An Overview of the Analytical Methods for the Determination of Organic Ultraviolet Filters in Cosmetic Products and Human Samples
Abstract
1. Introduction
2. Analytical Methods for UV Filter Determination in Cosmetic Samples
2.1. Sample Preparation
2.2. Analytical Techniques
3. Analytical Methods for UV Filter Determination in Biological Samples
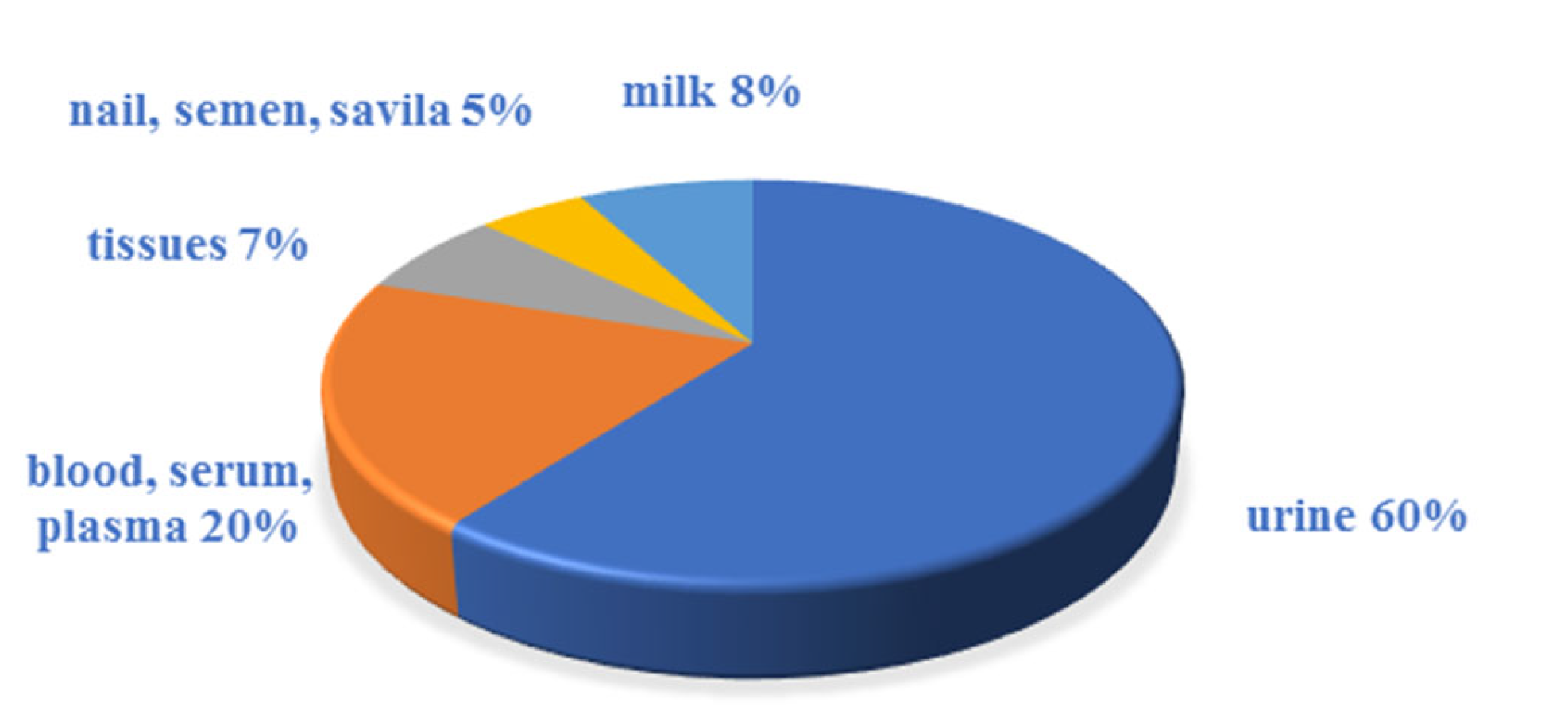
3.1. Sample Preparation
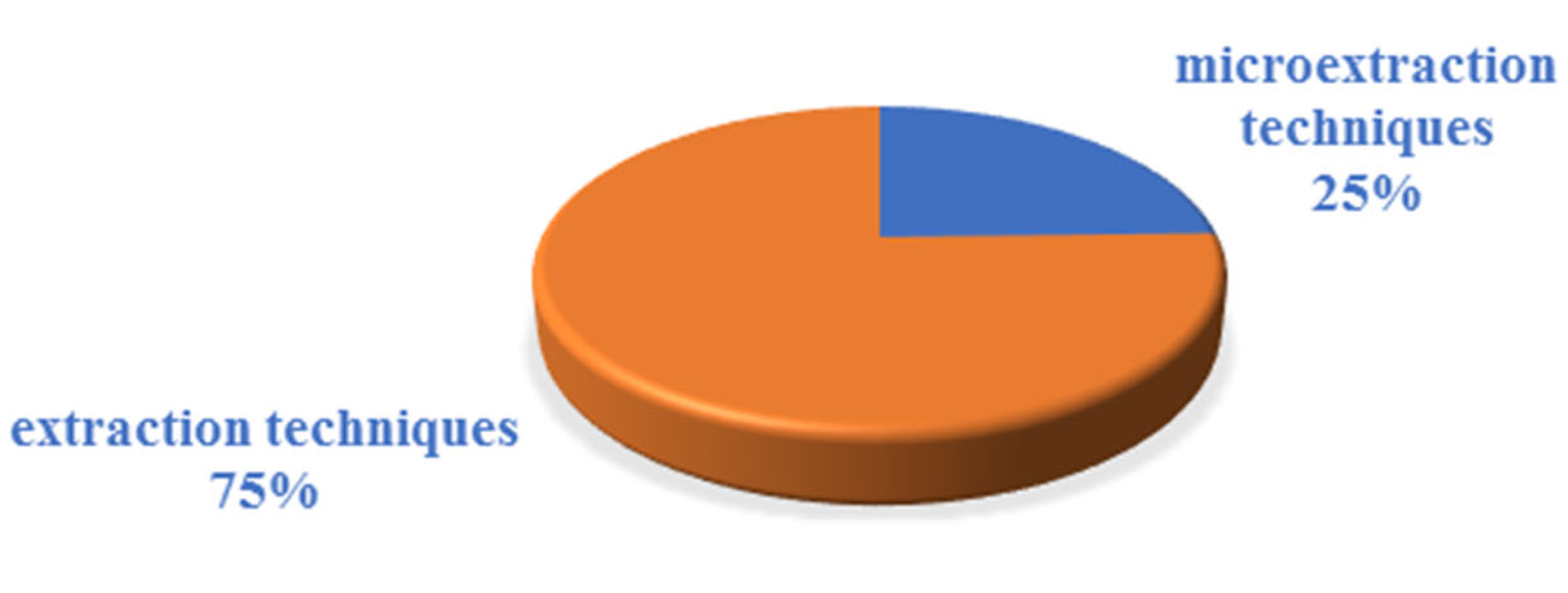
3.2. Analytical Techniques
3.3. Accuracy and Sensitivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| [C6MIM][PF6]: | hexyl-3-methylimidazolium hexafluorophosphate |
| 2-OH-BP: | 2-hydroxybenzophenone |
| 3-BC: | 3-benzophenone camphor |
| 4-AHA: | p-aminohippuric acid |
| 4-AMB: | p-acetamidobenzoic acid |
| 4-DHB: | 4,4-dihydroxybenzophenone |
| 4-MBC: | 3-(4-methylbenzylidene)-camphor |
| 4-OCH3-AHA: | p-acetamidohippuric acid |
| 4-OH-BP: | 4-hydroxybenzophenone |
| 5cx-EPS: | 5-(((2-hydroxybenzoyl)oxy)methyl)heptanoic acid |
| 5-OH-EHS: | 5-hydroxy-2-ethylhexyl salicylate |
| 5oxo-EHS: | 2-ethyl-5-oxohexyl 2-hydroxybenzoate |
| AALME: | air-assisted liquid–liquid microextraction |
| Ac: | Acetone |
| APCI: | atmosphere pressure chemical ionisation |
| API: | atmosphere pressure ionisation |
| APPI: | atmosphere pressure photoionisation |
| ASE: | accelerated solvent extraction |
| BMDBM: | butyl methoxydibenzoylmethane/avobenzene |
| BAµE: | bar adsorptive microextraction |
| BC: | benzyl cinnamate |
| BDM: | butyl methoxydibenzoylmethane |
| EMT: | bis-ethylhexyloxyphenol methoxyphenyl triazine |
| BP: | Benzophenone |
| BP-1: | 2,4-dihydroxybenzophenone |
| BP-10: | 2-hydroxy-4-methoxy-4′-methylbenzophenone |
| BP-12: | (2-hydroxy-4-octoxy-phenyl)-phenyl-methanone |
| BP-2: | 2,2′,4,4′-tetrahydroxybenzophenone |
| BP-3: | 2-hydroxy-4-methoxybenzophenone |
| BP-4: | 2-hydroxy-4-methoxybenzophenone-5-sulphonic acid |
| BP-6: | 2,2′-dihydroxy-4,4′-dimethoxybenzophenone |
| BP-7: | 5-chloro-2- hydroxybenzophenone |
| BP-8: | 2,2′-dihydroxy-4-methoxybenzophenone |
| BP-OH: | Benzhydrol |
| BS: | benzyl salicate |
| BZT: | Benzotriazole |
| C18: | Octadecyl |
| CDAA: | 2-cyano-3,3-diphenyl acrylic acid |
| CPE: | cloud point extraction |
| DAD: | diode-array detection |
| DART-MS: | direct-analysis-in-real-time mass spectrometry |
| DBT: | diethylhexyl butamino triazone |
| DCM: | Dichloromethane |
| DEA: | Diethylaminopropyl |
| DHHB: | diethyloamino hydroxybenzoyl hexyl benzoate |
| DLLME: | dispersive liquid–liquid microextraction |
| DMF: | n,n-dimethylformamide |
| DTS: | drometrizole trisiloxane |
| DS: | Densitometry |
| d-SPE: | dispersive solid-phase extraction |
| EA: | ethyl acetate |
| ECD: | electron captur detector |
| EDP: | 2-ethylhexyl 4-(n,n-dimethylamino)benzoate |
| EHC: | ethylhexyl cinnamate |
| EHS: | 2-ethylhexyl salicylate |
| EI: | electron impact |
| EMC: | ethylhexyl methoxycinnamate |
| EHS: | ethylhexyl salicylate |
| ESI: | electrospray ionisation |
| ET: | ethylhexyl triazone |
| EtOH: | Ethanol |
| EtPABA: | ethyl p-aminobenzoic acid |
| FL: | Fluorescence |
| FPSE: | fabric phase sorptive extraction |
| GC: | gas chromatography |
| HFLPME: | hollow-fiber liquid-phase microextraction |
| HPLC: | high-performance liquid chromatography |
| HS: | salicylic acid 3,3,5-trimethcyclohexyl ester |
| HTLC: | high-temperature liquid chromatographic |
| IMC: | isoamyl p-methoxycinnamate |
| LC: | liquid chromatography |
| LD: | liquid desorption |
| LLE: | liquid–liquid extraction |
| LOD: | limit of detection |
| log Ko/w: | log octanol/water partition coefficient |
| LOQ: | limit of quantification |
| LTP-MS: | low temperature plasma ionisation mass spectrometry |
| MA: | menthyl anthranilate |
| MAE: | microwave-assisted extraction |
| MBBT: | methylene bis-benzotriazolyl tetramethyl butyl phenol |
| MBC: | 4-methylbenzylidene camphor |
| MBP: | methylene bis-benzotriazoyl tetramethylbutylphenol |
| MeCN: | Acetonitrile |
| MEKC: | micellar electrokinetic capillary chromatography |
| MeOH: | Methanol |
| MEPS: | microextraction by packed sorbent |
| MMLLE: | microporous membrane liquid–liquid extraction |
| MS/MS: | tandem mass spectrometry |
| MS: | mass spectrometry |
| MSPD: | matrix solid phase dispersion |
| MTBE: | methyl tert-butyl ether |
| NaCl: | sodium chloride |
| OC: | 4-methylbenzilidene camphor/octocrylane |
| ODP: | octyl dimethyl PABA |
| ODPABA: | 2-ethylhexyl 4-(dimethylamino)benzoate |
| OMC: | 2-ethylhexyl p-methoxycinnamate |
| OS: | 2-ethylhexylsalicylate |
| PABA: | p-aminobenzoic acid |
| PMDSA: | 2-phenylbenzimidazole-5-sulphonic acid |
| PDA: | photodiode-array detection |
| PEG-25 PABA: | polyethylene glycol 25 paminobenzoic acid |
| PHBA: | 4-hydroxy benzoic acid |
| PLE: | pressurized liquid extraction |
| pKa | acid dissociation constant |
| PSA: | primary-secondary amine |
| QuEChERSExtraction: | Quick, Easy, Cheap, Effective, Rugged, and Safe Extraction |
| R: | Recovery |
| RSD: | relative standard deviation |
| SALLE: | salt-assisted liquid–liquid extraction |
| SBSE: | stir bar sorptive extraction |
| SDME: | single-drop microextraction |
| SFC: | supercritical fluid chromatography |
| SIA: | sequential injection analysis |
| SI SPE: | sequential injection solid-phase extraction |
| SLE: | solid–liquid extraction |
| SPE: | solid-phase extraction |
| SPME: | solid-phase microextraction |
| SWV: | squarewave voltammetry |
| TBHPBT: | 2-(5-tert-butyl-2-hydroxyphenyl)benzotriazole |
| TCM: | trichloroamine |
| TFA: | trifluoroacetic acid |
| TFC: | turbulent flow chromatography |
| THB: | 2,3,4-trihydroxybenzophenone |
| TLC: | thin-layer chromatography |
| UAE: | ultrasound-assisted extraction |
| UHPLC: | ultra-high-performance liquid chromatography |
| UHPSFC: | ultra-high performance supercritical fluid chromatography |
| UPLC: | ultra-performance liquid chromatography |
| USAD-SPE: | ultrasound-assisted dispersive solid phase extraction |
| UV/Vis: | ultraviolet/visible spectrometry |
| VADLLME: | vortex-assisted dispersive liquid–liquid microextraction |
References
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.M.; Watt, F.M. Contribution of stem cells and differentiated cells to epidermal tumours. Nat. Rev. Canc. 2003, 3, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Astel, A.; Stec, M.; Rykowska, I. Occurrence and distribution of UV filters in beach sediments of the Southern Baltic sea coast. Water 2020, 12, 3024. [Google Scholar] [CrossRef]
- Gasparro, F.P.; Mitchnick, M.; Nash, J.F. A review of sunscreen safety and efficacy. Photochem. Photobil. 1998, 68, 243–256. [Google Scholar] [CrossRef]
- Pafili, A.; Meikopoulos, T.; Kontogiannidou, E.; Papageorgiou, S.; Demiri, E.; Meimari, D.; Fatouros, D.G.; Gika, H.; Theodoridis, G. Development and validation of LC-MS/MS method for the determination of UV-filters across human skin in vitro. J. Chromatogr. B 2021, 1167, 122561. [Google Scholar] [CrossRef]
- Wang, A.; Hu, L.; Liu, J.; Tian, M.; Yang, L. Polyaniline-coated core-shell silica microspheres-based dispersive-solid phase extraction for detection of benzophenone-type UV filters in environmental water samples. Environ. Adv. 2021, 3, 100037. [Google Scholar] [CrossRef]
- EC. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. OJ L 2009, 342, 59–209. [Google Scholar]
- Chisvert, A.; Salvador, A. UV Filters in Sunscreens and Other Cosmetics. Regulatory Aspects and Analytical Methods. In Personal Care Products, 2nd ed.; Salvador, A., Chisvert, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 85–106. [Google Scholar]
- Martín-Pozo, L.; Gomez-Regalado, M.; Moscoso-Ruiz, I.; Zafra-Gomez, A. Analytical methods for the determination of endocrine discrupting chemicals in cosmetics and personal care products: A review. Talanta 2021, 234, 122642. [Google Scholar] [CrossRef]
- Chisvert, A.; Tarazona, I.; Salvador, A. A reliable and environmentally-friendly liquid-chromatographic method for multi-class determination of fat-soluble UV filters in cosmetic products. Anal. Chim. Acta 2013, 790, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Giokas, D.L.; Salvador, A.; Chisvert, A. UV filters: From sunscreens to human body and the environment. Trends Anal. Chem. 2007, 26, 360–374. [Google Scholar] [CrossRef]
- Lores, M.; Llampart, M.; Alvarez-Rivera, G.; Guerra, E.; Vila, M.; Celeiro, M.; Lamas, J.P.; Garcia-Jares, C. Positive lists of cosmetic ingredients: Analytical methodology for regulatory and safety controls—A review. Anal. Chim. Acta 2016, 915, 1–26. [Google Scholar] [CrossRef] [PubMed]
- De Orsi, D.; Giannini, G.; Gagliardi, L.; Porrà, R.; Berri, S.; Bolasco, A.; Carpani, I.; Tonelli, D. Simple Extraction and HPLC determination of UV-A and UV-B filters in sunscreen products. Chromatographia 2006, 64, 509–515. [Google Scholar] [CrossRef]
- Wu, Y.W.; Jiang, Y.Y.; Liu, J.F.; Xiong, K. Cloud point extraction combined with micellar electrokinetic capillary chromatography determination of benzophenones in cosmetic matrix. Electrophoresis 2008, 29, 819–826. [Google Scholar] [CrossRef]
- Liu, T.; Wu, D. Simultaneous determination of some ultraviolet-absorbing chemicals in sunscreen cosmetics using a high-performance liquid chromatography method. Int. J. Cosmet. Sci. 2011, 33, 408–415. [Google Scholar] [CrossRef]
- Nyeborg, M.; Pissavini, M.; Lemasson, Y.; Doucet, O. Validation of HPLC method for the simultaneous and quantitative determination of 12 UV-filters in cosmetics. Int. J. Cosmet. Sci. 2010, 32, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Haunschmidt, M.; Buchberger, W.; Klampfl, C.W.; Hertsens, R. Identification and semi-quantitative analysis of parabens and UV filters in cosmetic products by direct-analysis-in-real-time mass spectrometry and gas chromatography with mass spectrometric detection. Anal. Methods 2011, 3, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Mueller, J.; Park, H.R.; Kang, S.H.; Yoon, M.H.; Lee, J.B. Simultaneous Determination of nine UV filters and four preservatives in suncare products by High-Performance Liquid Chromatography. J. Chromatogr. Sci. 2011, 49, 554–559. [Google Scholar] [CrossRef]
- Yang, H.Y.; Li, H.F.; Ito, M.; Lin, J.M.; Guo, G.S.; Ding, M.Y. Combination of dynamic hollow fiber liquid-phase microextraction with HPLC analysis for the determination of UV filters in cosmetic products. Sci. China Chem. 2011, 10, 1627–1634. [Google Scholar] [CrossRef]
- Júnior, J.B.G.; Araujo, T.A.; Trindade, M.A.G.; Ferreira, V.S. Electroanalytical determination of the sunscreen agent octocrylene in cosmetic products. Int. J. Cosmet. Sci. 2012, 34, 91–94. [Google Scholar] [CrossRef]
- Yousef, A.N.; Haidar, S.; Al.-Khayat, M.A. Development and validation of RP-HPLC method for analysis of four UV filters in sunscreen products. Int. J. Pharm. Sci. Rev. Res. 2013, 23, 254–258. [Google Scholar]
- Kale, S.; Kulkarni, K.; Ugale, P.; Jadav, K. Application of HPTLC for the qualitative and quantitative analysis of avobenzone, oxybenzone, octinoxate in sunscreen cream. Int. J. Pharm. Pharm. Sci. 2014, 6, 391–394. [Google Scholar]
- Chang, N.I.; Yoo, M.; Lee, S. Determination of fourteen sunscreen agents in cosmetics using high-performance liquid chromatography. Int. J. Cosmet. Sci. 2015, 37, 175–180. [Google Scholar] [CrossRef]
- Lopez-Gazpio, J.; Garcia-Arrona, R.; Millán, E. Simultaneous determination of multiclass preservatives including isothiazolinones and benzophenone-type UV filters in household and personal care products by micellar electrokinetic chromatography. Electrophoresis 2015, 36, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, W.Y.; Jiang, S.J.; Feng, C.H.; Wang, S.W.; Chen, Y.L. Determining ultraviolet absorbents in sunscreen products by combining direct injection with micelle collapse on-line preconcentration capillary electrophoresis. J. Chromatogr. A 2015, 1383, 175–181. [Google Scholar] [CrossRef]
- Wharton, M.; Geary, M.; O’Connor, N.; Curtin, L.; Ketcher, K. Simultaneous liquid chromatographic determination of 10 ultra-violet filters in sunscreens. J. Chromatogr. Sci. 2015, 53, 1289–1295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, T.; Li, Z.; Niu, Q.; Li, Y.; Zhou, W. Double dispersant-assisted ionic liquid dispersive liquid–liquid microextraction coupled with capillary electrophoresis for the determination of benzophenone-type ultraviolet filters in sunscreen cosmetic product. Electrophoresis 2015, 36, 2530–2537. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Gerbig, S.; Spengler, B.; Schulz, S. Reactive low temperature plasma ionization mass spectrometry for the determination of organic UV filters in personal care products. Talanta 2018, 178, 780–787. [Google Scholar] [CrossRef]
- Meng, X.; Ma, Q.; Bai, H.; Wang, Z.; Han, C.; Wang, C. Simultaneous separation and determination of 15 organic UV filters in sunscreen cosmetics by HPLC-ESI-MS/MS. Int. J. Cosmet. Sci. 2016, 39, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Khalikova, M.A.; Leselllier, E.; Chapuzet, E.; Satínský, D.; West, C. Development and validation of ultra-high performance supercritical fluid chromatography method for quantitative determination of nine sunscreens in cosmetic samples. Anal. Chim. Acta 2018, 1034, 184–194. [Google Scholar] [CrossRef]
- Lu, S.; Long, F.; Lu, P.; Lei, B.; Jiang, Z.; Liu, G.; Hang, J.; Ma, S.; Yu, Y. Benzophenone-UV filters in personal care products and urine of schoolchildren from Shenzhen, China: Exposure assessment and possible source. Sci. Total Environ. 2018, 640–641, 1214–1220. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, P.G.; Wittenberg, J.B.; Rua, D.; Krynitsky, A.J. Simultaneous determination of cosmetics ingredients in nail products by fast gas chromatography with tandem mass spectrometry. J. Chromatogr. A 2016, 1446, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.Y.; Su, Y.L.; Weng, J.R.; Lin, Y.C.; Feng, C.H. Ultrasound–vortex-assisted dispersive liquid–liquid microextraction combined with high performance liquid chromatography–diode array detection for determining UV filters in cosmetics and the human stratum corneum. Molecules 2020, 25, 4642. [Google Scholar] [CrossRef]
- Imamović, B.; Šober, M.; Beĉić, E. Identification and determination butylmethoxydibenzoylmethane in the presence benzophenone-3 and ethylhexylmethoxycinnamate in suncare preparation. Int. J. Cosmet. Sci. 2009, 31, 383–389. [Google Scholar] [CrossRef]
- Vila, M.; Lamas, J.P.; Garcia-Jares, C.; Dagnac, T.; Llompart, M. Optimization of an analytical methodology for the simultaneous determination of different classes of ultraviolet filters in cosmetics by pressurized liquid extraction–gas chromatography tandem mass spectrometry. J. Chromatogr. A 2015, 1405, 12–22. [Google Scholar] [CrossRef]
- Sobańska, A.W.; Pyzowski, J. Quantification of sunscreen ethylhexyl triazone in topical skin-care products by Normal-Phase TLC/Densitometry. Sci. World J. 2012, 807516, 1–6. [Google Scholar] [CrossRef]
- Kapalavavi, B.; Marple, R.; Gamsky, C.; Yang, Y. Separation of sunscreens in skincare creams using greener high-temperature liquid chromatography and subcritical water chromatography. Int. J. Cosmet. Sci. 2012, 34, 169–175. [Google Scholar] [CrossRef]
- Vila, M.; Facorro, R.; Lamas, J.P.; Garcia-Jares, C.; Dagnac, T.; Llompart, M. Determination of fifteen water and fat-soluble UV filters in cosmetics by pressurized liquid extraction followed by liquid chromatography tandem mass spectrometry. Anal. Methods 2016, 8, 6787–6794. [Google Scholar] [CrossRef]
- Cadena-Aizaga, M.I.; Montedeoca-Esponda, S.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Santa Rodriguez, J.J. Organic UV filters in marine environments: An update of analytical methodologies, occurrence and distribution. Trends Environ. Anal. Chem. 2020, 25, e00079. [Google Scholar] [CrossRef]
- Dencausse, I.; Galland, A.; Clamou, J.L.; Basso, J. Validation of HPLC method for quantitative determination of Tinosorb®S and three other sunscreens in a high protection cosmetic product. Int. J. Cosmet. Sci. 2008, 30, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Sobańska, A.W.; Kałębasiak, K.; Pyzowski, J.; Brzezińska, E. Quantification of sunscreen Benzophenone-4 in hair shampoos by hydrophilic interactions Thin-Layer Chromatography/Densitometry or derivative UV spectrophotometry. J. Anal. Methods Chem. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lesellier, E.; Mith, D.; Dubrulle, I. Method developments approaches in supercritical fluid chromatography applied to the analysis of cosmetics. J. Chromatogr. A 2015, 1423, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Díaz, I.; Zafra-Gómez, A.; Ballesteros, O.; Navalón, A. Analytical methods for the determination of personal care products in human samples: An overview. Talanta 2014, 129, 448–458. [Google Scholar] [CrossRef]
- Gonzalez, H.G.; Farbrot, A.; Larkö, O. Percutaneous absorption of benzophenone-3, a common component of topical sunscreens. Clin. Exp. Dermatol. 2002, 27, 691–694. [Google Scholar] [CrossRef]
- Tarazona, I.; Chisvert, A.; Salvador, A. Determination of benzophenone-3 and its main metabolites in human serum by dispersive liquid–liquid microextraction followed by liquid chromatography tandem mass spectrometry. Talanta 2013, 116, 388–395. [Google Scholar] [CrossRef]
- Dewalquea, L.; Pirarda, C.; Dubois, N.; Charlier, C. Simultaneous determination of some phthalate metabolites, parabens and benzophenone-3 in urine by ultra-high pressure liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2014, 949–950, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Kuklenyik, Z.; Needham, L.L.; Calafat, A.M. Automated On-Line Column-Switching HPLC-MS/MS Method with Peak Focusing for the Determination of Nine Environmental Phenols in Urine. Anal. Chem. 2005, 77, 5407–5413. [Google Scholar] [CrossRef]
- Ye, X.; Kuklenyik, Z.; Needham, L.L.; Calafat, A.M. Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction–high performance liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2005, 383, 638–644. [Google Scholar] [CrossRef]
- Ye, X.; Bishop, A.M.; Reidy, J.A.; Needham, L.L.; Calafat, A.M. Temporal stability of the conjugated species of bisphenol A, parabens, and other environmental phenols in human urine. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 567–572. [Google Scholar] [CrossRef]
- Gavin, Q.W.; Ramage, R.T.; Waldman, J.M.; She, J. Development of HPLC-MS/MS method for the simultaneous determination of environmental phenols in human urine. Int. J. Environ. Anal. Chem. 2014, 94, 168–182. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Wanga, L.; Thomaidis, N.S.; Kannan, K. A multi-class bioanalytical methodology for the determination of bisphenol A diglycidyl ethers, p-hydroxybenzoic acid esters, benzophenone-type ultraviolet filters, triclosan, and triclocarban in human urine by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2014, 1324, 141–148. [Google Scholar] [PubMed]
- Vidal, L.; Chisvert, A.; Canals, A.; Salvador, A. Sensitive determination of free benzophenone-3 in human urine samples based on an ionic liquid as extractant phase in single-drop microextraction prior to liquid chromatography analysis. J. Chromatogr. A 2007, 1174, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Ito, R.; Honda, H.; Endo, N.; Okanouchi, N.; Saito, K.; Seto, Y.; Nakazawa, H. Measurement of Benzophenones in Human Urine Samples by Stir Bar Sorptive Extraction and Thermal Desorption-Gas Chromatography–Mass Spectrometry. Anal. Sci. 2008, 24, 1509–1512. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Ito, R.; Honda, H.; Koganei, Y.; Okanouchi, N.; Saito, K.; Seto, Y.; Nakazawa, H. Miniaturized hollow fiber assisted liquid-phase microextraction and gas chromatography–mass spectrometry for determination of benzophenone and derivates in human urine sample. J. Chromatogr. B 2009, 877, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Kunisue, T.; Wu, Q.; Tanabe, S.; Aldous, K.M.; Kannan, K. Analysis of five benzophenone-type UV filters in human urine by liquid chromatography-tandem mass spectrometry. Anal. Methods 2010, 2, 707–713. [Google Scholar] [CrossRef]
- León, Z.; Chisvert, A.; Tarazona, I.; Salvador, A. Solid-phase extraction liquid chromatography–tandem mass spectrometry analytical method for the determination of 2-hydroxy-4-methoxybenzophenone and its metabolites in both human urine and semen. Anal. Bioanal. Chem. 2010, 398, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Kunisue, T.; Chen, Z.; Buck Louis, G.M.; Sundaram, R.; Hediger, M.L.; Sun, L.; Kannan, K. Urinary concentrations of Benzophenone-type UV Filters in U.S. women and their association with endometriosis. Environ. Sci. Technol. 2012, 46, 4624–4632. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Nielsen, O.; Skakkebaek, N.E.; Juul, A.; Andersson, A.M. UV filters analyzed by isotope diluted TurboFlow-LC-MS/MS in urine from Danish children and adolescents. Int. J. Hyg. Environ. Health 2016, 12967, 1–10. [Google Scholar] [CrossRef] [PubMed]
- León-González, Z.; Ferreiro-Vera, C.; Priego-Capote, F.; Luque de Castro, M.D. Targeting metabolomics analysis of the sunscreen agent 2-ethylhexyl 4-(N,N-dimethylamino)benzoate in human urine by automated on-line solid-phase extraction–liquid chromatography–tandem mass spectrometry with liquid chromatography–time-of-flight/mass spectrometry confirmation. J. Chromatogr. A 2011, 1218, 3013–3021. [Google Scholar]
- Sarveiya, V.; Risk, S.; Benson, H.A.E. Liquid chromatographic assay for common sunscreen agents: Application to in vivo assessment of skin penetration and systemic absorption in human volunteers. J. Chromatogr. B 2004, 803, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Adoamnei, E.; Mendiola, J.; M oñino-García, M.; Vela-Soria, F.; Iribarne-Durán, L.M.; Fernández, M.F.; Olea, N.; Jørgensen, N.; Swan, S.H.; Torres-Cantero, A.M. Urinary concentrations of benzophenone-type ultra violet light filters and reproductive parameters in young men. Int. J. Hyg. Environ. Health 2018, 221, 531–540. [Google Scholar] [CrossRef]
- Ao, J.; Yuan, T.; Gu, J.; Ma, Y.; Shen, Z.; Tian, Y.; Shi, R.; Zhou, W.; Zhang, J. Organic UV filters in indoor dust and human urine: A study of characteristics, sources, associations and human exposure. Sci. Total Environ. 2018, 640–641, 1157–1164. [Google Scholar] [CrossRef]
- Klotz, K.; Hof, K.; Hiller, J.; Göen, T.; Drexler, H. Quantification of prominent organic UV filters and their metabolites in human urine and plasma samples. J. Chromatogr. B 2019, 1125, 1–8. [Google Scholar] [CrossRef]
- Bury, D.; Brüning, T.; Koch, H.M. Determination of metabolites of the UV filter 2-ethylhexyl salicylate in human urine by online-SPE-LC-MS/MS. J. Chromatogr B 2019, 1110–1111, 59–66. [Google Scholar] [CrossRef]
- Chang, F.K.; Shiea, J.; Tsai, H.J. Urinary concentrations of triclosan, benzophenone-3, and bisphenol a in Taiwanese children and adolescents. Int. J. Environ. Research Pub. Health 2017, 14, 1545. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Ma, X.; Huo, W.; Xu, S.; Cai, Z. Simultaneous determination of bisphenols, benzophenones and parabens in human urine by using UHPLC-TQMS. Chin. Chem. Lett. 2018, 29, 102–106. [Google Scholar] [CrossRef]
- Zuccherato Bocato, M.; Aparecida Cesila, C.; Favero Lataro, B.; de Oliveira, A.R.M.; Dobal Campíglia, A.; Barbosa Jr, F. A fast-multiclass method for the determination of 21 endocrine disruptors in human urine by using vortex-assisted dispersive liquid-liquid microextraction (VADLLME) and LC-MS/MS. Environ. Res. 2020, 189, 109883. [Google Scholar] [CrossRef]
- Rocha, B.A.; de Oliveira, A.R.M.; Barbosa, F. A fast and simple air-assisted liquid-liquid microextraction procedure for the simultaneous determination of bisphenols, parabens, benzophenones, triclosan, and triclocarban in human urine by liquid chromatography-tandem mass spectrometry. Talanta 2018, 183, 94–101. [Google Scholar] [CrossRef]
- Wang, L.H.; Huang, W.S.; Tai, H.M. Simultaneous determination of p-aminobenzoic acid and its metabolites in the urine of volunteers, treated with p-aminobenzoic acid sunscreen formulation. J. Pharm. Biomed. Anal. 2007, 43, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Jacobson, C.E.; Wennberg, A.M.; Larkö, O.; Farbrot, A. Solid-Phase Extraction and Reverse-Phase HPLC: Application to Study the Urinary Excretion Pattern of Benzophenone-3 and its Metabolite 2,4-Dihydroxybenzophenone in Human Urine. Anal. Chem. Insights 2008, 3, 1–7. [Google Scholar] [CrossRef]
- Vidal, M.T.; Chisvert, A.; Salvador, A. Sensitive sequential-injection system for the determination of 2-phenylbenzimidazole-5-sulphonic acid in human urine samples using on-line solid-phase extraction coupled with fluorimetric detection. Talanta 2003, 59, 591–599. [Google Scholar] [CrossRef]
- Balaguer, A.; Chisvert, A.; Salvador, A.; Herraez, M.; Diez, O. A solid-phase extraction and size-exclusion liquid chromatographic method for polyethylene glycol 25 p-aminobenzoic acid determination in urine: Validation for urinary excretion studies of users of sunscreens. Anal. Chim. Acta 2008, 611, 220–225. [Google Scholar] [CrossRef]
- Locatelli, M.; Furton, K.G.; Tartaglia, A.; Sperandio, E.; Ulusoy, H.I.; Kabir, A. An FPSE-HPLC-PDA method for rapid determination of solar UV filters in human whole blood, plasma and urine. J. Chromatogr. B 2019, 1118–1119, 40–50. [Google Scholar] [CrossRef]
- March, J.G.; Palou, J.; Chisvert, A.; Salvador, A. A simple novel configuration for in-vial microporous membrane liquid-liquid extraction. J. Chromatogr. A 2009, 1216, 5160–5163. [Google Scholar] [CrossRef]
- León, Z.; Chisvert, A.; Balaguer, Á.; Salvador, A. Development of a fully automated sequential injection solid-phase extraction procedure coupled to liquid chromatography to determine free 2-hydroxy-4-methoxybenzophenone and 2-hydroxy-4-methoxybenzophenone-5-sulphonic acid in human urine. Anal. Chim. Acta 2010, 664, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Stępkowska, A.; Alegre, A.; Nogueira, J.M.F. Determination of trace levels of benzophenone-type ultra-violet filters in real matrices by bar adsorptive micro-extraction using selective sorbent phases. J. Chromatogr. A 2013, 1311, 1–10. [Google Scholar] [CrossRef]
- Vosough, M.; Mojdehi, N.R.; Salemi, A. Chemometrics assisted dispersive liquid–liquid microextraction for quantification of seven UV filters in urine samples by HPLC-DAD. J. Sep. Sci. 2012, 35, 3575–3585. [Google Scholar] [CrossRef] [PubMed]
- Sanglard Silveira, R.; Rocha, B.A.; Rodrigues, J.L.; Barbosa Jr., F. Rapid, sensitive and simultaneous determination of 16 endocrine disrupting chemicals (parabens, benzophenones, bisphenols, and triclocarban) in human urine based on microextraction by packed sorbent combined with liquid chromatography tandem mass spectrometry (MEPS-LC-MS/MS). Chemosphere 2020, 240, 124951. [Google Scholar]
- Felix, T.; Hall, B.J.; Brodbelt, J.S. Determination of benzophenone-3 and metabolites in water and human urine by solid-phase microextraction and quadrupole ion trap GC-MS. Anal. Chim. Acta 1998, 371, 195–203. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Cárdenas, S. Nanostructured hybrid monolith with integrated stirring for the extraction of UV-filters from water and urine samples. Talanta 2018, 182, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Sunyer-Caldúa, A.; Peiróa, A.; Díaz, M.; Ibáñez, L.; Gago-Ferreroa, P.; Diaz-Cruz, M.S. Development of a sensitive analytical method for the simultaneous analysis of Benzophenone-type UV filters and paraben preservatives in umbilical cord blood. MethodsX 2021, 8, 1–13. [Google Scholar]
- Iribarne-Durán, L.M.; Domingo-Piñar, S.; Peinado, F.M.; Vela-Soria, F.; Jimėnez-Díaz, I.; Barranco, E.; Olea, N.; Freire, C.; Artacho-Cordón, F.; Ocón-Hernández, O. Menstrual blood concentrations of parabens and benzophenones and related factors in a sample of Spanish women: An exploratory study. Environ. Res. 2020, 183, 1–7. [Google Scholar] [CrossRef]
- Vela-Soria, F.; Ballesteros, O.; Zafra-Gómez, A.; Ballesteros, L.; Navalón, A. A new method for the determination of benzophenone-UV filters in human serum samples by dispersive liquid–liquid microextraction with liquid chromatography–tandem mass spectrometry. Talanta 2014, 121, 97–104. [Google Scholar] [CrossRef]
- Ye, X.; Wong, L.Y.; Jia, L.T.; Needham, L.L.; Calafat, A.M. Stability of the conjugated species of environmental phenols and parabens in human serum. Environ. Int. 2009, 35, 1160–1163. [Google Scholar] [CrossRef]
- Ye, X.; Tao, L.J.; Needham, L.L.; Calafat, A.M. Automated on-line column-switching HPLC–MS/MS method for measuring environmental phenols and parabens in serum. Talanta 2008, 76, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Teglia, C.M.; Santamaría, C.G.; Rodriguez, H.A.; Culzoni, M.J.; Goicoechea, H.C. Determination of 2-hydroxy-4-methoxybenzophenone in mice serum and human plasma by ultra-high-performance liquid chromatography enhanced by chemometrics. Microchem. J. 2019, 148, 35–41. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Mu, Y.; He, Y.; Qiu, T.; Li, W.; Zeng, L. Determination of 21 photoinitiators in human plasma by using high-performance liquid chromatography coupled with tandem mass spectrometry: A systemically validation and application in healthy volunteers. J. Chromatogr. A 2021, 1643, 462079. [Google Scholar] [CrossRef] [PubMed]
- Vela-Soria, F.; Iribarne-Durán, L.M.; Mustieles, V.; Jimėnez-Diaz, I.; Fernández, M.F.; Olea, N. QuEChERS and ultra-high performance liquid chromatography– tandem mass spectrometry method for the determination of parabens and ultraviolet filters in human milk samples. J. Chromatogr. A 2018, 1546, 1–9. [Google Scholar] [CrossRef]
- Ye, X.; Kuklenyik, Z.; Needham, L.L.; Calafat, A.M. Measuring environmental phenols and chlorinated organic chemicals in breast milk using automated on-line column-switching–high performance liquid chromatography–isotope dilution tandem mass spectrometry. J. Chromatogr. B 2006, 831, 110–115. [Google Scholar] [CrossRef]
- Molins-Delgado, D.; del Mar Olmo-Commpos, M.; Valeta-Juan, G.; Pleguezuelos-Hernández, V.; Barcelo, D.; Díaz-Cruz, M.S. Determination of UV filters in human breast milk using turbulent flow chromatography and babies’ daily intake estimation. Environ. Res. 2018, 161, 532–539. [Google Scholar] [CrossRef]
- Ye, X.; Bishop, A.M.; Needham, L.L.; Calafat, A.M. Automated on-line column-switching HPLC-MS/MS method with peak focusing for measuring parabens, triclosan, and other environmental phenols in human milk. Anal. Chim. Acta 2008, 622, 150–156. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, R.; Zafra-Gómez, A.; Dorival-García, N.; Ballesteros, O.; Navalón, A. Determination of benzophenone-UV filters in human milk samples using ultrasound-assisted extraction and clean-up with dispersive sorbents followed by UHPLC–MS/MS analysis. Talanta 2015, 134, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pozo, L.; del Carmen Gómez-Regalado, M.; Cantarero-Malagón, S.; Navalón, A.; Zafra-Gómez, A. Determination of ultraviolet filters in human nails using an acid sample digestion followed by ultra-high performance liquid chromatography mass spectrometry analysis. Chemosphere 2021, 273, 128603. [Google Scholar] [CrossRef]
- Vela-Soria, F.; Rodríguez, I.; Ballesteros, O.; Zafra-Gómez, A.; Ballesteros, L.; Cela, R.; Navalón, A. Simplified matrix solid phase dispersion procedure for the determination of parabens and benzophenone-ultraviolet filters in human placental tissue samples. J. Chromatogr. A 2014, 1371, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Valle-Sistac, J.; Molins-Delgado, D.; Díaz, M.; Ibáñez, L.; Barceló, D.; Díaz-Cruz, M.S. Determination of parabens and benzophenone-type UV filters in human placenta. First description of the existence of benzyl paraben and benzophenone-4. Environ. Int. 2016, 88, 243–249. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.L.; Rocha, B.A.; De Oliviera Souza, V.C.; Barbosa, F. Determination of 17 potential endocrine-disrupting chemicals in human saliva by dispersive liquid-liquid microextraction and liquid chromatography tandem mass spectrometry. Talanta 2019, 196, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Vela-Soria, F.; Gallardo-Torres, M.E.; Ballesteros, O.; Díaz, C.; Pėrez, J.; Navalón, A.; Fernández, M.F.; Olea, N. Assessment of parabens and ultraviolet filters in human placenta tissue by ultrasound-assisted extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2017, 1487, 153–161. [Google Scholar] [CrossRef] [PubMed]

| Chemical Name | INCI Name a | Abbreviation | CAS Number | Structure | Max. Concentration (%) | Log Ko/w a | pKa a | Solubility (g/L) a,b |
|---|---|---|---|---|---|---|---|---|
| Benzophenone derivatives | ||||||||
| 2-Hydroxy-4-methoxybenophenone/Oxybenzone | Benzophenone-3 | BP-3 | 131-57-7 |  | 10 | 3.79 | 7.56 | 0.21 |
| 2-Hydroxy-4-benzophenone-5-sulfonic acid and its sodium salt/Sulisobenzoate | Benzophenone-4, Benzophenone-5 | BP-4, BP-5 | 4065-45-6/6628-37-1 |  | 5 (as acid) | 0.37 | −0.70 | 0.65 |
| Benzoic acid, 2-[4-(diethylamino)-2-hydroxybenzoyl]-hexylester | Diethylamino Hydroxybenzoyl Hexyl Benzoate | DHHB | 302776-68-7 |  | 10 | 6.54 | 7.29 | 9.5 · 10−4 |
| p-Aminobenzoic acid derivatives | ||||||||
| Ethoxylated ethyl-4-aminobenzoate | PEG-25 PABA | PEG-25 PABA | 116242-27-4 |  | 10 | −0.66 | - | - |
| 2-Ethylhexyl-4-(dimethylamino)benzoate/Padimate O (USAN:BAN) | Ethylhexyl Dimethyl PABA | OD-PABA | 21245-02-3 | 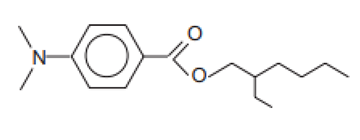 | 8 | 6.15 | 2.39 | 0.0021 |
| Salicylates | ||||||||
| Benzoic acid, 2-hydroxy-3,3,5-trimethylcyclohexyl ester/Homosalate | Homosalate | HS | 118-56-9 | 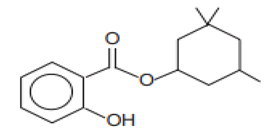 | 10 | 6.16 | 8.09 | 0.02 |
| 2-Ethylhexyl salicylate/Octisalate | Ethylhexyl Salicylate | EHS | 118-60-5 |  | 5 | 5.97 | 8.13 | 0.028 |
| Cinnamates | ||||||||
| 2-Ethylhexyl-4-methoxycinnamate/Octinoxate | Ethylhexyl Methoxycinnamate | OMC | 5466-77-3 |  | 10 | 5.8 | - | 0.15 |
| Isopentyl-4-methoxycinnamate/Amiloxate | Isoamyl p-Methoxycinnamate | IMC | 71617-10-2 | 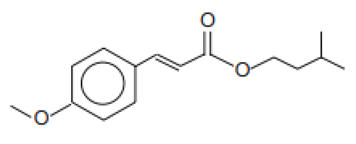 | 10 | 4.33 | - | 0.06 |
| Benzimidazole derivatives | ||||||||
| 2-Phenylbenzimidazole-5-sulfonic acid and its potassium, sodium, and triethanolamine salts/Ensulizole | Phenylbenzimidazole Sulfonic Acid | PMDSA | 27503-81-7 | 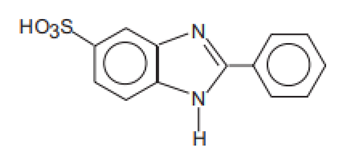 | 8 (as acid) | −0.16 | −0.87 | 0.26 |
| Sodium salt of 2,2′-bis(1,4-phenylene)-1H-benzimidazole-4,6-disulfonic acid)/Bisdisulizole disodium (USAN) | Disodium Phenyl Dibenzimidazole Tetrasulfonate | DPDT | 180898-37-7 |  | 10 (as acid) | −6.79 | −0.27 | 0.5 |
| Benzotriazole derivatives | ||||||||
| Phenol,2-(2H-benzotriazol-2-yl)-4-methyl-6-(2-methyl-3-(1,3,3,3-tetramethyl-1-(trimethylsilyl)oxy)-disiloxanyl)propyl) | Drometrizole Trisiloxane | DTS | 155633-54-8 |  | 15 | 10.38 | 1.2 | 5.5 · 10−10 |
| 2,2′-Methylene-bis(6-(2H-benzotriazol-2-yl)-4-(1,1,3,3-tetramethyl-butyl)phenol)/Bisoctrizole | Methylene Bis-Benzotriazolyl Tetramethylbutylphenol | MBP | 103597-45-1 |  | 10 | 12.46 | 7.56 | 3 · 10−8 |
| Camphor derivatives | ||||||||
| N,N,N-Trimethyl-4-(2-oxoborn-3-ylidenemethyl)anilinium methyl sulfate | Camphor Benzalkonium Methosulfate | CBM | 52793-97-2 | 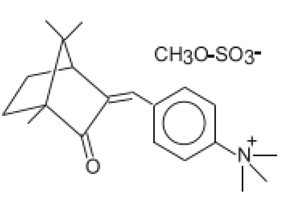 | 6 | 0.28 | - | 0.007 |
| 3,3′-(1,4-Phenylenedimethylene) bis(7,7-dimethyl-2-oxobicyclo-[2,2,1]hept-1-yl-methanesu fonic acid) and its salts/Ecamsule | Terephthalylidene Dicamphor Sulfonic Acid | PDSA | 92761-26-7, 90457-82-2 |  | 10 (as acid) | 3.83 | −1.05 | 0.014 |
| Alpha-(2-Oxoborn-3-ylidene)-toluene-4-sulphonic acid and its salts | Benzylidene Camphor Sulfonic Acid | BCSA | 56039-58-8 |  | 6 (as acid) | 2.22 | −0.7 | 0.038 |
| 3-(4-Methylbenzylidene)-d1 camphor/Enzacamene | 4-Methylbenzylidene Camphor | 4-MBC | 38102-62-4/ 36861-47-9 | 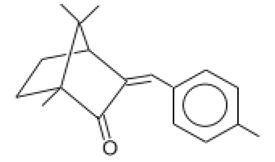 | 4 | 4.95 | - | 0.0051 |
| Polymer of N-{(2 and 4)-[(2-oxoborn-3-ylidene)methyl-]benzyl} acrylamide | Polyacrylamidomethyl Benzylidene Camphor | PBC | 113783-61-2 |  | 6 | - | - | - |
| Triazine derivatives | ||||||||
| Benzoic acid, 4,4-((6-((4-(((1,1-dimethylethyl)amino)carbonyl)phenyl)amino)-1,3,5-triazine-2,4-diyl)diimino)bis-, bis (2-ethylhexyl) ester/ Iscotrizinol (USAN) | Diethylhexyl Butamido Triazone | DBT | 154702-15-5 | 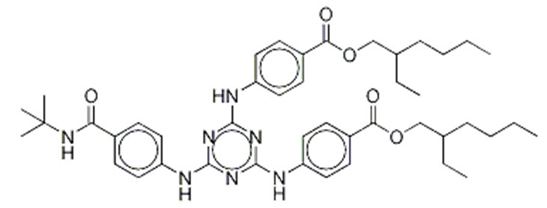 | 10 | 14.03 | 3.04 | 4.6 10−7 |
| 3,3′-(1,4-Phenylene)bis(5,6-diphenyl-1,2,4-triazine) | Phenylene Bis-Diphenyl triazine | - | 55514-22-2 |  | 5 | - | - | - |
| 2,4,6-Trianilino-(p-carbo-2′-ethylhexyl-1′-oxy)-1,3,5-triazine | Ethylhexyl Triazone | ET | 88122-99-0 | 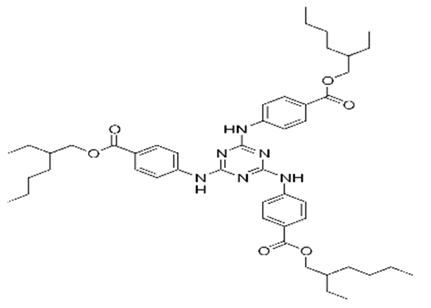 | 5 | 17.05 | 3.17 | - |
| 2,2′-(6-(4-Methoxyphenyl)-1,3,5-triazine-2,4-diyl)bis(5-((2-ethylhexyl)oxy)phenol)/Bemotrizinol | Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine | EMT | 187393-00-6 |  | 10 | 8.03 | 6.37 | 4.9 ·10−8 |
| Others | ||||||||
| 1-(4-tert-Butylphenyl)-3-(4-methoxyphenyl)propane-1,3-dione/Avobenzene | Butyl Methoxydibenzoyl-methane | BMDBM | 70356-09-1 |  | 5 | 4.51 | 9.74 | 0.037 |
| 2-Cyano-3,3-diphenyl acrylic acid, 2-ethylhexyl ester/Octocrilene | Octocrylene | OC | 6197-30-4 | 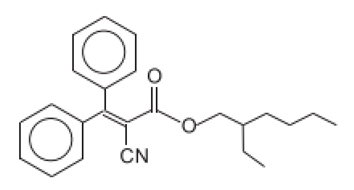 | 10 (as acid) | 6.88 | - | 2 · 10−4 |
| Dimethicodiethylbenzalmalonate | Polysilicone-15 | BMP | 207574-74-1 | 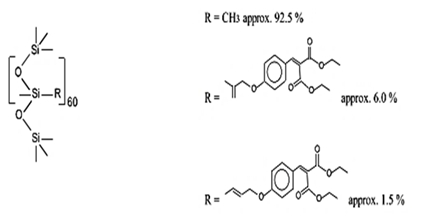 | 10 | - | - | - |
| 2-ethoxyethyl(2Z)-2-cyano-2-[3-(3-methoxy-propylamino) cyclohex-2-en-1-ylidene]acetate | Methoxypropylamino Cyclohexenylidene Ethoxyethylcyanoacetate | - | 1419401-88-9 |  | 3 | - | - | - |
| UV Filters | Matrix | Analytical Technique | Analytical Performance a | Ref. |
|---|---|---|---|---|
| BP-3, IMC, MBC, DHHB, OC, EDP, BDM, EMC, EHS, HS, DBT, ET, DTS, MBP, EMT | Sunscreens, facial creams, lip balms, aftershave creams | LC-UV/Vis; type of column: C18; column temperature: 60 °C; mobile phase: ethanol/formic acid (aq) mobile phase modifier: hydroxypropyl-β-cyclodextrin (HP-β-CD) | LOD: 0.02–0.22 µg mL−1 LOQ: 0.07–0.74 µg mL−1 R: 98–104% RSD: 0.9–7.1% | [10] |
| PMDSA, BP-4, BP-3, MBC, DHHB, EMC, OC, MBP, EMT, ET, BDM | Emulsion, oil | HPLC-UV/Vis; type of column: C8 or C18 or C16; column temp.: 35 °C; mobile phase: gradient acetonitrile/perchloric acid (aq) or isocratic methanol/acetonitrile or isocratic methanol/perchloric acid | LOD: 0.1–1.2 µg mL−1 LOQ: no data R: 93.9–103.4% RSD: 0.2–1% | [13] |
| BP-1, BP-2, BP-3 | Emulsion | MEKC-UV/Vis; type of capillary: a 51 cm uncoated fused-silica; surfactant: sodium tetraborate containing sodium dodecyl sulfate | 10−8–3.9010−7 mol/L LOQ: no data R: 89.5–102.5% RSD: 1.14–8.09% | [14] |
| PMDSA, PABA, BP-4, BP-3, IMC, MBC, OC, EMC, HS, EHS, MBBT | Creams, lotions, foundation, loose powder, lipstick | HPLC-UV/Vis; type of column: C18; column temp.: 30 °C; mobile phase: gradient methanol/tetrahydrofuran/perchloric acid (aq) | LOD: 200–500 ng mL−1 LOQ: 700–6700 ng mL−1 R: 98.5–102.2% RSD: 0.51–1.72% | [15] |
| PMDSA, BP-3, IMC, DHHB, OC, EMC, EHS, BDM, DBT, ET, MBP, EMT | Emulsion, sticks, powder | HPLC-UV/Vis; type of column: C18; column temp.: 40 °C; mobile phase: gradient ethanol/ 1% phosphoric acid (aq) | LOD: 0.04–1.66 µg mL−1 LOQ: 0.13–5.52 µg mL−1 R: 97–101.4% RSD: 0.38–2.42% | [16] |
| HS, EDP, EHC, EHS, MBC, BDM, BP-3, OC, PHBA, BC | Cream, milk, lotion, oil, lipstick | DART-MS (ESI+) | LOD: 2.5–460 µg g−1 LOQ: no data R: 71–120% RSD: 4–30% | [17] |
| EMC, IMC, EHS, MBC, BP-3, EDP, OC, BDM | Cream, lotion, spray | HPLC-UV/Vis; type of column: C18; column temp.: 30 °C; mobile phase: gradient acetonitrile/acetic acid (aq) | LOD: 0.03–1.5 mg L−1 LOQ: 0.08–4.6 mg L−1 R: 98–102% RSD: 0.97–6.1% | [18] |
| BP-4, BP-3, ODP, OMC, EHS | Cream, lotion, lipstick, foundation | HPLC-UV/Vis; type of column: C18; column temp.: 40 °C; mobile phase: gradient methanol/pure water (80:20; v/v) | LOD: 1–100 ng L−1 LOQ: 4–340 ng L−1 R: 98–102% RSD: 4–5.2% | [19] |
| OC | Emulsion | SWV/mercury electrode; a mixture of Britton–Robinson (BR) buffer and ethanol (7:3; v/v) as the supporting electrolyte | LOD: no data LOQ: no data R: 9.7–106% RSD: 1–3.42% | [20] |
| EMC, BP-3, EHS, OC | Emulsion | LC-UV/Vis; type of column: C18; mobile phase: methanol/water (85:15; v/v) | LOD: no data LOQ: no data R: 99.67–101% RSD: 0.044–1.5% | [21] |
| BDM, BP-3, EMC | Cream | HPTLC-DS.; type of column: C18 or silica gel; mobile phase: acetonitrile/water (18:2) or cyclohexane/diethyl ether/n-hexane/acetone (14:2:1:2) | LOD: no data LOQ: no data R: 92.7–102.4% RSD: no data | [22] |
| PABA, PMDSA, BP-3, MBC, BP-4, OC, EDP, EMC, BDM, HS, EHS, DBT, ET, DTS | Cream | HPLC-UV/Vis; type of column: C18; mobile phase: gradient ethanol/phosphate buffer | LOD: 0.01–1.99 mg L−1 LOQ: 0.02–6.02 mg L−1 R: 90.91–109.98% RSD: 0.16–12.69% | [23] |
| BP-3, BP-4 | Shampoo, gel, perfume, cream | MEKC-UV/Vis; type of capillary: a 64.5 cm uncoated fused-silica; surfactant: sodium dodecyl sulphate | LOD: 0.91–2.26 µg mL−1 LOQ: 2.72–6.79 µg mL−1 R: 90.4–107.4% RSD: 5.7–12% | [24] |
| BP-1, BP-2, BP-3, BP-4, BP-6, BP-8, OC, EMC, PABA | Lotion, cream | MEKC-UV/Vis; type of capillary: a 30.2 cm uncoated fused-silica; surfactant: sodium dodecyl sulfate/γ-cyclodextrin | LOD: no data LOQ: no data R: 95.08–104.57% RSD: no data | [25] |
| PABA, BP-3, IMC, MBC, OC, EDP, EMC, BDM, EHS, HS | Cream | HPLC-UV/Vis; type of column: C18; column temp.: 35 °C; mobile phase: isocratic ethanol/acetic acid (aq) (70:30; v/v) | LOD: 0.1–2 µg mL−1 LOQ: 0.5–5µg mL−1 R: no data RSD: no data | [26] |
| BP, BP-3, BP-1, HBP | Cream | MEKC-UV/Vis; type of capillary: a 60 cm uncoated fused-silica; surfactant: sodium dodecyl sulfate | LOD: 3.9–6.7 ng mL−1 LOQ: 13–22.3 ng mL−1 R: 80.2–117.7% RSD: no data | [27] |
| BP-3, EMC, OC, EHS, MBC, EDP | Cream, lipstick, blemish balm cream | LTP-MS | LOD: no data LOQ: no data R: no data RSD: 0.8–28.6% | [28] |
| PMDSA, BP-2, BP-1, BP-8, BP, BP-6, BP-3, EHS, BP-10, HS, IMC, MBC, DHHB, BDM, BP-12 | Lotion, cream, lipstick | HPLC-MS/MS (ESI); type of column: C18; column temp.: 30 °C; mobile phase: gradient methanol/0.1% ammonium hydroxide (aq) | LOD: 2–20 mg kg−1 LOQ: 5–50 mg kg−1 R: 86.9–103.5% RSD: 1–6.8% | [29] |
| EHS, EMC, BP-3, OC, EMT, BDM, DHHB, ET, DBT | Cream | UHPSFC-PDA; type of column: Torus 2-PIC; column temp.: 40 °C; mobile phase: gradient CO2/methanol/water/ammonium acetate | LOD: 0.2–1.7 mg kg−1 LOQ: 1–10.8 mg kg−1 R: 97.5–103.2% RSD: 0.7–1.6% | [30] |
| BP-1, BP-2, BP-3, BP-8, HBP | Toothpaste, shampoo, face cleansers, sunscreens, body lotions, gels, hair gels,lotions, mask, hand sanitizer | HPLC-MS/MS (ESI-); type of column: C18; column temp.: 40 °C; mobile phase: gradient methanol/acetonitrile/water | LOD: 0.002–0.197 ng mL−1 LOQ: 0.001–0.059 ng mL−1 R: 61.9–116% RSD: no data | [31] |
| BP-1 | Nail product | GC-MS/MS (EI+); type of column: ZB-SemiVolatiles; oven temp.: 40 °C/2 min—5 °C/1 min to 65 °C—50 °C/1 min to 300 °C/5 min | LOD: 18.3–2370 µg g−1 LOQ: no data R: 101–105% RSD: 0.69–1.13% | [32] |
| BDM, EMT, OMC, OC, ET | Lotion | HPLC-UV/Vis; type of column: C18; mobile phase: acetonitrile/0.25% formic acid (aq) | LOD: 15 ng mL−1 LOQ: no data R: 88.1–104.7% RSD: 0.8–5.4% | [33] |
| BDM | Emulsion | LC-UV/Vis; type of column: C18; column temp.: 42 °C; mobile phase: acetonitrile/0.5% phosphoric acid (aq) | LOD: 0.05796 µg mL−1 LOQ: 0.19322 µg mL−1 R: no data RSD: 0.46–2.83% | [34] |
| EMC, MBC, BP-1, BP-2, BP-6, BP-4, OC, PABA, EDP, EHS, HS, IMC, BP-3, BP-8, BS, MA | Cream, nail polish, lipstick, hair gel | GC-MS/MS (EI+); type of column: SLB-5 ms; oven temp.: 100 °C/1 min—25 °C/1 min—290 °C/5 min | LOD: 0.0027–0.56 µg g−1 LOQ: 0.009–1.9 µg g−1 R: 37.4–110.5% RSD: 3.9–9.1% | [35] |
| ET | Cream, lotion | TLC-DS.; type of layer: silica gel; mobile phase: cyclohexanediethyl ether (1:1) | LOD: 0.03 μg spot−1 LOQ: 0.1 μg spot−1 R: 95–105% RSD: 4.5–5% | [36] |
| PMDSA, BDM, OC, EHS | Cream | HTLC; type of column: C18; column temp.: 150–200 °C; mobile phase: isocratic methanol/water | LOD: no data LOQ: no data R: 90.3–113.2% RSD: 2.8–5% | [37] |
| EMC, MBC, BP-1, BP-2, BP-6, BDM, BP-4, PMDSA, MA, OC, EDP, IMC, BP-3, BP-8, | Lipsticks, hair gel, cream, nail polish | HPLC-MS/MS; type of column: C18; oven temp.: 30 °C; mobile phase: gradient methanol/0.1% formic acid/ammonia (aq) | LOD: 0.00039–0.031 µg g−1 LOQ: 0.0013–0.1 µg g−1 R: 81.7–102% RSD: 4.5–13% | [38] |
| BDM, BP-3, EMC, EMT | Emulsion | HPLC-UV/Vis; type of column: C18; column temp.: 25 °C; mobile phase: gradient tetrahydfofuran/acetonitrile/acetic acid (aq) | LOD: no data LOQ: no data R: 99.2–104.8% RSD: no data | [40] |
| BP-4 | Shampoo | TLC-UV/Vis; type of layer: silica gel 60 plates; mobile phase: acetate/ethanol/water/phosphate buffer (15:7:5:1; v/v/v/v) | LOD: 0.03 μg spot−1 LOQ: 0.1 μg spot−1 R: 100–103% RSD: 0.58–1.99% | [41] |
| EHS, EMC, BP-3, OC, BDM, DHHB, ET, DBT | Cream | SFC-UV/Vis; type of column: 2-ethyl pyridine; column temp.: 30 °C; mobile phase: gradient CO2/methanol/ethanol (97:1.5:1.5) | LOD: no data LOQ: no data R: no data RSD: 0.6–2% | [42] |
| UV Filters | Extraction Technique | Analytical Technique | Analytical Performance | Comments | Ref. |
|---|---|---|---|---|---|
| BP-3 | SPE (C8) | HPLC-UV/Vis; type of column: C18; mobile phase: isocratic methanol/water (70:30) | No data | Total content | [44] |
| BP-3 | SPE (Bond Elut Certify LRC) | UPLC-MS/MS (ESI-); type of column: Kinetex Phenyl-Hexyl; column temp.: 35 °C; mobile phase: water/acetonitrile/acetic acid (aq) | LOD: 0.3 ng mL−1 LOQ: 0.61–200 ng mL−1 R: 75.8–80.3% RSD: 0.3–8% | Total and free forms content | [46] |
| BP-3 | Online SPE (RP18) | HPLC-MS/MS (APCI−); type of column: RP18; mobile phase: gradient methanol/water | LOD: 0.3–0.5 ng mL−1 LOQ: no data R: 97–105% RSD: 1.7–20% | Total and forms content | [47,48,49] |
| BP-3 | SPE (C18) | HPLC-MS (APCI); type of column: C18-PFP; mobile phase: methanol/water | LOD: 0.2 ng mL−1 LOQ: no data R: 96% RSD: 9.03–11.7% | Total content | [50] |
| BP-1, BP-2, BP-8, 4-OH-BP | LLE (solvent: ethyl acetate) | HPLC-MS/MS (ESI+/ ESI−); type of column: C18; mobile phase: methanol/water (90:10; v/v) | LOD: no data LOQ: 0.7–2.0 ng mL−1 R: 84–112% RSD: no data | Total content | [51] |
| BP-3 | SDME (acceptor phase:[C6MIM][PF6]; 25 min; 900 rpm) | LC-UV; type of column: RP18; mobile phase: ethanol/1% acetic acid aq (60:40; v/v) | LOD: 1.3 ng mL−1 LOQ: no data R: no data RSD: 6% | Free forms | [52] |
| BP, BP-OH, 2-OH-BP, BP-3, BP-10 | SBSE (PDMS; 60 min; 500 rpm) | GC-MS; type of column: DB-5 ms; oven temp.: 40 °C/1 min—5 °C/1 min to 190 °C—15 °C/1 min to 280 °C/3 min | LOD: 0.05–0.1 ng mL−1 LOQ: 0.2–0.5 ng mL−1 R: 98.7–101.7% RSD: 1.5–4.8% | Free forms | [53] |
| BP, BP-OH, 2-OH-BP, BP-3, BP-10 | HFLPME (toluene; 15 min; 500 rpm) | GC-MS (EI); type of column: DB-5 ms; oven temp.: 40 °C/ 1 min—5 °C/ 1 min to 190 °C—15 °C/1 min to 280 °C/ 4 min | LOD: 5–10 pg mL−1 LOQ: 20–50 pg mL−1 R: 89.3–100.2% RSD: 2.5–9.3% | Total content | [54] |
| BP-1, BP-3, BP-8, BP-2, 4-OH-BP | LLE (solvent; 50% MTBE/ethyl acetate) | HPLC-MS/MS (ESI−); type of column: C18; mobile phase: gradient methanol/water | LOD: 0.08–0.28 mg mL−1 LOQ: 0.28–0.9 mg mL−1 R: 85.2–99.6% RSD: 2.8–4.5% | Total content | [55] |
| BP-1, BP-3, BP-8, THB | SPE (C18) | LC-MS/MS (ESI+); type of column: Mediterranean SEA 18; mobile phase: gradient methanol/water/0.1% formic acid aq | LOD: 1 ng mL−1 LOQ: 2–4 ng mL−1 R: 84–111% RSD: no data | Total content | [56] |
| BP-1, BP-2, BP-3, BP-8, 4-OH-BP | LLE (solvent; 50% MTBE/ethyl acetate) | HPLC-MS/MS (ESI); type of column: C18; mobile phase: gradient methanol/water | LOD: 0.013–0.28 ng mL−1 LOQ: no data R: 85.2–99.6% RSD: 1.4–4.5% | Total content | [57] |
| BP-1, BP-2, BP-3, BP-7, 4-OH-BP, 4-MBP, 4-MBC, 3-BC | LLE | On-line TurboFlow-LC–MS/MS; type of column: TurboFlow Cyclone P and Hypersil Gold aQ | LOD: 0.2–1.0 ng mL−1 LOQ: no data R: 77.1–108% RSD: 5.7–15.1% | Total and free form content | [58] |
| EDP | Automated SPE (C18 HD) | LC-MS/MS (ESI+); type of column: Mediterranean SEA C18; mobile phase: gradient methanol/ acetonitryle/water/0.2% formic acid | LOD: 0.3–1.1 ng mL−1 LOQ: 0.9–3.5 ng mL−1 R: 91–107% RSD: no data | Total and free forms content | [59] |
| BP-3, OMC, OS, HS | LLE (solvent: acetonitrile) | HPLC-DAD; type of column: C18; mobile phase: gradient methanol/water (75:25; v/v) | LOD: 0.03–0.2 µg mL−1 LOQ: 0.1–0.4 µg mL−1 R: 86.8–92.2% RSD: 3.0–4.4% | Total content | [60] |
| BP-1, BP-2, BP-3, BP-8, 4-OH-BP | DLLME (disperser solvent: acetone; extraction solvent: trichloromethane) | UHPLC-MS/MS | LOD: 0.1–0.2 ng mL−1 LOQ: 0.3–0.6 ng mL−1 R: 88–104% RSD: 0.5–22.5% | Total and free forms content | [61] |
| BP-3, 4-MBC, HS, OC | ASE & SPE | GC-MS/MS | LOD: 0.47–0.59 pg mL−1 LOQ: no data R: 70.5–110.7% RSD: <5.04% | Total and free forms content | [62] |
| BMDBM, CDAA, EHS, 5-OH-EHS, OC | LLE (solvent: actonitrile) | LC-LC-MS/MS (ESI); type of column: RP-18 ADS; | LOD: 0.1–1.5 µg L−1 LOQ: 0.2–4.1 µg L−1 R: 94.2–113.6% RSD: 2.6–16.5% | Total content | [63] |
| 5OH-EHS, 5oxo-EHS, 5cx-EPS | Online SPE (TurboFlow Phenyl) | HPLC-MS/MS (ESI); type of column: C18; mobile phase: gradient acetonitryle/water/0.05% acetic acid | LOD: no data LOQ: 0.01–0.15 µg L−1 R: 96–106% RSD: 1.2–2.4% | Total and free forms content | [64] |
| BP-3 | Online SPE (RP18) | HPLC-MS/MS (ESI); type of column: XDB-C18; mobile phase: gradient methanol/water | LOD: 0.16 µg L−1 LOQ: no data R: 101% RSD: 5% | Total and free forms content | [65] |
| BP-1, BP-2, BP-3, BP-8, 4-OH-BP | LLE (solvent: ethyl tert-butyl ether/ethyl acetate (5:1; v:v)) | UHPLC-TQMS (ESI−); type of column: C18; column temp.: 30 °C; mobile phase: water/acetonitrile | LOD: 0.01–0.2 ng mL−1 LOQ: no data R: 90.7–110.1% RSD: 6.9–14.2% | Total and free forms content | [66] |
| BP-1, BP-2, BP-3, BP-8, 4-OH-BP | VADLLME (disperser solvent: 2-propanol; extraction solvent: dichloromethane) | LC-MS/MS; type of column: C18; column temp.: 23 °C; mobile phase: water/methanol | LOD: 0.02–0.03 ng mL−1 LOQ: 0.05–0.4 ng mL−1 R: no data RSD: 1.2–12% | Total content | [67] |
| BP-1, BP-2, BP-3, BP-8, 4-OH-BP | AALLME (extraction solvent: 1,2-dichloroethane) | LC-MS/MS (ESI); type of column: C18; column temp.: 40 °C; mobile phase: water/methanol | LOD: 0.02–0.06 ng mL−1 LOQ: 0.05–0.20 ng mL−1 R: no data RSD: <15% | Total content | [68] |
| PABA, 4-AHA, 4-AMB, 4-OCH3-AHA | LLE & SPE (solvent: ethyl acetate; C18) | HPLC-ECD; type of column: C18; mobile phase: methanol/phosphate buffer (pH 5.5) (20:80; v/v) | LOD: no data LOQ: 0.04–0.18 ng mL−1 R: 96–99% RSD: 0.2–3.8% | Total content | [69] |
| BP-1, BP-3 | SPE (C8) | HPLC-UV; type of column: C18; mobile phase: acetonitryle/water | LOD: 2–40 ng mL−1 LOQ: no data R: no data RSD: 6.6–13% | Total and free form content | [70] |
| PMDSA | Online SPE | SIA-FL | LOD: 12 ng mL−1 LOQ: no data R: no data RSD: 2–13% | Free forms | [71] |
| PEG-25 PABA | SPE (C18) | LC-FL; mobile phase: dimethylfuran | LOD: 2.6 ng mL−1 LOQ: no data R: 91–100% RSD: 3–10% | Total content | [72] |
| BP-4, 4-DHB, BP-2, BP-1, BP-8, BZ | FPSE | HPLC-PDA; type of column: C18; mobile phase: methanol/phosphate buffer (pH 3) (45:55; v/v) | LOD: 0.03 µg mL−1 LOQ: 0.1 µg mL−1 R: no data RSD: 2.3–14.4% | Total content | [73] |
| EDP | In-vial MMLLE (hydrophobic PTFE membranes) | GC-MS; type of column: SPB-5; oven temp.: 60 °C/1.5 min—30 °C/1 min to 275 °C/20 min | LOD: no data LOQ: 0.11 µg L−1 R: no data RSD: 7.4% | Total content | [74] |
| BP-3, BP-4 | SI SPE (C18 and diethylaminopropyl) | LC/UV; type of column: RP18; mobile phase: ethanol/acetate buffer/1% acetic acid | LOD: 30–60 ng mL−1 LOQ: no data R: no data RSD: 6–13% | Free forms | [75] |
| BP-1, BP-2, BP-8, 4-OH-BP | MEPS (C18) | LC-MS/MS; mobile phase: water/methanol | LOD: 0.005–0.03 ng mL−1 LOQ: 0.02–0.10 ng mL−1 R: 18–118% RSD: 1–16% | Total and free forms content | [78] |
| BP-1, BP-3, BP-8 | SPME (Carbowax/DVB) | GC-MS; type of column: DB5-MS; Oven temp.: 50 °C/0.1 min—30 °C/1 min to 150 °C—18 °C/1 min to 250 °C/12 min | LOD: 5–10 ng mL−1 LOQ: no data R: no data RSD: 5–8% | Total content | [79] |
| BP, BP-1, BP-3, 4-OH-BP | BAµE | HPLC–DAD; type of column: Sea-18; mobile phase: methanol/water (75:25; v/v) | LOD(P2): <1.0 µg L−1 LOQ(P2): <0.3 µg L−1 LOD(AC4): <1.3 µg L−1 LOQ(AC4): <0.4 µg L−1 | Total content | [76] |
| OMC, BP-3, OC, OS, HS | DLLME (disperser solvent: carbon tetrachloride; extraction solvent: acetonitrile) | HPLC-DAD; type of column: C18; mobile phase: isocratic water/methanol/acetonitrile (8:42:50; v/v/v) | LOD: no data LOQ: 3–45 ng mL−1 R: 86.9–97.3% RSD: 0.1–6.4% | Total content | [77] |
| BP-1, BP-2, BP-3, BP-8, 4-OH-BP | Microextraction using a monolithic stirring extraction unit (150 min; 1100 rpm) | UPLC-DAD; mobile phase: acetonitrile/water | LOD: 1–10 µg L−1 LOQ: 5–20 µg L−1 R: 71–114 % RSD: 5.6–9.1% | Total content | [80] |
| UV Filters | Matrix | Extraction Technique | Analytical Technique | Analytical Performance | Comments | Ref. |
|---|---|---|---|---|---|---|
| BP-3, BP-1, BP-8 | Serum | DLLME (disperser solvent: acetone: extraction solvent: chloroform) | LC-MS/MS (ESI+); type of column: C18; mobile phase: gradient methanol/water/0.1% formic acid | LOD: 7–8 µg L−1 LOQ: 22–28 µg L−1 R: 77–104% RSD: 8–9% | Total content | [45] |
| BP-3, OMC, OS, HS | Plasma | LLE (solvent: acetonitrile) | HPLC-DAD; type of column: C18; mobile phase: gradient methanol/water (75:25; v/v) | LOD: 0.03–0.2 µg mL−1 LOQ: 0.1–0.4 µg mL−1 R: 90.8–103.8% RSD: 2.1–4.4% | Total content | [60] |
| BP-3, OMC, OS, HS | Bovine serum albumin | LLE (solvent; acetonitrile) | HPLC-DAD; type of column: C18; mobile phase: gradient methanol/ water (75:25; v/v) | LOD: 0.03–0.2 µg mL−1 LOQ: 0.1–0.4 µg mL−1 R: 97.9–102.3% RSD: 1.2–3.3% | Total content | [60] |
| BP-1, BP-2, BP-3, BP-6, BP-8, 4-OH-BP | Menstrual blood | DLLME (disperser solvent: acetone; extraction solvent: trichloromethane) | UHPLC-MS/MS (ESI); type of column: C18; | LOD: 0.2–0.3 ng mL−1 LOQ: no data R: no data RSD: 0.28–1.59% | Total and free forms content | [82] |
| BP-1, BP-2, BP-3, BP-6, BP-8, 4-OH-BP | Serum | DLLME (disperser solvent: acetone; extraction solvent: trichloromethane) | UPLC-MS/MS (ESI+); type of column: C18; mobile phase: gradient 0.1% ammoniacal aq/0.1% ammonia in methanol | LOD: 0.1–0.3 ng mL−1 LOQ: 0.4–0.9 ng mL−1 R: 97–106% RSD: 1.9–13.7% | Total and free forms content | [83] |
| BP-3 | Serum | Online SPE | HPLC-MS/MS (APPI-) | LOD: 0.5 ng mL−1 LOQ: no data R: 96% RSD: 7.7–8.7% | Total content | [84,85] |
| OC, BMDBM, CDAA | Plasma | LLE (solvent: acetonitrile) | LC-LC-MS/MS (ESI); type of column: C18; mobile phase: methanol/water | LOD: 1.1–6.5 µg L−1 LOQ: 3.5–20.7 µg L−1 R: 89.0–112.8% RSD: 3.0–4.9% | Total content | [63] |
| BP-3 | Plasma | LLE (solvent: acetonitrile) | UHPLC-DAD; type of column: C18; mobile phase: acetonitrile/water | LOD: no data LOQ: no data R: 94–99% RSD: 2.3–4.6% | Total content | [86] |
| BP-4, 4-DHB, BP-2, BP-1, BP-8, BZ | Whole blood | FPSE | HPLC-PDA; type of column: C18; mobile phase: methanol/phosphate buffer (pH 3) (45:55; v/v) | LOD: 0.03 µg mL−1 LOQ: 0.1 µg mL−1 R: no data RSD: 0.4–10.8% | Total content | [73] |
| BP-4, 4-DHB, BP-2, BP-1, BP-8, BZ | Plasma | FPSE | HPLC-PDA; type of column: C18; mobile phase: methanol/phosphate buffer (pH 3) (45:55; v/v) | LOD: 0.03 µg mL−1 LOQ: 0.1 µg mL−1 R: no data RSD: 3.6–11.1% | Total content | [73] |
| BP-3, BP-1, 4-OH-BP, BP-8, 4-DHB, BP-2, BP-4, BMDBM | Umbilical cord blood | LLE (solvent: MTBE) | LC-MS/MS (ESI+; ESI−); type of column: R18; mobile phase: methanol/water | LOD: 0.05–0.42 ng mL−1 LOQ: 0.18–1.39 ng mL−1 R: 14.3–146.4% RSD: 0.5–33.8% | Total content | [81] |
| BP, 4-MBP | Plasma | LLE-SPE (solvent: MTBE; Oasis Prime-HLB) | HPLC-MS/MS (ESI); type of column: C18; mobile phase: 0.1% formic acid in water/0.1% formic acid in methanol | LOD: 0.8–2 pg mL1 LOQ: 3.5–7 pg mL−1 R: 87–97% RSD: 3.1–9.1% | Total content | [87] |
| UV Filters | Matrix | Extraction Technique | Analytical Technique | Analytical Performance | Comments | Ref. |
|---|---|---|---|---|---|---|
| BP-1, BP-3, BP-8, THB | Semen | SPE (C18) | LC-MS/MS (ESI+); type of column: Mediterranean SEA 18; mobile phase: gradient mobile phase: 0.1% formic acid in water/0.1% formic acid in methanol | LOD: 0.03–0.04 ng mL−1 LOQ: 0.08–0.13 ng mL−1 R: 98–115% RSD: no data | Total content | [56] |
| BP-3, OMC, OS, HS | Epidermal membranes | LLE (solvent: acetonitrile) | HPLC-DAD; type of column: C18; mobile phase: gradient methanol/water (75:25; v/v) | LOD: 0.03–0.2 µg mL−1 LOQ: 0.1–0.4 µg mL−1 R: 98.5–99.5% RSD: 1.8–3.2% | Total content | [60] |
| OC, 3-BC, 4MBC, OMC, EDP, BP-1, BP-3, BP-6, BP-8, 4-OH-BP | Milk | QuEChERS Extraction; SALLE & d-SPE (sorbent: polysecondary amine and magnesium sulphate) | UHPLC-MS/MS (API); type of column: C18; mobile phase: gradient acetonirile/water/0.1% formic acid | LOD: 0.1–0.2 ng mL1 LOQ: 0.4–0.6 ng mL−1 R: 87–112% RSD: 8–14% | Total content | [88] |
| BP-3 | Breast milk | Online SPE (RP18) | HPLC-MS/MS (APCI-); type of column: RP18; mobile phase: gradient methanol/water | LOD: 0.51 ng mL−1 LOQ: no data R: 94.7% RSD: 12.7–18% | Total and free forms content | [89] |
| BP-1, BP-3, 4-OH-BP, 4DHB, 4MBC, ODPABA, EtPABA, TBHPBT | Breast milk | Online TFC | HPLC-MS/MS (ESI); type of column: Cyclone and C18; mobile phase: gradient methanol/water/0.1% formic acid | LOD: 0.1–1.5 ng g−1 LOQ: 0.3–5.1 ng g−1 R: no data RSD: 1–12% | Total content | [90] |
| BP-3 | Milk | Online SPE (RP18) | HPLC-MS/MS (APCI−); type of column: RP18; mobile phase: methanol/water | LOD: 0.4 ng mL−1 LOQ: no data R: 102% RSD: 8.8–12% | Total and free forms content | [91] |
| BP-1, BP-3, BP-6, BP-8, 4-OH-BP | Breast milk | USAD-SPE (15 min of sonification; sorbents: C18, polysecondary amine and magnesium sulphate) | UHPLC-MS/MS (ESI+); type of column: C18; mobile phase: gradient aqueous ammonium formate solution (pH 9)/0.025% ammonia in MeOH | LOD: 0.1–0.2 ng mL−1 LOQ: 0.3–0.6 ng mL−1 R: 90.9–109.5% RSD: 2.0–12.3% | Total content | [92] |
| BP-1, BP-2, BP-3, BP-6, BP-8, 4-OH-BP, THB, AVB | Nail | MAE (20 min, 1000 W of power) | UHPLC-MS/MS (ESI+); type of column: C18; mobile phase: gradient methanol/water/0.1% formic acid | LOD: 0.2–1.5 ng g−1 LOQ: 1.0–5.0 ng g−1 R: 90.2–112.2% RSD: 0.8–12.3% | Total content | [93] |
| BP-1, BP-2, BP-3, BP-6, BP-8, 4-OH-BP | Placental tissue | MSPD (solvent: ethyl acetate) | UHPLC-MS/MS (ESI); type of column: C18; mobile phase: gradient 0.1% ammoniacal aq solution/0.1% ammonia in methanol | LOD: 0.1 ng g−1 LOQ: 0.2–0.4 ng g−1 R: 95–106% RSD: 4.5–11.8% | Free forms | [94] |
| BP-1, BP-2, BP-3, BP-4, 4-OH-BP | Placental tissue | SLE (solvent: ethyl acetate) | LC-MS/MS (ESI−); type of column: RP18; mobile phase: gradient methanol/water | LOD: 0.02–0.36 ng mL−1 LOQ: 0.05–1.20 ng mL−1 R: 72–110% RSD: 4–40% | Total content | [95] |
| BP-1, BP-2, BP-3, BP-8, 4-OH-BP | Saliva | DLLME (disperser solvent: acetone; extraction solvent: trichloromethane) | LC-MS/MS; type of column: C18; mobile phase: gradient methanol/water | LOD: 0.01–0.15 ng mL−1 LOQ: 0.05–0.40 ng mL−1 R: no data RSD: 1–19% | Total content | [96] |
| EDP, 3-BC, MBC, OMC, OC, BP-1, BP-3, BP-6, BP-8, 4-OH-BP | Placenta tissue | UAE (disperser solvent: methanol; extraction solvent: anisole; 3 min of sonification) | UHPLC-MS/MS; type of column: C18; mobile phase: gradient acetonitrile/0.25% formic acid aq | LOD: 0.05–0.2 µg kg−1 LOQ: 0.15–0.5 µg kg−1 R: 90–112% RSD: 3–15% | Total content | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narloch, I.; Wejnerowska, G. An Overview of the Analytical Methods for the Determination of Organic Ultraviolet Filters in Cosmetic Products and Human Samples. Molecules 2021, 26, 4780. https://doi.org/10.3390/molecules26164780
Narloch I, Wejnerowska G. An Overview of the Analytical Methods for the Determination of Organic Ultraviolet Filters in Cosmetic Products and Human Samples. Molecules. 2021; 26(16):4780. https://doi.org/10.3390/molecules26164780
Chicago/Turabian StyleNarloch, Izabela, and Grażyna Wejnerowska. 2021. "An Overview of the Analytical Methods for the Determination of Organic Ultraviolet Filters in Cosmetic Products and Human Samples" Molecules 26, no. 16: 4780. https://doi.org/10.3390/molecules26164780
APA StyleNarloch, I., & Wejnerowska, G. (2021). An Overview of the Analytical Methods for the Determination of Organic Ultraviolet Filters in Cosmetic Products and Human Samples. Molecules, 26(16), 4780. https://doi.org/10.3390/molecules26164780






