Green Synthesis of Silver Nanoparticles Using Natural Extracts with Proven Antioxidant Activity
Abstract
:1. Introduction
2. Results
2.1. Determination of Antioxidant Potential of Examined Extracts
2.1.1. Reducing Power Activity by CUPRAC Assay
2.1.2. DPPH Free Radical Scavenging Activity
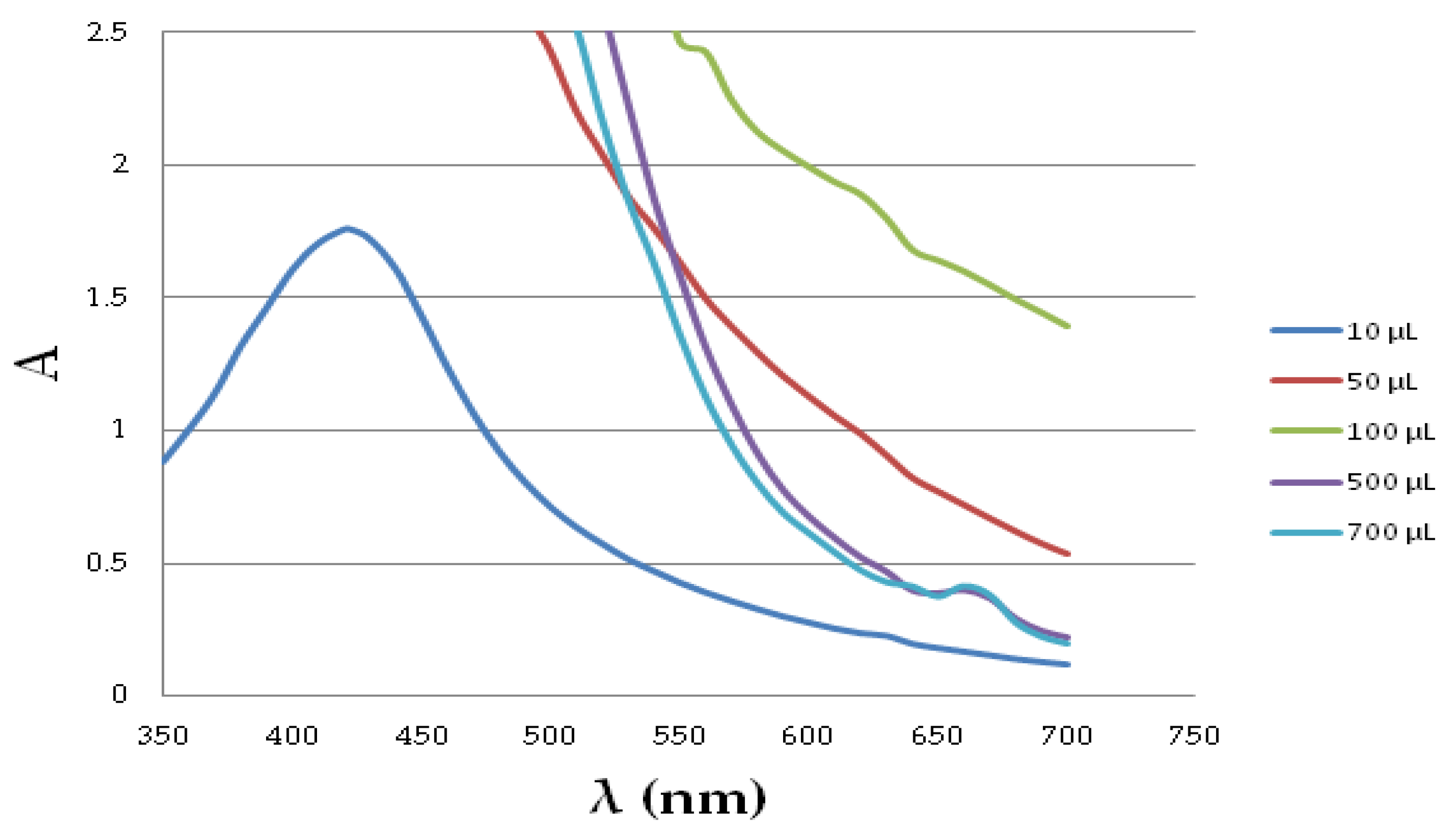
2.1.3. Silver NanoParticle Antioxidant Capacity (SNPAC) Measurements
2.2. Comparison of Methods for Antioxidant Potential Evaluation
2.3. Characteristics of AgNPs Obtained by Biogenic Synthesis with Examined Natural Extracts
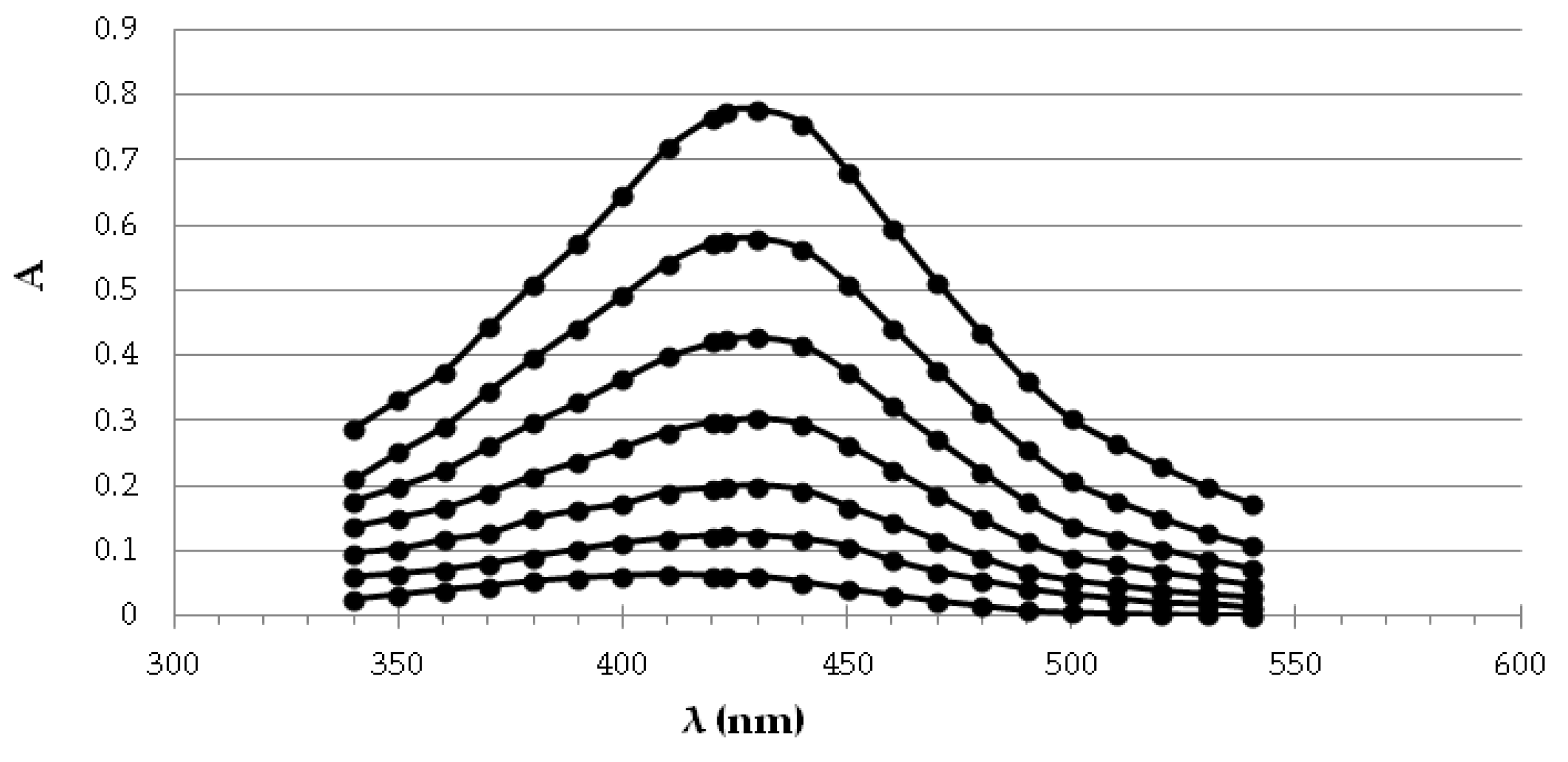
2.3.1. UV-Vis Spectroscopy
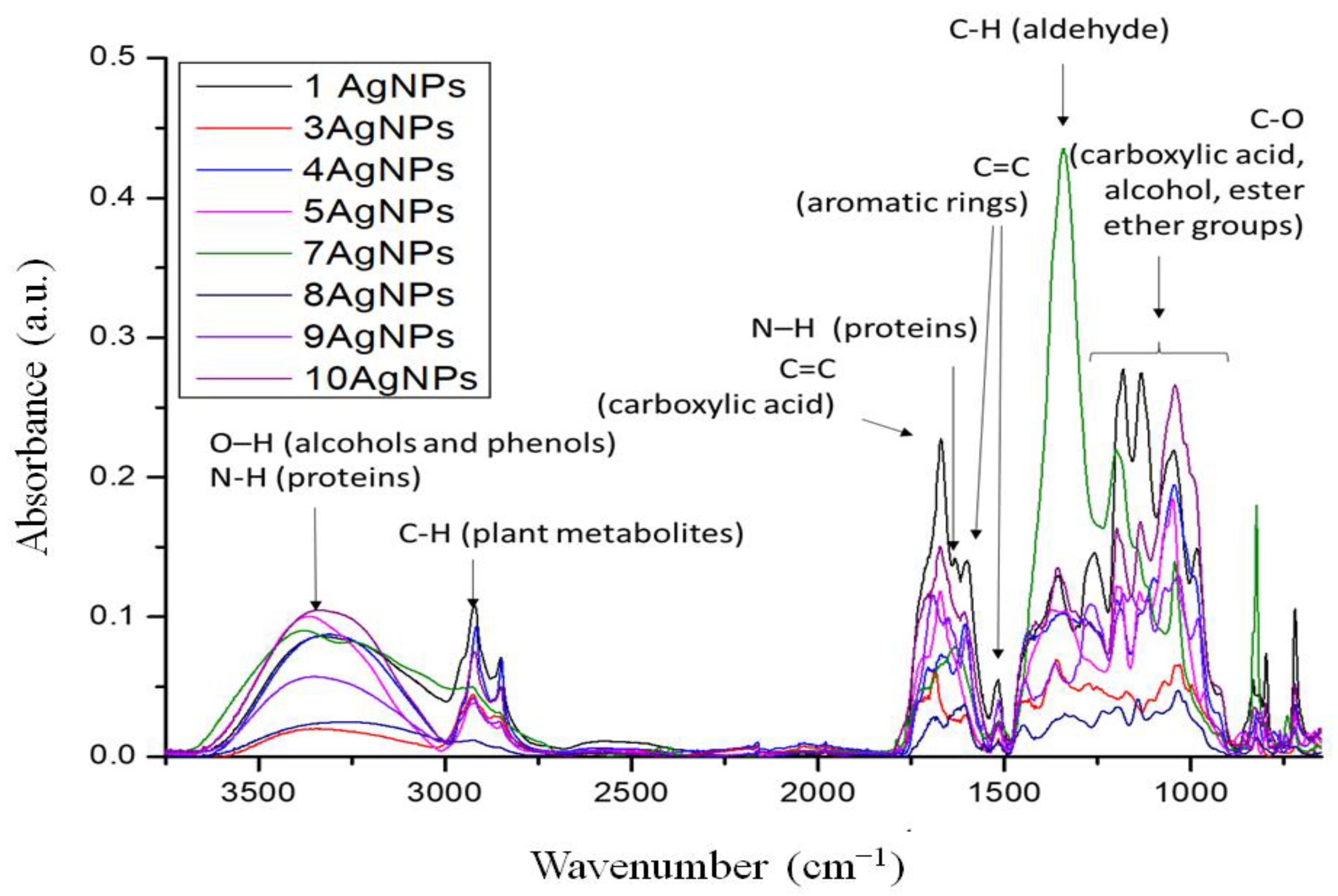
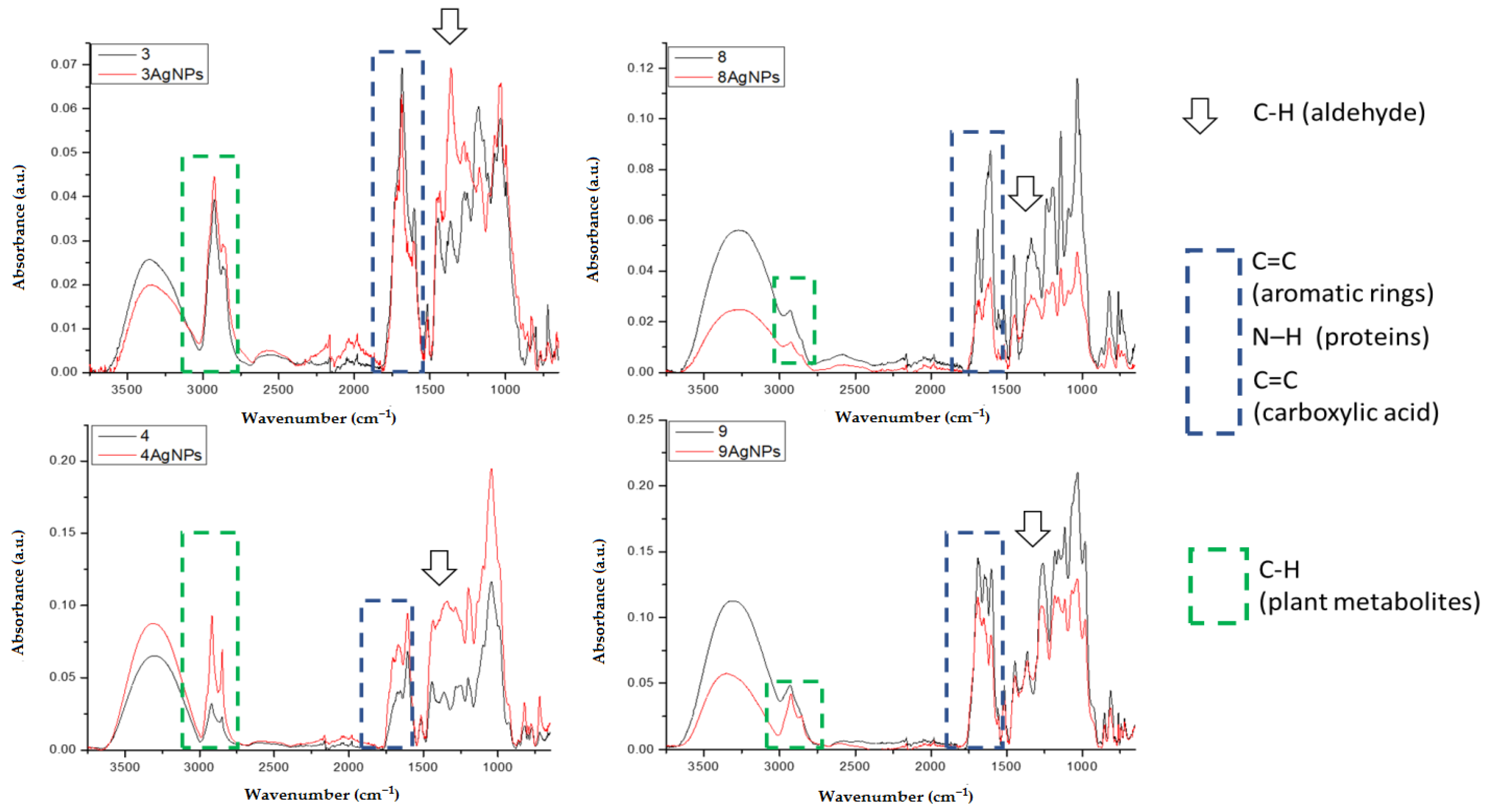
2.3.2. FTIR Spectroscopy
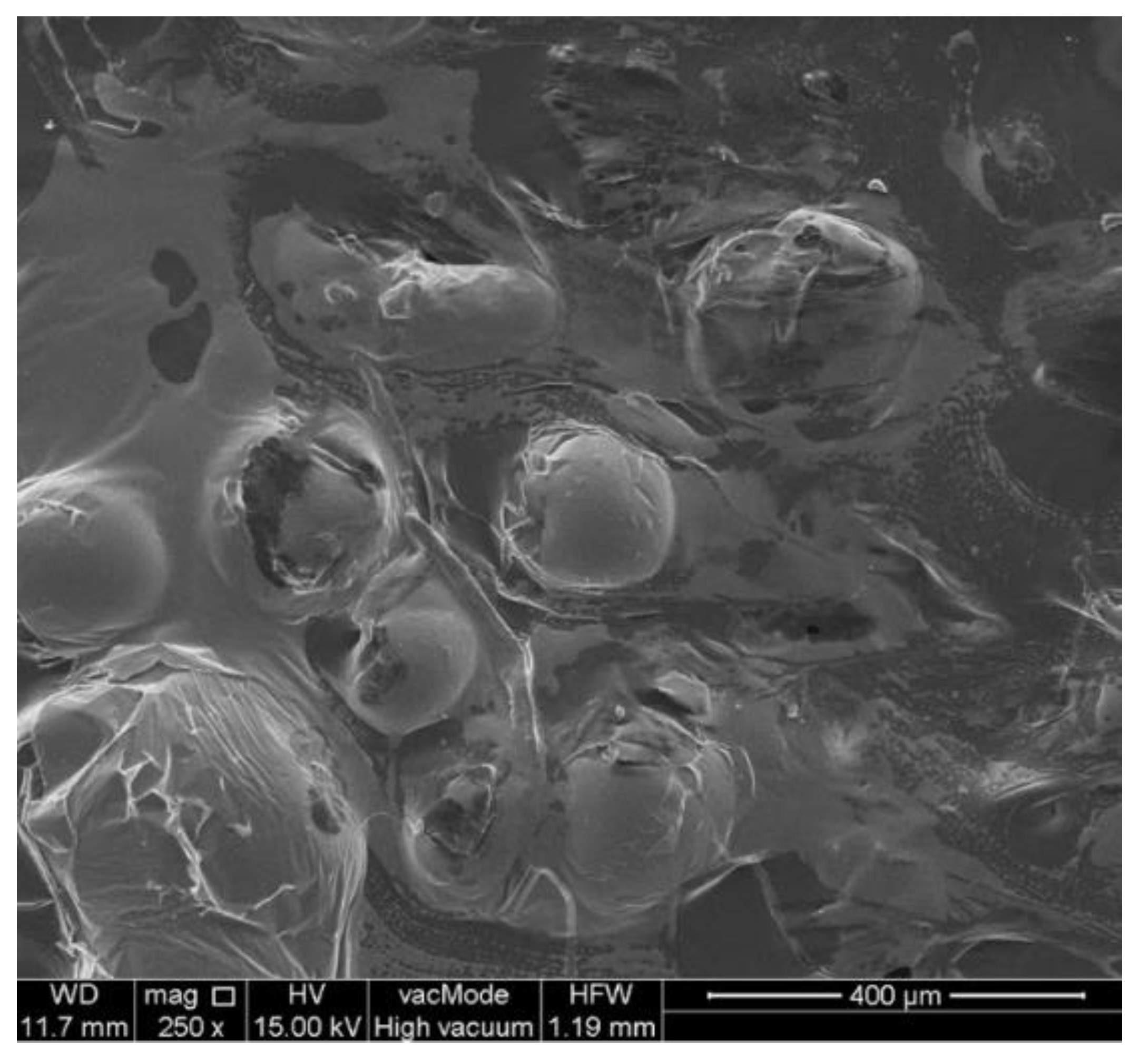
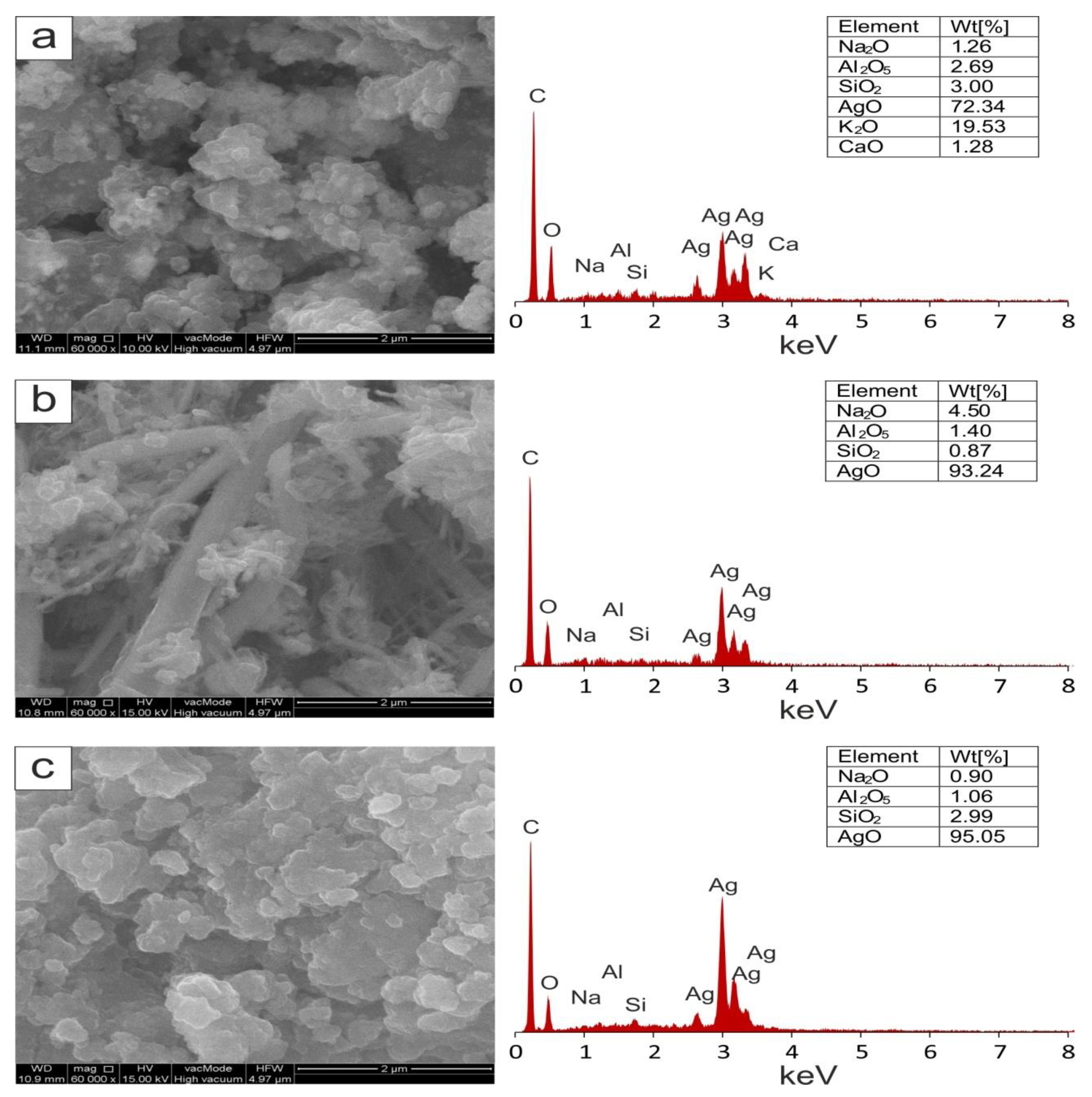
2.3.3. Scanning Electron Microscopy
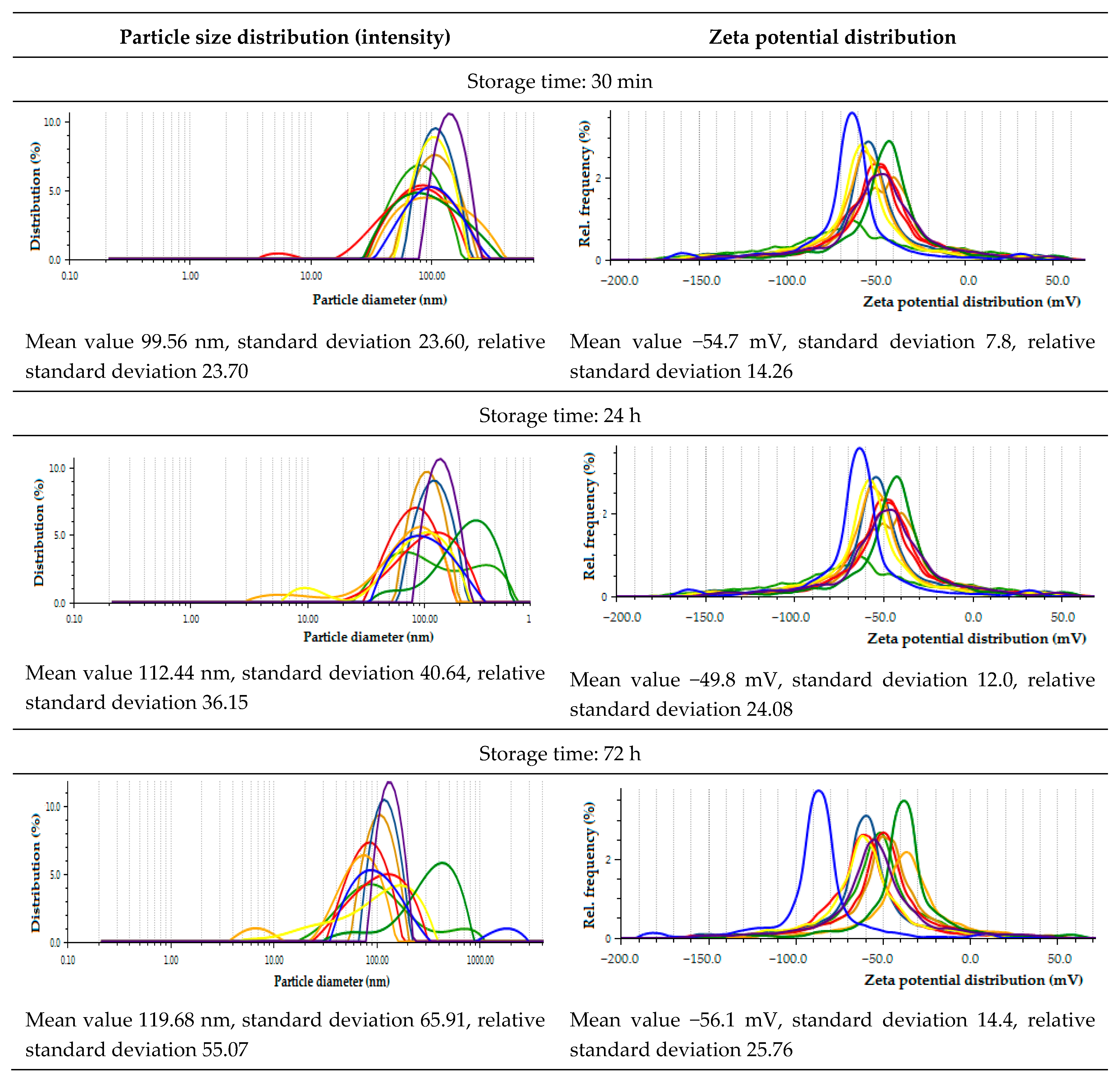
2.3.4. Zeta Potential and Size of AgNPs
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Collection of Plant Material and Sample Preparation
4.3. Reducing Antioxidant Power (CUPRAC)
4.4. DPPH Free Radical Scavenging Activity Assay
4.5. The Silver NanoParticle Antioxidant Capacity (SNPAC)
4.6. Synthesis of AgNPs
4.7. Fourier Transform Infrared (FTIR) Spectroscopy Measurements
4.8. SEM and EDS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Pimentel, B.; Andrade, V.; Zverev, V.; Gimaev, R.R.; Pomorov, A.S.; Pyatakov, A.; Alekhina, Y.; Komlev, A.; Makarova, L.; et al. Understanding the Dependence of Nanoparticles Magnetothermal Properties on Their Size for Hyperthermia Applications: A Case Study for La-Sr Manganites. Nanomaterials 2021, 11, 1826. [Google Scholar] [CrossRef]
- Di Foggia, M.; Tugnoli, V.; Ottani, S.; Dettin, M.; Zamuner, A.; Sanchez-Cortes, S.; Cesini, D.; Torreggiani, A. SERS Investigation on Oligopeptides Used as Biomimetic Coatings for Medical Devices. Biomolecules 2021, 11, 959. [Google Scholar] [CrossRef]
- Bila, D.; Radwan, Y.; Dobrovolskaia, M.A.; Panigaj, M.; Afonin, K.A. The Recognition of and Reactions to Nucleic Acid Nanoparticles by Human Immune Cells. Molecules 2021, 26, 4231. [Google Scholar] [CrossRef]
- Kukushkina, E.A.; Hossain, S.I.; Sportelli, M.C.; Ditaranto, N.; Picca, R.A.; Cioffi, N. Ag-Based Synergistic Antimicrobial Composites. A Critical Review. Nanomaterials 2021, 11, 1687. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Menon, S.; Kumar, S.V.; Tambuwala, M.M.; Bakshi, H.A.; Mehta, M.; Satija, S.; Gupta, G.; Chellappan, D.K.; Thangavelu, L.; et al. Antibacterial and antioxidant potential of biosynthesized copper nanoparticles mediated through Cissus arnotiana plant extract. J. Photochem. Photobiol. 2019, 197, 111531. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, H.; Manickam, M. Amaranthus spinosus leaf extract mediated FeO nanoparticles: Physicochemical traits, photocatalytic and antioxidant activity. ACS Sustain. Chem. Eng. 2015, 3, 3149–3156. [Google Scholar] [CrossRef]
- Babay, S.; Mhiri, T.; Toumi, M. Synthesis, structural and spectroscopic characterizations of maghemite-Fe2O3 prepared by one-step coprecipitation route. J. Mol. Struct. 2015, 1085, 286–293. [Google Scholar] [CrossRef]
- Mohamed, F.; Enaiet Allah, A.; Abu Al-Ola, K.A.; Shaban, M. Design and Characterization of a Novel ZnO–Ag/Polypyrrole Core–Shell Nanocomposite for Water Bioremediation. Nanomaterials 2021, 11, 1688. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, D.G.; Yoon, H.; Choi, Y.S.; Yoon, J.; Lee, J.C. Polyphenol/FeIII complex coated membranes having multifunctional properties prepared by a one-step fast assembly. Adv. Mater. Interfaces 2015, 2, 1500298. [Google Scholar] [CrossRef]
- Yang, L.; Cao, Z.; Sajja, H.K.; Mao, H.; Wang, L.; Geng, H.; Xu, H.; Jiang, T.; Wood, W.C.; Nie, S.; et al. Development of receptor targeted magnetic iron oxide nanoparticles for effcient drug delivery and tumor imaging. J. Biomed. Nanotechnol. 2008, 4, 439–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musino, D.; Devcic, J.; Lelong, C.; Luche, S.; Rivard, C.; Dalzon, B.; Landrot, G.; Rabilloud, T.; Capron, I. Impact of Physico-Chemical Properties of Cellulose Nanocrystal/Silver Nanoparticle Hybrid Suspensions on Their Biocidal and Toxicological Effects. Nanomaterials 2021, 11, 1862. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cabanas, M.; López-García, M.; Rodríguez-Barro, P.; Vilariño, T.; Lodeiro, P.; Herrero, R.; Barriada, J.L.; Sastre de Vicente, M.E. Antioxidant Capacity Assessment of Plant Extracts for Green Synthesis of Nanoparticles. Nanomaterials 2021, 11, 1679. [Google Scholar] [CrossRef] [PubMed]
- Zaharescu, T.; Blanco, I. Stabilization Effects of Natural Compounds and Polyhedral Oligomeric Silsesquioxane Nanoparticles on the Accelerated Degradation of Ethylene-Propylene-Diene Monomer. Molecules 2021, 26, 4390. [Google Scholar] [CrossRef]
- Alharthi, M.N.; Ismail, I.; Bellucci, S.; Khdary, N.H.; Abdel Salam, M. Biosynthesis Microwave-Assisted of Zinc Oxide Nanoparticles with Ziziphus jujuba Leaves Extract: Characterization and Photocatalytic Application. Nanomaterials 2021, 11, 1682. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Panwar, J.; Yun, Y.S. Biogenic Synthesis of Metallic Nanoparticles by Plant Extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A. Biological synthesis of metal nanoparticles. Hydrometallurgy 2006, 83, 132–140. [Google Scholar] [CrossRef]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Broich, M.L.; Moon, J.W.; Gu, B.; Allison, D.P.; Joy, D.C.; Phelps, T.J.; Doktycz, M.J. Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater. 2011, 7, 2148–2152. [Google Scholar] [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef]
- Patil Shriniwas, P.; Kumbhar Subhash, T. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem. Biophys. Rep. 2017, 10, 76–81. [Google Scholar] [CrossRef]
- Khandel, P.; Shahi, S.K.; Soni, D.K.; Yadaw, R.K.; Kanwar, L. Alpinia calcarata: Potential source for the fabrication of bioactive silver nanoparticles. Nano Converg. 2018, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Algebaly, A.S.; Mohammed, A.E.; Abutaha, N.; Elobeid, M.M. Biogenic synthesis of silver nanoparticles: Antibacterial and cytotoxic potential. Saudi J. Biol. Sci. 2020, 27, 1340–1351. [Google Scholar] [CrossRef]
- Suman, T.Y.; Radhika Rajasree, S.R.; Kanchana, A.; Elizabeth, S.B. Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extract. Colloids Surf. B Biointerfaces 2013, 106, 74–78. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Vennila, K.; Jayakumar, R.; Yusoff, A.R.; Hadibarata, T.; Palvannan, T. Phyto-synthesis of silver nanoparticles using Alternanthera tenella leaf extract: An effective inhibitor for the migration of human breast adenocarcinoma (MCF-7) cells. Bioprocess. Biosyst. Eng. 2016, 39, 651–659. [Google Scholar] [CrossRef]
- Gajendran, B.; Chinnasamy, A.; Durai, P.; Raman, J.; Ramar, M. Biosynthesis and characterization of silver nanoparticles from Datura inoxia and its apoptotic effect on human breast cancer cell line MCF7. Mater. Lett. 2014, 122, 98–102. [Google Scholar] [CrossRef]
- Csakvari, A.C.; Moisa, C.; Radu, D.G.; Olariu, L.M.; Lupitu, A.I.; Panda, A.O.; Pop, G.; Chambre, D.; Socoliuc, V.; Copolovici, L.; et al. Green Synthesis, Characterization, and Antibacterial Properties of Silver Nanoparticles Obtained by Using Diverse Varieties of Cannabis sativa Leaf Extracts. Molecules 2021, 26, 4041. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; López-Jiménez, A.J.; Abad-Jordá, M.; Rubio-Moraga, A.; Ahraz, O.; Gómez-Gómez, L.; Niza, E. Biogenic Silver Nanoparticles from Iris tuberosa as Potential Preservative in Cosmetic Products. Molecules 2021, 26, 4696. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.M.; Abdallah, B.M. Effective Inhibition of Candidiasis Using an Eco-Friendly Leaf Extract of Calotropis-gigantean-Mediated Silver Nanoparticles. Nanomaterials 2020, 10, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flieger, J.; Flieger, M. The [DPPH●/DPPH-H]-HPLC-DAD Method on Tracking the Antioxidant Activity of Pure Antioxidants and Goutweed (Aegopodium podagraria L.) Hydroalcoholic Extracts. Molecules 2020, 25, 6005. [Google Scholar] [CrossRef]
- Raimova, K.V.; Abdulladjanova, N.G.; Kurbanova, M.A.; Makhmanov, D.M.; Kadirova, S.O.; Tashpulatov, F.N.; Juraev, S.S.; Matchanov, A.D.; Rakhimov, R.N. Comprehensive study of the chemical composition of Urtica dioica L. J. Crit. Rev. 2020, 7, 750–755. [Google Scholar]
- Grauso, L.; de Falco, B.; Lanzotti, V.; Motti, R. Stinging nettle, Urtica dioica L.: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2020, 19, 1341–1377. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Ciocarlan, A.; Dragalin, I.; Aricu, A.; Lupascu, L.; Ciocarlan, N.; Popescu, V. Chemical Composition and Antimicrobial Activity of the Levisticum Officinale W.D.J. Koch Essential Oil. Chem. J. Mold. 2018, 13, 63–68. [Google Scholar] [CrossRef]
- Farid, M.; Aly, H.; Massoud, G.; Abdelgayed, S. Biochemical Studies on Bio Extracts as Antioxidant and Antibacterial Activity. J. Agricultural. Environ. Vet. Sci. 2017, 1, 45–69. [Google Scholar]
- Zimmer, A.R.; Leonardi, B.; Miron, D.; Schapoval, E.; Rodrigues de Oliveira, J.; Gosmann, G. Antioxidant and anti-inflammatory properties of Capsicum baccatum: From traditional use to scientific approach. J. Ethnopharmacol. 2012, 139, 228–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazaruk, J.; Orlikowski, P. Phytochemical profile and therapeutic potential of Viscum album L. Nat. Product Res. 2015, 30, 1–13. [Google Scholar] [CrossRef]
- Koch, W.; Zagórska, J.; Marzec, Z.; Kukula-Koch, W. Applications of Tea (Camellia sinensis) and its Active Constituents in Cosmetics. Molecules 2019, 24, 4277. [Google Scholar] [CrossRef] [Green Version]
- Burris, K.; Harte, F.M.; Davidson, P.; Jr, C.N.; Zivanovic, S. Composition and bioactive properties of Yerba Mate (Ilex paraguariensis A. St.-Hil.): A review. Chilean J. Agric. Res. 2012, 72, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Holdt, S.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Quero, J.; Osada, J.; Martín-Belloso, O.; Rodríguez-Yoldi, M.J. Phenolic-Rich Extracts from Avocado Fruit Residues as Functional Food Ingredients with Antioxidant and Antiproliferative Properties. Biomolecules 2021, 11, 977. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Kostić, A.Ž.; Gašić, U.M.; Lević, S.; Stanojević, S.P.; Barać, M.B.; Tešić, Ž.L.; Nedović, V.; Pešić, M.B. Skimmed Goat’s Milk Powder Enriched with Grape Pomace Seed Extract: Phenolics and Protein Characterization and Antioxidant Properties. Biomolecules 2021, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef] [PubMed]
- Scampicchio, M.; Wang, J.; Blasco, A.J.; Sanchez Arribas, A.; Mannino, S.; Escarpa., A. Nanoparticle-Based Assays of Antioxidant Activity. Anal. Chem. 2006, 78, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, M.; Güngör, N.; Baki, S.; Güclü, K.; Apak, R. Development of a Silver Nanoparticle-Based Method for the Antioxidant Capacity Measurement of Polyphenols. Anal. Chem. 2012, 84, 8052–8059. [Google Scholar] [CrossRef] [PubMed]
- Noipa, T.; Srijaranai, S.; Tuntulani, T.; Ngeontae, W. New approach for evaluation of the antioxidant capacity based on scavenging DPPH free radical in micelle systems. Food Res. Int. 2011, 44, 798–806. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- GonÇalves, S.; Gomes, D.; Costa, P.; Romano, A. The phenolic content and antioxidant activity of infusions from Mediterranean medicinal plants. Ind. Crop. Prod. 2013, 43, 465–471. [Google Scholar] [CrossRef]
- Srinivasan, N.; Perumal, S.; Francis, G. A promosing approach to enhance the total phenolic content and antioxidant activity of raw and processed Jatropha curcas L. kernel meal extracts. Ind. Crop. Prod. 2013, 43, 261–269. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Blois, M. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Kumar Chaudhury, N. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Dungchai, W.; Sameenoi, Y.; Chailapakul, O.; Volckens, J.; Henry, C.S. Determination of aerosol oxidative activity using silver nanoparticle aggregation on paper-based analytical devices. Analyst 2013, 138, 6766–6773. [Google Scholar] [CrossRef] [Green Version]
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess. Biosyst. Eng. 2009, 32, 79–84. [Google Scholar] [CrossRef]
- Mock, J.J.; Barbic, M.; Smith, D.R.; Schultz, D.A.; Schultz, S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002, 116, 6755–6759. [Google Scholar] [CrossRef]
- Sriramulu, M.; Sumathi, S. Photocatalytic, antioxidant, antibacterial and anti-inflammatory activity of silver nanoparticles synthesised using forest and edible mushroom. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 045012. [Google Scholar] [CrossRef] [Green Version]
- Rao, M.L.; Savithramma, N. Biological synthesis of silver nanoparticles using Svensonia hyderobadensis leaf extract and evaluation of their antimicrobial efficacy. J. Pharm. Sci. Res. 2011, 3, 1117–1121. [Google Scholar]
- Bala, M.; Arya, V. Biological synthesis of silver nanoparticles from aqueous extract of endophytic fungus Aspergillus fumigates and its antibacterial action. Int. J. Nanomat. Biostruct. 2013, 3, 37–41. [Google Scholar]
- Akbal, A.; Turkdemir, M.H.; Cicek, A.; Ulug, B. Relation between Silver Nanoparticle Formation Rate and Antioxidant Capacity of Aqueous Plant Leaf Extracts. J. Spectrosc. 2016, 2016, 4083421. [Google Scholar] [CrossRef] [Green Version]
- Thirunavoukkarasu, M.; Balaji, U.; Behera, S.; Panda, P.K.; Mishra, B.K. Biosynthesis of silver nanoparticle from leaf extract of Desmodium gangeticum (L.) DC. and its biomedical potential. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 116, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, B.; Subramanian, V.; Tumala, A.; Vellaichamy, E. Rapid synthesis of biocompatible silver nanoparticles using aqueous extract of Rosa damascena petals and evaluation of their anticancer activity. Asian Pac. J. Trop. Med. 2014, 7 (Suppl. 1), S294–S300. [Google Scholar] [CrossRef] [Green Version]
- Manikandan, R.; Manikandan, B.; Raman, T.; Arunagirinathan, K.; Prabhu, N.M.; Jothi Basu, M.; Perumal, M.; Palanisamy, S.; Munusamy, A. Biosynthesis of silver nanoparticles using ethanolic petals extract of Rosa indica and characterization of its antibacterial. Anticancer and anti-inflammatory activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 138, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shen, Y.; Xie, A.; Yu, X.; Qiu, L.; Zhang, L.; Zhang, Q. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007, 9, 852–858. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Iannone, A.; Natali, S.; Lavecchia, R. Green synthesis of silver nanoparticles using bilberry and red currant waste extracts. Processes 2019, 7, 193. [Google Scholar] [CrossRef] [Green Version]
- Bastos-Arrieta, J.; Florido, A.; Pérez-Ràfols, C.; Serrano, N.; Fiol, N.; Poch, J.; Villaescusa, I. Green synthesis of Ag nanoparticles using grape stalk waste extract for the modification of screen-printed electrodes. Nanomaterials 2018, 8, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amini, S.M.; Akbari, A. Metal nanoparticles synthesis through natural phenolic acids. IET Nanobiotechnol. 2019, 13, 771–777. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Vilela, D.; González, M.C.; Escarpa, A. Nanoparticles as analytical tools for in-vitro antioxidant-capacity assessment and beyond. TrAC, Trends Anal. Chem. 2015, 64, 1–16. [Google Scholar] [CrossRef]
- Teixeira, A.M.; Sousa, C. A review on the biological activity of camellia species. Molecules 2021, 26, 2178. [Google Scholar] [CrossRef]
- Gupta, D. Methods for determination of antioxidant capacity: A review. Int. J. Pharm. Sci. Res. 2015, 6, 546–566. [Google Scholar] [CrossRef]
- Bhutto, A.A.; Kalay, S.; Sherazi, S.T.H.; Culha, M. Quantitative structure–activity relationship between antioxidant capacity of phenolic compounds and the plasmonic properties of silver nanoparticles. Talanta 2018, 189, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Litwinienko, G.; Ingold, K.U. Abnormal solvent effects on hydrogen atom abstraction. 2. Resolution of the curcumin antioxidant controversy. The role of sequential proton loss electron transfer. J. Org. Chem. 2004, 69, 5888–5896. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Abnormal solvent effects on hydrogen atom abstraction. 3.Novel kinetics in sequential proton loss electron transfer chemistry. J. Org. Chem. 2005, 70, 8982–8990. [Google Scholar] [CrossRef] [Green Version]
- Mashwani, Z.-U.-R.; Khan, T.; Khan, M.A.; Nadhman, A. Synthesis in plants and plant extracts of silver nanoparticles with potent antimicrobial properties: Current status and future prospects. Appl. Microbiol. Biotechnol. 2015, 99, 9923–9934. [Google Scholar] [CrossRef]
- Yuan, C.-G.; Huo, C.; Gui, B.; Liu, P.; Zhang, C. Green synthesis of silver nanoparticles using Chenopodium aristatum L. stem extract and their catalytic/antibacterial activities. J. Clust. Sci. 2017, 28, 1319–1333. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Gopal, K. Biosynthesis of silver and gold nanoparticles using chenopodium album leaf extract. Colloids Surf. A 2010, 369, 27–33. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological properties, bioactive constituents, and pharmacokinetics of some capsicum spp. and capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef] [PubMed]
- Aza-González, C.; Núñez-Palenius, H.G.; Ochoa-Alejo, N. Molecular biology of capsaicinoid biosynthesis in chili pepper (Capsicum spp.). Plant. Cell Rep. 2011, 30, 695–706. [Google Scholar] [CrossRef]
- Yilmaz, M.; Turkdemir, H.; Kilic, M.A.; Bayramd, E.; Cicekeg, A.; Metef, A.; Ulugg, B. Biosynthesis of silver nanoparticles using leaves of Stevia rebaudiana. Mater. Chem. Phys. 2011, 130, 1195–1202. [Google Scholar] [CrossRef]
- Garcia, M.A. Corrigendum: Surface plasmons in metallic nanoparticles: Fundamentals and applications. J. Phys. D Appl. Phys. 2011, 44, 283001. [Google Scholar] [CrossRef]
| Reference Chemical | Linearity Range (µM) | Slope ± SD | Intercept ± SD | Standard Error of Estimate (se) | LOD (µM) | Fisher F Statistic (F) | Coefficient of Determination (R2) |
|---|---|---|---|---|---|---|---|
| Trolox | 300–1000 | 0.00057 ± 0.00002 | 0.00331 ± 0.01470 | 0.018 | 86.84 | 624.29 | 0.9968 |
| Chlorogenic acid | 300–1000 | 0.00126 ± 0.00007 | 0.10900 ± 0.04400 | 0.055 | 115.23 | 332.97 | 0.9940 |
| Ascorbic acid | 300–1000 | 0.00065 ± 0.00002 | −0.06010 ± 0.0127 | 0.016 | 65.48 | 646.32 | 0.9969 |
| Investigated Extracts | Absorbance [Mean Value] N = 2, dill. 1:100 | TAC (mM) | ||
|---|---|---|---|---|
| Trolox | Ascorbic Acid | Chlorogenic Acid | ||
| Aegopodium podagraria | 0.108 | 18.881 | 16.744 | 8.571 |
| Ilex paraguariensis | 0.271 | 47.377 | 42.015 | 21.507 |
| Porphyra Yezoensis1 | 0.114 1 | 0.199 1 | 0.176 1 | 0.090 1 |
| Urtica dioica | 0.005 | 0.874 | 0.775 | 0.396 |
| Camellia sinensis | 0.645 | 112.762 | 100.000 | 51.190 |
| Viscum album | 0.009 | 1.573 | 1.395 | 0.714 |
| Capsicum baccatum | 0.007 | 1.136 | 1.007 | 0.515 |
| Tilia cordata | 0.089 | 15.559 | 13.798 | 7.063 |
| Salvia officinalis | 0.152 | 26.573 | 23.565 | 12.063 |
| Levisticum officinale | 0.036 | 6.293 | 5.581 | 2.857 |
| Reference Chemicals | Linearity Range (mM) | Slope ± SD | Intercept ± SD | Standard Error of Estimate (se) | LOD (µM) | Fisher F Statistic (F) | Coefficient of Determination (R2) |
|---|---|---|---|---|---|---|---|
| Trolox | 0.005–0.03 | 2757.764 ± 37.169 | −1.1065 ± 0.7077 | 0.963 | 0.8498 | 5504.78 | 0.9993 |
| Chlorogenic acid | 0.01–0.025 | 2940.649 ± 95.967 | −1.2686 ± 1.4789 | 1.730 | 1.6500 | 938.94 | 0.9978 |
| Ascorbic acid | 0.01–0.03 | 2756.221 ± 115.521 | −3.3061 ± 2.2370 | 2.790 | 1.8044 | 569.26 | 0.9930 |
| Investigated Extracts | % I | Absorbance | TAC (mM) | ||
|---|---|---|---|---|---|
| Trolox | Ascorbic Acid | Chlorogenic Acid | |||
| Aegopodium podagraria 2 | 48.26 | 0.445 | 0.0614 | 0.1997 | 0.0578 |
| Ilex paraguariensis 1 | 77.06 | 0.197 | 0.1252 | 0.4089 | 0.1177 |
| Porphyra Yezoensis 3,4 | 25.84 | 0.709 | 0.0017 | 0.0054 | 0.0016 |
| Urtica dioica 2 | 30.93 | 0.594 | 0.0446 | 0.1439 | 0,0420 |
| Camellia sinensis 1,5 | 64.02 | 0.344 | 1.8893 | 6.1434 | 1.7763 |
| Viscum album 2 | 20.23 | 0.631 | 0.0388 | 0.1249 | 0.0366 |
| Capsicum baccatum 2 | 26.36 | 0.704 | 0.0276 | 0.0897 | 0.0259 |
| Tilia cordata 1 | 62.09 | 0.326 | 0.1833 | 0.5958 | 0.1724 |
| Salvia officinalis 2 | 78.26 | 0.187 | 0.1151 | 0.3755 | 0.1082 |
| Levisticum officinale 2 | 30.23 | 0.600 | 0.0514 | 0.1666 | 0.0484 |
| Reference Chemical | Linearity Range (µM) | Slope ± SD | Intercept ± SD | Standard Error of Estimate (se) | LOD (µM) | Fisher F Statistic (F) | Coefficient of Determination (R2) |
|---|---|---|---|---|---|---|---|
| Trolox | 3.5–160.0 | 0.006189 ± 0.000177 | 0.067507 ± 0.01139 | 0.03013 | 5.8548 | 1224.18 | 0.9919 |
| Chlorogenic acid | 3.5–25.0 | 0.02975 ± 0.001567 | 0.0181 ± 0.02156 | 0.03307 | 2.5034 | 360.49 | 0.9863 |
| Ascorbic acid | 3.5–25.0 | 0.0169 ± 0.000571 | 0.0715 ± 0.007861 | 0.01206 | 1.5141 | 878.25 | 0.9943 |
| Investigated Extracts | Absorbance (Mean Value) | TAC (mM) | ||
|---|---|---|---|---|
| Trolox | Ascorbic Acid | Chlorogenic Acid | ||
| Aegopodium podagraria 1 | 1.172 | 9.9938 | 3.6460 | 2.1721 |
| Ilex paraguariensis 2 | 1.244 | 53.2262 | 19.4260 | 11.5379 |
| Porphyra Yezoensis 1 | 0.249 | 1.6422 | 0.5882 | 0.4346 |
| Urtica dioica 1 | 0.899 | 7.5236 | 2.7420 | 1.6582 |
| Camellia sinensis 2 | 1.696 | 73.6754 | 26.9148 | 15.7920 |
| Viscum album 1 | 0.318 | 2.2665 | 0.8168 | 0.5645 |
| Capsicum baccatum 1 | 0.495 | 3.8681 | 1.4033 | 0.8977 |
| Tilia mordata 2 | 0.584 | 23.3668 | 8.4911 | 5.3261 |
| Salvia officinalis 2 | 0.712 | 29.1577 | 10.6118 | 6.5308 |
| Levisticum officinale 1 | 1.397 | 12.0296 | 4.3922 | 2.5956 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flieger, J.; Franus, W.; Panek, R.; Szymańska-Chargot, M.; Flieger, W.; Flieger, M.; Kołodziej, P. Green Synthesis of Silver Nanoparticles Using Natural Extracts with Proven Antioxidant Activity. Molecules 2021, 26, 4986. https://doi.org/10.3390/molecules26164986
Flieger J, Franus W, Panek R, Szymańska-Chargot M, Flieger W, Flieger M, Kołodziej P. Green Synthesis of Silver Nanoparticles Using Natural Extracts with Proven Antioxidant Activity. Molecules. 2021; 26(16):4986. https://doi.org/10.3390/molecules26164986
Chicago/Turabian StyleFlieger, Jolanta, Wojciech Franus, Rafał Panek, Monika Szymańska-Chargot, Wojciech Flieger, Michał Flieger, and Przemysław Kołodziej. 2021. "Green Synthesis of Silver Nanoparticles Using Natural Extracts with Proven Antioxidant Activity" Molecules 26, no. 16: 4986. https://doi.org/10.3390/molecules26164986
APA StyleFlieger, J., Franus, W., Panek, R., Szymańska-Chargot, M., Flieger, W., Flieger, M., & Kołodziej, P. (2021). Green Synthesis of Silver Nanoparticles Using Natural Extracts with Proven Antioxidant Activity. Molecules, 26(16), 4986. https://doi.org/10.3390/molecules26164986








