Modification of Hemodialysis Membranes for Efficient Circulating Tumor Cell Capture for Cancer Therapy
Abstract
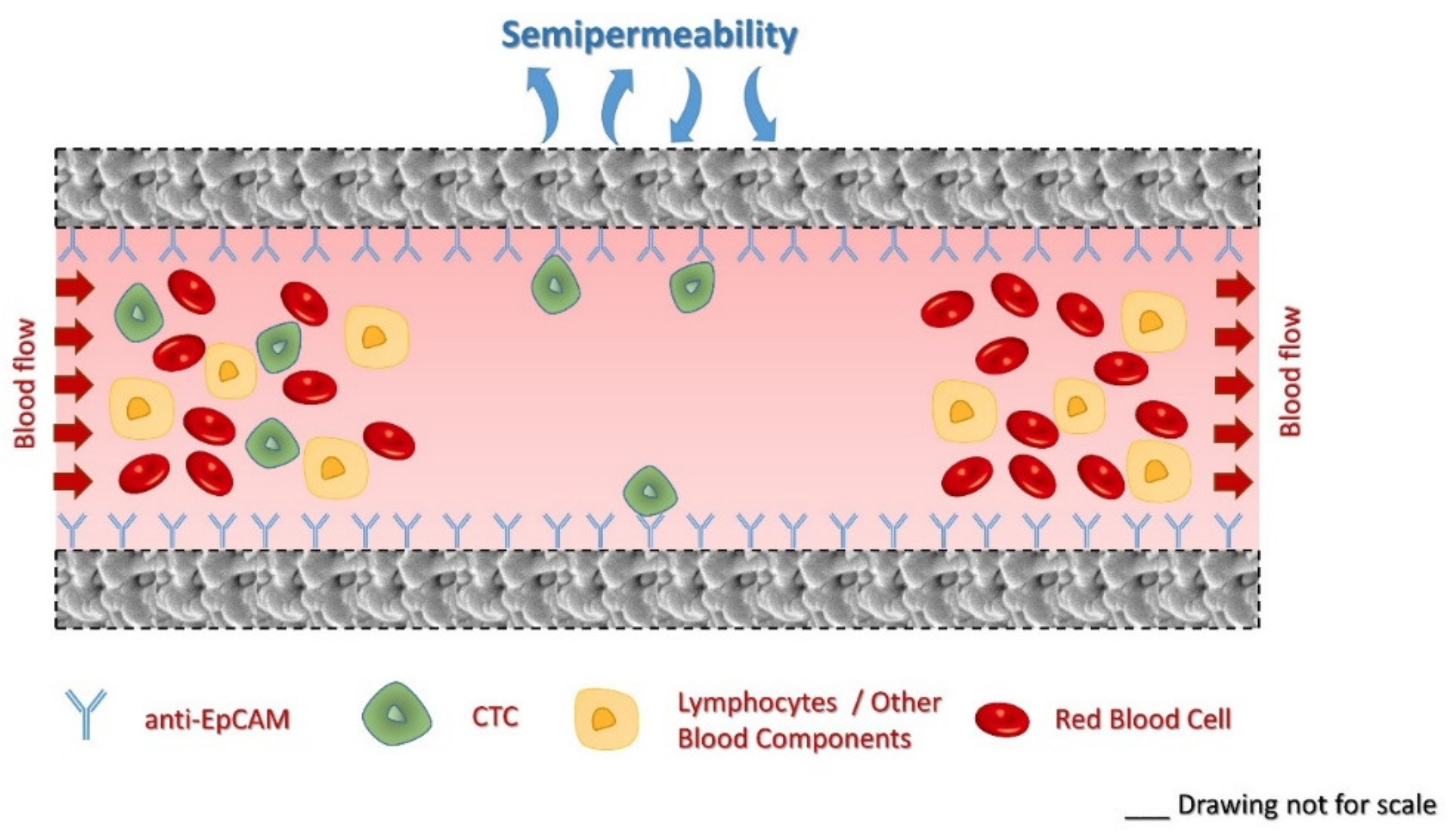
1. Introduction
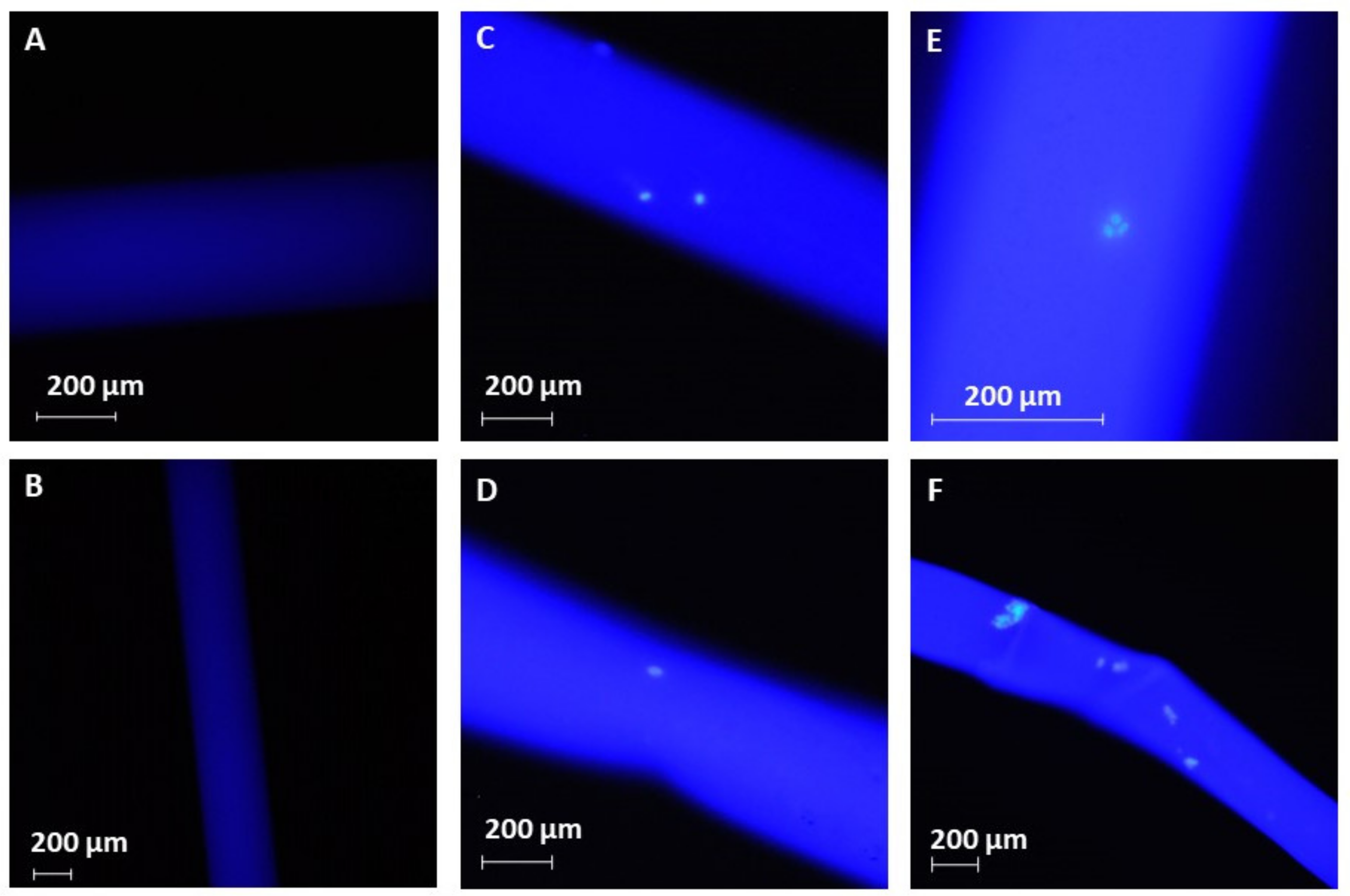
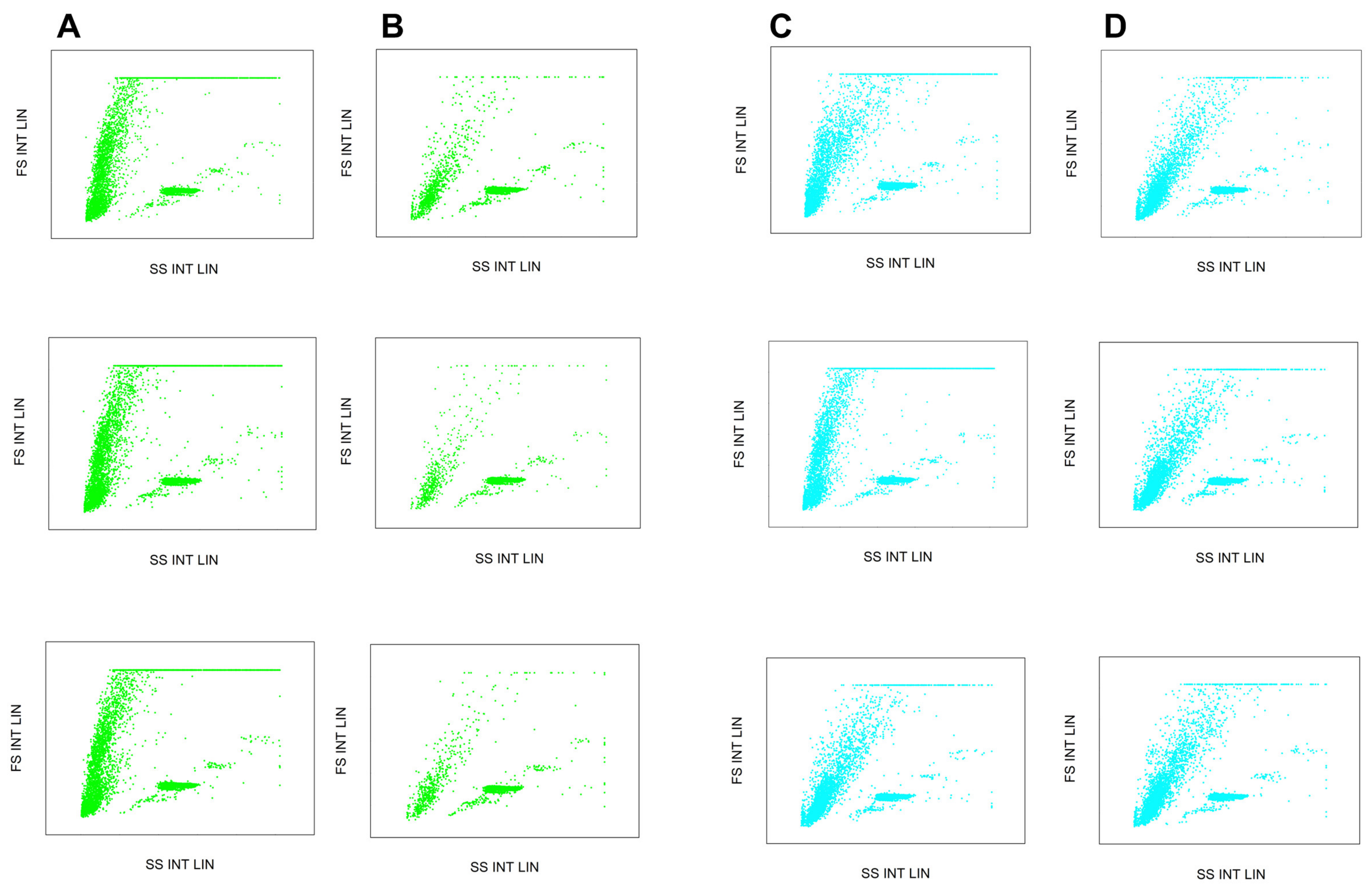
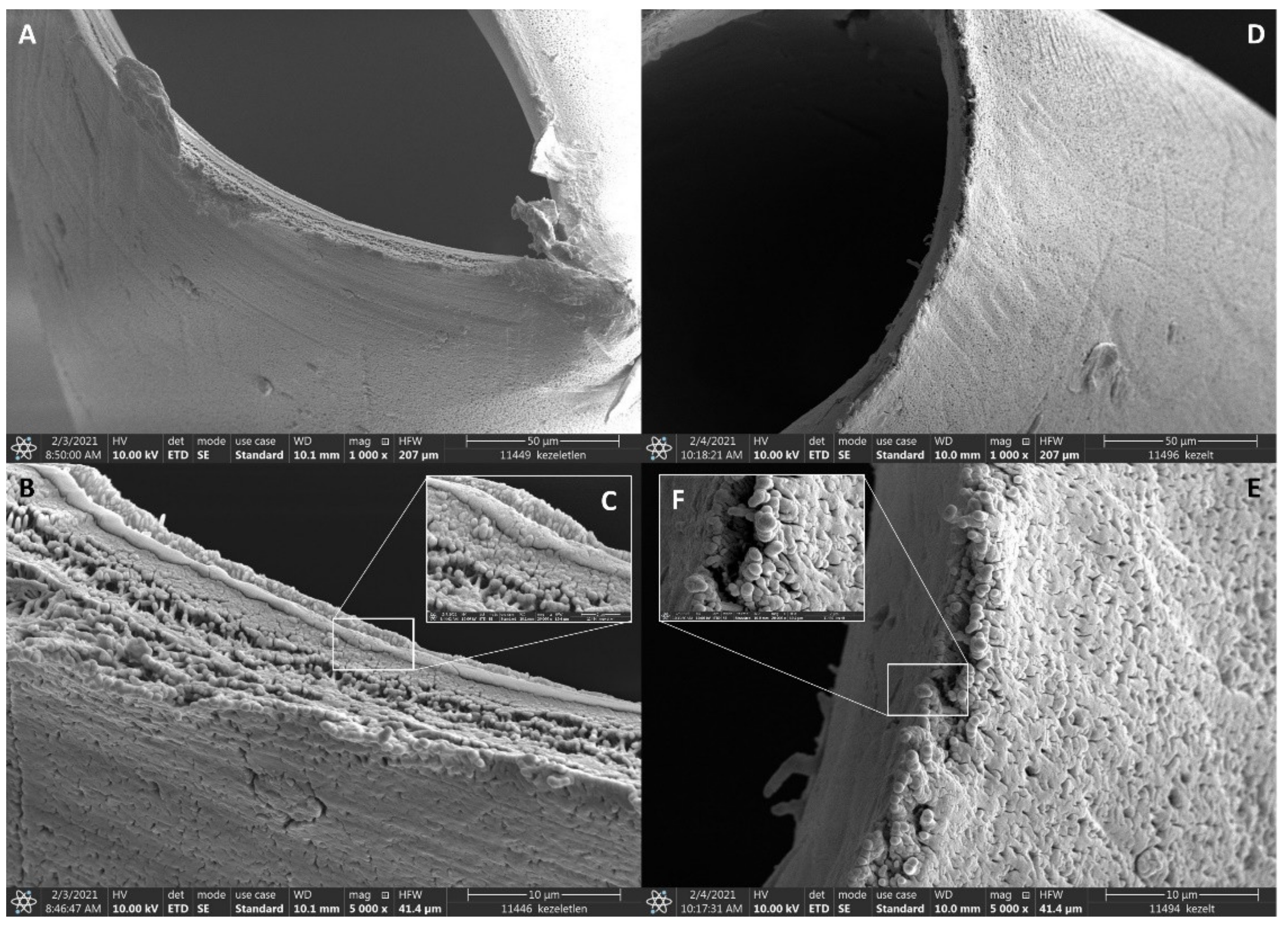
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Immobilization of Anti-EpCAM onto the Surface of Hollow Fibers
3.3. Cell Culturing
3.4. Flow Cytometry
3.5. Cell Capture
3.6. Water Permeability Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- GLOBOCAN. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf (accessed on 20 January 2021).
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Miller, M.C.; Doyle, G.V.; Terstappen, L.W. Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J. Oncol. 2010, 2010, 617421. [Google Scholar] [CrossRef]
- Barradas, A.M.; Terstappen, L.W. Towards the Biological Understanding of CTC: Capture Technologies, Definitions and Potential to Create Metastasis. Cancers 2013, 5, 1619–1642. [Google Scholar] [CrossRef]
- Bailey, P.C.; Martin, S.S. Insights on CTC Biology and Clinical Impact Emerging from Advances in Capture Technology. Cells 2019, 8, 553. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nature reviews. Cancer 2002, 2, 442–454. [Google Scholar]
- Balakrishnan, A.; Koppaka, D.; Anand, A.; Deb, B.; Grenci, G.; Viasnoff, V.; Thompson, E.W.; Gowda, H.; Bhat, R.; Rangarajan, A.; et al. Circulating Tumor Cell cluster phenotype allows monitoring response to treatment and predicts survival. Sci. Rep. 2019, 9, 7933. [Google Scholar] [CrossRef]
- Hsieh, J.C.; Chen, G.Y.; Jhou, D.D.; Chou, W.C.; Yeh, C.N.; Hwang, T.L.; Lin, H.C.; Chu, H.C.; Wang, H.M.; Yen, T.C.; et al. The Prognostic Value of Circulating Tumor Cells in Asian Neuroendocrine Tumors. Sci. Rep. 2019, 9, 19917. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, A.; Kiavue, N.; Bidard, F.C.; Pierga, J.Y.; Cabel, L. Clinical utility of circulating tumor cells: An update. Mol. Oncol. 2020, 15, 1647–1666. [Google Scholar] [CrossRef] [PubMed]
- De Wit, S.; Van Dalum, G.; Terstappen, L.W.M.M. Detection of Circulating Tumor Cells. Science 2014, 2014, 819362. [Google Scholar] [CrossRef] [PubMed]
- Bankó, P.; Lee, S.Y.; Nagygyörgy, V.; Zrínyi, M.; Chae, C.H.; Cho, D.H.; Telekes, A. Technologies for circulating tumor cell separation from whole blood. J. Hematol. Oncol. 2019, 12, 48. [Google Scholar] [CrossRef]
- Dolfus, C.; Piton, N.; Toure, E.; Sabourin, J.C. Circulating tumor cell isolation: The assets of filtration methods with polycarbonate track-etched filters. Chin. J. Cancer Res. 2015, 27, 479–487. [Google Scholar]
- Kowalik, A.; Kowalewska, M.; Góźdź, S. Current approaches for avoiding the limitations of circulating tumor cells detection methods—implications for diagnosis and treatment of patients with solid tumors. Transl. Res. 2017, 185, 58–84.e15. [Google Scholar] [CrossRef]
- Guzman, N.A.; Guzman, D.E. A Two-Dimensional Affinity Capture and Separation Mini-Platform for the Isolation, Enrichment, and Quantification of Biomarkers and Its Potential Use for Liquid Biopsy. Biomedicines 2020, 8, 255. [Google Scholar] [CrossRef]
- Tura, A.; Lüke, J.; Merz, H.; Reinsberg, M.; Lüke, M.; Jager, M.J.; Grisanti, S. Identification of circulating melanoma cells in uveal melanoma patients by dual-marker immunoenrichment. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4395–4404. [Google Scholar] [CrossRef]
- Lim, S.B.; Di Lee, W.; Vasudevan, J.; Lim, W.T.; Lim, C.T. Liquid biopsy: One cell at a time. NPJ Precis. Oncol. 2019, 3, 23. [Google Scholar] [CrossRef]
- Ribeiro-Samy, S.; Oliveira, M.I.; Pereira-Veiga, T.; Muinelo-Romay, L.; Carvalho, S.; Gaspar, J.; Freitas, P.P.; López-López, R.; Costa, C.; Diéguez, L. Fast and efficient microfluidic cell filter for isolation of circulating tumor cells from unprocessed whole blood of colorectal cancer patients. Sci. Rep. 2019, 9, 8032. [Google Scholar] [CrossRef]
- Zhang, Z.; King, M.R. Nanomaterials for the Capture and Therapeutic Targeting of Circulating Tumor Cells. Cell. Mol. Bioeng. 2017, 10, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Gaitas, A. Extracorporeal photo-immunotherapy for circulating tumor cells. PLoS ONE 2015, 10, e0127219. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, C.; Del Rio, A.; Martini, C.; Manoni, E.; Varchi, G. Light-Induced Therapies for Prostate Cancer Treatment. Front. Chem. 2019, 7, 719. [Google Scholar] [CrossRef] [PubMed]
- Vieyra-Garcia, P.A.; Wolf, P. Extracorporeal Photopheresis: A Case of Immunotherapy Ahead of Its Time. Transfus. Med. Hemother. 2020, 47, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Garban, F.; Carras, S.; Drillat, P.; Jacob, M.C.; Fabre, B.; Callanan, M.; Courby, S.; Makowski, C.; Cahn, J.Y.; Gressin, R. Extracorporeal photopheresis as a curative treatment strategy in non epidermotropic T-cell lymphoma and large granular lymphocyte leukemia. Ann. Oncol. 2012, 23, 2386–2390. [Google Scholar] [CrossRef] [PubMed]
- Edelman, M.J.; Potter, P.; Mahaffey, K.G.; Frink, R.; Leidich, R.B. The potential for reintroduction of tumor cells during intraoperative blood salvage: Reduction of risk with use of the RC-400 leukocyte depletion filter. Urology 1996, 47, 179–181. [Google Scholar] [CrossRef]
- Frühauf, N.R.; Dumpich, O.; Kaudel, C.P.; Kasimir-Bauer, S.; Oldhafer, K.J. Filtration of malignant cells: Tumour cell depletion in an ex vivo model using a leukocyte adhesion filter. Perfusion 2001, 16, 51–55. [Google Scholar] [CrossRef]
- Gaitas, A.; Kim, G. Chemically Modified Plastic Tube for High Volume Removal and Collection of Circulating Tumor Cells. PLoS ONE 2015, 10, e0133194. [Google Scholar] [CrossRef]
- Petruzzelli, M.; Wagner, E.F. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 2016, 30, 489–501. [Google Scholar] [CrossRef]
- Porporato, P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 2004, 4, 11–22. [Google Scholar] [CrossRef]
- Bellomo, R.; Tipping, P.; Boyce, N. Continuous veno-venous hemofiltration with dialysis removes cytokines from the circulation of septic patients. Crit. Care Med. 1993, 21, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Reis, T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat. Rev. Nephrol. 2020, 16, 308–310. [Google Scholar] [CrossRef]
- Harm, S.; Schildböck, C.; Hartmann, J. Cytokine Removal in Extracorporeal Blood Purification: An in vitro Study. Blood Purif. 2020, 49, 33–43. [Google Scholar] [CrossRef]
- David, S.; Thamm, K.; Schmidt, B.M.W.; Falk, C.S.; Kielstein, J.T. Effect of extracorporeal cytokine removal on vascular barrier function in a septic shock patient. J. Intensive Care 2017, 5, 12. [Google Scholar] [CrossRef]
- Prado, B.L.; Qian, Y. Anti-cytokines in the treatment of cancer cachexia. Ann. Palliat. Med. 2019, 8, 67–79. [Google Scholar] [CrossRef]
- Yu, D.; Tang, L.; Dong, Z.; Loftis, K.A.; Ding, Z.; Cheng, J.; Qin, B.; Yan, J.; Li, W. Effective reduction of non-specific binding of blood cells in a microfluidic chip for isolation of rare cancer cells. Biomater. Sci. 2018, 6, 2871–2880. [Google Scholar] [CrossRef]
- Finelli, L.; Miller, J.T.; Tokars, J.I.; Alter, M.J.; Arduino, M.J. National surveillance of dialysis-associated diseases in the United States, 2002. Semin. Dial. 2005, 18, 52–61. [Google Scholar] [CrossRef]
- Sheth, R.A.; Sheth, A.U. A Primer on Hemodialysis From an Interventional Radiology Perspective. Tech. Vasc. Interv. Radiol. 2017, 20, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Datta-Mannan, A.; Lu, J.; Witcher, D.R.; Leung, D.; Tang, Y.; Wroblewski, V.J. The interplay of non-specific binding, target-mediated clearance and FcRn interactions on the pharmacokinetics of humanized antibodies. mAbs 2015, 7, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabières, C.; Riethdorf, S. Cancer micrometastases. Nature reviews. Clin. Oncol. 2009, 6, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.C.; Proudhon, C.; Pierga, J.Y. Circulating tumor cells in breast cancer. Mol. Oncol. 2016, 10, 418–430. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Gires, O. EpCAM (CD326) finding its role in cancer. Br. J. Cancer 2007, 96, 417–423. [Google Scholar] [CrossRef]
- Thimsen, V.; Hölsken, A.; Buchfelder, M.; Flitsch, J.; Fahlbusch, R.; Stefanits, H.; Losa, M.; Jones, D.T.W.; Buslei, R. EpCAM (CD326) is differentially expressed in craniopharyngioma subtypes and Rathke’s cleft cysts. Sci. Rep. 2016, 6, 29731. [Google Scholar] [CrossRef]
- Gires, O.; Pan, M.; Schinke, H.; Canis, M.; Baeuerle, P.A. Expression and function of epithelial cell adhesion molecule EpCAM: Where are we after 40 years? Cancer Metastasis Rev. 2020, 39, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Nicolazzo, C.; Raimondi, C.; Francescangeli, F.; Ceccarelli, S.; Trenta, P.; Magri, V.; Marchese, C.; Zeuner, A.; Gradilone, A.; Gazzaniga, P. EpCAM-expressing circulating tumor cells in colorectal cancer. Int. J. Biol. Markers 2017, 32, e415–e420. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, F.; Pinzani, P.; Orlando, C.; Gasperoni, S.; Vannini, L.; Antonuzzo, L.; Mario, P. Circulating tumour cells in colorectal cancer. Eur. J. Cancer 2008, 6, 52–59. [Google Scholar] [CrossRef][Green Version]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Cronin, R.E.; Reilly, R.F. Unfractionated heparin for hemodialysis: Still the best option. Semin. Dial. 2010, 23, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.E.; Murawski, J.; Tabani, Y.; Wolff, S.H.; Zydney, A.L.; Funderburk, F.R.; Huang, Z.; Kapoian, T.; Sherman, R.A. Water permeability of high-flux dialyzer membranes after Renalin reprocessing. Kidney Int. 2007, 71, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
| Initial | Flow Through | Efficiency (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample ID | Cell Concentration (pcs/µL) | Average Cell Concentration (pcs/µL) | Absolute Cell Count (pcs) | Sample ID | Cell Concentration (pcs/µL) | Average Cell Concentration (pcs/µL) | Absolute Cell Count (pcs) | |
| PBS-EpCAM 1/1 | 844 | 842 | 420808 | PBS-EpCAM 1/1 | 175 | 167 | 83432 | 80.17 |
| PBS-EpCAM 1/2 | 848 | PBS-EpCAM 1/2 | 164 | |||||
| PBS-EpCAM 1/3 | 832 | PBS-EpCAM 1/3 | 162 | |||||
| PBS-EpCAM 2/1 | 771 | 778 | 389155 | PBS-EpCAM 2/1 | 112 | 108 | 54022 | 86.12 |
| PBS-EpCAM 2/2 | 777 | PBS-EpCAM 2/2 | 111 | |||||
| PBS-EpCAM 2/3 | 786 | PBS-EpCAM 2/3 | 101 | |||||
| PBS-EpCAM 3/1 | 807 | 795 | 397403 | PBS-EpCAM 3/1 | 115 | 109 | 54593 | 86.26 |
| PBS-EpCAM 3/2 | 798 | PBS-EpCAM 3/2 | 103 | |||||
| PBS-EpCAM 3/3 | 780 | PBS-EpCAM 3/3 | 109 | |||||
| PBS-Control 1/1 | 869 | 827 | 413533 | PBS-Control 1/1 | 561 | 535 | 267630 | 35.28 |
| PBS-Control 1/2 | 811 | PBS-Control 1/2 | 532 | |||||
| PBS-Control 1/3 | 801 | PBS-Control 1/3 | 513 | |||||
| PBS-Control 2/1 | 700 | 778 | 389153 | PBS-Control 2/1 | 483 | 504 | 252238 | 35.18 |
| PBS-Control 2/2 | 811 | PBS-Control 2/2 | 521 | |||||
| PBS-Control 2/3 | 825 | PBS-Control 2/3 | 509 | |||||
| PBS-Control 3/1 | 805 | 818 | 408873 | PBS-Control 3/1 | 538 | 533 | 266692 | 34.77 |
| PBS-Control 3/2 | 816 | PBS-Control 3/2 | 537 | |||||
| PBS-Control 3/3 | 833 | PBS-Control 3/3 | 525 | |||||
| Blood-EpCAM 1/1 | 811 | 824 | 412200 | Blood-EpCAM 1/1 | 257 | 257 | 128500 | 68.83 |
| Blood-EpCAM 1/2 | 812 | Blood-EpCAM 1/2 | 265 | |||||
| Blood-EpCAM 1/3 | 850 | Blood-EpCAM 1/3 | 249 | |||||
| Blood-EpCAM 2/1 | 791 | 802 | 401058 | Blood-EpCAM 2/1 | 211 | 217 | 108270 | 73.00 |
| Blood-EpCAM 2/2 | 805 | Blood-EpCAM 2/2 | 217 | |||||
| Blood-EpCAM 2/3 | 811 | Blood-EpCAM 2/3 | 222 | |||||
| Blood-EpCAM 3/1 | 836 | 840 | 419902 | Blood-EpCAM 3/1 | 271 | 269 | 134253 | 68.03 |
| Blood-EpCAM 3/2 | 840 | Blood-EpCAM 3/2 | 266 | |||||
| Blood-EpCAM 3/3 | 844 | Blood-EpCAM 3/3 | 269 | |||||
| Blood-Control 1/1 | 853 | 846 | 423233 | Blood-Control 1/1 | 635 | 635 | 317323 | 25.02 |
| Blood-Control 1/2 | 841 | Blood-Control 1/2 | 641 | |||||
| Blood-Control 1/3 | 846 | Blood-Control 1/3 | 629 | |||||
| Blood-Control 2/1 | 813 | 822 | 410932 | Blood-Control 2/1 | 666 | 671 | 335482 | 18.36 |
| Blood-Control 2/2 | 822 | Blood-Control 2/2 | 676 | |||||
| Blood-Control 2/3 | 830 | Blood-Control 2/3 | 672 | |||||
| Blood-Control 3/1 | 805 | 808 | 404175 | Blood-Control 3/1 | 647 | 645 | 322735 | 20.15 |
| Blood-Control 3/2 | 810 | Blood-Control 3/2 | 650 | |||||
| Blood-Control 3/3 | 810 | Blood-Control 3/3 | 640 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarvas, G.; Szerenyi, D.; Tovari, J.; Takacs, L.; Guttman, A. Modification of Hemodialysis Membranes for Efficient Circulating Tumor Cell Capture for Cancer Therapy. Molecules 2021, 26, 4845. https://doi.org/10.3390/molecules26164845
Jarvas G, Szerenyi D, Tovari J, Takacs L, Guttman A. Modification of Hemodialysis Membranes for Efficient Circulating Tumor Cell Capture for Cancer Therapy. Molecules. 2021; 26(16):4845. https://doi.org/10.3390/molecules26164845
Chicago/Turabian StyleJarvas, Gabor, Dora Szerenyi, Jozsef Tovari, Laszlo Takacs, and Andras Guttman. 2021. "Modification of Hemodialysis Membranes for Efficient Circulating Tumor Cell Capture for Cancer Therapy" Molecules 26, no. 16: 4845. https://doi.org/10.3390/molecules26164845
APA StyleJarvas, G., Szerenyi, D., Tovari, J., Takacs, L., & Guttman, A. (2021). Modification of Hemodialysis Membranes for Efficient Circulating Tumor Cell Capture for Cancer Therapy. Molecules, 26(16), 4845. https://doi.org/10.3390/molecules26164845







