Identification and Characterization of Glucosyltransferase That Forms 1-Galloyl-β-d-Glucogallin in Canarium album L., a Functional Fruit Rich in Hydrolysable Tannins
Abstract
1. Introduction
2. Results
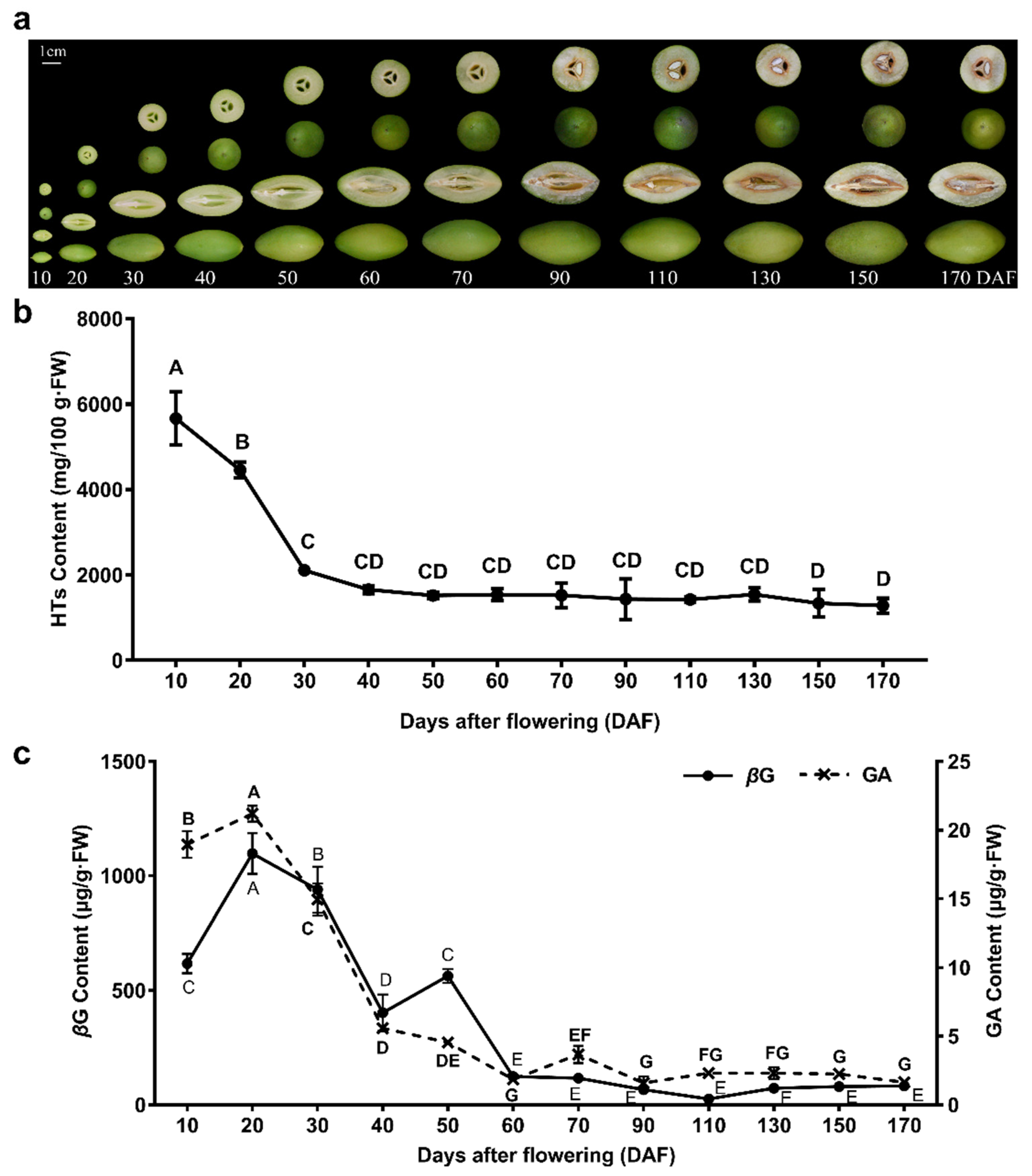
2.1. Analysis of Total HTs, GA, and βG Contents in C. album
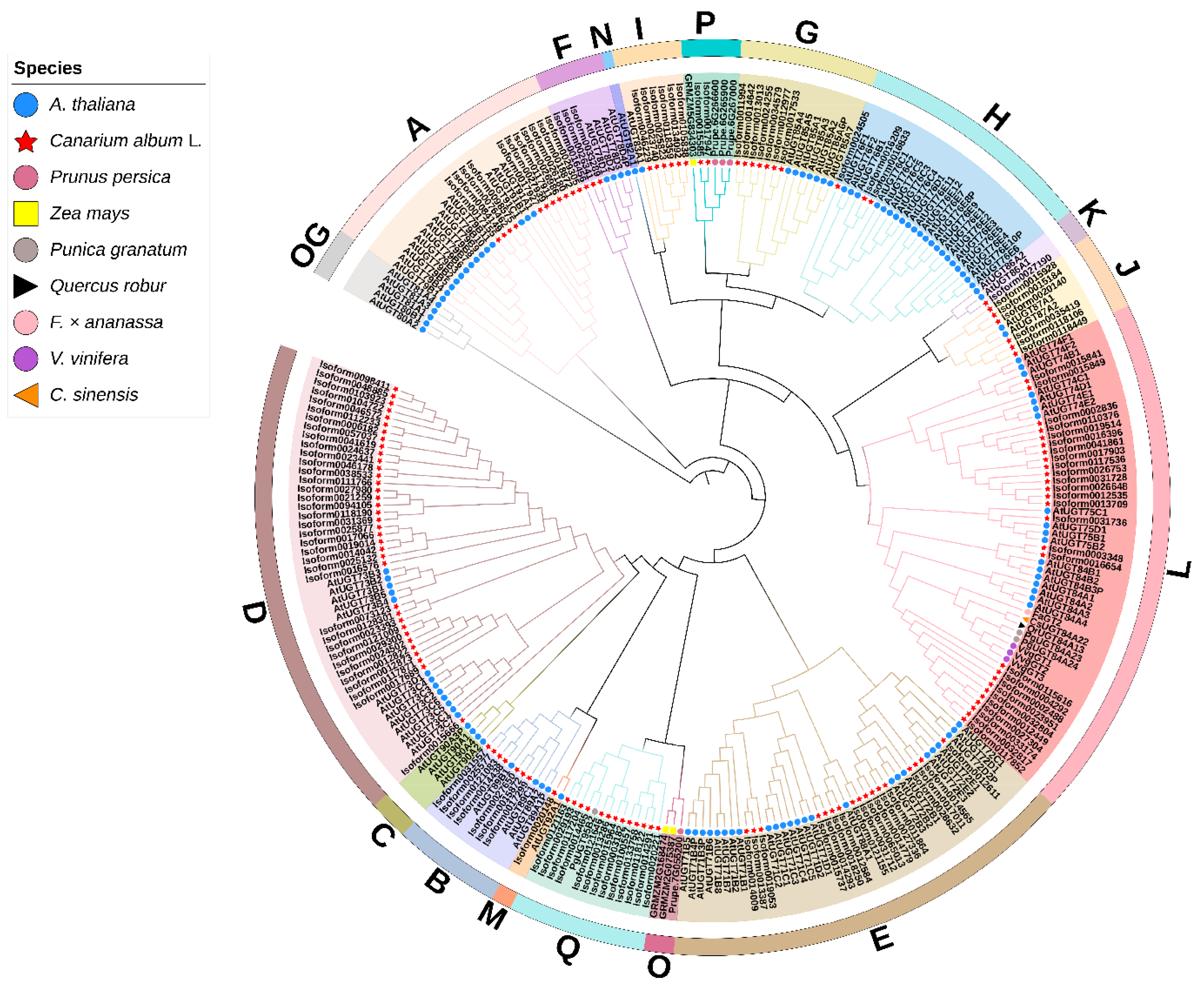
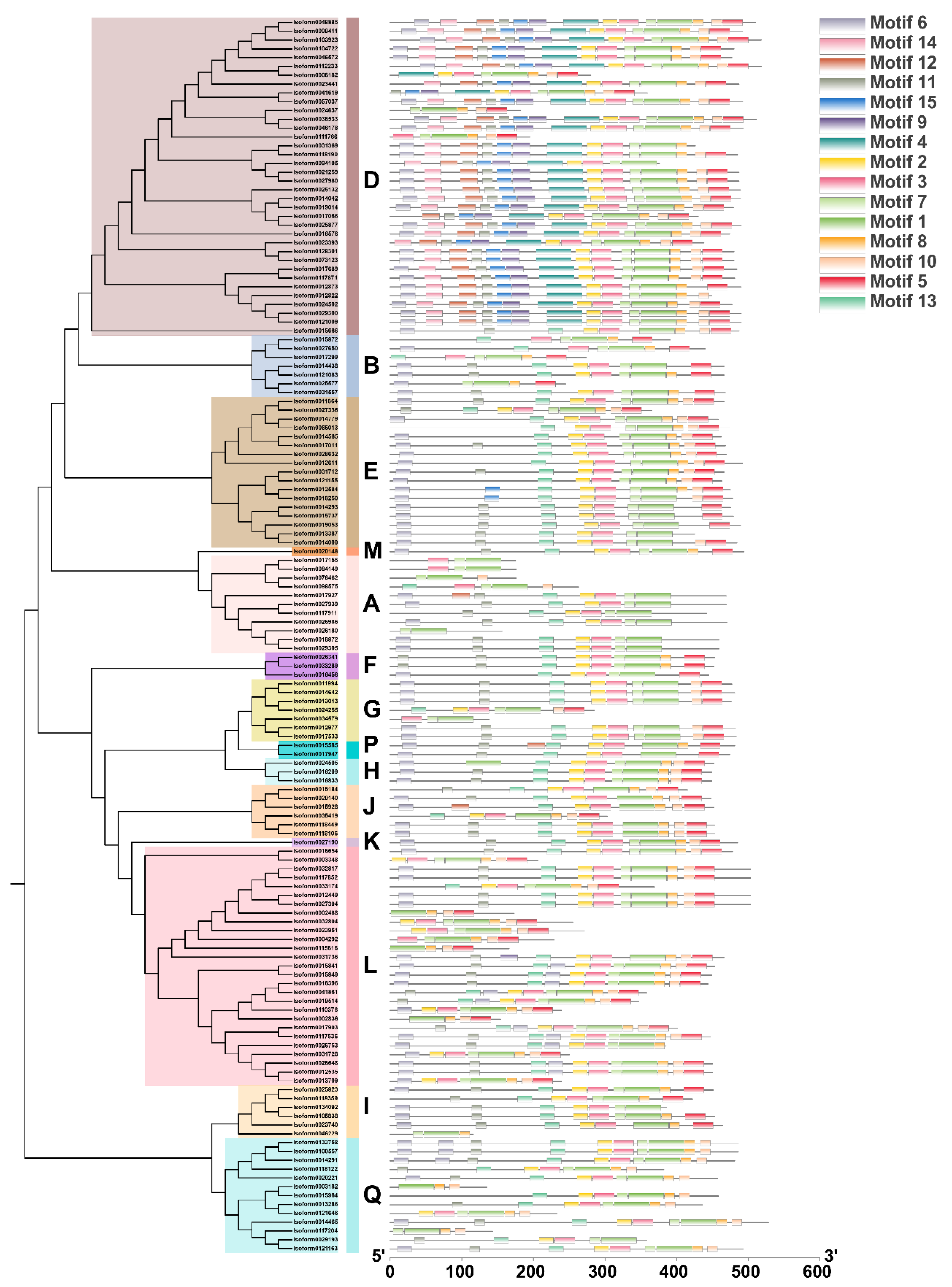
2.2. Identification and Phylogenetic Analysis of C. album UGT Family Members
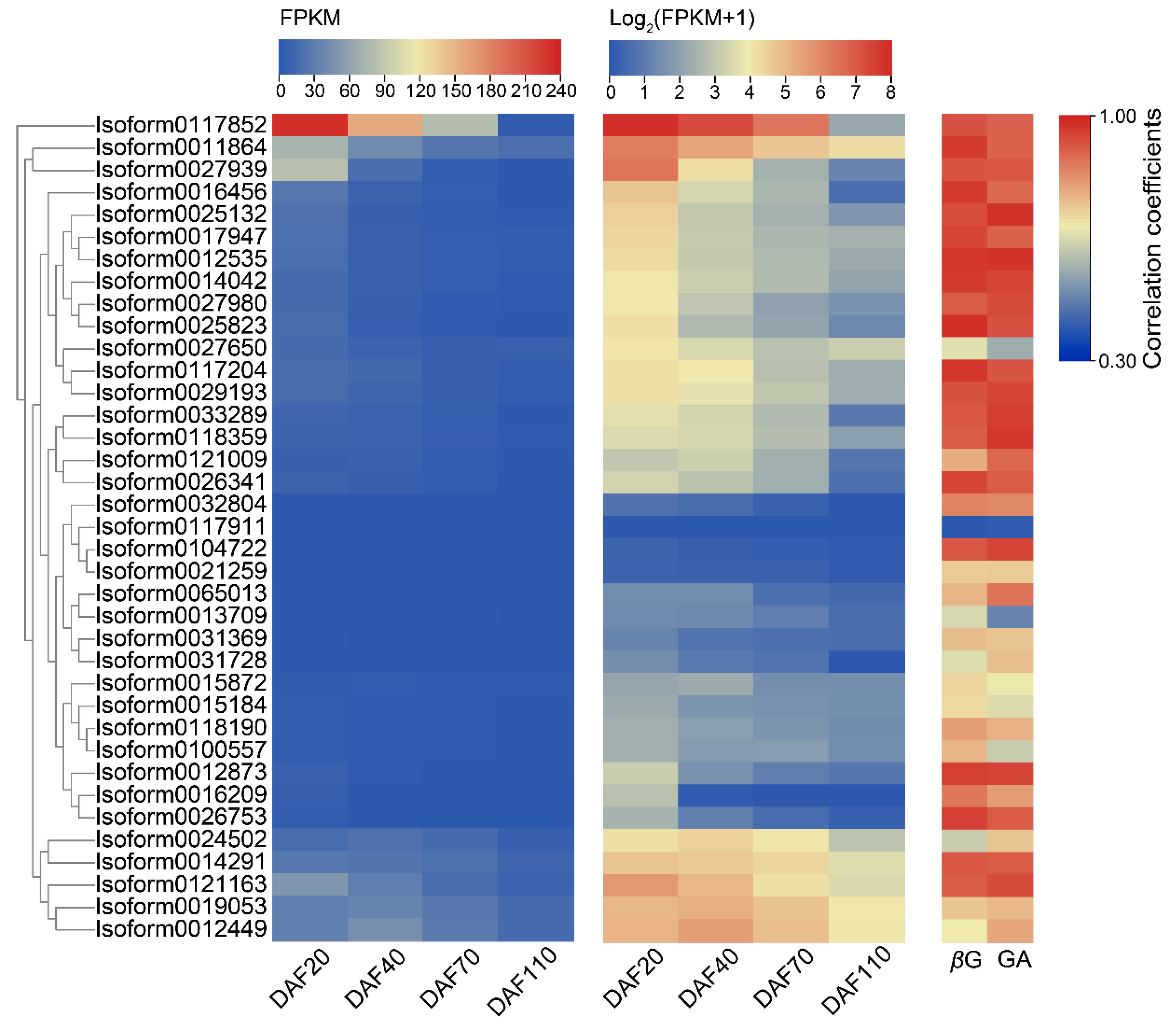
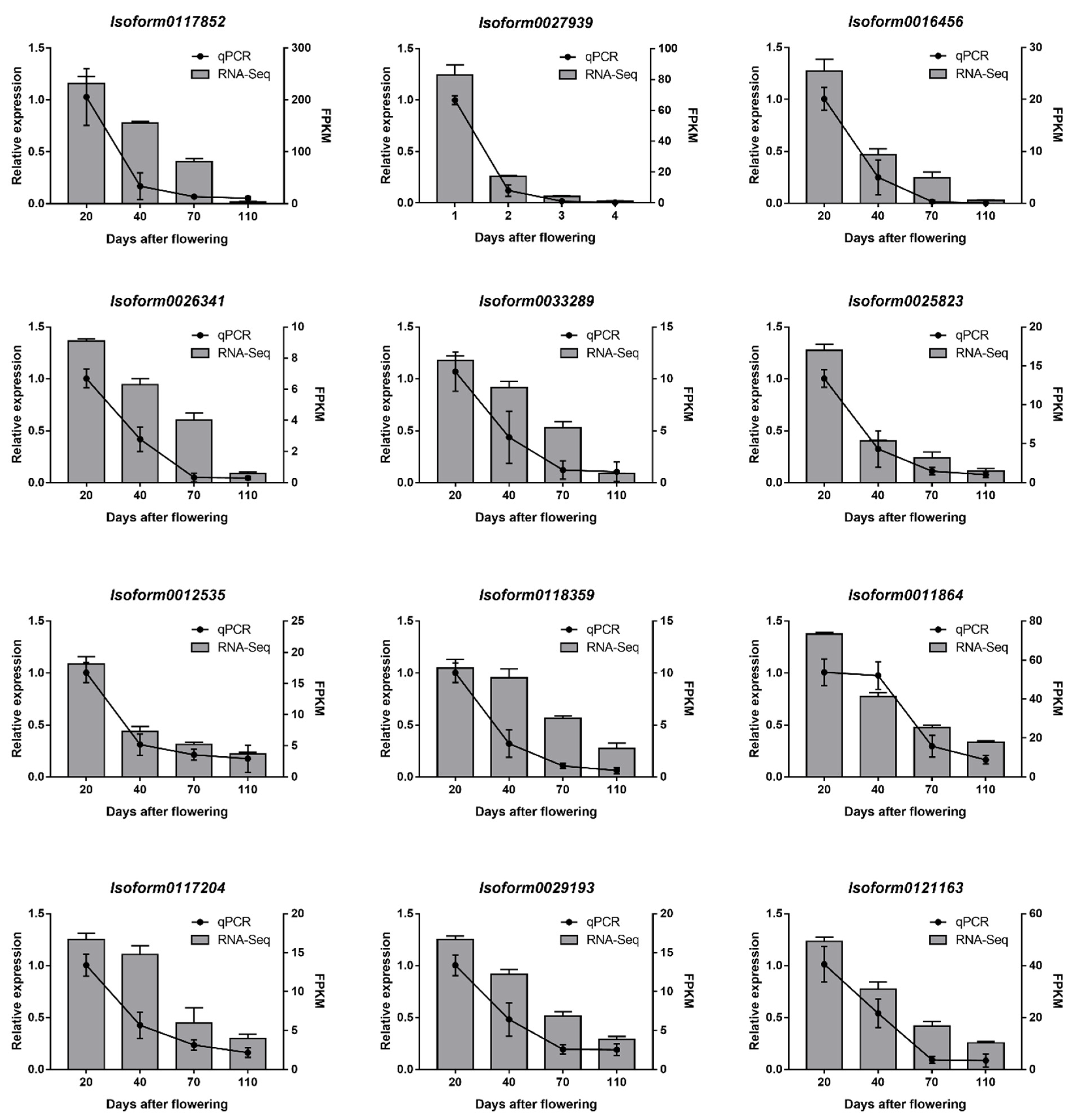
2.3. Expression Patterns of Candidate UGTs in the Developmental Stages of C. album Fruits and Selection of Key CaUGT Genes
2.4. Real-Time Quantitative RT-PCR (RT-qPCR) Verification
2.5. The Full-Length CDS Analysis of UGT84A77
2.6. Subcellular Localization
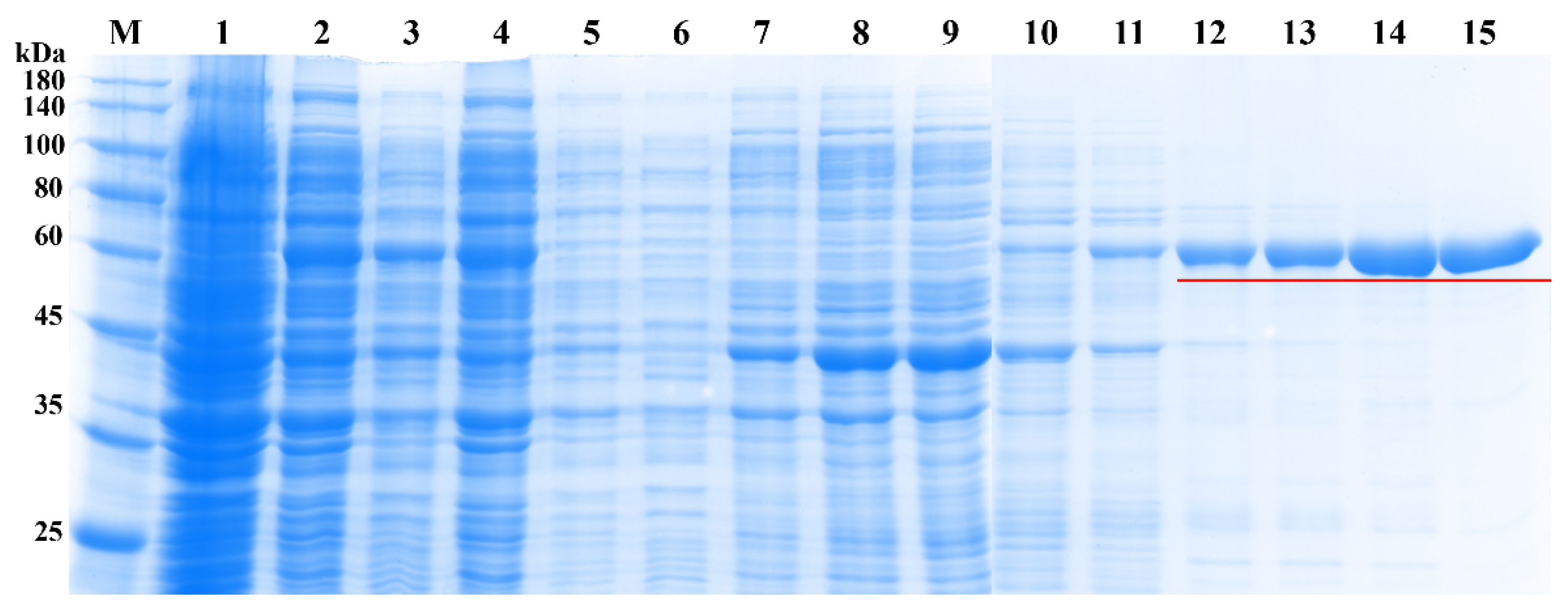
2.7. Expression and Purification of Recombinant UGT84A77 Protein
2.8. Enzymatic Properties of Recombinant UGT84A77 Protein
3. Discussion
3.1. The C. album Fruit, Especially the Young Fruit, Is Rich in HTs
3.2. Analysis and Screening of the UGT Gene Family in C. album
3.3. UGT84A77 Catalyzes the Formation of βG
4. Materials and Methods
4.1. Plant Materials and Sampling
4.2. Chemicals
4.3. Measurement and Quantification of Total HTs, GA, and βG
4.4. Identification and Characterization of C. album UGT Genes
4.5. Phylogenetic and Classification Analysis of CaUGTs
4.6. Expression Pattern and Correlation Analysis of Candidate CaUGTs
4.7. RNA Extraction and RT-qPCR
4.8. Cloning and Subcellular Localization
4.9. Prokaryotic Expression of UGT84A77
4.10. Enzymatic Activity Assay of Recombinant Protein
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taveira de Jesus, N.Z.; Falcao, H.d.S.; Gomes, I.F.; de Almeida Leite, T.J.; de Morais Lima, G.R.; Barbosa-Filho, J.M.; Tavares, J.F.; da Silva, M.S.; de Athayde-Filho, P.F.; Batista, L.M. Tannins, Peptic Ulcers and Related Mechanisms. Int. J. Mol. Sci. 2012, 13, 3203–3228. [Google Scholar] [CrossRef]
- Valverde Malaver, C.L.; Colmenares Dulcey, A.J.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Macias, F.A.; Isaza Martinez, J.H. Hydrolysable Tannins and Biological Activities of Meriania hernandoi and Meriania nobilis (Melastomataceae). Molecules 2019, 24, 746. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; He, X.; Teng, B.; McRae, J.M. Valonea Tannin: Tyrosinase Inhibition Activity, Structural Elucidation and Insights into the Inhibition Mechanism. Molecules 2021, 26, 2747. [Google Scholar] [CrossRef]
- Olchowik-Grabarek, E.; Sekowski, S.; Bitiucki, M.; Dobrzynska, I.; Shlyonsky, V.; Ionov, M.; Burzynski, P.; Roszkowska, A.; Swiecicka, I.; Abdulladjanova, N.; et al. Inhibition of interaction between Staphylococcus aureus alpha-hemolysin and erythrocytes membrane by hydrolysable tannins: Structure-related activity study. Sci. Rep. 2020, 10, 11168. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, I.; Zhu, W.; Nafie, M.S.; Dutta, K.; Li, C. Anti-COVID-19 effects of ten structurally different hydrolysable tannins through binding with the catalytic-closed sites of COVID-19 main protease: An in-silico approach. Preprints 2020 2020, 2020030277. [Google Scholar] [CrossRef]
- Maatsola, S.; Kurkinen, S.; Engström, M.T.; Nyholm, T.K.M.; Pentikäinen, O.; Salminen, J.-P.; Haataja, S. Inhibition of pneumolysin cytotoxicity by hydrolysable tannins. Antibiotics 2020, 9, 930. [Google Scholar] [CrossRef]
- Noce, A.; Di Daniele, F.; Campo, M.; Di Lauro, M.; Pietroboni Zaitseva, A.; Di Daniele, N.; Marrone, G.; Romani, A. Effect of hydrolysable tannins and anthocyanins on recurrent urinary tract infections in nephropathic patients: Preliminary data. Nutrients 2021, 13, 591. [Google Scholar] [CrossRef]
- Ajebli, M.; Eddouks, M. The promising role of plant tannins as bioactive antidiabetie agents. Curr. Med. Chem. 2019, 26, 4852–4884. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A. Scope of hydrolysable tannins as possible antimicrobial agent. Phytother. Res. 2016, 30, 1035–1045. [Google Scholar] [CrossRef]
- Khan, H.; Saeedi, M.; Nabavi, S.M.; Mubarak, M.S.; Bishayee, A. Glycosides from medicinal plants as potential anticancer agents: Emerging trends towards future drugs. Curr. Med. Chem. 2019, 26, 2389–2406. [Google Scholar] [CrossRef]
- Soares, S.; Brandao, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in food: Insights into the molecular perception of astringency and bitter taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Liu, Y.; Zhuang, J.; Yao, S.; Liu, L.; Jiang, X.; Zhou, K.; Wang, Y.; Xie, D.; Bennetzen, J.L.; et al. Discovery and characterization of tannase genes in plants: Roles in hydrolysis of tannins. New Phytol. 2020, 226, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Arapitsas, P. Hydrolyzable tannin analysis in food. Food Chem. 2012, 135, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Niemetz, R.; Gross, G.G. Enzymology of gallotannin and ellagitannin biosynthesis. Phytochemistry 2005, 66, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Nishiguchi, M.; Funke, E.; Miyazawa, S.-I.; Miyama, T.; Milkowski, C. Dehydroquinate dehydratase/shikimate dehydrogenases involved in gallate biosynthesis of the aluminum-tolerant tree species Eucalyptus camaldulensis. Planta 2021, 253, 1–18. [Google Scholar] [CrossRef]
- Osmani, S.A.; Bak, S.; Moller, B.L. Substrate specificity of plant UDP-dependent glycosyltransferases predicted from crystal structures and homology modeling. Phytochemistry 2009, 70, 325–347. [Google Scholar] [CrossRef]
- Lim, E.K.; Doucet, C.J.; Li, Y.; Elias, L.; Worrall, D.; Spencer, S.P.; Ross, J.; Bowles, D.J. The activity of Arabidopsis glycosyltransferases toward salicylic acid, 4-hydroxybenzoic acid, and other benzoates. J. Biol. Chem. 2002, 277, 586–592. [Google Scholar] [CrossRef]
- Thompson, A.M.G.; Iancu, C.V.; Neet, K.E.; Dean, J.V.; Choe, J.Y. Differences in salicylic acid glucose conjugations by UGT74F1 and UGT74F2 from Arabidopsis thaliana. Sci. Rep. 2017, 7, 46629. [Google Scholar] [CrossRef]
- Khater, F.; Fournand, D.; Vialet, S.; Meudec, E.; Cheynier, V.; Terrier, N. Identification and functional characterization of cDNAs coding for hydroxybenzoate/hydroxycinnamate glucosyltransferases co-expressed with genes related to proanthocyanidin biosynthesis. J. Exp. Bot. 2012, 63, 1201–1214. [Google Scholar] [CrossRef][Green Version]
- Mittasch, J.; Bottcher, C.; Frolova, N.; Bonn, M.; Milkowski, C. Identification of UGT84A13 as a candidate enzyme for the first committed step of gallotannin biosynthesis in pedunculate oak (Quercus robur). Phytochemistry 2014, 99, 44–51. [Google Scholar] [CrossRef]
- Tahara, K.; Nishiguchi, M.; Frolov, A.; Mittasch, J.; Milkowski, C. Identification of UDP glucosyltransferases from the aluminum-resistant tree Eucalyptus camaldulensis forming β-glucogallin, the precursor of hydrolyzable tannins. Phytochemistry 2018, 152, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.L.; Yao, S.B.; Dai, X.L.; Yin, Q.G.; Liu, Y.J.; Jiang, X.L.; Wu, Y.H.; Qian, Y.M.; Pang, Y.Z.; Gao, L.P.; et al. Identification of UDP-glycosyltransferases involved in the biosynthesis of astringent taste compounds in tea (Camellia sinensis). J. Exp. Bot. 2016, 67, 2285–2297. [Google Scholar] [CrossRef] [PubMed]
- Schulenburg, K.; Feller, A.; Hoffmann, T.; Schecker, J.H.; Martens, S.; Schwab, W. Formation of β-glucogallin, the precursor of ellagic acid in strawberry and raspberry. J. Exp. Bot. 2016, 67, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.N.; Qin, X.Q.; Wilson, A.E.; Li, G.; Tian, L. Two UGT84 family glycosyltransferases catalyze a critical reaction of hydrolyzable tannin biosynthesis in pomegranate (Punica granatum). PLoS ONE 2016, 11, e0156319. [Google Scholar] [CrossRef]
- Ono, N.N.; Bandaranayake, P.C.; Tian, L. Establishment of pomegranate (Punica granatum) hairy root cultures for genetic interrogation of the hydrolyzable tannin biosynthetic pathway. Planta 2012, 236, 931–941. [Google Scholar] [CrossRef]
- Mei, Z.; Zhang, X.; Liu, X.; Imani, S.; Fu, J. Genetic analysis of Canarium album in different areas of China by improved RAPD and ISSR. C. R. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- He, C.N. Canarium album (Lour.) Raeusch. (Qingguo, Chinese Olive). Diet. Chin. Herbs 2015, 307–313. [Google Scholar] [CrossRef]
- Raven, P.H.; Zhang, L.B.; Al-Shehbaz, I.A. Flora of China; Science Press & Missouri Botanical Garden Press, 2008. Available online: http://www.iplant.cn/foc (accessed on 1 September 2019).
- He, Z.Y.; Xia, W.S.; Chen, J. Isolation and structure elucidation of phenolic compounds in Chinese olive (Canarium album L.) fruit. Eur. Food Res. Technol. 2008, 226, 1191–1196. [Google Scholar] [CrossRef]
- Xiang, Z.B.; Chen, H.S.; Jin, Y.S.; Wang, G.L.; Xiang, L.X.; Chen, W. Phenolic constituents of Canarium album. Chem. Nat. Compd. 2010, 46, 119–120. [Google Scholar] [CrossRef]
- He, Z.Y.; Xia, W.S.; Liu, Q.H.; Chen, J. Identification of a new phenolic compound from Chinese olive (Canarium album L.) fruit. Eur. Food Res. Technol. 2009, 228, 339–343. [Google Scholar] [CrossRef]
- He, Z.Y.; Xia, W.S. Analysis of phenolic compounds in Chinese olive (Canarium album L.) fruit by RPHPLC-DAD-ESI-MS. Food Chem. 2007, 105, 1307–1311. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, N.; Ding, H.; Yao, R. Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res. Int. 2008, 41, 363–370. [Google Scholar] [CrossRef]
- Kuo, C.T.; Liu, T.H.; Hsu, T.H.; Lin, F.Y.; Chen, H.Y. Antioxidant and antiglycation properties of different solvent extracts from Chinese olive (Canarium album L.) fruit. Asian Pac. J. Trop. Med. 2015, 8, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.T.; Cho, Y.Y.; Hsieh, S.C.; Chiang, A.N. Chinese olive extract ameliorates hepatic lipid accumulation in vitro and in vivo by regulating lipid metabolism. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Yeh, Y.T.; Chiang, A.N.; Hsieh, S.C. Chinese olive (Canarium album L.) fruit extract attenuates metabolic dysfunction in diabetic Rats. Nutrients 2017, 9, 1123. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chu, Y.F.; Wu, X.Z.; Liu, R.H. Antioxidant and anti proliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
- He, F.; Chen, J.; Dong, K.; Leng, Y.; Xu, J.; Hu, P.; Yao, Y.; Xiong, J.; Pei, X. Multi-technical analysis on the antioxidative capacity and total phenol contents of 94 traditional Chinese dietary medicinal herbs. Food Sci. Nutr. 2018, 6, 1358–1369. [Google Scholar] [CrossRef]
- Chang, Q.; Su, M.; Chen, Q.; Zeng, B.; Li, H.; Wang, W. Physicochemical properties and antioxidant capacity of Chinese olive (Canarium album L.) cultivars. J. Food Sci. 2017, 82, 1369–1377. [Google Scholar] [CrossRef]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Vasco, C.; Riihinen, K.; Ruales, J.; Kamal-Eldin, A. Phenolic compounds in Rosaceae fruits from Ecuador. J. Agric. Food Chem. 2009, 57, 1204–1212. [Google Scholar] [CrossRef]
- Wilson, A.E.; Tian, L. Phylogenomic analysis of UDP-dependent glycosyltransferases provides insights into the evolutionary landscape of glycosylation in plant metabolism. Plant J. 2019, 100, 1273–1288. [Google Scholar] [CrossRef]
- Caputi, L.; Malnoy, M.; Goremykin, V.; Nikiforova, S.; Martens, S. A genome-wide phylogenetic reconstruction of family 1 UDP-glycosyltransferases revealed the expansion of the family during the adaptation of plants to life on land. Plant J. 2012, 69, 1030–1042. [Google Scholar] [CrossRef]
- Li, Y.; Baldauf, S.; Lim, E.K.; Bowles, D.J. Phylogenetic analysis of the UDP-glycosyltransferase multigene family of Arabidopsis thaliana. J. Biol. Chem. 2001, 276, 4338–4343. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, P.; Wang, Y.; Dong, R.; Yu, H.; Hou, B. Genome-wide identification and phylogenetic analysis of Family-1 UDP glycosyltransferases in maize (Zea mays). Planta 2014, 239, 1265–1279. [Google Scholar] [CrossRef]
- Cheng, X.; Muhammad, A.; Li, G.; Zhang, J.; Cheng, J.; Qiu, J.; Jiang, T.; Jin, Q.; Cai, Y.; Lin, Y. Family-1 UDP glycosyltransferases in pear (Pyrus bretschneideri): Molecular identification, phylogenomic characterization and expression profiling during stone cell formation. Mol. Biol. Rep. 2019, 46, 2153–2175. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, P.I.; Owens, I.S.; Burchell, B.; Bock, K.W.; Bairoch, A.; Belanger, A.; Fournel-Gigleux, S.; Green, M.; Hum, D.W.; Iyanagi, T.; et al. The UDP glycosyltransferase gene superfamily: Recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 1997, 7, 255–269. [Google Scholar] [CrossRef]
- Gachon, C.M.; Langlois-Meurinne, M.; Saindrenan, P. Plant secondary metabolism glycosyltransferases: The emerging functional analysis. Trends Plant Sci. 2005, 10, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Khorolragchaa, A.; Kim, Y.J.; Rahimi, S.; Sukweenadhi, J.; Jang, M.G.; Yang, D.C. Grouping and characterization of putative glycosyltransferase genes from Panax ginseng Meyer. Gene 2014, 536, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Bisswanger, H. Enzyme assays. Perspect. Sci. 2014, 1, 41–55. [Google Scholar] [CrossRef]
- Cleland, W.W. [6] Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979, 63, 103–138. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.J.; Tan, S.Y.; Chen, J.; Liu, S.W.; Jiang, S.B.; Xiang, H.Y.; Xie, Y. Isolation of anti-HIV components from Canarium album fruits by high-speed counter-current chromatography. Anal. Lett. 2013, 46, 1057–1068. [Google Scholar] [CrossRef]
- Hsieh, S.C.; Hsieh, W.J.; Chiang, A.N.; Su, N.W.; Yeh, Y.T.; Liao, Y.C. The methanol-ethyl acetate partitioned fraction from Chinese olive fruits inhibits cancer cell proliferation and tumor growth by promoting apoptosis through the suppression of the NF-κB signaling pathway. Food Funct. 2016, 7, 4797–4803. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects-A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plant Sci. 2016, 7, 735. [Google Scholar] [CrossRef]
- Cherepanova, N.; Shrimal, S.; Gilmore, R. N-linked glycosylation and homeostasis of the endoplasmic reticulum. Curr. Opin. Cell Biol. 2016, 41, 57–65. [Google Scholar] [CrossRef]
- Mrudulakumari Vasudevan, U.; Lee, E.Y. Flavonoids, terpenoids, and polyketide antibiotics: Role of glycosylation and biocatalytic tactics in engineering glycosylation. Biotechnol. Adv. 2020, 41, 107550. [Google Scholar] [CrossRef]
- Louveau, T.; Osbourn, A. The sweet side of plant-specialized metabolism. Cold Spring Harbor Perspect. Biol. 2019, 11, a034744. [Google Scholar] [CrossRef]
- Tiwari, P.; Sangwan, R.S.; Sangwan, N.S. Plant secondary metabolism linked glycosyltransferases: An update on expanding knowledge and scopes. Biotechnol. Adv. 2016, 34, 714–739. [Google Scholar] [CrossRef]
- Wu, B.P.; Gao, L.X.; Gao, J.; Xu, Y.Y.; Liu, H.R.; Cao, X.M.; Zhang, B.; Chen, K.S. Genome-wide identification, expression patterns, and functional analysis of UDP glycosyltransferase family in peach (Prunus persica L. Batsch). Front. Plant Sci. 2017, 8, 389. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhuo, X.K.; Yan, X.L.; Zhang, Q.X. Comparative genomic and transcriptomic analyses of family-1 UDP glycosyltransferase in Prunus mume. Int. J. Mol. Sci. 2018, 19, 3382. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.M.; Nawaz, M.A.; Shah, Z.H.; Ludwig-Muller, J.; Chung, G.; Ahmad, M.Q.; Yang, S.H.; Lee, S.I. Comparative genomic and transcriptomic analyses of family-1 UDP glycosyltransferase in three Brassica species and Arabidopsis indicates stress-responsive regulation. Sci. Rep. 2018, 8, 1875. [Google Scholar] [CrossRef] [PubMed]
- Paquette, S.; Møller, B.L.; Bak, S. On the origin of family 1 plant glycosyltransferases. Phytochemistry 2003, 62, 399–413. [Google Scholar] [CrossRef]
- Singh, R.; Rastogi, S.; Dwivedi, U.N. Phenylpropanoid metabolism in ripening fruits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 398–416. [Google Scholar] [CrossRef]
- Brazier-Hicks, M.; Gershater, M.; Dixon, D.; Edwards, R. Substrate specificity and safener inducibility of the plant UDP-glucose-dependent family 1 glycosyltransferase super-family. Plant Biotechnol. J. 2018, 16, 337–348. [Google Scholar] [CrossRef]
- Wilson, A.E.; Feng, X.X.; Ono, N.N.; Holland, D.; Amir, R.; Tian, L. Characterization of a UGT84 family glycosyltransferase provides new insights into substrate binding and reactivity of galloylglucose ester-forming UGTs. Biochemistry 2017, 56, 6389–6400. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Pang, C.; Fan, S.; Song, M.; Yu, J.; Wei, H.; Ma, Q.; Li, L.; Zhang, C.; Yu, S. Genome-wide analysis of the family 1 glycosyltransferases in cotton. Mol. Genet. Genom. 2015, 290, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- Mashima, K.; Hatano, M.; Suzuki, H.; Shimosaka, M.; Taguchi, G. Identification and Characterization of Apigenin 6-C-Glucosyltransferase Involved in Biosynthesis of Isosaponarin in Wasabi (Eutrema japonicum). Plant Cell Physiol. 2019, 60, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Han, X.; Chen, J.; Han, Z.; Shen, L.; Sun, W.; Chen, S. Identification of specific glycosyltransferases involved in flavonol glucoside biosynthesis in Ginseng using integrative metabolite profiles, DIA proteomics, and phylogenetic analysis. J. Agric. Food Chem. 2021, 69, 1714–1726. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, M.; Zhang, W.; Cao, Y.; Li, C.; Wang, W. Identification and characterization of two new 1-O-Acyl-glucose-ester forming glucosyltransferases from Erigeron breviscapus. J. Agric. Food Chem. 2019, 67, 2848–2855. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Sun, F.; Suttapitugsakul, S.; Wu, R. Global and site-specific analysis of protein glycosylation in complex biological systems with mass spectrometry. Mass Spectrom. Rev. 2019, 38, 356–379. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Analysis of hydrolysable tannins. Anim. Feed Sci. Technol. 2001, 91, 3–20. [Google Scholar] [CrossRef]
- Saha, S.; Imran, I.B. Isolation, detection, and quantification of hydrolyzable tannins of the biosynthetic pathway by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2021, 35, e9005. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Leo, D.J.N.A.R. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Liu, S.; Ma, J.; Liu, H.; Guo, Y.; Li, W.; Niu, S. An efficient system for Agrobacterium-mediated transient transformation in Pinus tabuliformis. Plant Methods 2020, 16, 52. [Google Scholar] [CrossRef]
- Chang, L.J.; Wu, S.; Tian, L. Effective genome editing and identification of a regiospecific gallic acid 4-O-glycosyltransferase in pomegranate (Punica granatum L.). Hortic. Res. 2019, 6, 123. [Google Scholar] [CrossRef]


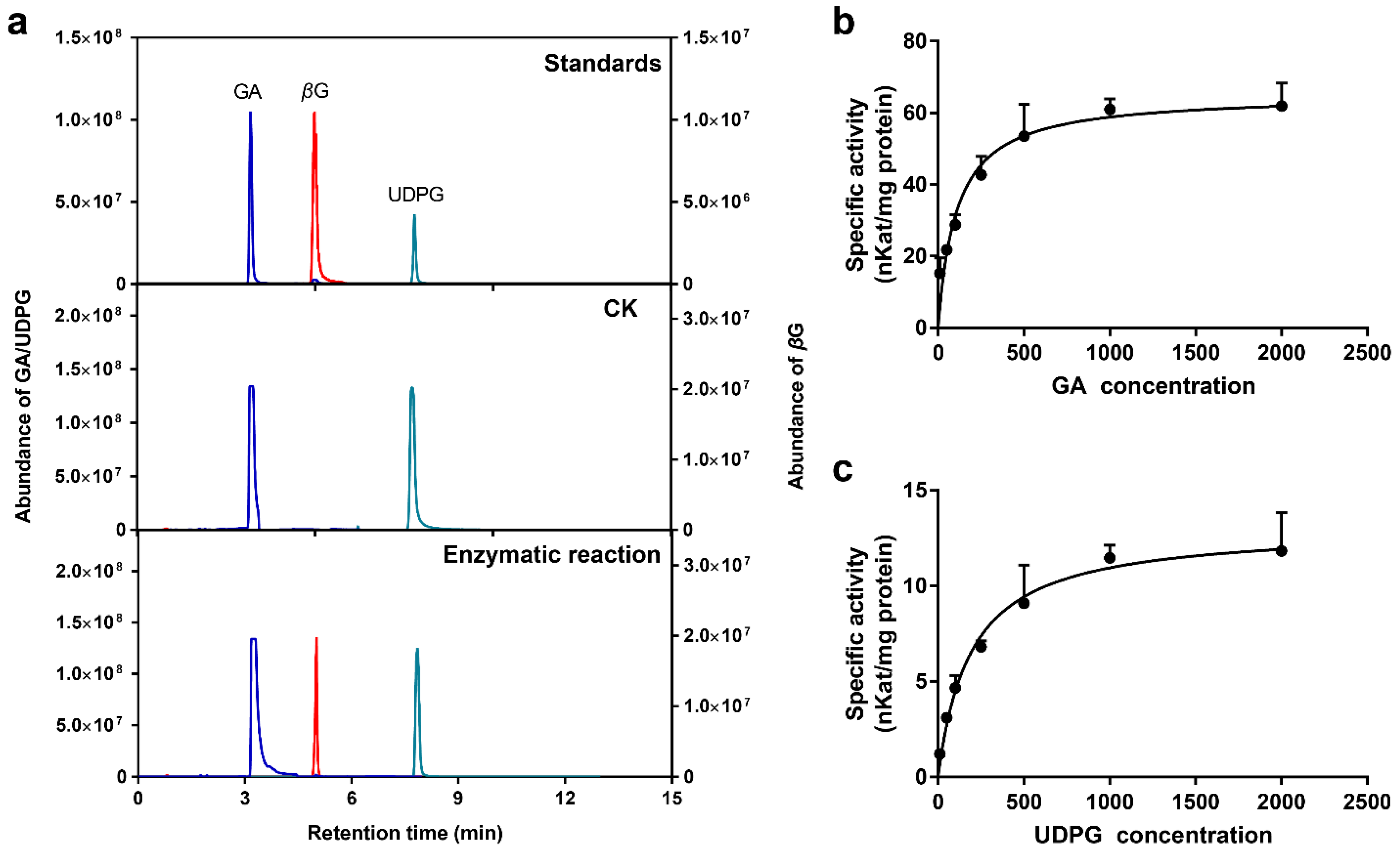
| Substrate | Vmax (nKat/mg) | Km (μM) | Kcat (s−1) | Kcat/Km (s−1·M−1) |
|---|---|---|---|---|
| GA a | 65.21 ± 3.22 | 108.90 ± 21.06 | 3.71 ± 0.18 | 34,076.64 |
| UDPG b | 13.06 ± 0.66 | 193.30 ± 34.33 | 0.74 ± 0.04 | 3844.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Q.; Zhang, S.; Qiu, N.; Liu, L.; Wang, W.; Xie, Q.; Chang, Q.; Chen, Q. Identification and Characterization of Glucosyltransferase That Forms 1-Galloyl-β-d-Glucogallin in Canarium album L., a Functional Fruit Rich in Hydrolysable Tannins. Molecules 2021, 26, 4650. https://doi.org/10.3390/molecules26154650
Ye Q, Zhang S, Qiu N, Liu L, Wang W, Xie Q, Chang Q, Chen Q. Identification and Characterization of Glucosyltransferase That Forms 1-Galloyl-β-d-Glucogallin in Canarium album L., a Functional Fruit Rich in Hydrolysable Tannins. Molecules. 2021; 26(15):4650. https://doi.org/10.3390/molecules26154650
Chicago/Turabian StyleYe, Qinghua, Shiyan Zhang, Nana Qiu, Linmin Liu, Wei Wang, Qian Xie, Qiang Chang, and Qingxi Chen. 2021. "Identification and Characterization of Glucosyltransferase That Forms 1-Galloyl-β-d-Glucogallin in Canarium album L., a Functional Fruit Rich in Hydrolysable Tannins" Molecules 26, no. 15: 4650. https://doi.org/10.3390/molecules26154650
APA StyleYe, Q., Zhang, S., Qiu, N., Liu, L., Wang, W., Xie, Q., Chang, Q., & Chen, Q. (2021). Identification and Characterization of Glucosyltransferase That Forms 1-Galloyl-β-d-Glucogallin in Canarium album L., a Functional Fruit Rich in Hydrolysable Tannins. Molecules, 26(15), 4650. https://doi.org/10.3390/molecules26154650






