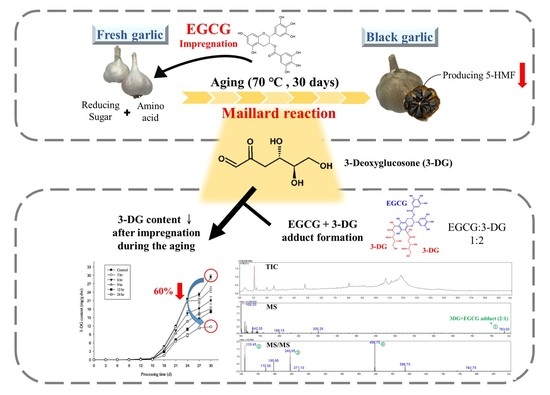
Reduction of 3-Deoxyglucosone by Epigallocatechin Gallate Results Partially from an Addition Reaction: The Possible Mechanism of Decreased 5-Hydroxymethylfurfural in Epigallocatechin Gallate-Treated Black Garlic
Abstract
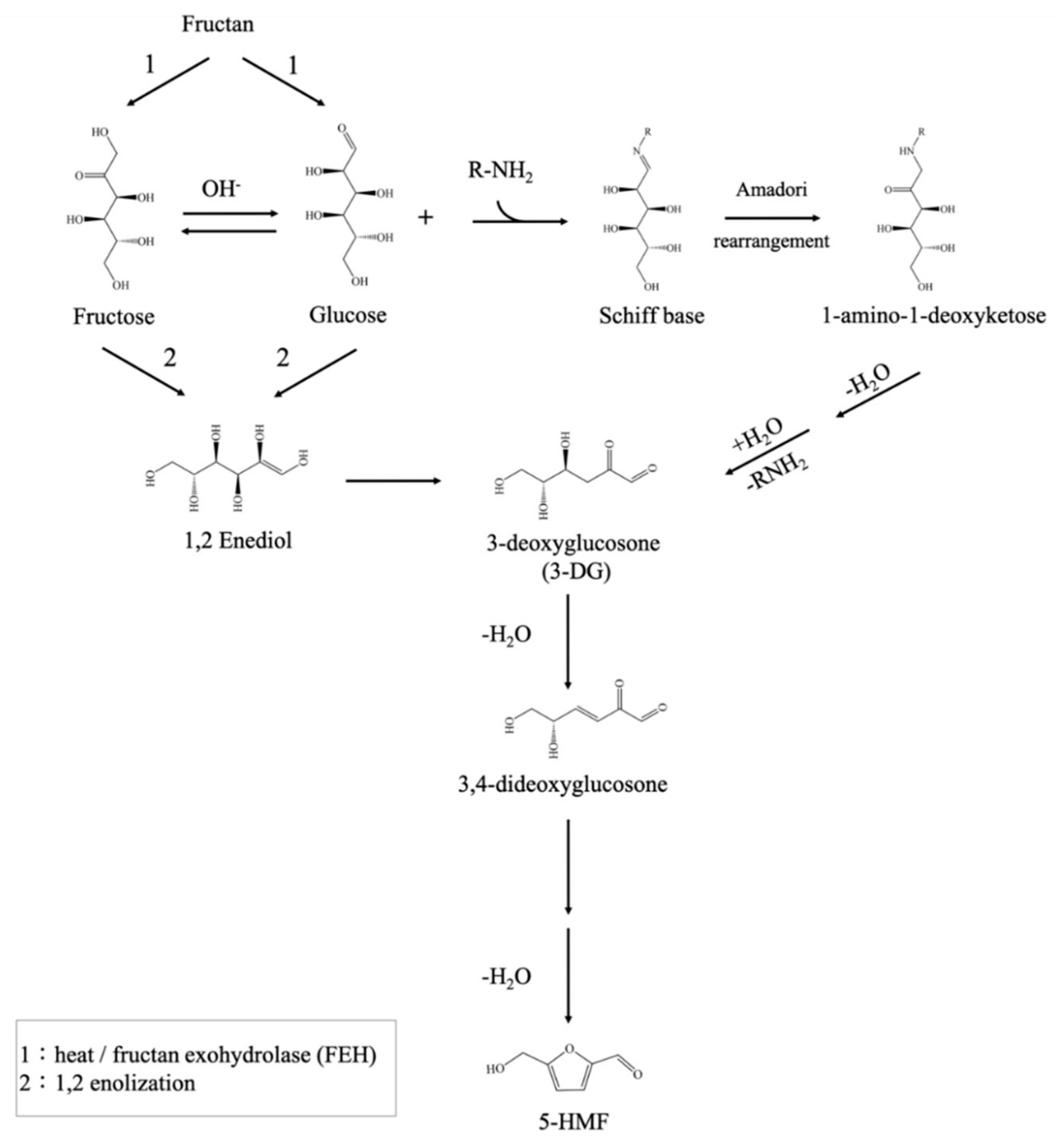
:1. Introduction
2. Results and Discussion
2.1. Effect of Various Impregnation Conditions on 3-DG Content during the Aging Process
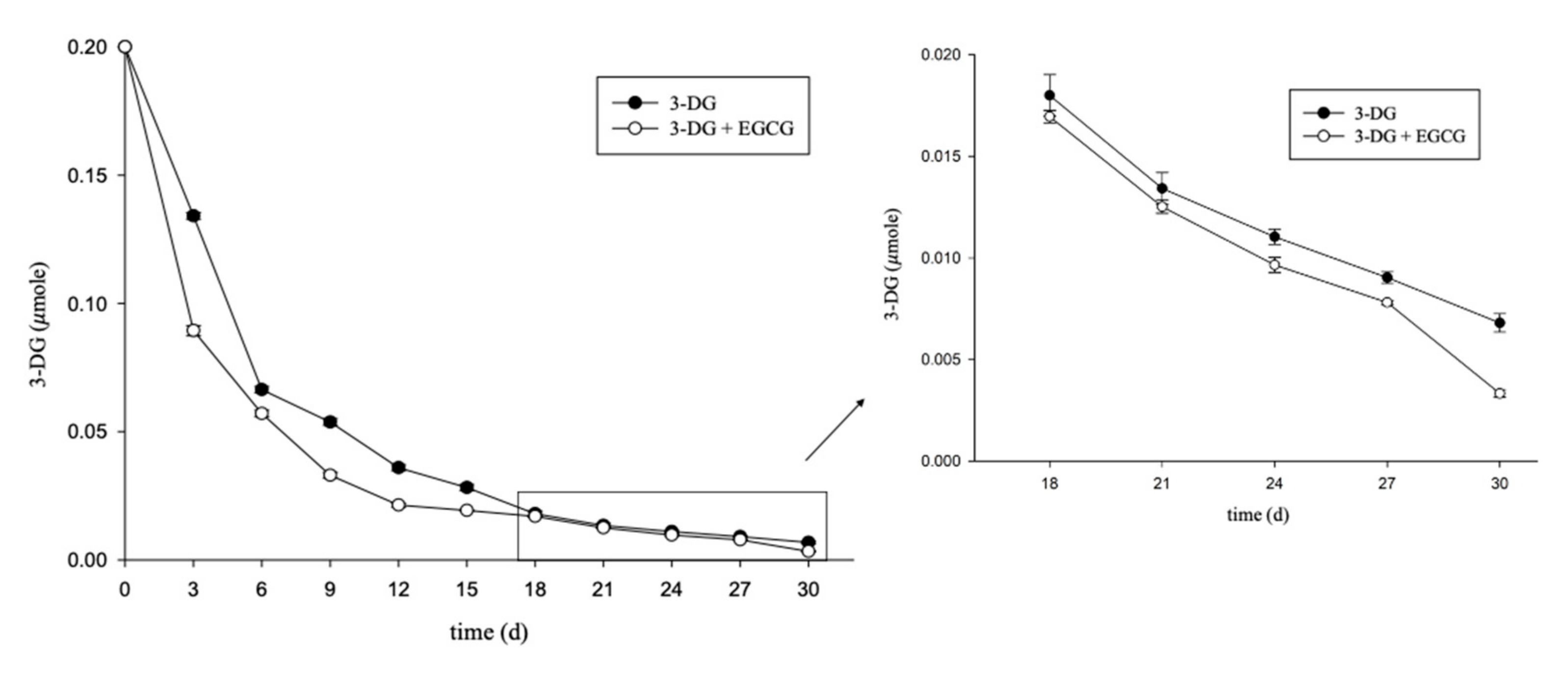
2.2. Kinetic Study of Various Impregnation Conditions on 3-DG Content during Aging of Garlic
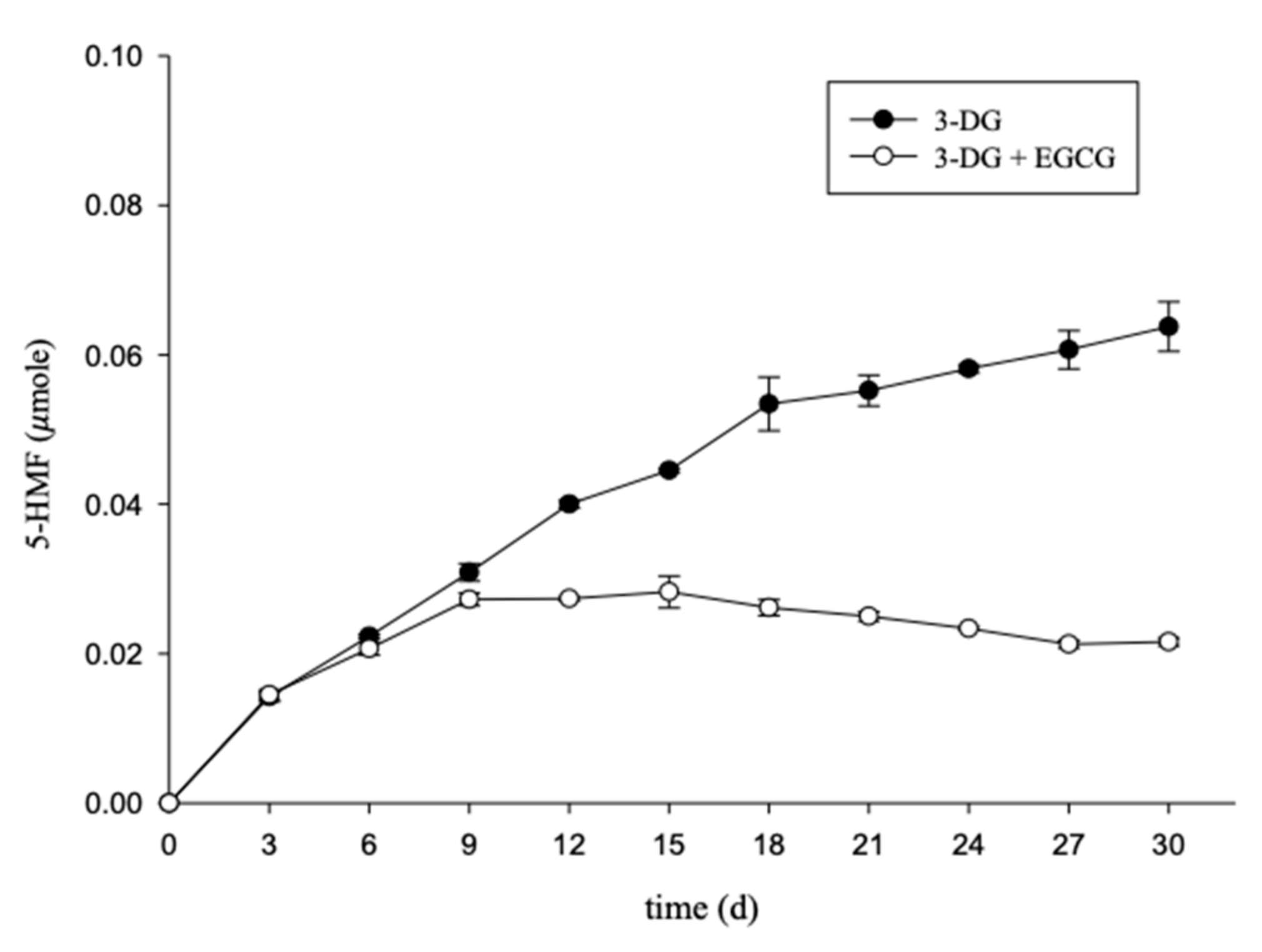
2.3. Effects of EGCG on 3-DG and 5-HMF Content in an Aging-Mimicking Model System
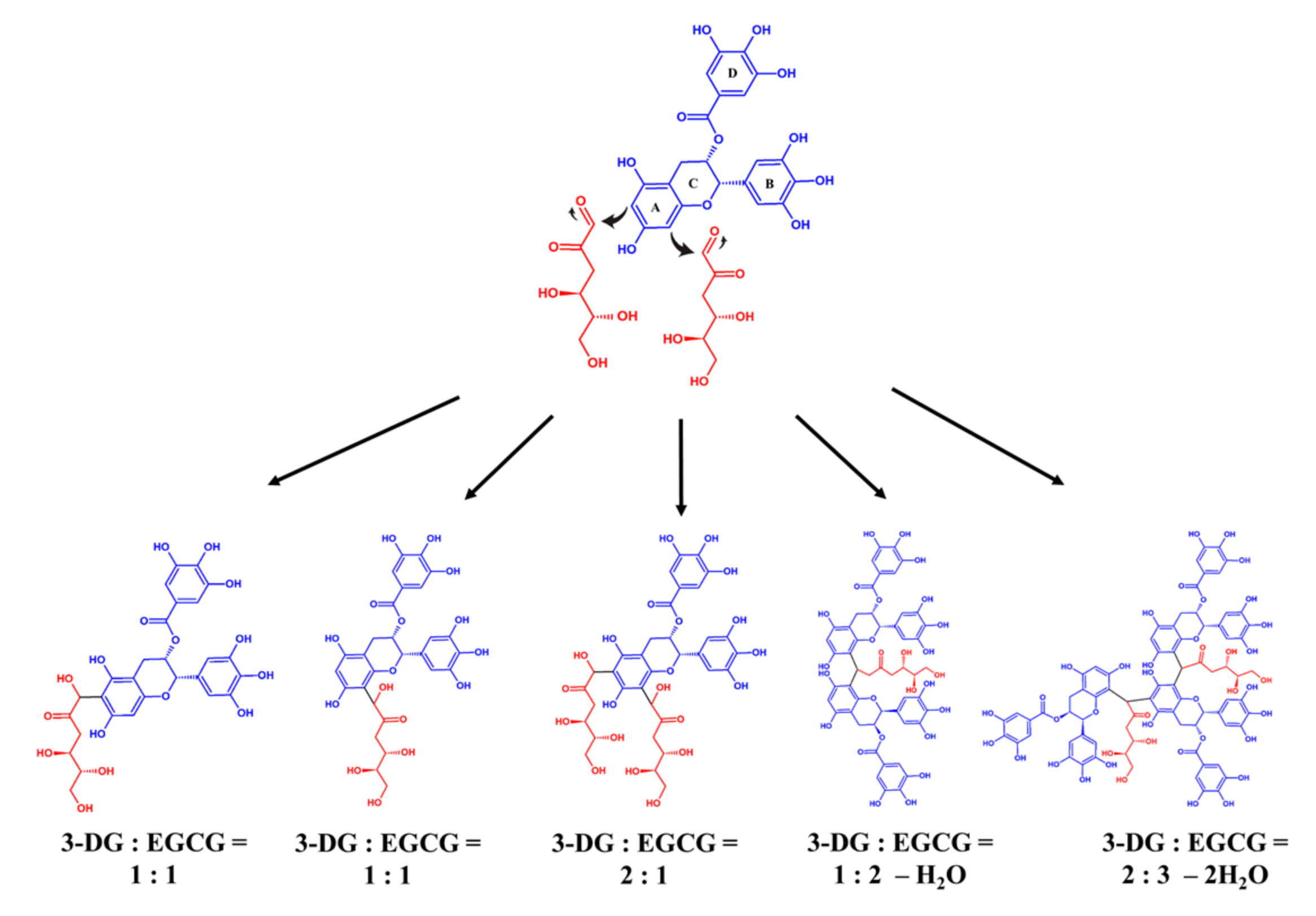
2.4. Identification of the Adducts from the Reaction of EGCG and 3-DG in an Aging-Mimicking Model System
3. Materials and Methods
3.1. Materials and Sample Preparation
3.2. Measurement of 3-DG and 5-HMF Content
3.3. Kinetic Study
3.4. Identification of Adducts from the Reaction of EGCG and 3-DG in the Aging-Mimicking Model Using HPLC-MS/MS
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, X.; Li, N.; Lu, X.; Liu, P.; Qiao, X. Effects of temperature on the quality of black garlic. J. Sci. Food Agric. 2016, 96, 2366–2372. [Google Scholar] [CrossRef]
- Monien, B.H.; Frank, H.; Seidel, A.; Glatt, H. Conversion of the common food constituent 5-hydroxymethylfurfural into a mutagenic and carcinogenic sulfuric acid ester in the mouse in vivo. Chem. Res. Toxicol. 2009, 22, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Severin, I.; Dumont, C.; Jondeau-Cabaton, A.; Graillot, V.; Chagnon, M.-C. Genotoxic activities of the food contaminant 5-hydroxymethylfurfural using different in vitro bioassays. Toxicol. Lett. 2010, 192, 189–194. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Shlyankevich, M.; Jeong, H.-K.; Douglas, J.S.; Surh, Y.-J. Bioactivation of 5-hydroxymethyl-2-furaldehyde to an electrophilic and mutagenic allylic sulfuric acid ester. Biochem. Biophys. Res. Commun. 1995, 209, 996–1002. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chen, K.-T.; Lin, J.-A.; Chen, Y.-T.; Chen, Y.-A.; Wu, J.-T.; Hsieh, C.-W. Recent advances in processing technology to reduce 5-hydroxymethylfurfural in foods. Trends Food Sci. Technol. 2019, 93, 271–280. [Google Scholar] [CrossRef]
- Ros-Polski, V.; Popović, V.; Koutchma, T. Effect of ultraviolet-C light treatment on Hydroxymethylfurfural (5-HMF) content in high fructose corn syrup (HFCS) and model syrups. J. Food Eng. 2016, 179, 78–87. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Wu, G.; Zhang, H.; Wang, L.; Qian, H.; Qi, X. Reduction of 5-hydroxymethylfurfural formation by flavan-3-ols in Maillard reaction models and fried potato chips. J. Sci. Food Agric. 2018, 98, 5294–5301. [Google Scholar] [CrossRef]
- Zhang, Y.; An, X. Inhibitory mechanism of quercetin against the formation of 5-(hydroxymethyl)-2-furaldehyde in buckwheat flour bread by ultra-performance liquid chromatography coupled with high-resolution tandem mass spectrometry. Food Res. Int. 2017, 95, 68–81. [Google Scholar] [CrossRef]
- Akıllıoglu, H.G.; Mogol, B.A.; Gökmen, V. Degradation of 5-hydroxymethylfurfural during yeast fermentation. Food Addit. Contam. A 2011, 28, 1629–1635. [Google Scholar] [CrossRef]
- Anese, M.; Suman, M. Mitigation strategies of furan and 5-hydroxymethylfurfural in food. Food Res. Int. 2013, 51, 257–264. [Google Scholar] [CrossRef]
- Akkarachaneeyakorn, S.; Laguerre, J.; Tattiyakul, J.; Neugnot, B.; Boivin, P.; Morales, F.; Birlouez-Aragon, I. Optimization of Combined Microwave–Hot Air Roasting of Malt Based on Energy Consumption and Neo-Formed Contaminants Content. J. Food Sci. 2010, 75, E201–E207. [Google Scholar] [CrossRef]
- Mtaoua, H.; Sánchez-Vega, R.; Ferchichi, A.; Martín-Belloso, O. Impact of high-intensity pulsed electric fields or thermal treatment on the quality attributes of date juice through storage. J. Food Process. Preserv. 2017, 41, e13052. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Gao, J.; Li, J.; Zhang, Y.; Yang, Y.; Wang, S. Mitigation of 3-deoxyglucosone and 5-hydroxymethylfurfural in brown fermented milk via an alternative browning process based on the hydrolysis of endogenous lactose. Food Funct. 2019, 10, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.; Troise, A.D.; Ataç Mogol, B.E.; Roullier, V.; Gourdon, A.; El Mafadi Jian, S.; Hamzalıoğlu, B.A.l.; Gόkmen, V.; Fogliano, V. Controlling the Maillard reaction by reactant encapsulation: Sodium chloride in cookies. J. Agric. Food Chem. 2012, 60, 10808–10814. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Effects of thermal treatment on polysaccharide degradation during black garlic processing. LWT Food Sci. Technol. 2018, 95, 223–229. [Google Scholar] [CrossRef]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Pan, Z.; Shao, H.-X.; Liu, T.; Lu, X.-Y.; Fan, X.-H. Research progress of 5-hydroxymethylfurfural, a safety-related substance in traditional Chinese medicine injections. Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2017, 42, 1842–1846. [Google Scholar]
- Perez Locas, C.; Yaylayan, V.A. Isotope labeling studies on the formation of 5-(hydroxymethyl)-2-furaldehyde (HMF) from sucrose by pyrolysis-GC/MS. J. Agric. Food Chem. 2008, 56, 6717–6723. [Google Scholar] [CrossRef] [PubMed]
- Nobis, A.; Röhrig, A.; Hellwig, M.; Henle, T.; Becker, T.; Gastl, M. Formation of 3-deoxyglucosone in the malting process. Food Chem. 2019, 290, 187–195. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Santos, S.A.; Silvestre, A.J.; Barros, A.S.; Ferreira, A.C.; Silva, A.M. Quantification of 3-deoxyglucosone (3DG) as an aging marker in natural and forced aged wines. J. Food Compos. Anal. 2016, 50, 70–76. [Google Scholar] [CrossRef]
- Bruhns, P.; Kanzler, C.; Degenhardt, A.G.; Koch, T.J.; Kroh, L.W. Basic structure of melanoidins formed in the Maillard reaction of 3-deoxyglucosone and γ-aminobutyric acid. J. Agric. Food Chem. 2019, 67, 5197–5203. [Google Scholar] [CrossRef]
- Ashraf, J.M.; Shahab, U.; Tabrez, S.; Lee, E.J.; Choi, I.; Yusuf, M.A.; Ahmad, S. DNA glycation from 3-deoxyglucosone leads to the formation of AGEs: Potential role in cancer auto-antibodies. Cell Biochem. Biophys. 2016, 74, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Atzenbeck, L.; Pischetsrieder, M.; Morales, F.J. Investigations on the Reaction of C3 and C6 α-dicarbonyl compounds with hydroxytyrosol and related compounds under competitive conditions. J. Argric. Food Chem. 2016, 64, 6327–6332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokkinidou, S.; Peterson, D.G. Response surface methodology as optimization strategy for reduction of reactive carbonyl species in foods by means of phenolic chemistry. Food Funct. 2013, 4, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-Y.; Li, S.; Wang, Y.; Tan, D.; Pan, M.-H.; Sang, S.; Ho, C.-T. Reactive dicarbonyl compounds and 5-(hydroxymethyl)-2-furfural in carbonated beverages containing high fructose corn syrup. Food Chem. 2008, 107, 1099–1105. [Google Scholar] [CrossRef]
- Taş, N.G.; Gökmen, V. Effect of alkalization on the Maillard reaction products formed in cocoa during roasting. Food Res. Int. 2016, 89, 930–936. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chen, Y.-T.; Hsieh, H.-J.; Chen, K.-T.; Chen, Y.-A.; Wu, J.-T.; Tsai, M.-S.; Lin, J.-A.; Hsieh, C.-W. Exploring epigallocatechin gallate impregnation to inhibit 5-hydroxymethylfurfural formation and the effect on antioxidant ability of black garlic. LWT Food Sci. Technol. 2020, 117, 108628. [Google Scholar] [CrossRef]
- Totlani, V.M.; Peterson, D.G. Influence of epicatechin reactions on the mechanisms of Maillard product formation in low moisture model systems. J. Agric. Food Chem. 2007, 55, 414–420. [Google Scholar] [CrossRef]
- Arena, E.; Ballistreri, G.; Fallico, B. Kinetics of 3-Deoxy-D-Erythro-Hexos-2-Ulose in Unifloral Honeys. J. Food Sci. 2011, 76, C1044–C1049. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Y.; Pu, D.; Zhang, Y.; Sun, B.; Zhao, Z. Kinetics of α-dicarbonyl compounds formation in glucose-glutamic acid model of Maillard reaction. Food Sci. Nutr. 2021, 9, 290–302. [Google Scholar] [CrossRef]
- Sang, S.; Shao, X.; Bai, N.; Lo, C.-Y.; Yang, C.S.; Ho, C.-T. Tea polyphenol (−)-epigallocatechin-3-gallate: A new trapping agent of reactive dicarbonyl species. Chem. Res. Toxicol. 2007, 20, 1862–1870. [Google Scholar] [CrossRef]
- Totlani, V.M.; Peterson, D.G. Epicatechin carbonyl-trapping reactions in aqueous Maillard systems: Identification and structural elucidation. J. Agric. Food Chem. 2006, 54, 7311–7318. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.Y.; Li, S.; Tan, D.; Pan, M.H.; Sang, S.; Ho, C.T. Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions. Mol. Nutr. Food Res. 2006, 50, 1118–1128. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, S.; Chen, Y.; Zhang, R.; Zhou, M.; Wang, C.; Feng, N.; Wu, Q. Structure-activity relationship of procyanidins on advanced glycation end products formation and corresponding mechanisms. Food Chem. 2019, 272, 679–687. [Google Scholar] [CrossRef]
- Yu, J.; Cui, H.; Zhang, Q.; Hayat, K.; Zhan, H.; Yu, J.; Jia, C.; Zhang, X.; Ho, C.-T. Adducts Derived from (−)-Epigallocatechin Gallate-Amadori Rearrangement Products in Aqueous Reaction Systems: Characterization, Formation, and Thermolysis. J. Agric. Food Chem. 2020, 68, 10902–10911. [Google Scholar] [CrossRef]
- Oral, R.A.; Mortas, M.; Dogan, M.; Sarioglu, K.; Yazici, F. New approaches to determination of HMF. Food Chem. 2014, 143, 367–370. [Google Scholar] [CrossRef]
- Yu, X.; Cui, H.; Hayat, K.; Hussain, S.; Jia, C.; Zhang, S.-L.; Tahir, M.U.; Zhang, X.; Ho, C.-T. Effective mechanism of (−)-epigallocatechin gallate indicating the critical formation conditions of Amadori compound during an aqueous Maillard reaction. J. Agric. Food Chem. 2019, 67, 3412–3422. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; de Araújo Calado, V.M.; Jarvis, B. Observations on the use of statistical methods in food science and technology. Food Res. Int. 2014, 55, 137–149. [Google Scholar] [CrossRef]


| Group | Zero-Order Equation | First-Order Equation | ||
|---|---|---|---|---|
| k | R2 | k | R2 | |
| Control | 1.5514 | 0.9217 | 0.3014 | 0.9095 |
| 3 h | 1.3792 | 0.9160 | 0.3466 | 0.8689 |
| 6 h | 1.1717 | 0.9189 | 0.3727 | 0.8482 |
| 9 h | 1.0075 | 0.9288 | 0.3090 | 0.8979 |
| 12 h | 0.9029 | 0.9308 | 0.3028 | 0.8600 |
| 24 h | 0.6716 | 0.9127 | 0.3180 | 0.8514 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, A.-T.; Lin, J.-A.; Lee, C.-H.; Chen, Y.-A.; Wu, J.-T.; Tsai, M.-S.; Cheng, K.-C.; Hsieh, C.-W. Reduction of 3-Deoxyglucosone by Epigallocatechin Gallate Results Partially from an Addition Reaction: The Possible Mechanism of Decreased 5-Hydroxymethylfurfural in Epigallocatechin Gallate-Treated Black Garlic. Molecules 2021, 26, 4746. https://doi.org/10.3390/molecules26164746
Tu A-T, Lin J-A, Lee C-H, Chen Y-A, Wu J-T, Tsai M-S, Cheng K-C, Hsieh C-W. Reduction of 3-Deoxyglucosone by Epigallocatechin Gallate Results Partially from an Addition Reaction: The Possible Mechanism of Decreased 5-Hydroxymethylfurfural in Epigallocatechin Gallate-Treated Black Garlic. Molecules. 2021; 26(16):4746. https://doi.org/10.3390/molecules26164746
Chicago/Turabian StyleTu, An-Ting, Jer-An Lin, Chieh-Hsiu Lee, Yi-An Chen, Jung-Tsung Wu, Ming-Shiun Tsai, Kuan-Chen Cheng, and Chang-Wei Hsieh. 2021. "Reduction of 3-Deoxyglucosone by Epigallocatechin Gallate Results Partially from an Addition Reaction: The Possible Mechanism of Decreased 5-Hydroxymethylfurfural in Epigallocatechin Gallate-Treated Black Garlic" Molecules 26, no. 16: 4746. https://doi.org/10.3390/molecules26164746
APA StyleTu, A.-T., Lin, J.-A., Lee, C.-H., Chen, Y.-A., Wu, J.-T., Tsai, M.-S., Cheng, K.-C., & Hsieh, C.-W. (2021). Reduction of 3-Deoxyglucosone by Epigallocatechin Gallate Results Partially from an Addition Reaction: The Possible Mechanism of Decreased 5-Hydroxymethylfurfural in Epigallocatechin Gallate-Treated Black Garlic. Molecules, 26(16), 4746. https://doi.org/10.3390/molecules26164746