Taxonomic Insights and Its Type Cyclization Correlation of Volatile Sesquiterpenes in Vitex Species and Potential Source Insecticidal Compounds: A Review
Abstract
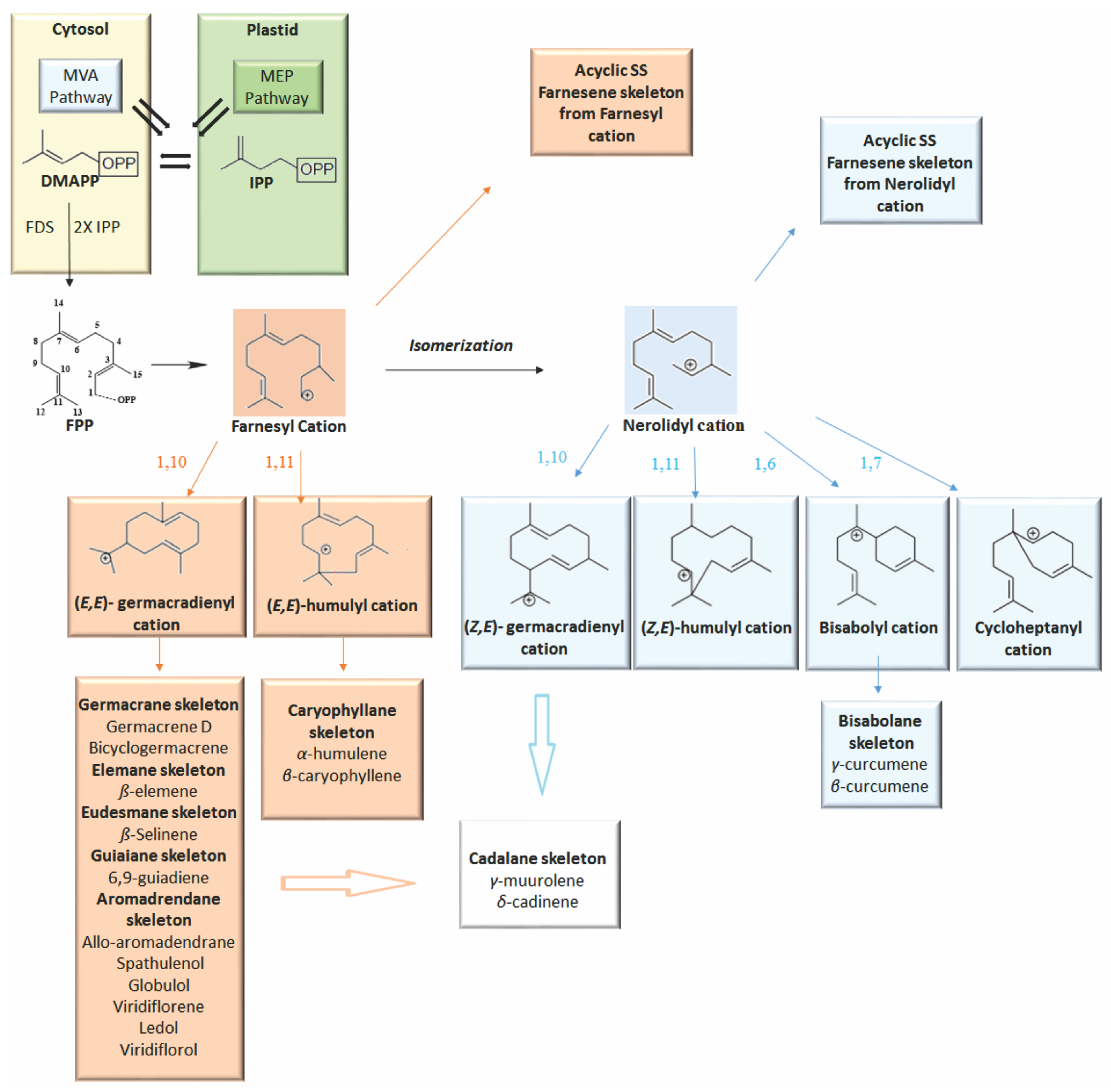
1. Introduction
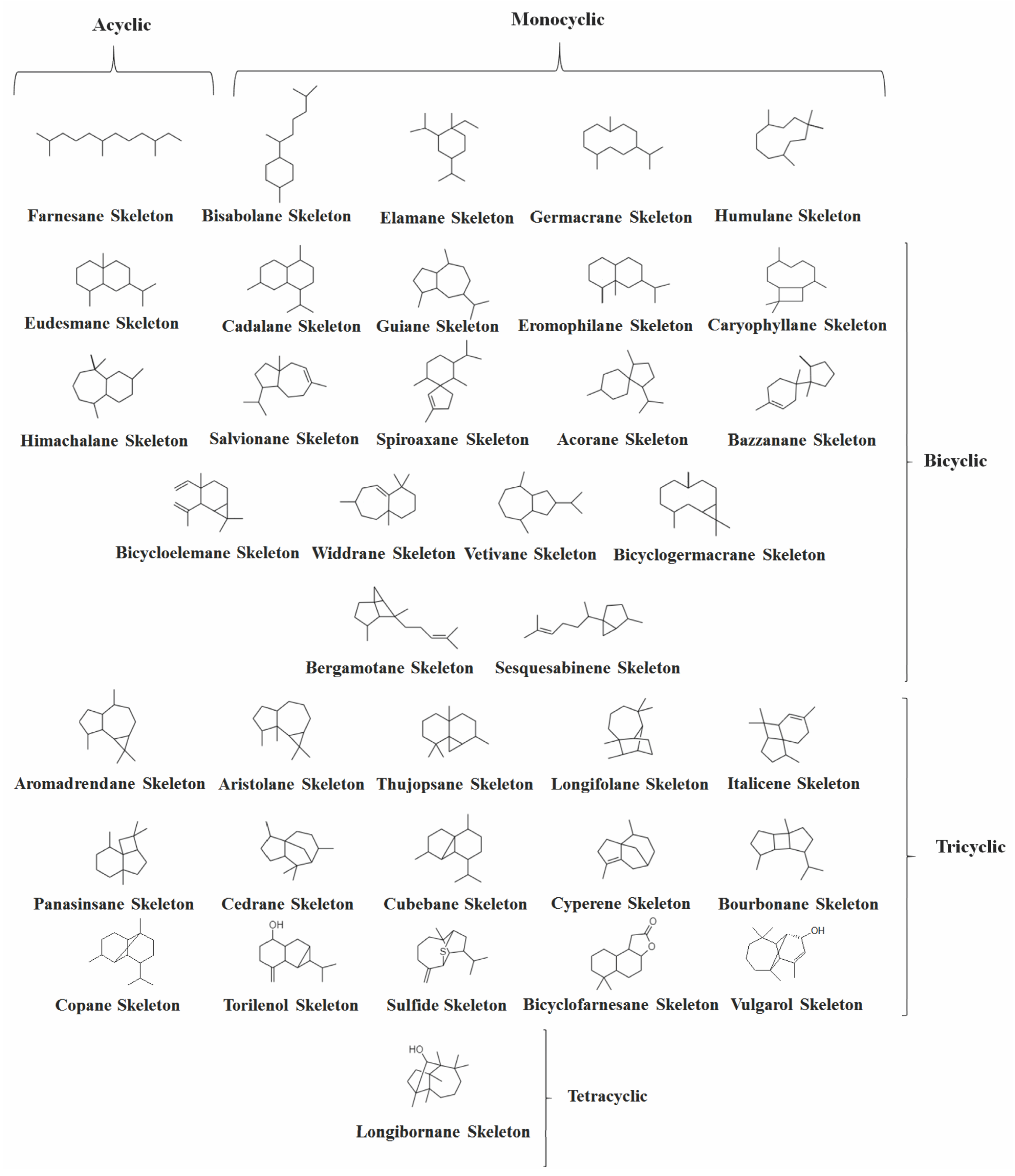
2. Volatile Sesquiterpenes in Vitex Genus
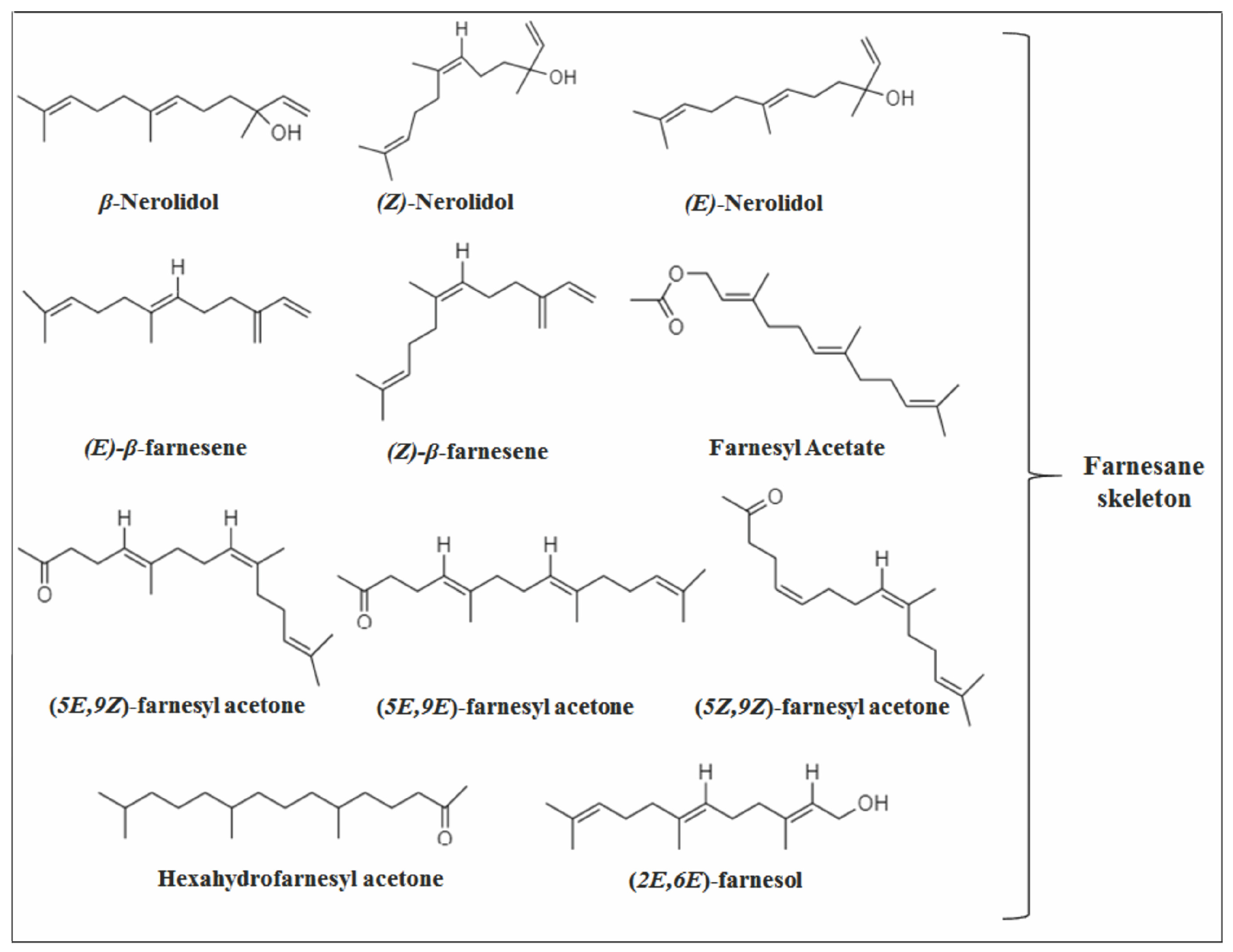
2.1. Acyclic Sesquiterpenes
2.2. Monocyclic Sesquiterpenes
2.2.1. Humulane Skeleton
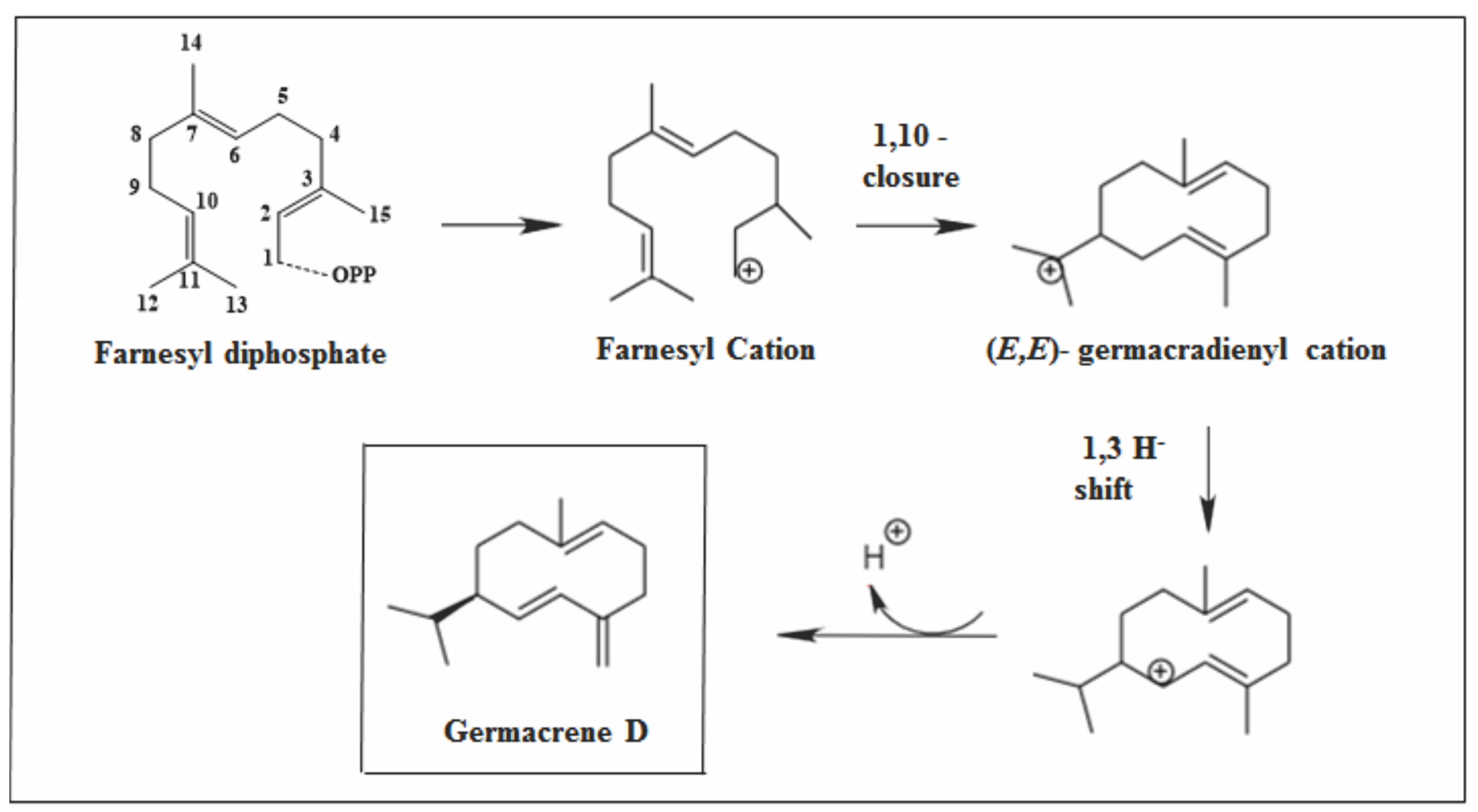
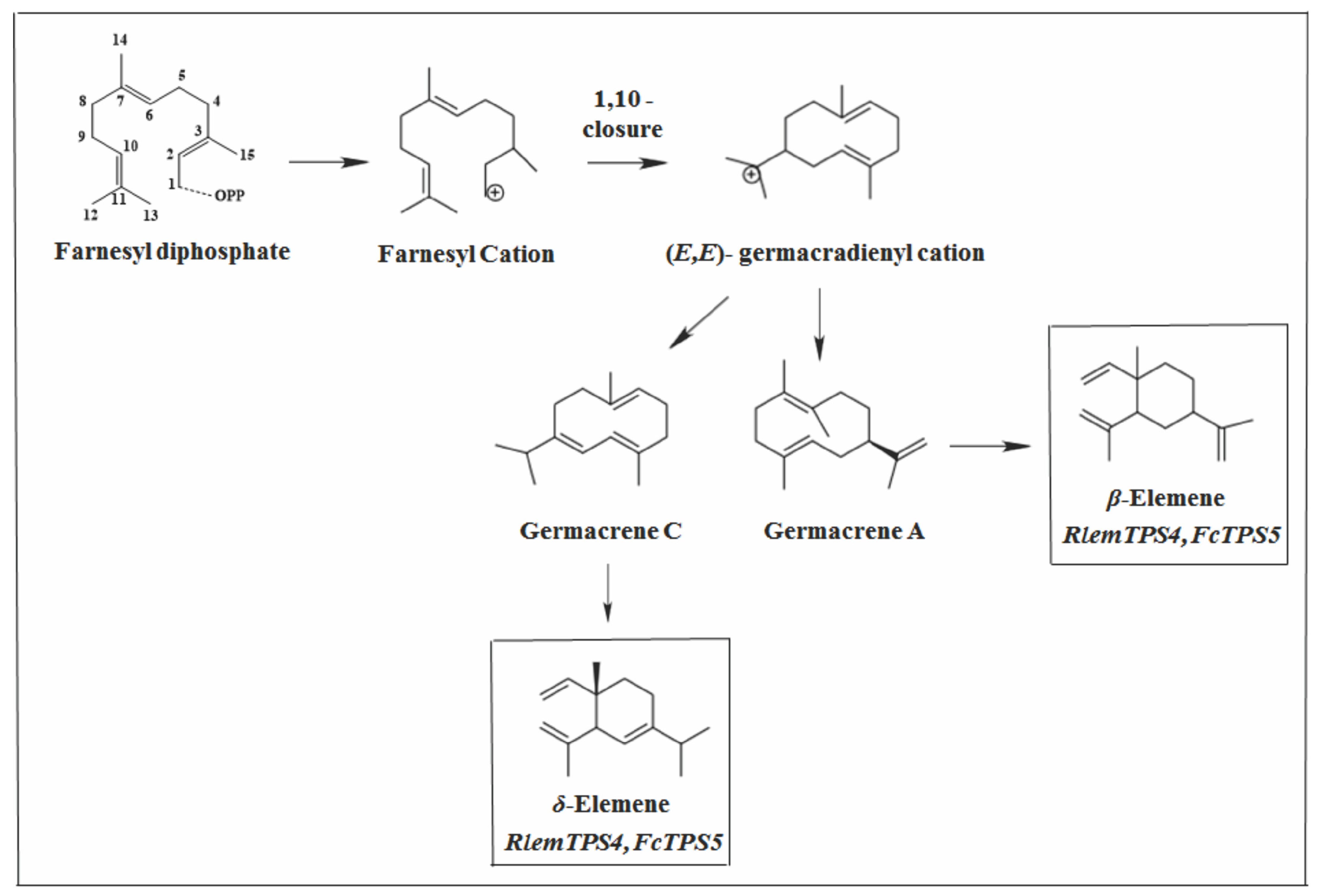
2.2.2. Germacrane Skeleton
2.2.3. Bisabolane Skeleton
2.2.4. Elemane Skeleton
2.3. Bicyclic Sesquiterpenes
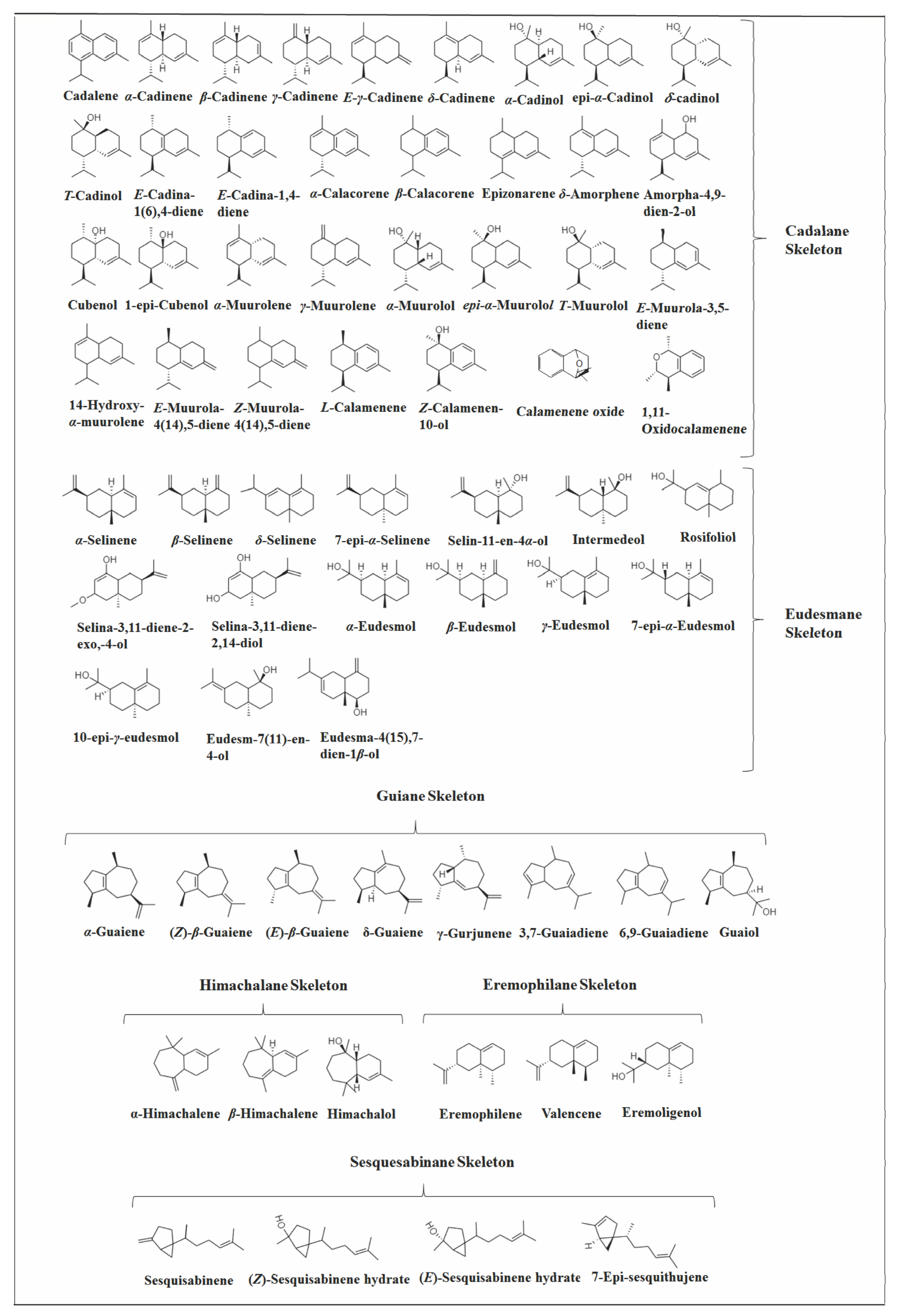
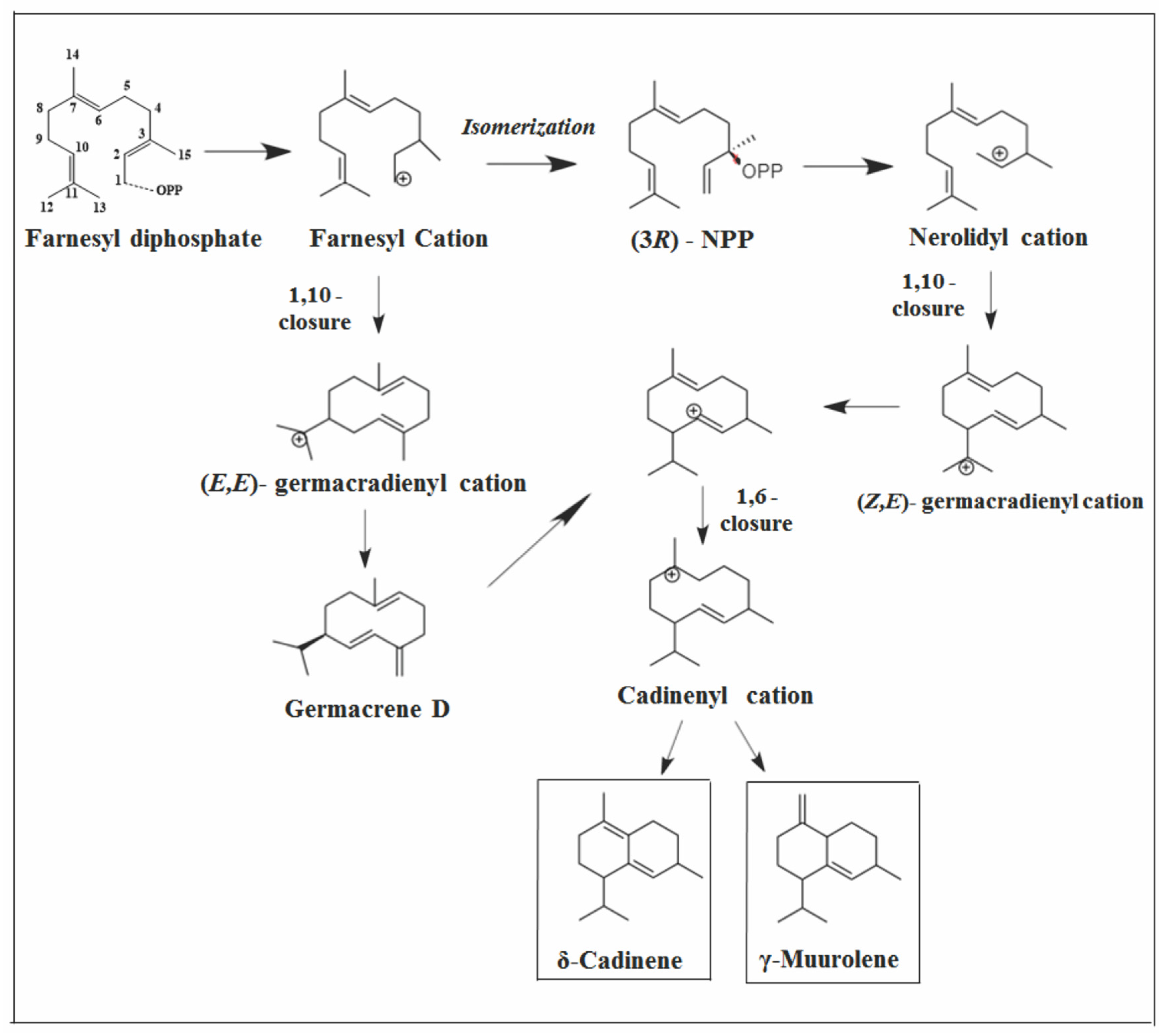
2.3.1. Cadalane Skeleton
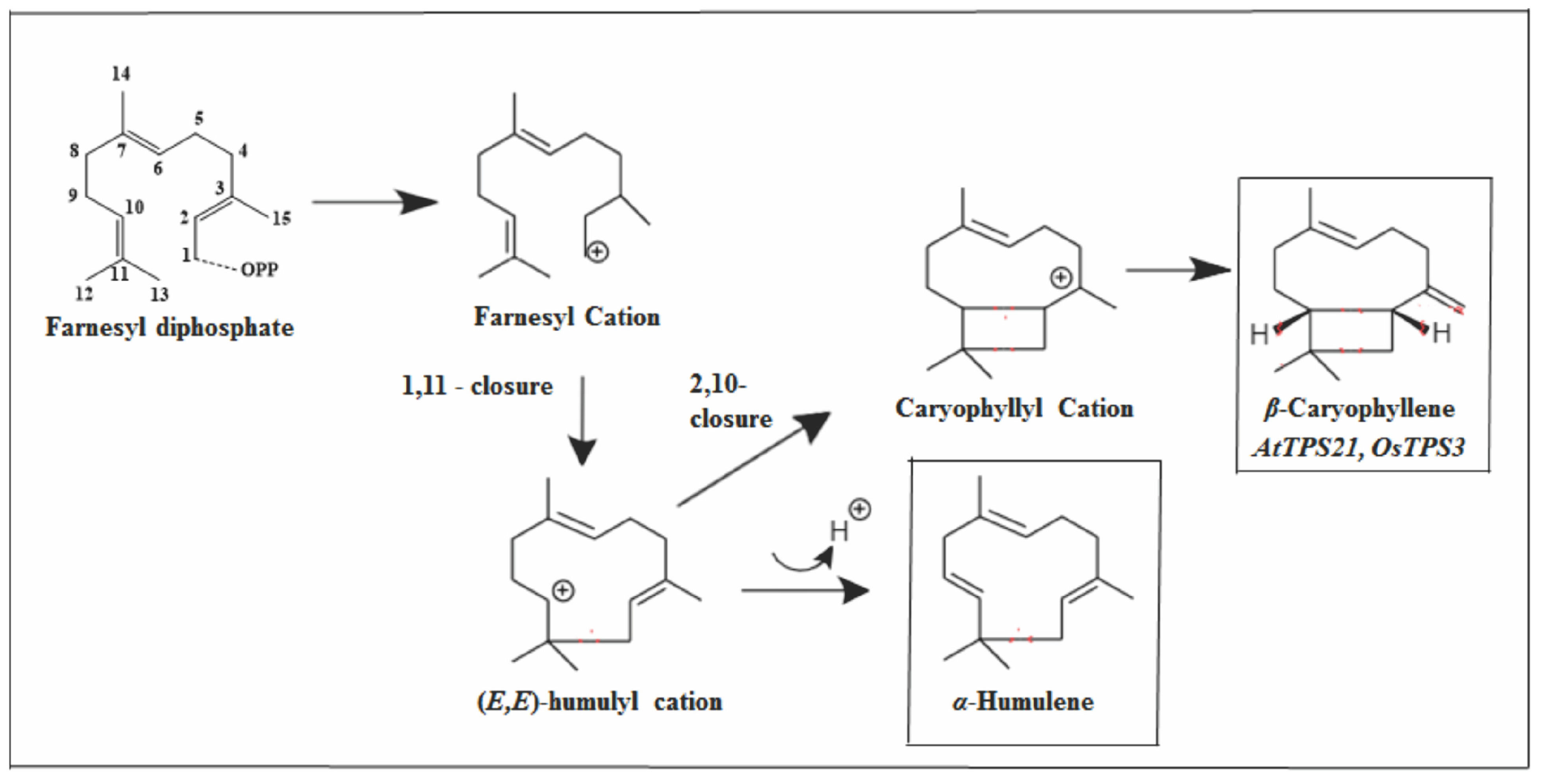
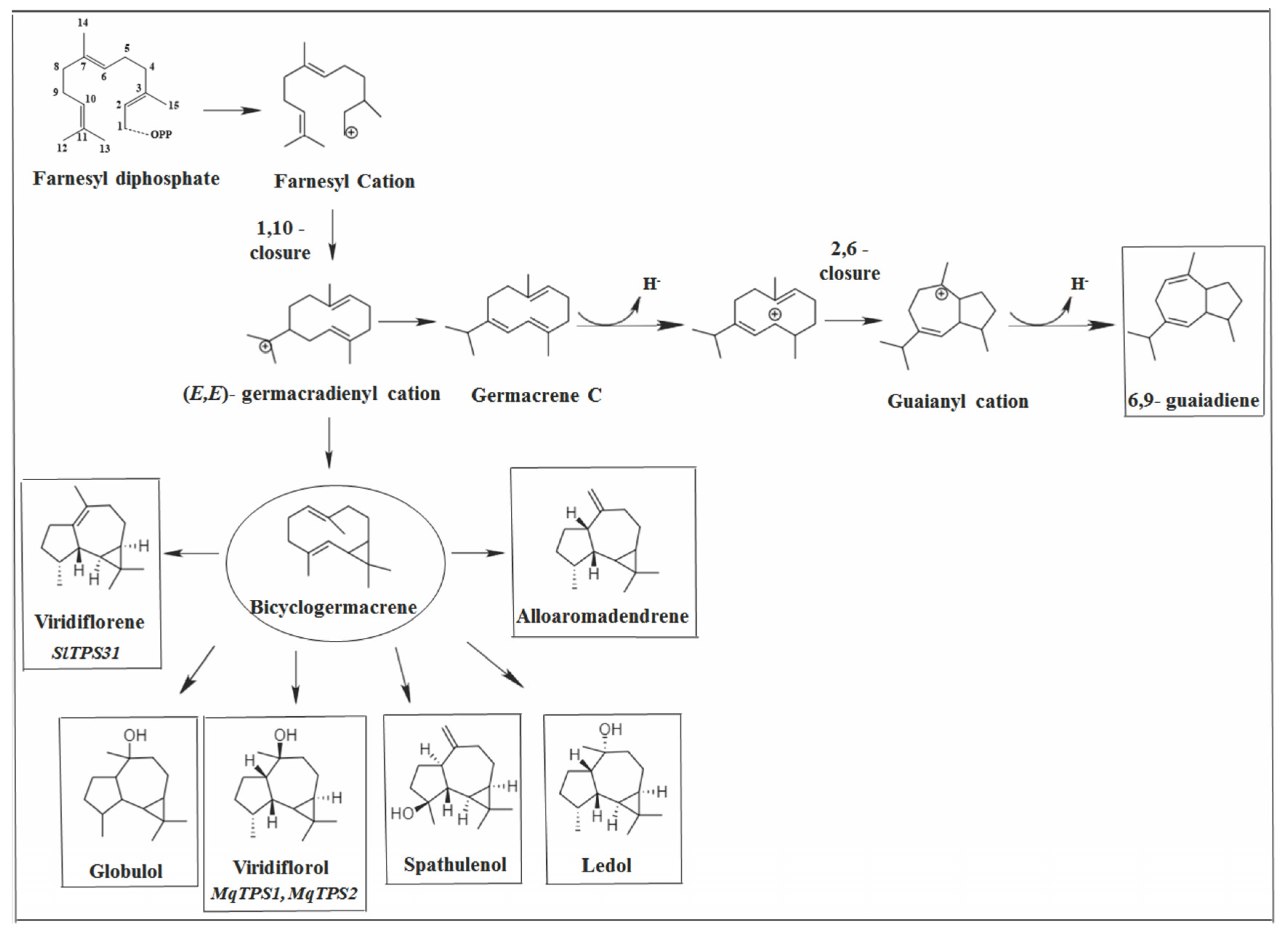
2.3.2. Caryophyllane Skeleton
2.3.3. Eudesmane Skeleton
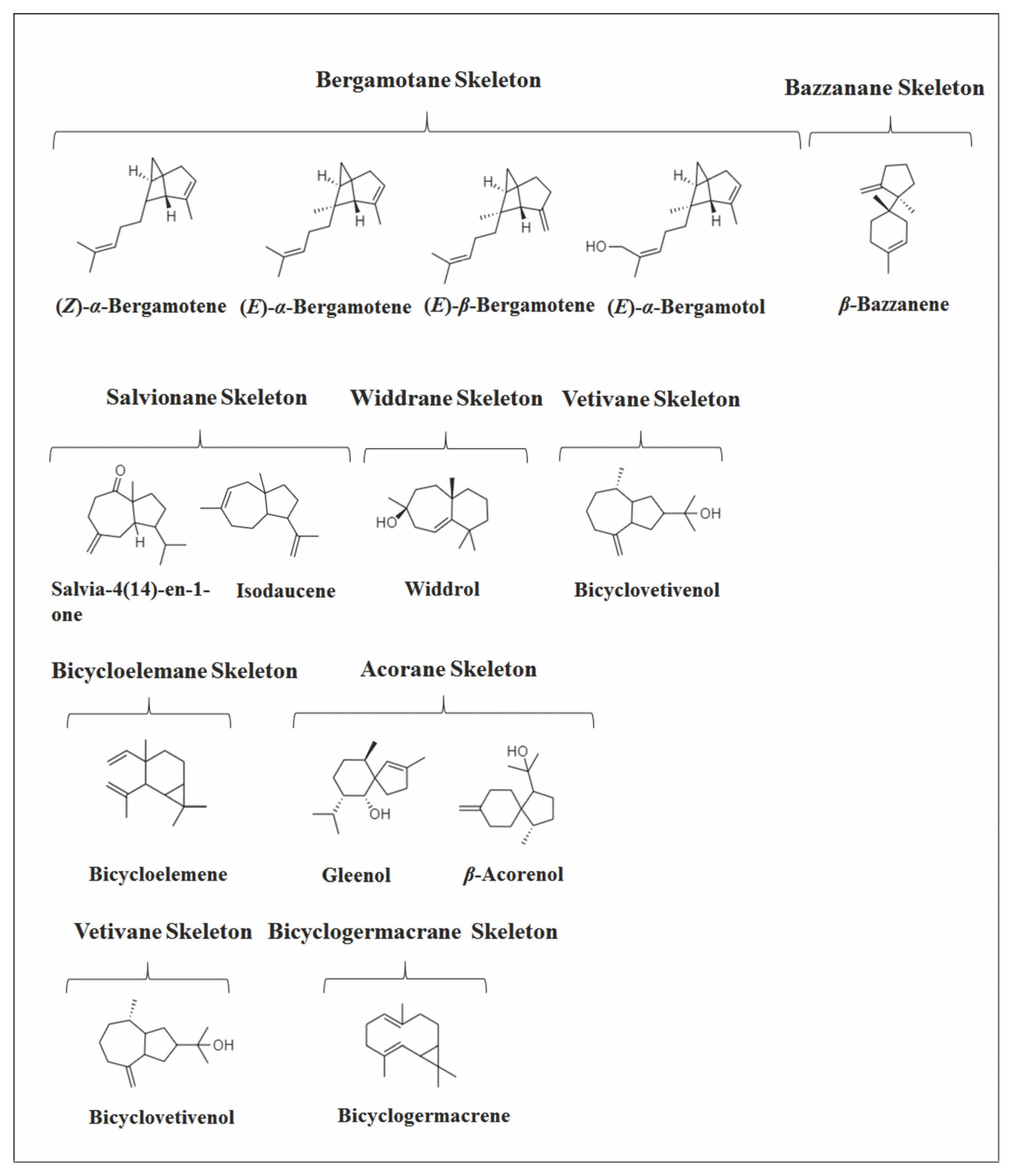
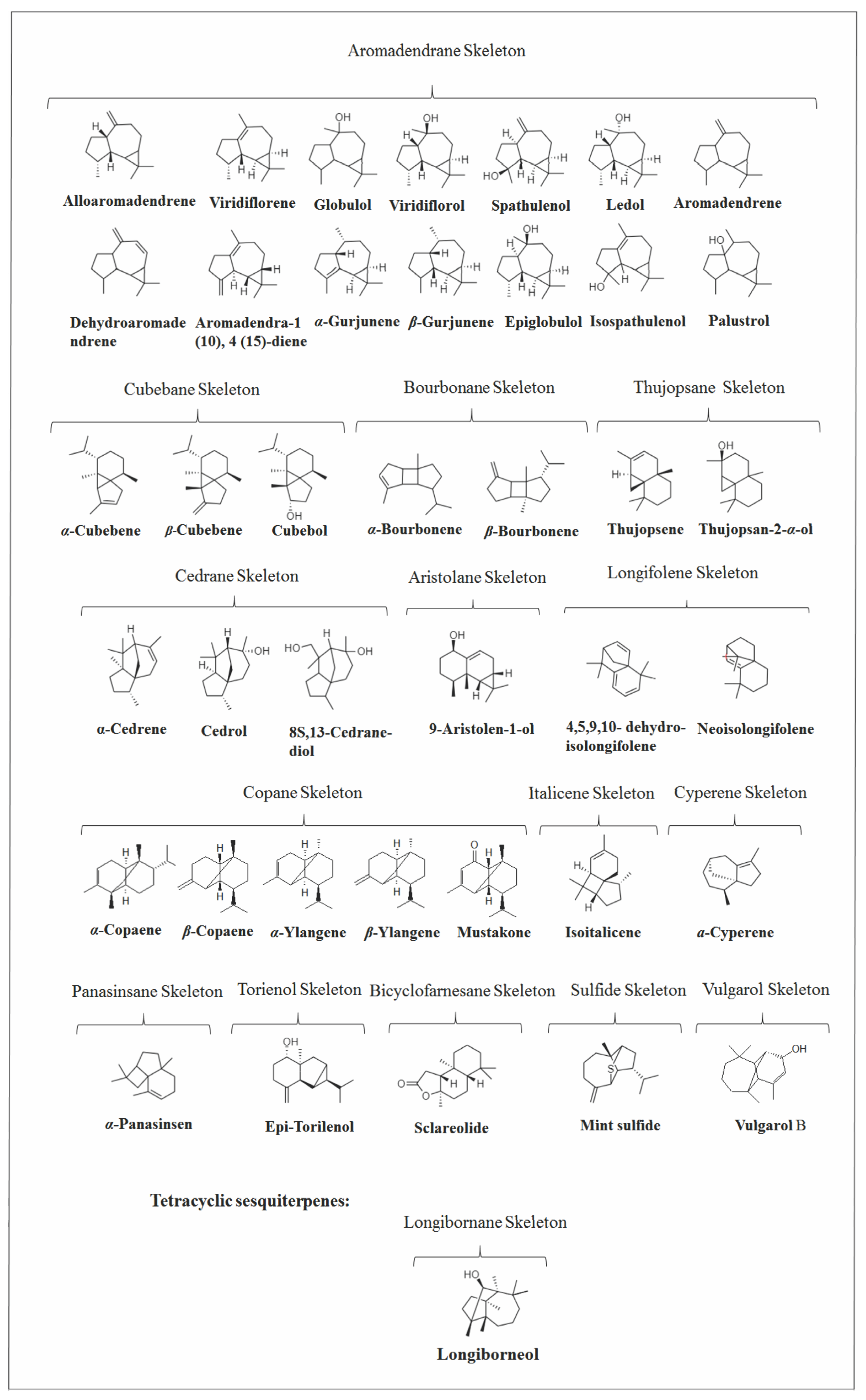
2.4. Other Bicyclic Sesquiterpenes
2.5. Tricyclic and Tetracyclic Sesquiterpenes
3. Insecticide and Response Activity of Sesquiterpenes Identified in Vitex Species
3.1. Acyclic Sesquiterpenes
3.2. Monocyclic Sesquiterpenes
3.3. Bicyclic Sesquiterpenes
3.4. Tricyclic Sesquiterpenes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christianson, D.W. Unearthing the roots of the terpenome. Curr. Opin. Chem. Biol. 2008, 12, 141–150. [Google Scholar] [CrossRef]
- Lange, B.M.; Rujan, T.; Martin, W.; Croteau, R. Isoprenoid biosynthesis: The evolution of two ancient and distinct pathways across genomes. Proc. Natl. Acad. Sci. USA 2000, 97, 13172–13177. [Google Scholar] [CrossRef] [PubMed]
- Sapir-Mir, M.; Mett, A.; Belausov, E.; Tal-Meshulam, S.; Frydman, A.; Gidoni, D.; Eya, Y. Peroxisomal localization of arabidopsis isopentenyl diphosphate isomerases suggests that part of the plant isoprenoid mevalonic acid pathway is compartmentalized to peroxisomes. Plant Physiol. 2008, 148, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Hamberger, B. P450s controlling metabolic bifurcations in plant terpene specialized metabolism. Phytochem. Rev. 2018, 17, 81–111. [Google Scholar] [CrossRef]
- Nelson, D.; Werck-Reichhart, D. A P450-centric view of plant evolution. Plant J. 2011, 66, 194–211. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Hare, S.R.; Tantillo, D.J. Dynamic behavior of rearranging carbocations—Implications for terpene biosynthesis. Beilstein J. Org. Chem. 2016, 12, 377–390. [Google Scholar] [CrossRef]
- Harms, V.; Schröder, B.; Oberhauser, C.; Tran, C.D.; Winkler, S.; Dräger, G.; Kirschning, A. Methyl-Shifted Farnesyldiphosphate Derivatives Are Substrates for Sesquiterpene Cyclases. Org. Lett. 2020, 22, 4360–4365. [Google Scholar] [CrossRef]
- Cane, D.E. Enzymatic Formation of Sesquiterpenes. Chem. Rev. 1990, 90, 1089–1103. [Google Scholar] [CrossRef]
- Durairaj, J.; Di Girolamo, A.; Bouwmeester, H.J.; de Ridder, D.; Beekwilder, J.; van Dijk, A.D.J. An Analysis of Characterized Plant Sesquiterpene Synthases. Phytochemistry 2018, 158, 157–165. [Google Scholar] [CrossRef]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef]
- Zhang, C.; Li, M.; Zhao, G.R.; Lu, W. Harnessing Yeast Peroxisomes and Cytosol Acetyl-CoA for Sesquiterpene α-Humulene Production. J. Agric. Food Chem. 2020, 68, 1382–1389. [Google Scholar] [CrossRef]
- Morse, A.; Kevan, P.; Shipp, L.; Khosla, S.; McGarvey, B. The impact of greenhouse tomato (Solanales: Solanaceae) floral volatiles on bumble bee (Hymenoptera: Apidae) pollination. Environ. Entomol. 2012, 41, 855–864. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, Y.; Köllner, T.G.; Zhang, W.; Wu, J.; Wu, J.; Guo, Y.; Zhang, Y. Identification and characterization of (E)-β-caryophyllene synthase and α/β-pinene synthase potentially involved in constitutive and herbivore-induced terpene formation in cotton. Plant Physiol. Biochem. 2013, 73, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.X.; Xiang, C.Y.; Li, J.X.; Yang, C.Q.; Hu, W.L.; Wang, L.J.; Lou, Y.G.; Chen, X.Y. The rice (E)-β-caryophyllene synthase (OsTPS3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry 2007, 68, 1632–1641. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef]
- Schnee, C.; Köllner, T.G.; Held, M.; Turlings, T.C.J.; Gershenzon, J.; Degenhardt, J. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc. Natl. Acad. Sci. USA 2006, 103, 1129–1134. [Google Scholar] [CrossRef]
- Pazouki, L.; Memari, H.R.; Kännaste, A.; Bichele, R.; Niinemets, Ü. Germacrene A synthase in yarrow (Achillea millefolium) is an enzyme with mixed substrate specificity: Gene cloning, functional characterization and expression analysis. Front. Plant Sci. 2015, 6, 111. [Google Scholar] [CrossRef]
- Azizul, N.H.; Ahmad, W.A.N.W.; Rosli, N.L.; Azmi, M.A.H.M.; Liang, C.E.; Mazlan, N.W.; Assaw, S. The coastal medicinal plant Vitex rotundifolia: A mini-review on its bioactive compounds and pharmacological activity. Tradit. Med. Res. 2021, 6, 11. [Google Scholar] [CrossRef]
- Pereira, E.J.P.; Silva, H.C.; Holanda, C.L.; de Menezes, J.E.S.A.; Siqueira, S.M.C.; Rodrigues, T.H.S.; Fontenelle, R.O.S.; do Vale, J.P.C.; da Silva, P.T.; Santiago, G.M.P.; et al. Chemical composition, cytotoxicity and larvicidal activity against Aedes aegypti of essential oils from Vitex gardineriana Schauer. Bol. Latinoam. Caribe Plantas Med. Aromat. 2018, 17, 302–309. [Google Scholar]
- Jokić, S.; Jerković, I.; Rajić, M.; Aladić, K.; Bilić, M.; Vidović, S. SC-CO2 extraction of Vitex agnus-castus L. fruits: The influence of pressure, temperature and water presoaking on the yield and GC–MS profiles of the extracts in comparison to the essential oil composition. J. Supercrit. Fluids 2017, 123, 50–57. [Google Scholar] [CrossRef]
- De Sena Filho, J.G.; Barreto, I.C.; Soares Filho, A.O.; Nogueira, P.C.L.; Teodoro, A.V.; Cruz Da Silva, A.V.; Xavier, H.S.; Rabbani, A.R.C.; Spakowicz, D.J.; Duringer, J.M. Volatile metabolomic composition of vitex species: Chemodiversity insights and acaricidal activity. Front. Plant Sci. 2017, 8, 1931. [Google Scholar] [CrossRef] [PubMed]
- Neves, R.C.S.; Da Camara, C.A.G. Chemical composition and acaricidal activity of the essential oils from Vitex agnus-castus L. (Verbenaceae) and selected monoterpenes. An. Acad. Bras. Cienc. 2016, 88, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Chang, T.Y.; Chang, L.Z.; Wang, H.F.; Yih, K.H.; Hsieh, W.Y.; Chang, T.M. Inhibition of melanogenesis Versus antioxidant properties of essential oil extracted from leaves of Vitex negundo linn and chemical composition analysis by GC-MS. Molecules 2012, 17, 3902–3916. [Google Scholar] [CrossRef]
- Sonibare, O.O.; Effiong, I.; Oladosu, I.A.; Ekundayo, O. Chemical constituents and antimicrobial activity of the essential oil of vitex doniana sweet (verbernaceae). J. Essent. Oil-Bear. Plants 2009, 12, 185–188. [Google Scholar] [CrossRef]
- Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Sales, F.; Boyom, F.; Salgueiro, L. Composition and anti-fungal activity of the essential oil from cameroonian Vitex rivularis gurke. Nat. Prod. Res. 2009, 23, 1478–1484. [Google Scholar] [CrossRef]
- Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L.; Antunes, T.; Sevinate-Pinto, I.; Sales, F. Vitex ferruginea schumach. Et. Thonn. Subsp. Amboniensis (gürke) verdc: Glandular trichomes micromorphology, composition and antifungal activity of the essential oils. J. Essent. Oil Res. 2008, 20, 86–90. [Google Scholar] [CrossRef]
- Yilar, M.; Bayan, Y.; Onaran, A. Chemical composition and antifungal effects of Vitex agnus-castus L. and Myrtus communis L. plants. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 466–471. [Google Scholar] [CrossRef]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Chanotiya, C.S.; Thul, S. Phytochemical diversity in essential oil of Vitex negundo L. populations from India. Rec. Nat. Prod. 2016, 10, 452–464. [Google Scholar]
- Nyiligira, E.; Viljoen, A.M.; Başer, K.H.C.; Ózek, T.; Van Vuuren, S.F. Essential oil composition and in vitro antimicrobial and anti-inflammatory activity of South African Vitex species. S. Afr. J. Bot. 2004, 70, 611–617. [Google Scholar] [CrossRef]
- Hadj Mohammadi, M.R.; Afif, A.A.; Rezaee, M.B. Chemical composition of leaf, flower and fruit oil of Vitex pseudo-negundo (hausskn.) hand.-mzt. fro Iran. J. Essent. Oil Res. 2006, 18, 308–309. [Google Scholar] [CrossRef]
- Nagegowda, D.A. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef]
- Bohlmann, J.; Keeling, C.I. Terpenoid biomaterials. Plant J. 2008, 54, 656–669. [Google Scholar] [CrossRef]
- Block, A.K.; Vaughan, M.M.; Schmelz, E.A.; Christensen, S.A. Biosynthesis and function of terpenoid defense compounds in maize (Zea mays). Planta 2019, 249, 21–30. [Google Scholar] [CrossRef]
- Boutanaev, A.M.; Moses, T.; Zi, J.; Nelson, D.R.; Mugford, S.T.; Peters, R.J.; Osbourn, A. Investigation of terpene diversification across multiple sequenced plant genomes. Proc. Natl. Acad. Sci. USA 2015, 112, E81–E88. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, P.M.; Mirabella, R.; Diergaarde, P.J.; VanDoorn, A.; Tissier, A.; Kant, M.R.; Prins, M.; De Vos, M.; Haring, M.A.; Schuurink, R.C. Improved herbivore resistance in cultivated tomato with the sesquiterpene biosynthetic pathway from a wild relative. Proc. Natl. Acad. Sci. USA 2012, 109, 20124–20129. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, Y.; Heinstein, P.; Davisson, V.J. Cloning, expression, and characterization of (+)-δ-cadinene synthase: A catalyst for cotton phytoalexin biosynthesis. Arch. Biochem. Biophys. 1995, 324, 255–266. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, S.H.; Hua, J.; Li, D.S.; Ling, Y.; Luo, Q.; Li, S.H. Characterization of defensive cadinenes and a novel sesquiterpene synthase responsible for their biosynthesis from the invasive Eupatorium adenophorum. New Phytol. 2021, 229, 1740–1754. [Google Scholar] [CrossRef]
- Muchlinski, A.; Chen, X.; Lovell, J.T.; Köllner, T.G.; Pelot, K.A.; Zerbe, P.; Ruggiero, M.; Callaway, L.M.; Laliberte, S.; Chen, F.; et al. Biosynthesis and Emission of Stress-Induced Volatile Terpenes in Roots and Leaves of Switchgrass (Panicum virgatum L.). Front. Plant Sci. 2019, 10, 1144. [Google Scholar] [CrossRef] [PubMed]
- Kiryu, M.; Hamanaka, M.; Yoshitomi, K.; Mochizuki, S.; Akimitsu, K.; Gomi, K. Rice terpene synthase 18 (OsTPS18) encodes a sesquiterpene synthase that produces an antibacterial (E)-nerolidol against a bacterial pathogen of rice. J. Gen. Plant Pathol. 2018, 84, 221–229. [Google Scholar] [CrossRef]
- Keasling, J.D. Synthetic biology and the development of tools for metabolic engineering. Metab. Eng. 2012, 14, 189–195. [Google Scholar] [CrossRef]
- Da Costa, F.B.; Terfloth, L.; Gasteiger, J. Sesquiterpene lactone-based classification of three Asteraceae tribes: A study based on self-organizing neural networks applied to chemosystematics. Phytochemistry 2005, 66, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Cincotta, F.; Verzera, A.; Tripodi, G.; Condurso, C. Determination of Sesquiterpenes in Wines by HS-SPME Coupled with GC-MS. Chromatography 2015, 2, 410–421. [Google Scholar] [CrossRef]
- Li, Z.; Howell, K.; Fang, Z.; Zhang, P. Sesquiterpenes in grapes and wines: Occurrence, biosynthesis, functionality, and influence of winemaking processes. Compr. Rev. Food Sci. Food Saf. 2020, 19, 247–281. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Çiftçi, G.A.; Yildirim, Ş.U.; Demirci, B.; Kirimer, N. The cytotoxic activity of Vitex agnus castus L. essential oils and their biochemical mechanisms. Ind. Crops Prod. 2014, 55, 33–42. [Google Scholar] [CrossRef]
- Pantelić, J.; Filipović, B.; Šošić-Jurjević, B.; Ajdžanović, V.; Trifunović, S.; Medigović, I.; Milošević, V. Vitex agnus-castus essential oil affects thyroid C cells and bone metabolism in middle-aged male rats. Acta Vet. Brno. 2013, 63, 23–35. [Google Scholar] [CrossRef]
- Ignjatović, D.; Tovilović, G.; Šošić-Jurjević, B.; Filipović, B.; Janać, B.; Milošević, V.; Tomić, M. Bioactivity of the essential oil from berries of Vitex agnus castus in middle aged male rats. Dig. J. Nanomater. Biostructures 2012, 7, 1727–1734. [Google Scholar]
- Ajdžanović, V.; Spasojević, I.; Pantelić, J.; Sǒšić-Jurjević, B.; Filipović, B.; Milošević, V.; Severs, W. Vitex agnus-castus L. essential oil increases human erythrocyte membrane fluidity. J. Med. Biochem. 2012, 31, 222–227. [Google Scholar] [CrossRef]
- Stojković, D.; Soković, M.; Glamočlija, J.; Džamić, A.; Ćirić, A.; Ristić, M.; Grubišić, D. Chemical composition and antimicrobial activity of Vitex agnus-castus L. fruits and leaves essential oils. Food Chem. 2011, 128, 1017–1022. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Phytochemistry and nematicidal activity of the essential oils from 8 greek lamiaceae aromatic plants and 13 terpene components. J. Agric. Food Chem. 2010, 58, 7856–7863. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.A.; El-Hefny, M.; Salem, M.Z.M.; Ali, H.M. The biofungicide activity of some plant essential oils for the cleaner production of model linen fibers similar to those used in ancient Egyptian mummification. Processes 2020, 8, 79. [Google Scholar] [CrossRef]
- Khoury, M.; Stien, D.; Eparvier, V.; Ouaini, N.; El Beyrouthy, M. Report on the Medicinal Use of Eleven Lamiaceae Species in Lebanon and Rationalization of Their Antimicrobial Potential by Examination of the Chemical Composition and Antimicrobial Activity of Their Essential Oils. Evid.-Based Complement. Altern. Med. 2016, 2016, 2547169. [Google Scholar] [CrossRef] [PubMed]
- Abdelgaleil, S.A.M.; Mohamed, M.I.E.; Shawir, M.S.; Abou-Taleb, H.K. Chemical composition, insecticidal and biochemical effects of essential oils of different plant species from Northern Egypt on the rice weevil, Sitophilus oryzae L. J. Pest Sci. 2016, 89, 219–229. [Google Scholar] [CrossRef]
- Borges, A.R.; Aires, J.R.D.A.; Higino, T.M.M.; de Medeiros, M.D.G.F.; Citó, A.M.D.G.L.; Lopes, J.A.D.; de Figueiredo, R.C.B.Q. Trypanocidal and cytotoxic activities of essential oils from medicinal plants of Northeast of Brazil. Exp. Parasitol. 2012, 132, 123–128. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Çenet, M.; Öztürk, B.; Bozok, F.; Karabörklü, S.; Demirci, S.C. Chemical Characterization, Phytotoxic, Antimicrobial and Insecticidal Activities of Vitex agnus-castus’ Essential Oil from East Mediterranean Region. J. Essent. Oil-Bear. Plants 2015, 18, 1500–1507. [Google Scholar] [CrossRef]
- Toplan, G.G.; Kurkcuoglu, M.; Husnu Can Baser, K.; Sariyar, G. Composition of the essential oils from samples of Vitex agnus-castus L. growing in Turkey. J. Essent. Oil Res. 2015, 27, 337–342. [Google Scholar] [CrossRef]
- Eryigit, T.; Çig, A.; Okut, N.; Yildirim, B.; Ekici, K. Evaluation of chemical composition and antimicrobial activity of Vitex agnus castus L. fruits’ essential oils from West Anatolia, Turkey. J. Essent. Oil-Bear. Plants 2015, 18, 208–214. [Google Scholar] [CrossRef]
- Crock, J.; Wildung, M.; Croteau, R. Isolation and bacterial expression of a sesquiterpene synthase cDNA clone from peppermint (Mentha × piperita, L.) that produces the aphid alarm pheromone (E)-β-farnesene. Proc. Natl. Acad. Sci. USA 1997, 94, 12833–12838. [Google Scholar] [CrossRef]
- Maruyama, T.; Ito, M.; Honda, G. Molecular cloning, functional expression and characterization of (E)-β-farnesene synthase from Citrus junos. Biol. Pharm. Bull. 2001, 24, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.P.W.; Philippe, R.N.; Godard, K.A.; Sturrock, R.N.; Bohlmann, J. Characterization of four terpene synthase cDNAs from methyl jasmonate-induced Douglas-fir, Pseudotsuga menziesii. Phytochemistry 2005, 66, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Liu, X.; Pan, G.; Hou, X.; Zhang, H.; Yuan, Y. In vitro characterization of a (E)-β-farnesene synthase from Matricaria recutita L. and its up-regulation by methyl jasmonate. Gene 2015, 571, 58–64. [Google Scholar] [CrossRef]
- Yu, X.; Jones, H.D.; Ma, Y.; Wang, G.; Xu, Z.; Zhang, B.; Zhang, Y.; Ren, G.; Pickett, J.A.; Xia, L. (E)-β-Farnesene synthase genes affect aphid (Myzus persicae) infestation in tobacco (Nicotiana tabacum). Funct. Integr. Genom. 2012, 12, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Picaud, S.; Brodelius, M.; Brodelius, P.E. Expression, purification and characterization of recombinant (E)-β-farnesene synthase from Artemisia annua. Phytochemistry 2005, 66, 961–967. [Google Scholar] [CrossRef]
- De Brum, T.F.; Boligon, A.A.; Frohlich, J.K.; Schwanz, T.G.; Zadra, M.; Piana, M.; Froeder, A.L.F.; Athayde, M.L. Composition and antioxidant capacity of the essential oil of leaves of Vitex megapotamica (Sprengel) Moldenke. Nat. Prod. Res. 2013, 27, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Ouoba, A.M.; Koudou, J.; Somé, N.; Guissou, I.P.; Figueredo, G.; Chaixhat, J.C. Volatile components of the leaves of Vitex simplicifolia oliv. Asian J. Chem. 2009, 21, 3304–3306. [Google Scholar]
- Alemdar, S.; Hartwig, S.; Frister, T.; König, J.C.; Scheper, T.; Beutel, S. Heterologous Expression, Purification, and Biochemical Characterization of α-Humulene Synthase from Zingiber zerumbet Smith. Appl. Biochem. Biotechnol. 2016, 178, 474–489. [Google Scholar] [CrossRef]
- Keeling, C.I.; Weisshaar, S.; Ralph, S.G.; Jancsik, S.; Hamberger, B.; Dullat, H.K.; Bohlmann, J. Transcriptome mining, functional characterization, and phylogeny of a large terpene synthase gene family in spruce (Picea spp.). BMC Plant Biol. 2011, 11, 43. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; D’Auria, J.C.; Farooq, A.; Pichersky, E.; Gershenzon, J. Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell 2003, 15, 481–494. [Google Scholar] [CrossRef]
- Dhandapani, S.; Kim, M.J.; Chin, H.J.; Leong, S.H.; Jang, I.C. Identification and functional characterization of tissue-specific terpene synthases in Stevia rebaudiana. Int. J. Mol. Sci. 2020, 21, 8566. [Google Scholar] [CrossRef]
- Hattan, J.I.; Shindo, K.; Sasaki, T.; Misawa, N. Isolation and functional characterization of new terpene synthase genes from traditional edible plants. J. Oleo Sci. 2018, 67, 1235–1246. [Google Scholar] [CrossRef]
- Jayaramaiah, R.H.; Anand, A.; Beedkar, S.D.; Dholakia, B.B.; Punekar, S.A.; Kalunke, R.M.; Gade, W.N.; Thulasiram, H.V.; Giri, A.P. Functional characterization and transient expression manipulation of a new sesquiterpene synthase involved in β-caryophyllene accumulation in Ocimum. Biochem. Biophys. Res. Commun. 2016, 473, 265–271. [Google Scholar] [CrossRef]
- Kumeta, Y.; Ito, M. Characterization of α-humulene synthases responsible for the production of sesquiterpenes induced by methyl jasmonate in Aquilaria cell culture. J. Nat. Med. 2016, 70, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Nawade, B.; Shaltiel-Harpaz, L.; Yahyaa, M.; Kabaha, A.; Kedoshim, R.; Bosamia, T.C.; Ibdah, M. Characterization of terpene synthase genes potentially involved in black fig fly (Silba adipata) interactions with Ficus carica. Plant Sci. 2020, 298, 110549. [Google Scholar] [CrossRef]
- Bülow, N.; König, W.A. The role of germacrene D as a precursor in sesquiterpene biosynthesis: Investigations of acid catalyzed, photochemically and thermally induced rearrangements. Phytochemistry 2000, 55, 141–168. [Google Scholar] [CrossRef]
- Rinkel, J.; Rabe, P.; Garbeva, P.; Dickschat, J.S. Lessons from 1,3-Hydride Shifts in Sesquiterpene Cyclizations. Angew. Chem. Int. Ed. 2016, 55, 13593–13596. [Google Scholar] [CrossRef] [PubMed]
- Prosser, I.; Altug, I.G.; Phillips, A.L.; König, W.A.; Bouwmeester, H.J.; Beale, M.H. Enantiospecific (+)- and (−)-germacrene D synthases, cloned from goldenrod, reveal a functionally active variant of the universal isoprenoid-biosynthesis aspartate-rich motif. Arch. Biochem. Biophys. 2004, 432, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.M.; Croteau, R. Cyclization Enzymes in the Biosynthesis of Monoterpenes, Sesquiterpenes, and Diterpenes. In Biosynthesis; Springer: Berlin/Heidelberg, Germany, 2000; Volume 209, pp. 53–95. [Google Scholar] [CrossRef]
- Deguerry, F.; Pastore, L.; Wu, S.; Clark, A.; Chappell, J.; Schalk, M. The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases. Arch. Biochem. Biophys. 2006, 454, 123–136. [Google Scholar] [CrossRef]
- Blank, P.N.; Barrow, G.H.; Christianson, D.W. Crystal structure of F95Q epi-isozizaene synthase, an engineered sesquiterpene cyclase that generates biofuel precursors β- and γ-curcumene. J. Struct. Biol. 2019, 207, 218–224. [Google Scholar] [CrossRef]
- Li, R.; Chou, W.K.W.; Himmelberger, J.A.; Litwin, K.M.; Harris, G.G.; Cane, D.E.; Christianson, D.W. Reprogramming the chemodiversity of terpenoid cyclization by remolding the active site contour of epi-isozizaene synthase. Biochemistry 2014, 53, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Tantillo, D.J. Is a 1,4-Alkyl Shift Involved in the Biosynthesis of Ledol and Viridiflorol? J. Org. Chem. 2017, 82, 3957–3959. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.N.; Thang, T.D.; Ogunwande, I.A.; Lawal, O.A. Study on essential oils from the leaves of two Vietnamese plants: Jasminum subtriplinerve C.L. Blume and Vitex quinata (Lour) F.N. Williams. Nat. Prod. Res. 2016, 30, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Miyoshi, S.; Tamaoki, D.; Yamada, S.; Tanaka, K.; Uji, Y.; Tanaka, S.; Akimitsu, K.; Gomi, K. Isolation of jasmonate-induced sesquiterpene synthase of rice: Product of which has an antifungal activity against Magnaporthe oryzae. J. Plant Physiol. 2014, 171, 625–632. [Google Scholar] [CrossRef]
- Ling, C.; Zheng, L.; Yu, X.; Wang, H.; Wang, C.; Wu, H.; Zhang, J.; Yao, P.; Tai, Y.; Yuan, Y. Cloning and functional analysis of three aphid alarm pheromone genes from German chamomile (Matricaria chamomilla L.). Plant Sci. 2020, 294, 110463. [Google Scholar] [CrossRef]
- Wang, G.R.; Wang, H. Cell suspension culture of Rhizoma zedoariae in a two-stage perfusion bioreactor system for β-elemene production. In Vitro Cell. Dev. Biol.-Plant 2019, 55, 209–220. [Google Scholar] [CrossRef]
- Kumeta, Y.; Ito, M. Genomic organization of δ-guaiene synthase genes in Aquilaria crassna and its possible use for the identification of Aquilaria species. J. Nat. Med. 2011, 65, 508–513. [Google Scholar] [CrossRef]
- Adio, A.M. (−)-trans-β-Elemene and related compounds: Occurrence, synthesis, and anticancer activity. Tetrahedron 2009, 65, 5145–5159. [Google Scholar] [CrossRef]
- Rinkel, J.; Dickschat, J.S. Addressing the chemistry of germacrene A by isotope labeling experiments. Org. Lett. 2019, 21, 2426–2429. [Google Scholar] [CrossRef]
- Faraldos, J.A.; Wu, S.; Chappell, J.; Coates, R.M. Conformational analysis of (+)-germacrene A by variable-temperature NMR and NOE spectroscopy. Tetrahedron 2007, 63, 7733–7742. [Google Scholar] [CrossRef]
- De Kraker, J.W.; Franssen, M.C.R.; De Groot, A.; König, W.A.; Bouwmeester, H.J. (+)-Germacrene A biosynthesis—The committed step in the biosynthesis of bitter sesquiterpene lactones in chicory. Plant Physiol. 1998, 117, 1381–1392. [Google Scholar] [CrossRef]
- De Kraker, J.W.; Franssen, M.C.R.; De Groot, A.; Shibata, T.; Bouwmeester, H.J. Germacrenes from fresh costus roots. Phytochemistry 2001, 58, 481–487. [Google Scholar] [CrossRef]
- Tressl, R.; Engel, K.H.; Kossa, M.; Köppler, H. Characterization of Tricyclic Sesquiterpenes in Hop (Humulus lupulus, var. Hersbrucker Spät). J. Agric. Food Chem. 1983, 31, 892–897. [Google Scholar] [CrossRef]
- Uji, Y.; Ozawa, R.; Shishido, H.; Taniguchi, S.; Takabayashi, J.; Akimitsu, K.; Gomi, K. Isolation of a sesquiterpene synthase expressing in specialized epithelial cells surrounding the secretory cavities in rough lemon (Citrus jambhiri). J. Plant Physiol. 2015, 180, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Arigoni, D. Stereochemical aspects of sesquiterpene biosynthesis. Pure Appl. Chem. 1975, 41, 219–245. [Google Scholar] [CrossRef]
- Boland, W.; Garms, S. Induced volatiles of Medicago truncatula: Molecular diversity and mechanistic aspects of a multiproduct sesquiterpene synthase from M. truncatula. Flavour Fragr. J. 2010, 25, 114–116. [Google Scholar] [CrossRef]
- Yan, X.; Li, W.; Liang, D.; Zhao, G.; Caiyin, Q.; Qiao, J. Comparative transcriptome analysis of sesquiterpene biosynthesis and functional characterization of sesquiterpene synthases in Leonurus sibiricus L. Planta 2021, 253, 71. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.M.; Tsuji, J.; Davis, G.D.; Pierce, M.L.; Essenberg, M. Purification of (+)-δ-cadinene synthase, a sesquiterpene cyclase from bacteria-inoculated cotton foliar tissue. Phytochemistry 1996, 41, 1047–1055. [Google Scholar] [CrossRef]
- Loizzi, M.; Miller, D.J.; Allemann, R.K. Silent catalytic promiscuity in the high-fidelity terpene cyclase δ-cadinene synthase. Org. Biomol. Chem. 2019, 17, 1206–1214. [Google Scholar] [CrossRef]
- González, V.; Grundy, D.J.; Faraldos, J.A.; Allemann, R.K. The amino-terminal segment in the β-domain of δ-cadinene synthase is essential for catalysis. Org. Biomol. Chem. 2016, 14, 7451–7454. [Google Scholar] [CrossRef] [PubMed]
- Faraldos, J.A.; Miller, D.J.; González, V.; Yoosuf-Aly, Z.; Cascón, O.; Li, A.; Allemann, R.K. A 1,6-ring closure mechanism for (+)-δ-cadinene synthase? J. Am. Chem. Soc. 2012, 134, 5900–5908. [Google Scholar] [CrossRef]
- Benedict, C.R.; Lu, J.L.; Pettigrew, D.W.; Liu, J.; Stipanovic, R.D.; Williams, H.J. The cyclization of farnesyl diphosphate and nerolidyl diphosphate by a purified recombinant δ-cadinene synthase. Plant Physiol. 2001, 125, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Prakash, O.; Jain, S.; Ali, M. Volatile constituents of the fruits of Vitex negundo linn. J. Essent. Oil-Bear. Plants 2007, 10, 247–250. [Google Scholar] [CrossRef]
- Suksamrarn, A.; Werawattanametin, K.; Brophy, J.J. Variation of essential oil constituents in Vitex trifolia species. Flavour Fragr. J. 1991, 6, 97–99. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, X. Chemical composition and antioxidant activity of the seeds oil of Vitex kwangsiensis C. Pei. Rec. Nat. Prod. 2018, 12, 630–633. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Rakotosaona, R.; Nzekoue, F.K.; Canale, A.; Nicoletti, M.; Maggi, F. Insecticidal and mosquito repellent efficacy of the essential oils from stem bark and wood of Hazomalania voyronii. J. Ethnopharmacol. 2020, 248, 112333. [Google Scholar] [CrossRef] [PubMed]
- Nararak, J.; Sathantriphop, S.; Kongmee, M.; Mahiou-Leddet, V.; Ollivier, E.; Manguin, S.; Chareonviriyaphap, T. Excito-repellent activity of β-caryophyllene oxide against Aedes aegypti and Anopheles minimus. Acta Trop. 2019, 197, 105030. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. Β-Caryophyllene and Β-Caryophyllene Oxide—Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Ding, Y.; Huffaker, A.; Köllner, T.G.; Weckwerth, P.; Robert, C.A.M.; Spencer, J.L.; Lipka, A.E.; Schmelz, E.A. Selinene volatiles are essential precursors for maize defense promoting fungal pathogen resistance. Plant Physiol. 2017, 175, 1455–1468. [Google Scholar] [CrossRef] [PubMed]
- Starks, C.M.; Back, K.; Chappell, J.; Noel, J.P. Structural basis for cyclic terpene biosynthesis by tobacco 5-epi-aristolochene synthase. Science 1997, 277, 1815–1820. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Badawy, M.E.I.; Shawir, M.S.; Mohamed, M.I.E. Chemical composition, fumigant and contact toxicities of essential oils isolated from egyptian plants against the stored grain insects; Sitophilus oryzae L. and Tribolium castaneum (Herbst). Int. Med. J. 2015, 25, 639–647. [Google Scholar]
- Movahhed Haghighi, T.; Saharkhiz, M.J.; Khosravi, A.R.; Raouf Fard, F.; Moein, M. Essential oil content and composition of Vitex pseudo-negundo in Iran varies with ecotype and plant organ. Ind. Crops Prod. 2017, 109, 53–59. [Google Scholar] [CrossRef]
- Crocoll, C.; Asbach, J.; Novak, J.; Gershenzon, J.; Degenhardt, J. Terpene synthases of oregano (Origanum vulgare L.) and their roles in the pathway and regulation of terpene biosynthesis. Plant Mol. Biol. 2010, 73, 587–603. [Google Scholar] [CrossRef]
- Külheim, C.; Padovan, A.; Hefer, C.; Krause, S.T.; Köllner, T.G.; Myburg, A.A.; Degenhardt, J.; Foley, W.J. The Eucalyptus terpene synthase gene family. BMC Genom. 2015, 16, 450. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, B.; Cao, X.; Zhang, B.; Chen, K. Citrus CmTPS1 is associated with formation of sesquiterpene bicyclogermacrene. Sci. Hortic. 2017, 226, 133–140. [Google Scholar] [CrossRef]
- Booth, J.K.; Page, J.E.; Bohlmann, J. Terpene synthases from Cannabis sativa. PLoS ONE 2017, 12, e0173911. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.; Kim, S.U.; Ro, D.K. Molecular cloning and characterization of (+)-epi-α-bisabolol synthase, catalyzing the first step in the biosynthesis of the natural sweetener, hernandulcin, in Lippia dulcis. Arch. Biochem. Biophys. 2012, 527, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.T.; Santos, H.S.; Teixeira, A.M.R.; Bandeira, P.N.; Holanda, C.L.; Vale, J.P.C.; Pereira, E.J.P.; Menezes, J.E.S.A.; Rodrigues, T.H.S.; Souza, E.B.; et al. Seasonal variation in the chemical composition and larvicidal activity against Aedes aegypti of essential oils from Vitex gardneriana Schauer. S. Afr. J. Bot. 2019, 124, 329–332. [Google Scholar] [CrossRef]
- Steele, C.L.; Crock, J.; Bohlmann, J.; Croteau, R. Sesquiterpene synthases from grand fir (Abies grandis): Comparison of constitutive and wound-induced activities, and cDNA isolation, characterization, and bacterial expression of δ-selinene synthase and γ- humulene synthase. J. Biol. Chem. 1998, 273, 2078–2089. [Google Scholar] [CrossRef]
- An, T.; Li, L.; Lin, Y.; Zeng, F.; Lin, P.; Zi, J. Characterization of Guaiene Synthases from Stellera chamaejasme L. Flowers and Their Application in de novo Production of (−)-Rotundone in Yeast. J. Agric. Food Chem. 2020, 68, 3214–3219. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.P.; Andersen, T.B.; Sweetman, C.; Møller, B.L.; Ford, C.; Simonsen, H.T. Two key polymorphisms in a newly discovered allele of the Vitis vinifera TPS24 gene are responsible for the production of the rotundone precursor α-guaiene. J. Exp. Bot. 2016, 67, 799–808. [Google Scholar] [CrossRef]
- Lee, J.B.; Hirohashi, S.; Yamamura, Y.; Taura, F.; Kurosaki, F. Induction, cloning and functional expression of a sesquiterpene biosynthetic enzyme, δ-guaiene synthase, of Aquilaria microcarpa cell cultures. Nat. Prod. Commun. 2014, 9, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Z.; Wang, M.; Wei, J.; Chen, H.; Gao, Z.; Sui, C.; Luo, H.; Zhang, X.; Yang, Y.; et al. Identification of genes related to agarwood formation: Transcriptome analysis of healthy and wounded tissues of Aquilaria sinensis. BMC Genom. 2013, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Kumeta, Y.; Ito, M. Characterization of δ-guaiene synthases from cultured cells of Aquilaria, responsible for the formation of the sesquiterpenes in agarwood. Plant Physiol. 2010, 154, 1998–2007. [Google Scholar] [CrossRef]
- Kaul, P.N.; Rao, B.R.R.; Bhattacharya, A.K.; Singh, K.; Syamasundar, K.V.; Ramesh, S. Essential Oil Composition of Vitex negundo L. Flowers. J. Essent. Oil Res. 2005, 17, 483–484. [Google Scholar] [CrossRef]
- Sharma, K.; Guleria, S.; Razdan, V.K.; Babu, V. Synergistic antioxidant and antimicrobial activities of essential oils of some selected medicinal plants in combination and with synthetic compounds. Ind. Crops Prod. 2020, 154, 112569. [Google Scholar] [CrossRef]
- Schmidt, C.O.; Bouwmeester, H.J.; Bülow, N.; König, W.A. Isolation, characterization, and mechanistic studies of (−)-α-gurjunene synthase from Solidago canadensis. Arch. Biochem. Biophys. 1999, 364, 167–177. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Ma, L.T.; Wang, S.Y.; Chu, F.H. Cloning and expression of a sesquiterpene synthase gene from Taiwania cryptomerioides. Holzforschung 2015, 69, 1041–1048. [Google Scholar] [CrossRef]
- Padovan, A.; Keszei, A.; Köllner, T.G.; Degenhardt, J.; Foley, W.J. The molecular basis of host plant selection in Melaleuca quinquenervia by a successful biological control agent. Phytochemistry 2010, 71, 1237–1244. [Google Scholar] [CrossRef]
- Bleeker, P.M.; Spyropoulou, E.A.; Diergaarde, P.J.; Volpin, H.; De Both, M.T.J.; Zerbe, P.; Bohlmann, J.; Falara, V.; Matsuba, Y.; Pichersky, E.; et al. RNA-seq discovery, functional characterization, and comparison of sesquiterpene synthases from Solanum lycopersicum and Solanum habrochaites trichomes. Plant Mol. Biol. 2011, 77, 323–336. [Google Scholar] [CrossRef]
- Durán-Peña, M.J.; Botubol Ares, J.M.; Hanson, J.R.; Collado, I.G.; Hernández-Galán, R. Biological activity of natural sesquiterpenoids containing a gem-dimethylcyclopropane unit. Nat. Prod. Rep. 2015, 32, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Mcmurry, J.E.; Bosch, G.K. Synthesis of Macrocyclic Terpenoid Hydrocarbons by Intramolecular Carbonyl Coupling: Bicyclogermacrene, Lepidozene, and Casbene. J. Org. Chem. 1987, 52, 4885–4893. [Google Scholar] [CrossRef]
- Tran, D.N.; Cramer, N. Biomimetic synthesis of (+)-ledene, (+)-viridiflorol, (−)-palustrol, (+)-spathulenol, and psiguadial A, C, and D via the platform terpene (+)-bicyclogermacrene. Chem.—Eur. J. 2014, 20, 10654–10660. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.d.C.; Góis Ruiz, A.L.T.; Ann Foglio, M.; de Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Ghisalberti, E.L. Bioactive acylphloroglucinol derivatives from Eucalyptus species. Phytochemistry 1996, 41, 7–22. [Google Scholar] [CrossRef]
- Ntana, F.; Bhat, W.W.; Johnson, S.R.; Jørgensen, H.J.L.; Collinge, D.B.; Jensen, B.; Hamberger, B. A sesquiterpene synthase from the endophytic fungus serendipita indica catalyzes formation of viridiflorol. Biomolecules 2021, 11, 898. [Google Scholar] [CrossRef]
- Shukal, S.; Chen, X.; Zhang, C. Systematic engineering for high-yield production of viridiflorol and amorphadiene in auxotrophic Escherichia coli. Metab. Eng. 2019, 55, 170–178. [Google Scholar] [CrossRef]
- Li, R.; Tee, C.S.; Jiang, Y.L.; Jiang, X.Y.; Venkatesh, P.N.; Sarojam, R.; Ye, J. A terpenoid phytoalexin plays a role in basal defense of Nicotiana benthamiana against Potato virus X. Sci. Rep. 2015, 5, 9682. [Google Scholar] [CrossRef] [PubMed]
- Himanen, S.J.; Blande, J.D.; Klemola, T.; Pulkkinen, J.; Heijari, J.; Holopainen, J.K. Birch (Betula spp.) leaves adsorb and re-release volatiles specific to neighbouring plants—A mechanism for associational herbivore resistance? New Phytol. 2010, 186, 722–732. [Google Scholar] [CrossRef]
- Huang, X.Z.; Xiao, Y.T.; Köllner, T.G.; Jing, W.X.; Kou, J.F.; Chen, J.Y.; Liu, D.F.; Gu, S.H.; Wu, J.X.; Zhang, Y.J.; et al. The terpene synthase gene family in Gossypium hirsutum harbors a linalool synthase GhTPS12 implicated in direct defence responses against herbivores. Plant Cell Environ. 2018, 41, 261–274. [Google Scholar] [CrossRef]
- Karban, R. Associational resistance for mule’s ears with sagebrush neighbors. Plant Ecol. 2007, 191, 295–303. [Google Scholar] [CrossRef]
- Kessler, A.; Halitschke, R.; Diezel, C.; Baldwin, I.T. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 2006, 148, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Dreher, D.; Athmer, B.; Porzel, A.; Gavrin, A.; Baldermann, S.; Tissier, A.; Hause, B. Medicago TERPENE SYNTHASE 10 is involved in defense against an oomycete root pathogen. Plant Physiol. 2019, 180, 1598–1613. [Google Scholar] [CrossRef]
- Kamolsukyunyong, W.; Sukhaket, W.; Ruanjaichon, V.; Toojinda, T.; Vanavichit, A. Single-feature polymorphism mapping of isogenic rice lines identifies the influence of terpene synthase on brown planthopper feeding preferences. Rice 2013, 6, 18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, B.; Liu, Q.; Zhou, Z.; Yin, H.; Xie, Y.; Wei, Y. Two terpene synthases in resistant Pinus massoniana contribute to defence against Bursaphelenchus xylophilus. Plant Cell Environ. 2021, 44, 257–274. [Google Scholar] [CrossRef]
- Liu, G.; Yang, M.; Fu, J. Identification and characterization of two sesquiterpene synthase genes involved in volatile-mediated defense in tea plant (Camellia sinensis). Plant Physiol. Biochem. 2020, 155, 650–657. [Google Scholar] [CrossRef]
- Cui, L.L.; Dong, J.; Francis, F.; Liu, Y.J.; Heuskin, S.; Lognay, G.; Chen, J.L.; Bragard, C.; Tooker, J.F.; Liu, Y. E-β-farnesene synergizes the influence of an insecticide to improve control of cabbage aphids in China. Crop Prot. 2012, 35, 91–96. [Google Scholar] [CrossRef]
- Francis, F.; Vandermoten, S.; Verheggen, F.; Lognay, G.; Haubruge, E. Is the (E)-β-farnesene only volatile terpenoid in aphids? J. Appl. Entomol. 2005, 129, 6–11. [Google Scholar] [CrossRef]
- Gibson, R.W.; Pickett, J.A. Wild potato repels aphids by release of aphid alarm pheromone. Nature 1983, 302, 608–609. [Google Scholar] [CrossRef]
- Verheggen, F.J.; Arnaud, L.; Bartram, S.; Gohy, M.; Haubruge, E. Aphid and plant volatiles induce oviposition in an aphidophagous hoverfly. J. Chem. Ecol. 2008, 34, 301–307. [Google Scholar] [CrossRef]
- Xu, Q.; Hatt, S.; Han, Z.; Francis, F.; Chen, J. Combining E-β-farnesene and methyl salicylate release with wheat-pea intercropping enhances biological control of aphids in North China. Biocontrol Sci. Technol. 2018, 28, 883–894. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Birkett, M.A.; Blande, J.; Hooper, A.M.; Martin, J.L.; Khambay, B.; Prosser, I.; Smart, L.E.; Wadhams, L.J. Response of economically important aphids to components of Hemizygia petiolata essential oil. Pest Manag. Sci. 2005, 61, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Maisnam, J.; Jain, A.; Sharma, K.K.; Bhattacharya, R. Aphid-repellent pheromone E-β-farnesene is generated in transgenic Arabidopsis thaliana over-expressing farnesyl diphosphate synthase2. Ann. Bot. 2015, 115, 581–591. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, X.; Ning, Y.; Jing, W.; Bruce, T.J.A.; Qi, F.; Xu, Q.; Wu, K.; Zhang, Y.; Guo, Y. TPS46, a rice terpene synthase conferring natural resistance to bird cherry-oat aphid, Rhopalosiphum padi (Linnaeus). Front. Plant Sci. 2017, 8, 110. [Google Scholar] [CrossRef]
- Paventi, G.; de Acutis, L.; De Cristofaro, A.; Pistillo, M.; Germinara, G.S.; Rotundo, G. Biological activity of Humulus lupulus (L.) essential oil and its main components against Sitophilus granarius (L.). Biomolecules 2020, 10, 1108. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.C.S.; Milet-Pinheiro, P.; Da Silva, P.C.B.; Da Silva, A.G.; Da Silva, M.V.; Do Amaral Ferraz Navarro, D.M.; Da Silva, N.H. (E)-Caryophyllene and α-humulene: Aedes aegypti oviposition deterrents elucidated by gas chromatography-electrophysiological assay of Commiphora leptophloeos leaf oil. PLoS ONE 2015, 10, e0144586. [Google Scholar] [CrossRef] [PubMed]
- You, C.X.; Guo, S.S.; Zhang, W.J.; Yang, K.; Wang, C.F.; Geng, Z.F.; Du, S.S.; Deng, Z.W.; Wang, Y.Y. Chemical Constituents and Activity of Murraya microphylla Essential Oil against Lasioderma serricorne. Nat. Prod. Commun. 2015, 10, 1934578X1501000936. [Google Scholar] [CrossRef]
- Araújo, M.J.C.; Câmara, C.A.G.; Born, F.S.; Moraes, M.M.; Badji, C.A. Acaricidal activity and repellency of essential oil from Piper aduncum and its components against Tetranychus urticae. Exp. Appl. Acarol. 2012, 57, 139–155. [Google Scholar] [CrossRef]
- Røstelien, T.; Borg-Karlson, A.K.; Fäldt, J.; Jacobsson, U.; Mustaparta, H. The plant sesquiterpene germacrene D specifically activates a major type of antennal receptor neuron of the tobacco budworm moth Heliothis virescens. Chem. Senses 2000, 25, 141–148. [Google Scholar] [CrossRef][Green Version]
- Yamasaki, T.; Sato, M.; Sakoguchi, H. (−)-Germacrene D: Masking substance of attractants for the cerambycid beetle, Monochamus alternatus (Hope). Appl. Entomol. Zool. 1997, 32, 423–429. [Google Scholar] [CrossRef]
- Birkett, M.A.; Bruce, T.J.A.; Pickett, J.A. Repellent activity of Nepeta grandiflora and Nepeta clarkei (Lamiaceae) against the cereal aphid, Sitobion avenae (Homoptera: Aphididae). Phytochem. Lett. 2010, 3, 139–142. [Google Scholar] [CrossRef]
- Birkett, M.A.; Al Abassi, S.; Kröber, T.; Chamberlain, K.; Hooper, A.M.; Guerin, P.M.; Pettersson, J.; Pickett, J.A.; Slade, R.; Wadhams, L.J. Antiectoparasitic activity of the gum resin, gum haggar, from the East African plant, Commiphora holtziana. Phytochemistry 2008, 69, 1710–1715. [Google Scholar] [CrossRef]
- Tozin, L.R.d.S.; Marques, M.O.M.; Rodrigues, T.M. Herbivory by leaf-cutter ants changes the glandular trichomes density and the volatile components in an aromatic plant model. AoB Plants 2017, 9, plx057. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Trumble, J.T.; Millar, J.G.; White, K.K. Topical Toxicity of Tomato Sesquiterpenes to the Beet Armyworm and the Role of These Compounds in Resistance Derived from an Accession of Lycopersicon hirsutum f. typicum. J. Agric. Food Chem. 1994, 42, 807–810. [Google Scholar] [CrossRef]
- Townsend, B.J.; Poole, A.; Blake, C.J.; Llewellyn, D.J. Antisense suppression of a (+)-δ-cadinene synthase gene in cotton prevents the induction of this defense response gene during bacterial blight infection but not its constitutive expression. Plant Physiol. 2005, 138, 516–528. [Google Scholar] [CrossRef]
- Zebelo, S.; Song, Y.; Kloepper, J.W.; Fadamiro, H. Rhizobacteria activates (+)-δ-cadinene synthase genes and induces systemic resistance in cotton against beet armyworm (Spodoptera exigua). Plant Cell Environ. 2016, 39, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Saha, S.; Walia, S.; Shakil, N.A.; Kumar, J.; Annapurna, K. Cadinene sesquiterpenes from Eupatorium adenophorum and their antifungal activity. J. Environ. Sci. Health—Part B Pestic. Food Contam. Agric. Wastes 2013, 48, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zhou, Q.M.; Peng, C.; Xie, X.F.; Guo, L.; Li, X.H.; Liu, J.; Liu, Z.H.; Dai, O. Sesquiterpenoids from the herb of Leonurus japonicus. Molecules 2013, 18, 5051–5058. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Molisso, D.; Digilio, M.C.; Giorgini, M.; Corrado, G.; Bruce, T.J.A.; D’Agostino, N.; Rao, R. Zucchini Plants Alter Gene Expression and Emission of (E)-β-Caryophyllene Following Aphis gossypii Infestation. Front. Plant Sci. 2021, 11, 592603. [Google Scholar] [CrossRef]
- Köllner, T.G.; Held, M.; Lenk, C.; Hiltpold, I.; Turlings, T.C.J.; Gershenzon, J.; Degenhardta, J. A maize (E)-β-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell 2008, 20, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, G.; Huang, X.; Guo, H.; Su, X.; Han, L.; Zhang, Y.; Qi, Z.; Xiao, Y.; Cheng, H. Overexpression of the caryophyllene synthase gene GhTPS1 in cotton negatively affects multiple pests while attracting parasitoids. Pest Manag. Sci. 2020, 76, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xin, Z.; Li, J.; Hu, L.; Lou, Y.; Lu, J. (E)-β-caryophyllene functions as a host location signal for the rice white-backed planthopper Sogatella furcifera. Physiol. Mol. Plant Pathol. 2015, 91, 106–112. [Google Scholar] [CrossRef]
- Zhuang, X.; Köllner, T.G.; Zhao, N.; Li, G.; Jiang, Y.; Zhu, L.; Ma, J.; Degenhardt, J.; Chen, F. Dynamic evolution of herbivore-induced sesquiterpene biosynthesis in sorghum and related grass crops. Plant J. 2012, 69, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Sabulal, B.; Dan, M.; Kurup, R.; Pradeep, N.S.; Valsamma, R.K.; George, V. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry 2006, 67, 2469–2473. [Google Scholar] [CrossRef]
- Gunasena, G.H.; Vinson, S.B.; Williams, H.J.; Stipanovic, R.D. Effects of Caryophyllene, Caryophyllene Oxide, and Their Interaction with Gossypol on the Growth and Development of Heliothis virescens (F.) (Lepidoptera: Noctuidae). J. Econ. Entomol. 1988, 81, 93–97. [Google Scholar] [CrossRef]
- Langenhheim, J.H. Higher plant terpenoids: A phytocentric overview of their ecological roles. J. Chem. Ecol. 1994, 20, 1223–1280. [Google Scholar] [CrossRef]
- Petrakis, E.A.; Kimbaris, A.C.; Perdikis, D.C.; Lykouressis, D.P.; Tarantilis, P.A.; Polissiou, M.G. Responses of Myzus persicae (Sulzer) to three Lamiaceae essential oils obtained by microwave-assisted and conventional hydrodistillation. Ind. Crops Prod. 2014, 62, 272–279. [Google Scholar] [CrossRef]
- Suleiman, R.; Mgani, Q.; Nyandoro, S. Chemical compositions and mosquito repellency of essential oils from Artabotrys hexapetalus and Artabotrys rupestris. Int. J. Biol. Chem. Sci. 2015, 8, 2804. [Google Scholar] [CrossRef]
- Becker, E.M.; Herrfurth, C.; Irmisch, S.; Köllner, T.G.; Feussner, I.; Karlovsky, P.; Splivallo, R. Infection of corn ears by Fusarium spp. induces the emission of volatile sesquiterpenes. J. Agric. Food Chem. 2014, 62, 5226–5236. [Google Scholar] [CrossRef]
- Sowbhagya, H.B. Chemistry, Technology, and Nutraceutical Functions of Celery (Apium graveolens L.): An Overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 389–398. [Google Scholar] [CrossRef]
- Lanubile, A.; Ferrarini, A.; Maschietto, V.; Delledonne, M.; Marocco, A.; Bellin, D. Functional genomic analysis of constitutive and inducible defense responses to Fusarium verticillioides infection in maize genotypes with contrasting ear rot resistance. BMC Genom. 2014, 15, 710. [Google Scholar] [CrossRef]
- Chu, S.S.; Jiang, G.H.; Liu, Z.L. Insecticidal compounds from the essential oil of Chinese medicinal herb Atractylodes chinensis. Pest Manag. Sci. 2011, 67, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.C.; Ran, X.H.; Chen, R.; Luo, H.R.; Liu, Y.Q.; Zhou, J.; Zhao, Y.X. Germacrane-type sesquiterpenoids from the roots of Valeriana officinalis var. latifolia. J. Nat. Prod. 2010, 73, 1563–1567. [Google Scholar] [CrossRef]
- Maatooq, G.T.; Hoffmann, J.J. Fungistatic sesquiterpenoids from Parthenium. Phytochemistry 1996, 43, 67–69. [Google Scholar] [CrossRef]
- Li, R.S.; Zhu, J.H.; Guo, D.; Li, H.L.; Wang, Y.; Ding, X.P.; Mei, W.L.; Chen, Z.B.; Dai, H.F.; Peng, S.Q. Genome-wide identification and expression analysis of terpene synthase gene family in Aquilaria sinensis. Plant Physiol. Biochem. 2021, 164, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Azzarina, A.B.; Mohamed, R.; Lee, S.Y.; Nazre, M. Temporal and spatial expression of terpene synthase genes associated with agarwood formation in Aquilaria malaccensis Lam. N. Z. J. For. Sci. 2016, 46, 12. [Google Scholar] [CrossRef]
- Lackus, N.D.; Morawetz, J.; Xu, H.; Gershenzon, J.; Dickschat, J.S.; Köllner, T.G. The sesquiterpene synthase pttps5 produces (1s,5s,7r,10r)-guaia-4(15)-en-11-ol and (1s,7r,10r)-guaia-4-en-11-ol in oomycete-infected poplar roots. Molecules 2021, 26, 555. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Drenaggi, E.; Desneux, N.; Maggi, F. Phytol, (E)-nerolidol and spathulenol from Stevia rebaudiana leaf essential oil as effective and eco-friendly botanical insecticides against Metopolophium dirhodum. Ind. Crops Prod. 2020, 155, 112844. [Google Scholar] [CrossRef]
- Luo, C.; Li, D.L.; Wang, Y.; Guo, S.S.; Du, S.S. Bioactivities of 3-butylidenephthalide and n-butylbenzene from the essential oil of ligusticum jeholense against stored-product insects. J. Oleo Sci. 2019, 68, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, C.L.; Klun, J.A.; Bryson, C.T.; Kobaisy, M.; Duke, S.O. Isolation and Identification of Mosquito Bite Deterrent Terpenoids from Leaves of American (Callicarpa americana) and Japanese (Callicarpa japonica) Beautyberry. J. Agric. Food Chem. 2005, 53, 5948–5953. [Google Scholar] [CrossRef]
- Hubert, T.D.; Wiemer, D.F. Ant-repellent terpenoids from Melampodium divaricatum. Phytochemistry 1985, 24, 1197–1198. [Google Scholar] [CrossRef]
- Gijsen, H.J.; Wijnberg, J.B.; de Groot, A. Structure, occurrence, biosynthesis, biological activity, synthesis, and chemistry of aromadendrane sesquiterpenoids. In Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer: Berlin/Heidelberg, Germany, 1995; Volume 64, pp. 149–193. [Google Scholar] [CrossRef]
- Sun, Z.H.; Hu, C.Q.; Wang, J.Y. A new sesquiterpene from Caragana intermediia and its anti-Pyricularia oryzae P-2b activity. Chin. J. Chem. 2008, 26, 831–834. [Google Scholar] [CrossRef]
- Goldsby, G.; Burke, B.A. Sesquiterpene lactones and a sesquiterpene diol from jamaican ambrosia peruviana. Phytochemistry 1987, 26, 1059–1063. [Google Scholar] [CrossRef]
- Scher, J.M.; Speakman, J.B.; Zapp, J.; Becker, H. Bioactivity guided isolation of antifungal compounds from the liverwort Bazzania trilobata (L.) S.F. Gray. Phytochemistry 2004, 65, 2583–2588. [Google Scholar] [CrossRef]
- Wheeler, G.S. Chemotype variation of the weed Melaleuca quinquenervia influences the biomass and fecundity of the biological control agent Oxyops vitiosa. Biol. Control 2006, 36, 121–128. [Google Scholar] [CrossRef]
- Wheeler, G.S.; Ordung, K.M. Secondary metabolite variation affects the oviposition preference but has little effect on the performance of Boreioglycaspis melaleucae: A biological control agent of Melaleuca quinquenervia. Biol. Control 2005, 35, 115–123. [Google Scholar] [CrossRef]
- Martins, C.B.C.; Zarbin, P.H.G. Volatile Organic Compounds of Conspecific-Damaged Eucalyptus benthamii Influence Responses of Mated Females of Thaumastocoris peregrinus. J. Chem. Ecol. 2013, 39, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Harley, R.M. Labiatae. In Families and Genera of Vascular Plants; Kubitzki, K., Kadereit, J.W., Eds.; Springer: Berlin, Germany, 2004; pp. 167–275. [Google Scholar]
- Li, B.; Cantino, P.D.; Olmstead, R.G.; Bramley, G.L.C.; Xiang, C.L.; Ma, Z.H.; Tan, Y.H.; Zhang, D.X. A large-scale chloroplast phylogeny of the Lamiaceae sheds new light on its subfamilial classification. Sci. Rep. 2016, 6, 34343. [Google Scholar] [CrossRef]
- Hashimoto, M.Y.; Costa, D.P.; Faria, M.T.; Ferreira, H.D.; Santos, S.C.; Paula, J.R.; Seraphin, J.C.; Ferri, P.H. Chemotaxonomy of marsypianthes mart. ex benth. based on essential oil variability. J. Braz. Chem. Soc. 2014, 25, 1504–1511. [Google Scholar] [CrossRef]
- Faria, M.T.; Costa, D.P.; Vilela, E.C.; Ribeiro, D.G.; Ferreira, H.D.; Santos, S.C.; Seraphin, J.C.; Ferri, P.H. Chemotaxonomic Markers in Essential Oils of Hypenia (Mart. Ex Benth.) R. Harley. J. Braz. Chem. Soc. 2012, 23, 1844–1852. [Google Scholar] [CrossRef]
- Wawrzyn, G.T.; Quin, M.B.; Choudhary, S.; López-Gallego, F.; Schmidt-Dannert, C. Draft genome of omphalotus olearius provides a predictive framework for sesquiterpenoid natural product biosynthesis in basidiomycota. Chem. Biol. 2012, 19, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Quin, M.B.; Flynn, C.M.; Wawrzyn, G.T.; Choudhary, S.; Schmidt-Dannert, C. Mushroom hunting using bioinformatics: Application of a predictive framework facilitates the selective identification of sesquiterpene synthases in Basidiomycota. ChemBioChem 2013, 14, 2480–2491. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, I.C.; de Almeida, A.S.; Sena Filho, J.G. Taxonomic Insights and Its Type Cyclization Correlation of Volatile Sesquiterpenes in Vitex Species and Potential Source Insecticidal Compounds: A Review. Molecules 2021, 26, 6405. https://doi.org/10.3390/molecules26216405
Barreto IC, de Almeida AS, Sena Filho JG. Taxonomic Insights and Its Type Cyclization Correlation of Volatile Sesquiterpenes in Vitex Species and Potential Source Insecticidal Compounds: A Review. Molecules. 2021; 26(21):6405. https://doi.org/10.3390/molecules26216405
Chicago/Turabian StyleBarreto, Ighor C., Anderson S. de Almeida, and José G. Sena Filho. 2021. "Taxonomic Insights and Its Type Cyclization Correlation of Volatile Sesquiterpenes in Vitex Species and Potential Source Insecticidal Compounds: A Review" Molecules 26, no. 21: 6405. https://doi.org/10.3390/molecules26216405
APA StyleBarreto, I. C., de Almeida, A. S., & Sena Filho, J. G. (2021). Taxonomic Insights and Its Type Cyclization Correlation of Volatile Sesquiterpenes in Vitex Species and Potential Source Insecticidal Compounds: A Review. Molecules, 26(21), 6405. https://doi.org/10.3390/molecules26216405





