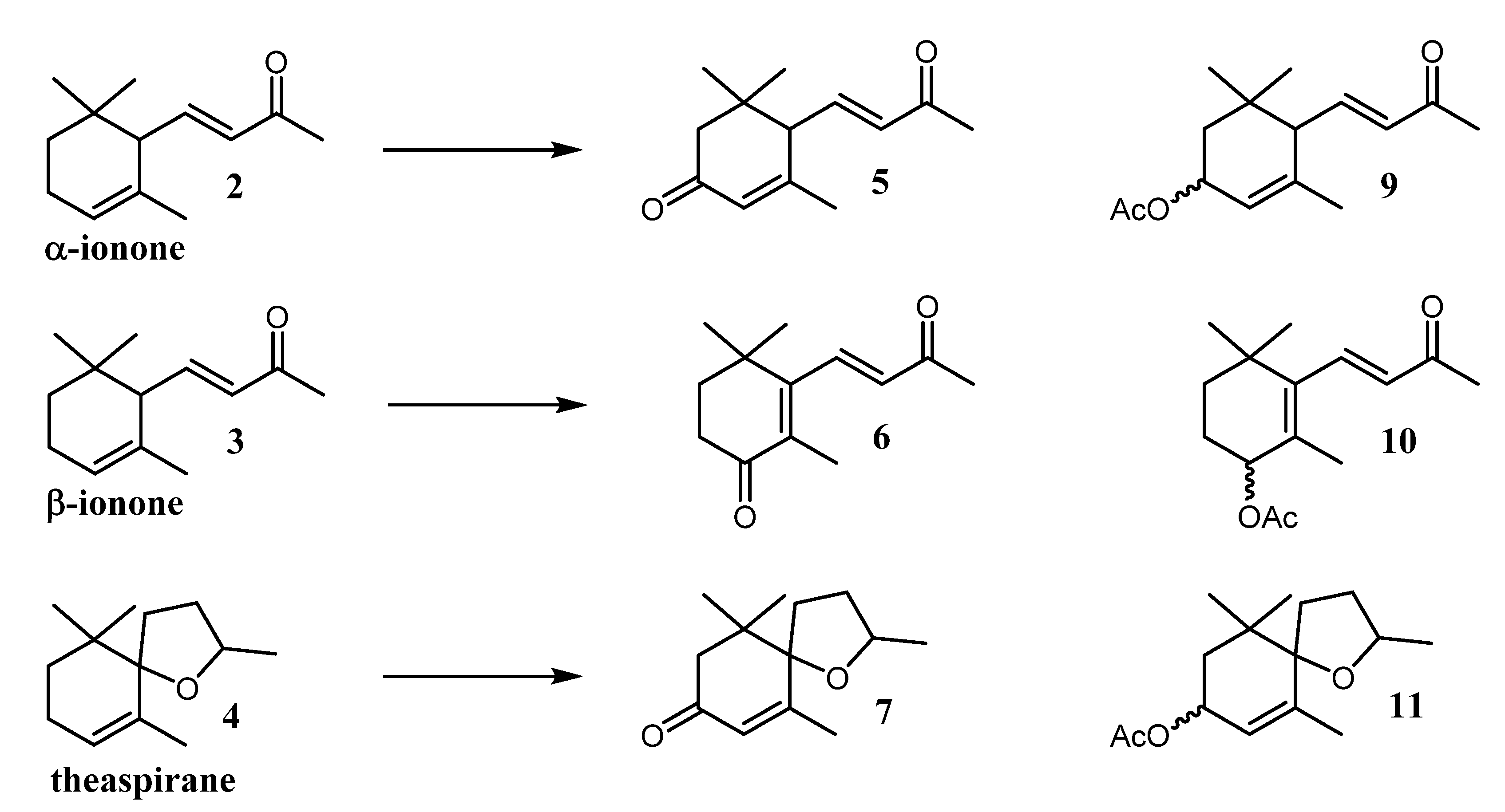
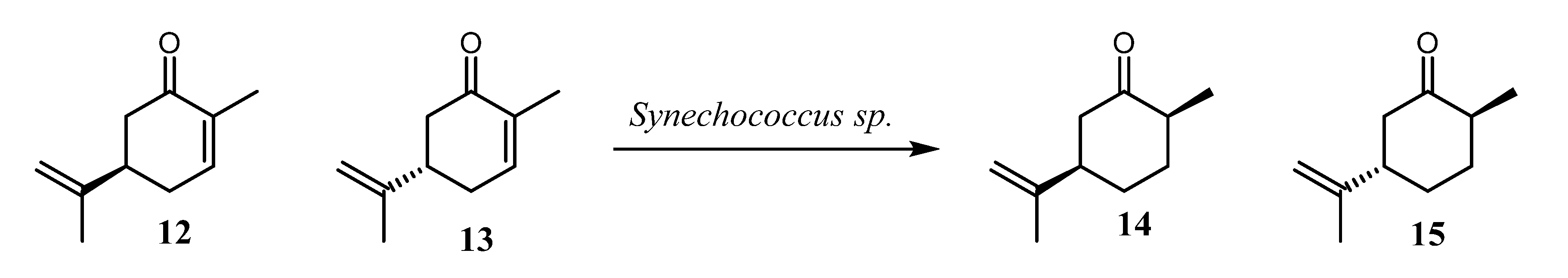
3.2. Biotransformation Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandal, N. Global Markets for Flavors and Fragrances; BCC Research: Wellesley, MA, USA, 2014. [Google Scholar]
- Van Wyk, N.; Kroukamp, H.; Pretorius, I.S. The smell of synthetic biology: Engineering strategies for aroma compound pro-duction in yeast. Fermentation 2018, 4, 54. [Google Scholar] [CrossRef] [Green Version]
- Serra, S. Recent Advances in the Synthesis of Carotenoid-Derived Flavours and Fragrances. Molecules 2015, 20, 12817–12840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, P.J.; Bugg, T.D. Enzymology of the carotenoid cleavage dioxygenases: Reaction mechanisms, inhibition and biochemical roles. Arch. Biochem. Biophys. 2014, 544, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Serra, S.; De Simeis, D. Fungi-Mediated Biotransformation of the Isomeric Forms of the Apocarotenoids Ionone, Damascone and Theaspirane. Molecules 2019, 24, 19. [Google Scholar] [CrossRef] [Green Version]
- Lee, X.J.; Ong, H.C.; Gan, Y.Y.; Chen, W.-H.; Mahlia, T.M.I. State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers. Manag. 2020, 210, 112707. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Q. Microalgae-based nitrogen bioremediation. Algal Res. 2020, 46, 101775. [Google Scholar] [CrossRef]
- Della Greca, M.; Pinto, G.; Pistillo, P.; Pollio, A.; Previtera, L.; Temussi, F. Biotransformation of ethinylestradiol by microalgae. Chemosphere 2008, 70, 2047–2053. [Google Scholar] [CrossRef]
- Odjadjare, E.C.; Mutanda, T.; Olaniran, A.O. Potential biotechnological application of microalgae: A critical review. Crit. Rev. Biotechnol. 2017, 37, 37–52. [Google Scholar] [CrossRef]
- Mathimani, T.; Baldinelli, A.; Rajendran, K.; Prabakar, D.; Matheswaran, M.; van Leeuwen, R.P.; Pugazhendhi, A. Review on cultivation and thermochemical conversion of microalgae to fuels and chemicals: Process evaluation and knowledge gaps. J. Clean. Prod. 2019, 208, 1053–1064. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Rasoul-Amini, S.; Fotooh-Abadi, E.; Ghasemi, Y. Biotransformation of monoterpenes by immobilized microalgae. Environ. Boil. Fishes 2011, 23, 975–981. [Google Scholar] [CrossRef]
- Tripathi, U.; Rao, S.R.; Ravishankar, G. Biotransformation of phenylpropanoid compounds to vanilla flavor metabolites in cul-tures of Haematococcus pluvialis. Process Biochem. 2002, 38, 419–426. [Google Scholar] [CrossRef]
- De Carvalho, C.C.; da Fonseca, M.M.R. Biotransformation of terpenes. Biotechnol. Adv. 2006, 24, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Jia, Q.; Chen, X.; Köllner, T.G.; Bhattacharya, D.; Wong, G.K.-S.; Gershenzon, J.; Chen, F. Terpene Biosynthesis in Red Algae Is Catalyzed by Microbial Type But Not Typical Plant Terpene Synthases. Plant Physiol. 2019, 179, 382–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattanaik, B.; Lindberg, P. Terpenoids and Their Biosynthesis in Cyanobacteria. Life 2015, 5, 269–293. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.L. The use of microalgae and cyanobacteria in the improvement of agricultural practices: A review on their biofertilising, biostimulating and biopesticide roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
- Lee, J.-W.; Trinh, C.T. Towards renewable flavors, fragrances, and beyond. Curr. Opin. Biotechnol. 2020, 61, 168–180. [Google Scholar] [CrossRef]
- Janssens, L.; De Pooter, H.; Schamp, N.; Vandamme, E. Production of flavors by microorganisms. Process Biochem. 1992, 27, 195–215. [Google Scholar] [CrossRef]
- Fraatz, M.A.; Berger, R.G.; Zorn, H. Nootkatone—A biotechnological challenge. Appl. Microbiol. Biotechnol. 2009, 83, 35–41. [Google Scholar] [CrossRef]
- Shimoda, K.; Kubota, N.; Hamada, H.; Kaji, M.; Hirata, T. Asymmetric reduction of enones with Synechococcus sp. PCC 7942. Tetrahedron Asymmetry 2004, 15, 1677–1679. [Google Scholar] [CrossRef]
- Balcerzak, L.; Lipok, J.; Strub, D.; Lochyński, S. Biotransformations of monoterpenes by photoautotrophic micro-organisms. J. Appl. Microbiol. 2014, 117, 1523–1536. [Google Scholar] [CrossRef] [Green Version]
- Furusawa, M.; Hashimoto, T.; Noma, Y.; Asakawa, Y. Highly Efficient Production of Nootkatone, the Grapefruit Aroma from Valencene, by Biotransformation. Chem. Pharm. Bull. 2005, 53, 1513–1514. [Google Scholar] [CrossRef] [Green Version]
- Furusawa, M.; Hashimoto, T.; Noma, Y.; Asakawa, Y. Biotransformation of citrus aromatics nootkatone and valencene by mi-croorganisms. Chem. Pharm. Bull. 2005, 53, 1423–1429. [Google Scholar] [CrossRef] [Green Version]
- Asakawa, Y.; Hashimoto, T.; Noma, Y.; Furusawa, M. Modification of Valencene by Bio- and Chemical Transformation. Nat. Prod. Commun. 2013, 8, 859–862. [Google Scholar] [CrossRef] [Green Version]
- Serra, S. MnO2/TBHP: A versatile and user-friendly combination of reagents for the oxidation of allylic and benzylic methylene functional groups. Eur. J. Org. Chem. 2015, 29, 6472–6478. [Google Scholar] [CrossRef]
- Tu, V.A.; Kaga, A.; Gericke, K.-H.; Watanabe, N.; Narumi, T.; Toda, M.; Brueckner, B.; Baldermann, S.; Mase, N. Synthesis and Characterization of Quantum Dot Nanoparticles Bound to the Plant Volatile Precursor of Hydroxy-apo-10′-carotenal. J. Org. Chem. 2014, 79, 6808–6815. [Google Scholar] [CrossRef]
- Murray-Gulde, C.L.; Heatley, J.E.; Schwartzman, A.L.; Rodgers, J.J.H. Algicidal Effectiveness of Clearigate, Cutrine-Plus, and Copper Sulfate and Margins of Safety Associated with Their Use. Arch. Environ. Contam. Toxicol. 2002, 43, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation. Environ. Int. 2013, 51, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Görs, M.; Schumann, R.; Hepperle, D.; Karsten, U. Quality analysis of commercial Chlorella products used as dietary supplement in human nutrition. Environ. Boil. Fishes 2009, 22, 265–276. [Google Scholar] [CrossRef]
- Rzymski, P.; Jaśkiewicz, M. Microalgal food supplements from the perspective of Polish consumers: Patterns of use, adverse events, and beneficial effects. Environ. Boil. Fishes 2017, 29, 1841–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.-P.; Chen, F.; Liu, X.; Li, X.-Z. Carotenoid composition in the green microalga Chlorococcum. Food Chem. 2002, 76, 319–325. [Google Scholar] [CrossRef]
- Zhang, D.-H.; Lee, Y.-K. Ketocarotenoid production by a mutant of Chlorococcum sp. in an outdoor tubular photobioreactor. Biotechnol. Lett. 1999, 21, 7–10. [Google Scholar] [CrossRef]
- Liu, B.-H.; Lee, Y.-K. Composition and biosynthetic pathways of carotenoids in the astaxanthin-producing green alga Chloro-coccum sp. Biotechnol. Lett. 1999, 21, 1007–1010. [Google Scholar] [CrossRef]
- Da Luz, D.S.; Da Silva, D.G.; Souza, M.M.; Giroldo, D.; Martins, C.D.M.G. Efficiency of neutral red, E vans Blue and MTT to assess vi-ability of the freshwater microalgae Desmodesmus communis and Pediastrum boryanum. Phycol. Res. 2016, 64, 56–60. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.W., II; Shaw, P.E. Synthesis of nootkatone from valencene. J. Agr. Food Chem. 1978, 26, 1430–1432. [Google Scholar] [CrossRef]
- Hong, B.; Lebeuf, R.; Delbaere, S.; Alsters, P.L.; Nardello-Rataj, V. One-Pot Synthesis of (+)-Nootkatone via Dark Singlet Oxygenation of Valencene: The Triple Role of the Amphiphilic Molybdate Catalyst. Catalysts 2016, 6, 184. [Google Scholar] [CrossRef] [Green Version]
- Prein, M.; Adam, W. The schenck ene reaction: Diastereoselective oxyfunctionalization with singlet oxygen in synthetic ap-plications. Angew. Chem. Int. Ed. Engl. 1996, 35, 477–494. [Google Scholar] [CrossRef]



| Experimental Condition |
|---|
| Light + Demineralized water |
| Dark + Demineralized water |
| Light + BG-11 medium |
| Dark + BG-11 medium |
| Microalgae + Light + BG-11 medium + Glucose |
| Microalgae + Dark + BG-11 medium + Glucose |
| Entry | Experimental Condition | % of Compound 8 Found in Chlorococcum sp. (JB3) * | % of Compound 8 Found in Chlorella sp. (211.8b) * | % of Compound 8 Found in Chlorella sp. (211.8p) * |
|---|---|---|---|---|
| 1 | light + demineralized water | 56 ± 3 | 56 ± 3 | 56 ± 3 |
| 2 | dark + demineralized water | 17 ± 2 | 17 ± 2 | 17 ± 2 |
| 3 | light + BG-11 medium | 50 ± 5 | 50 ± 5 | 50 ± 5 |
| 4 | dark + BG-11 medium | 20 ± 2 | 20 ± 2 | 20 ± 2 |
| 5 | microalgae + light + BG-11 medium + glucose | 61 ± 3 | 58 ± 5 | 64 ± 3 |
| 6 | microalgae + dark + BG-11 medium + glucose | 8 ± 1 | 10 ± 2 | 7 ± 1 |
| Entry | Experimental Condition | Substrate | Compound Observed | % Observed in the Crude Mixture | Oxidation Products Observed in the Crude Mixture (%) * |
|---|---|---|---|---|---|
| A | light + BG-11 medium | Theaspirane | 7 | 7 | 82 |
| 11 | 3 | ||||
| B | light + BG-11 medium | β-ionone | 6 | 2 | 86 |
| 10 | - | ||||
| C | light + BG-11 medium | α-ionone | 5 | 1 | 80 |
| 9 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Simeis, D.; Serra, S.; Di Fonzo, A.; Secundo, F. Oxidation of Terpenoids to Achieve High-Value Flavor and Fragrances—Questioning Microalgae Oxidative Capabilities in the Biotransformation of the Sesquiterpene Valencene and of Selected Natural Apocarotenoids. Chemistry 2021, 3, 821-830. https://doi.org/10.3390/chemistry3030059
De Simeis D, Serra S, Di Fonzo A, Secundo F. Oxidation of Terpenoids to Achieve High-Value Flavor and Fragrances—Questioning Microalgae Oxidative Capabilities in the Biotransformation of the Sesquiterpene Valencene and of Selected Natural Apocarotenoids. Chemistry. 2021; 3(3):821-830. https://doi.org/10.3390/chemistry3030059
Chicago/Turabian StyleDe Simeis, Davide, Stefano Serra, Alessandro Di Fonzo, and Francesco Secundo. 2021. "Oxidation of Terpenoids to Achieve High-Value Flavor and Fragrances—Questioning Microalgae Oxidative Capabilities in the Biotransformation of the Sesquiterpene Valencene and of Selected Natural Apocarotenoids" Chemistry 3, no. 3: 821-830. https://doi.org/10.3390/chemistry3030059
APA StyleDe Simeis, D., Serra, S., Di Fonzo, A., & Secundo, F. (2021). Oxidation of Terpenoids to Achieve High-Value Flavor and Fragrances—Questioning Microalgae Oxidative Capabilities in the Biotransformation of the Sesquiterpene Valencene and of Selected Natural Apocarotenoids. Chemistry, 3(3), 821-830. https://doi.org/10.3390/chemistry3030059