1H-NMR-Based Metabolomics: An Integrated Approach for the Detection of the Adulteration in Chicken, Chevon, Beef and Donkey Meat
Abstract
:1. Introduction
2. Results
2.1. 1H-NMR Identification of Metabolites in Different Meat Samples
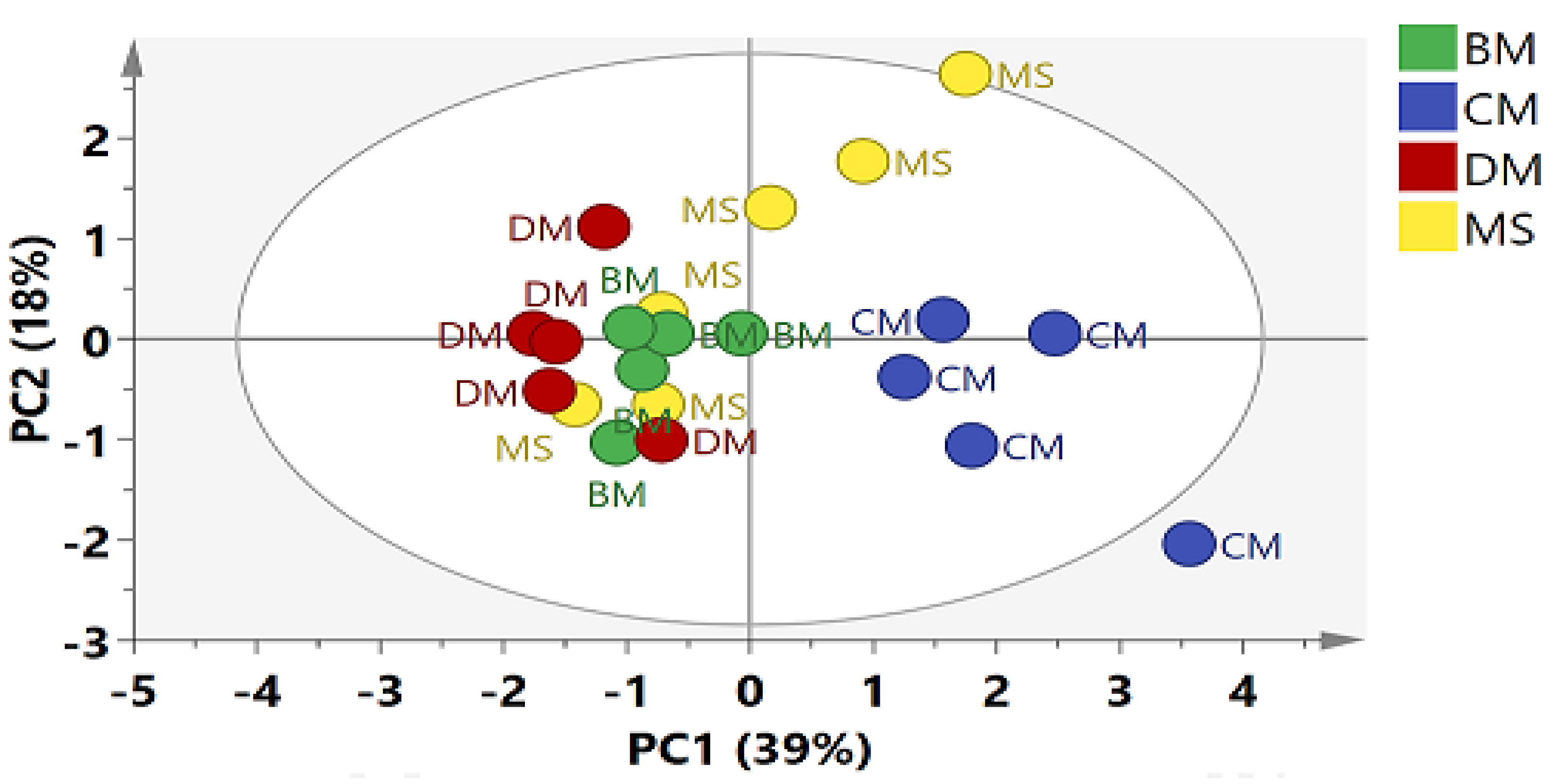
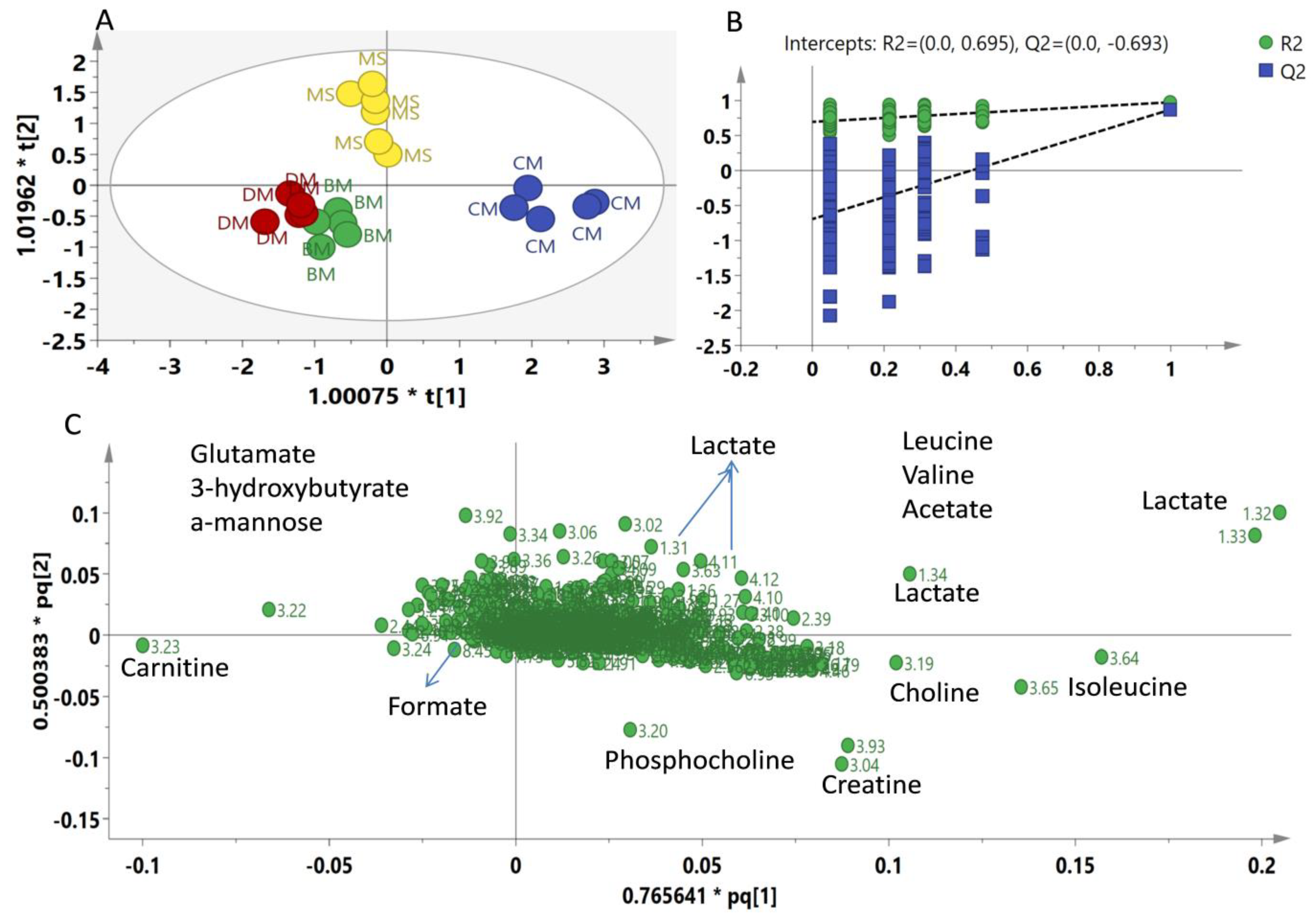
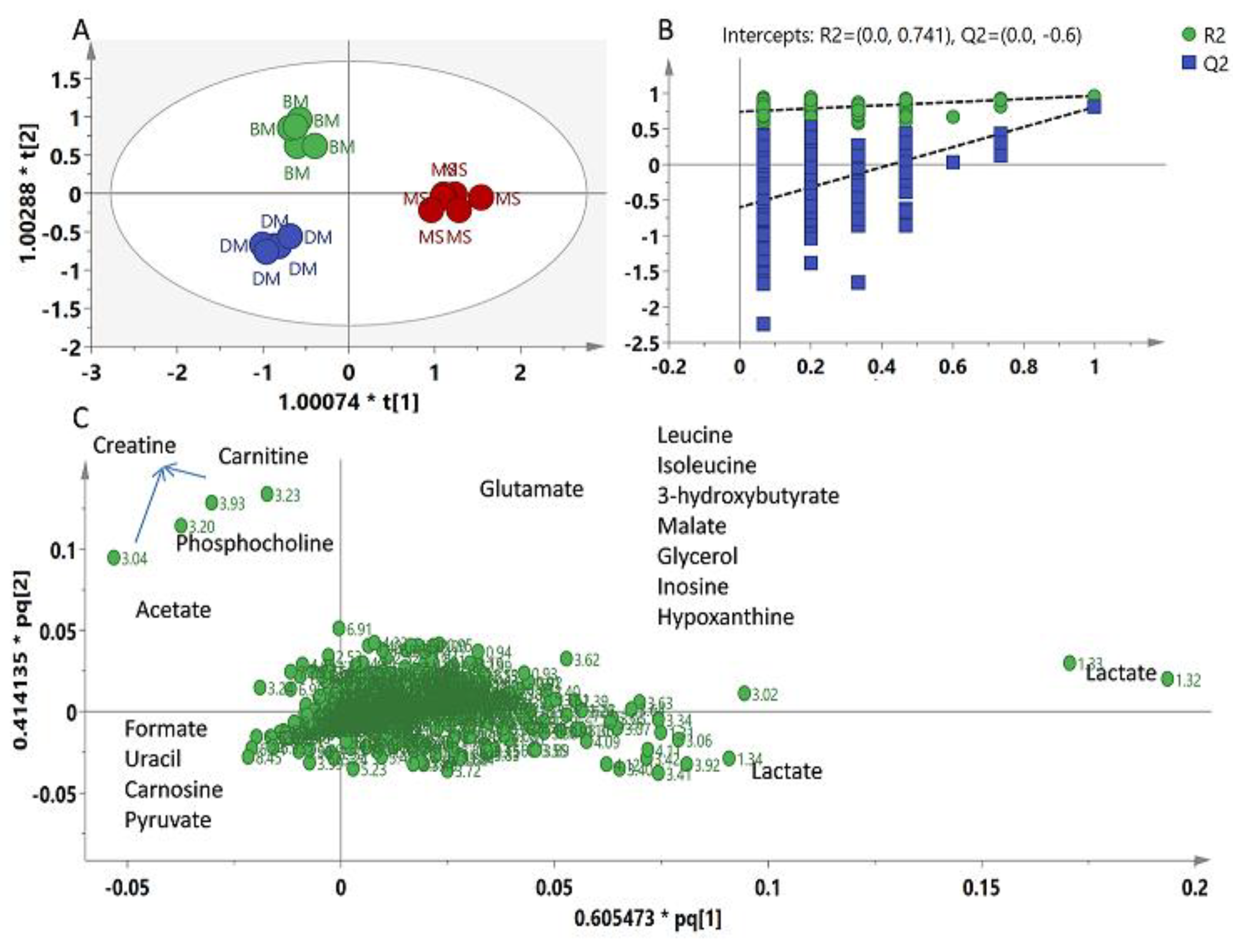
2.2. Multivariate Data Analysis
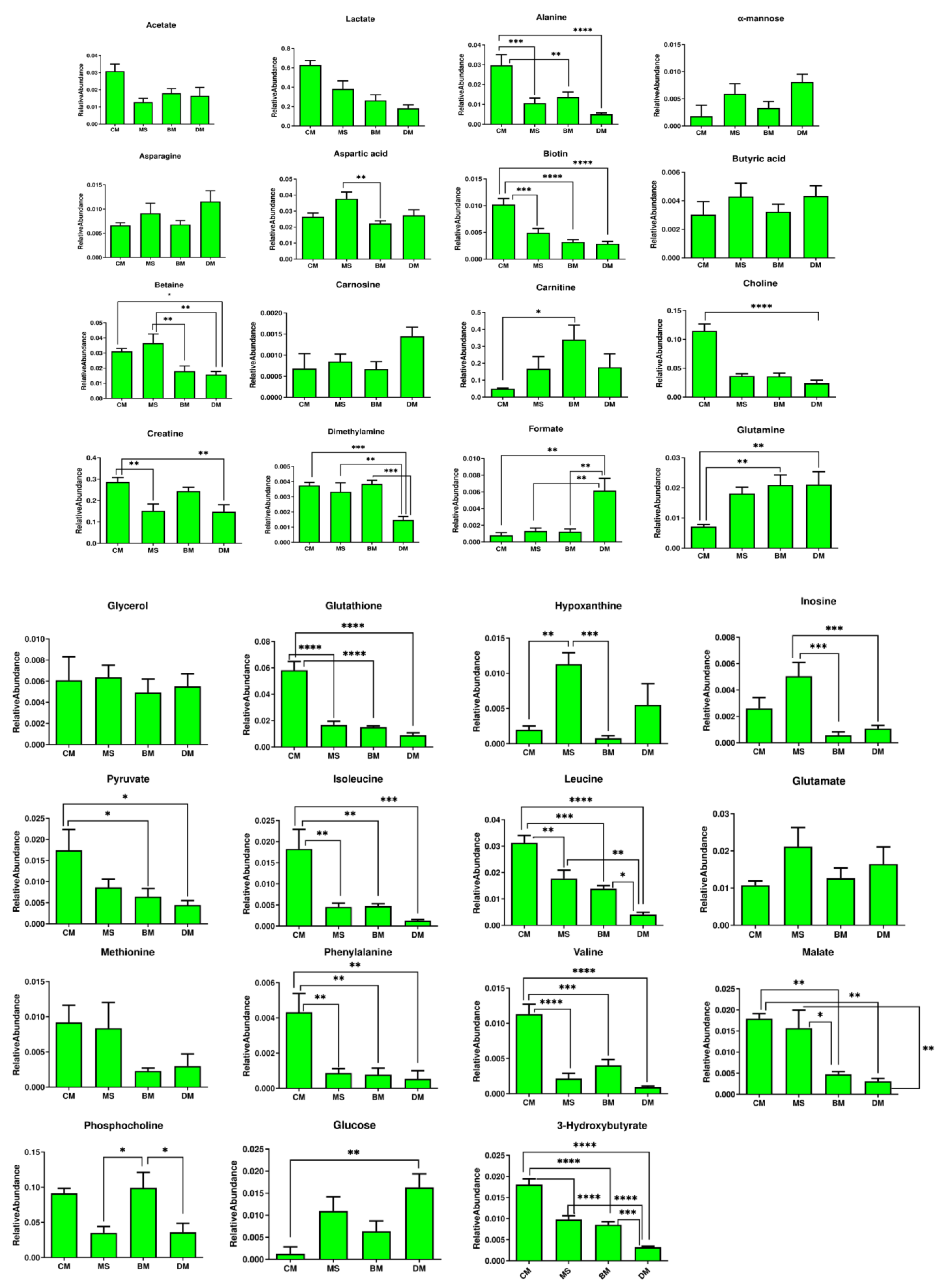
2.3. Relative Quantification
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Biological Samples Collection
4.3. Sample Preparation for NMR Spectroscopy
4.4. NMR Spectroscopy
4.5. NMR Spectral Data Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.W.; Bonham, M.P.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef]
- Jalil, H.; Hussain, S.S.; Siddiqi, A.F. An empirical study of meat supply chain and prices pattern in Lahore (Pakistan): A case study. Int. J. Supply Chain Manag. 2019, 2, 44–52. [Google Scholar]
- Ahmad, R.S.; Imran, A.; Hussain, M.B. Nutritional Composition of Meat. In Meat Science and Nutrition; Arshad, M.S., Ed.; IntechOpen: London, UK, 2018; pp. 61–77. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Aziz, D.M. Molecular and histological investigation of adulterated ready-to-eat heated meat products with chicken substances. Int. Food Res. J. 2018, 25, 1948–1952. [Google Scholar]
- Guntarti, A.; Martono, S.; Yuswanto, A.; Rohman, A. Analysis of beef meatball adulteration with wild boar meat using real-time polymerase chain reaction. Int. Food Res. J. 2017, 24, 2451–2455. [Google Scholar]
- Findus Beef Lasagne Contained up to 100% Horsemeat, FSA Says. Available online: https://www.bbc.com/news/uk-21375594 (accessed on 15 August 2020).
- Traces of Pork DNA Found in Halal Prison Meat. Available online: https://www.bbc.com/news/uk-21302925 (accessed on 22 August 2020).
- Ulca, P.; Balta, H.; Çağın, I.; Senyuva, H.Z. Meat species identification and Halal authentication using PCR analysis of raw and cooked traditional Turkish foods. Meat Sci. 2013, 94, 280–284. [Google Scholar] [CrossRef]
- Karabasanavar, N.S.; Singh, S.P.; Kumar, D.; Shebannavar, S.N. Detection of pork adulteration by highly-specific PCR assay of mitochondrial D-loop. Food Chem. 2014, 145, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Cevallos, J.M.C.; Reyes-De-Corcuera, J.I.; Etxeberria, E.; Danyluk, M.D.; Rodrick, G.E. Metabolomic analysis in food science: A review. Trends Food Sci. Technol. 2009, 20, 557–566. [Google Scholar] [CrossRef]
- Hu, C.; Xu, G. Mass spectrometry based metabolomics analysis for foodomics. Trends Anal. Chem. 2013, 52, 36–46. [Google Scholar] [CrossRef]
- Brennan, L. NMR-based metabolomics: From sample preparation to applications in nutrition research. Prog. Nucl. Magn. Reason. Spectrosc. 2014, 83, 42–49. [Google Scholar] [CrossRef]
- Jakes, W.; Gerdova, A.; Defernez, M.; Watson, A.D.; McCallum, C.; Limer, E.; Colquhoun, I.J.; Williamson, D.C.; Kemsley, E.K. Authentication of beef versus horse meat using 60 MHz 1H NMR spectroscopy. Food Chem. 2015, 175, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castejon, D.; García-Segura, J.M.; Escudero, R.; Herrera, A.; Cambero, M.I. Metabolomics of meat exudate: It’s potential to evaluate beef meat conservation and aging. Anal. Chim. Acta 2015, 901, 1–11. [Google Scholar] [CrossRef]
- Zanardi, E.; Caligiani, A.; Palla, L.; Mariani, M.; Ghidini, S.; Di Ciccio, P.A.; Palla, G.; Ianieri, A. Metabolic profiling by 1H NMR of ground beef irradiated at different irradiation doses. Meat Sci. 2015, 103, 83–89. [Google Scholar] [CrossRef]
- Xiao, Z.; Ge, C.; Zhoua, G.; Zhanga, W.; Liao, G. 1H NMR-based metabolic characterization of Chinese Wuding chicken meat. Food Chem. 2019, 274, 574–582. [Google Scholar] [CrossRef]
- Bischof, G.; Witte, F.; Terjung, N.; Januschewski, E.; Heinz, V.; Juadjur, A.; Gibis, M. Analysis of aging type and aging time-related changes in the polar fraction of metabolome of beef by 1H NMR spectroscopy. Food Chem. 2020, 342, 128353. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Petracci, M.; Li, C.; Fiore, E.; Laghi, L. An Untargeted Metabolomics Investigation of Jiulong Yak (Bos grunniens) Meat by 1H-NMR. Foods 2020, 9, 481. [Google Scholar] [CrossRef]
- Cônsolo, N.R.B.; Rosa, A.F.; Barbosa, L.C.G.S.; Maclean, P.H.; Padilla, A.H.; Colnago, L.A.; Titto, E.A.L. Preliminary study on the characterization of Longissimus lumborum dark cutting meat in Angus x Nellore crossbreed cattle using NMR-based metabolomics. Meat Sci. 2020, 172, 108350. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Ko, Y.J.; Jo, C. Potential of 2D qNMR spectroscopy for distinguishing chicken breeds based on the metabolic differences. Food Chem. 2020, 342, 128316. [Google Scholar] [CrossRef] [PubMed]
- Muroya, S.; Ueda, S.; Komatsu, T.; Miyakawa, T.; Ertbjerg, P. MEATabolomics: Muscle and Meat Metabolomics in Domestic Animals. Metabolites 2020, 10, 188. [Google Scholar] [CrossRef]
- Shintu., L.; Caldarelli, S.; Franke, B.M. Pre-selection of potential molecular markers for the geographic origin of dried beef by HR-MAS NMR spectroscopy. Meat Sci. 2007, 76, 700–707. [Google Scholar] [CrossRef]
- Straadt, I.K.; Aaslyng, M.D.; Bertram, H.C. An NMR-based metabolomics study of pork from different crossbreeds and relation to sensory perception. Meat Sci. 2013, 96, 719–728. [Google Scholar] [CrossRef]
- Behan, A.A.; Akhtar, M.T.; Chwen, L.T.; Fakurazi, S.; Kaka, U.; Muhamad, A.; Samsudin, A.A. Meat quality, fatty acid content and NMR metabolic profile of dorper sheep supplemented with bypass fats. Foods 2021, 10, 1133. [Google Scholar] [CrossRef]
- Graham, S.F.; Kennedy, T.; Chevallier, O.; Gordon, A.; Farmer, L.; Elliott, C.; Moss, B. The application of NMR to study changes in polar metabolite concentrations in beef longissimus dorsi stored for different periods post mortem. Metabolomics 2010, 6, 395–404. [Google Scholar] [CrossRef]
- Simmler, C.; Napolitano, J.G.; McAlpine, J.B.; Chen, S.N.; Pauli, G.F. Universal quantitative NMR analysis of complex natural samples. Curr. Opin. Biotechnol. 2014, 25, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S. Quantitative metabolomics using NMR. Trends Anal. Chem. 2008, 27, 228–237. [Google Scholar] [CrossRef]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.D.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- Witowski, N.; Lusczek, E.; Determan, C.; Lexcen, D.; Mulier, K.; Ostrowski, B.; Beilman, G. A Four-Compartment Metabolomics Analysis of the Liver, Muscle, Serum, and Urine Response to Polytrauma with Hemorrhagic Shock following Carbohydrate Prefeed. PLoS ONE 2015, 10, e0124467. [Google Scholar] [CrossRef] [Green Version]
- Sacco, D.; Brescia, M.A.; Buccolieri, A.; Jambrenghi, A.C. Geographical origin and breed discrimination of Apulian lamb meat samples by means of analytical and spectroscopic determinations. Meat Sci. 2005, 71, 542–548. [Google Scholar] [CrossRef]
- Straadt, I.K.; Aaslyng, M.D.; Bertram, H.C. Assessment of meat quality by NMR-an investigation of pork products originating from different breeds. Magn. Reson. Chem. 2011, 49, S71–S78. [Google Scholar] [CrossRef]
- Egan, A.F. Lactic acid bacteria of meat and meat products. Antonie Van Leeuwenhoek 1983, 49, 327–336. [Google Scholar] [CrossRef]
- Puolanne, E. Lactic acid in muscle and its effects on meat quality. In Proceedings of the 55th Annual Reciprocal Meats Conference; American Meat Science Association: Kearney, MO, USA, 2002; Volume 55, pp. 57–62. [Google Scholar]
- Rosenvold, K.; Essen-Gustavsson, B.; Andersen, H.J. Dietary manipulation of pro-and macroglycogen in porcine skeletal muscle. J. Anim. Sci. 2002, 81, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Halestrap, A.P.; Price, N.T. The proton-linked monocarboxylate transporter (MCT) family: Structure, function and regulation. Biochem. J. 1999, 343, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Batlle, N.; Aristoy, M.C.; Toldrá, F. Early postmortem detection of exudative pork meat based on nucleotide content. J. Food Sci. 2000, 65, 413–416. [Google Scholar] [CrossRef]
- Tikk, M.; Tikk, K.; Tørngren, M.A.; Meinert, L.; Aaslyng, M.D.; Karlsson, A.H.; Andersen, H.J. Development of inosine monophosphate and its degradation products during aging of pork of different qualities in relation to basic taste and retronasal flavor perception of the meat. J. Agric. Food Chem. 2006, 54, 7769–7777. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shtarkman, I.N.; Smirnova, V.S.; Chernikov, A.V.; Bruskov, V.I. Guanosine and inosine display antioxidant activity, protect DNA in vitro from oxidative damage induced by reactive oxygen species, and serve as radio protectors in mice. Radiat. Res. 2006, 165, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Dalle Zotte, A.; Gleeson, E.; Franco, D.; Cullere, M.; Lorenzo, J.M. Proximate composition, amino acid profile, and oxidative stability of slow-growing indigenous chickens compared with commercial broiler chickens. Foods 2020, 9, 546. [Google Scholar] [CrossRef]
- Sforza, S.; Galaverna, G.; Schivazappa, C.; Marchelli, R.; Dossena, A.; Virgili, R. Effect of extended aging of Parma dry-cured ham on the content of oligopeptides and free amino acids. J. Agric. Food Chem. 2006, 54, 9422–9429. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, V.; Song, Z.; Hwang, I. The potential role of secondary metabolites in modulating the flavor and taste of the meat. Int. Food Res. J. 2019, 122, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched chain amino acids: Beyond nutrition metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, S.; Gatlin III, D.M. Dietary requirement for leucine, isoleucine and valine (branched chain amino acids) by juvenile red drum sciaenops ocellatus. Aquac. Nutr. 2018, 24, 1056–1065. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, L.; Qiao, S.; Mao, X.; Zeng, X. Effects of dietary leucine supplementation in low crude protein diets on performance, nitrogen balance, whole-body protein turnover, carcass characteristics and meat quality of finishing pigs. J. Anim. Sci. 2016, 87, 911–920. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Zeisel, S.H.; da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettigrew, J.; Esnaola, M. Swine nutrition and pork quality: A review. J. Anim. Sci. 2001, 79, 316–342. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Mar, M.H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Alagawany, M.; Arain, M.A.; Abd, M.E.; El-Hack, K.D. Beneficial impacts of choline in animal and human with special reference to its role against fatty liver syndrome. J. Exp. Biol. 2017, 5, 589–598. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Yu, L.; Wang, M.; Liu, S.; Sun, L.; Chen, Q. Effects of supplementation of rumen-protected choline on growth performance, meat quality and gene expression in longissimus dorsi muscle of lambs. Arch. Anim. Nutr. 2015, 69, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Dobenecker, B.; Braun, U. Creatine and creatinine contents in different diet types for dogs-Effects of source and processing. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1017–1024. [Google Scholar] [CrossRef]
- Lee, H.J.; Jayasena, D.D.; Kim, S.H.; Kim, H.J.; Heo, K.N.; Song, J.E.; Jo, C. Comparison of bioactive compounds and quality traits of breast meat from Korean native ducks and commercial ducks. Korean J. Food Sci. Anim. Resour. 2015, 35, 114–120. [Google Scholar] [CrossRef] [Green Version]
- James, B.W.; Goodband, R.D.; Unruh, J.A.; Tokach, M.D.; Nelssen, J.L.; Dritz, S.S. A review of creatine supplementation and its potential to improve pork quality. J. Appl. Anim. Res. 2002, 21, 1–16. [Google Scholar] [CrossRef]
- Li, J.L.; Guo, Z.Y.; Li, Y.J.; Zhang, L.; Gao, F.; Zhou, G.H. Effect of creatine monohydrate supplementation on carcass traits, meat quality and postmortem energy metabolism of finishing pigs. Anim. Prod. Sci. 2016, 56, 48–54. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Klebanov, G.I.; Teselkin, Y.O.; Babenkova, I.V.; Popov, I.N.; Levin, G.; Tyulina, O.V.; Boldyrev, A.A.; Vladimirov, Y.A. Evidence for a direct interaction of superoxide anion radical with carnosine. Biochem. Mol. Biol. Educ. 1997, 43, 99–106. [Google Scholar] [CrossRef]
- Hu, X.; Hongtrakul, K.; Ji, C.; Ma, Q.; Guan, S.; Song, C.; Zhang, Y.; Zhao, L. Effect of carnosine on growth performance, carcass characteristics, meat quality and oxidative stability in broiler chickens. J. Poult. Sci. 2009, 46, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Cong, J.; Zhang, L.; Li, J.; Wang, S.; Gao, F.; Zhou, G. Effects of dietary supplementation with carnosine on growth performance, meat quality, antioxidant capacity and muscle fiber characteristics in broiler chickens. J. Sci. Food Agric. 2017, 97, 3733–3741. [Google Scholar] [CrossRef] [PubMed]
- Antonelo, D.S.; Cônsolo, N.R.B.; Gómez, J.F.M.; Beline, M.; Pavan, B.; Souza, C.; Goulart, R.S.; Colnago, L.A.; Silva, S.L. NMR-based metabolomics to assess metabolites correlated with beef sensory properties. Proceedings of 65th International Congress of Meat Science and Technology (ICoMST 2019), Potsdam, Germany, 4–9 August 2019; pp. 801–803. [Google Scholar]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta Mol. Cell. Res. 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, J.; Josephkwon, K.S.; Dohyunryu, L.; Hwang, G.S. Discrimination of the Geographical Origin of Beef by 1H NMR-Based Metabolomics. J. Agric. Food Chem. 2010, 58, 10458–10466. [Google Scholar] [CrossRef] [PubMed]
- Ritota, M.; Casciani, L.; Failla, S.; Valentini, M. HRMAS-NMR spectroscopy and multivariate analysis meat characterization. Meat Sci. 2012, 92, 754–761. [Google Scholar] [CrossRef]
- Kim, Y.H.B.; Kemp, R.; Samuelsson, L.M. Effects of dry-aging on meat quality attributes and metabolite profiles of beef loins. Meat Sci. 2015, 111, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Kodani, Y.; Miyakawa, T.; Komatsu, T.; Tanokura, M. NMR-based metabolomics for simultaneously evaluating multiple determinants of primary beef quality in Japanese Black cattle. Sci. Rep. 2017, 7, 1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Jiang, G.; Kebreab, E.; Li, J.; Feng, X.; Li, C.; Zhang, X.; Huang, X.; Fang, C.; Fang, R.; et al. 1H NMR-based metabolomics study of breast meat from Pekin and Linwu duck of different ages and relation to meat quality. Int. Food Res. J. 2020, 133, 109126. [Google Scholar] [CrossRef]
- Liu, C.; Pan, D.; Ye, Y.; Cao, J. 1H NMR and multivariate data analysis of the relationship between the age and quality of duck meat. Food Chem. 2013, 141, 1281–1286. [Google Scholar] [CrossRef]
- Wang, X.; Fang, C.; He, J.; Dai, Q.; Fang, R. Comparison of the meat metabolite composition of Linwu and Pekin ducks using 600 MHz 1H nuclear magnetic resonance spectroscopy. Poult. Sci. J. 2017, 96, 192–199. [Google Scholar] [CrossRef]
- Beauclercq, S.; Desbarats, L.N.; Antier, C.H.; Collin, A.; Tesseraud, S.; Bourin, M.; Duval, E.L.B.; Berri, C. Serum and Muscle Metabolomics for the Prediction of Ultimate pH, a Key Factor for Chicken-Meat Quality. J. Proteome Res. 2016, 15, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.I.L.; Mappley, L.J.; Ragione, R.M.L.; Woodward, M.J.; Claus, S.P. NMR-based metabolic characterization of chicken tissues and biofluids: A model for avian research. Metabolomics 2016, 12, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Z.; Luo, Y.; Wang, G.; Ge, C.; Zhou, G.; Zhanga, W.; Liao, G. 1H-NMR-based water-soluble low molecular weight compound characterization and fatty acid composition of boiled Wuding chicken during processing. J. Sci. Food Agric. 2018, 99, 429–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundekilde, U.K.; Rasmussen, M.K.; Young, J.F.; Bertram, H.C. High resolution magic angle spinning NMR spectroscopy reveals that pectoralis muscle dystrophy in chicken is associated with reduced muscle content of anserine and carnosine. Food Chem. 2016, 217, 151–154. [Google Scholar] [CrossRef] [PubMed]
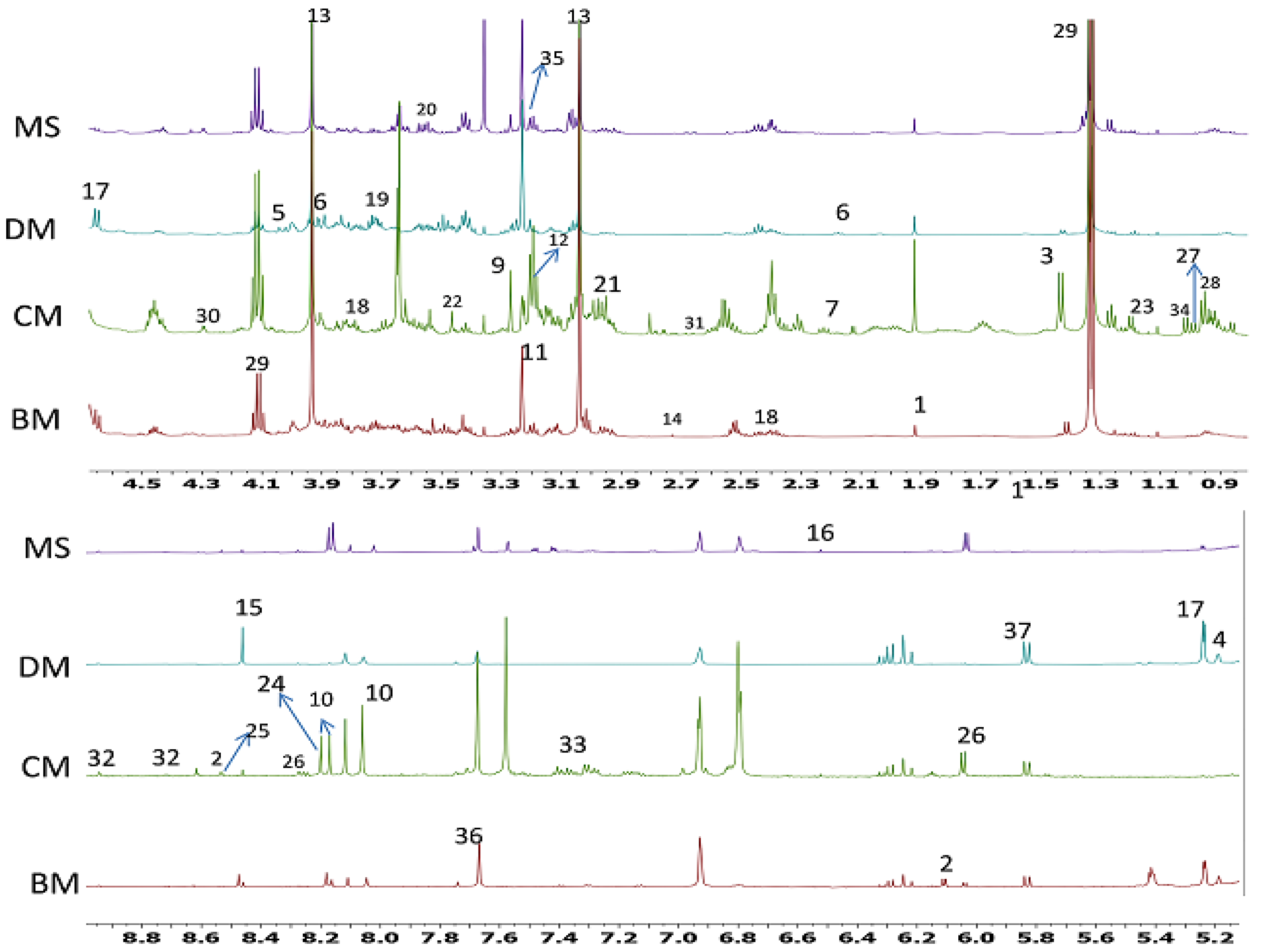
| Number | Metabolites | Multiplicity |
|---|---|---|
| 1 | Acetate | 1.92 (s) |
| 2 | ADP/AMP/ATP | 6.11(d), 8.27(s); 8.54(s) |
| 3 | Alanine | 1.48/1.43 (d) |
| 4 | α -Mannose | 5.18 (d) |
| 5 | Asparagine | 2.94 (m), 4.00 (dd) |
| 6 | Aspartic acid | 3.90 (dd) |
| 7 | Biotin | 2.22 (t), 3.10 (m) |
| 8 | Butyric acid | 0.860 (t); 2.175 (t) |
| 9 | Betaine | 3.27 (s), 3.90 (s) |
| 10 | Carnosine | 8.18 (s), 8.06 (s), 7.12 (s), 2.7 (m), 4.5 (m), 2.97 (dd) |
| 11 | Carnitine | 2.40 (m), 3.23 (s) |
| 12 | Choline | 3.18 (s), 4.06 (m) |
| 13 | Creatine | 3.04 (s), 3.93 (s) |
| 14 | Dimethylamine | 2.73 (s) |
| 15 | Formate | 8.45 (s) |
| 16 | fumarate | 6.51 (s) |
| 17 | Glucose | 4.64 (d), 5.24 (d) |
| 18 | Glutamate | 2.06 (m), 2.37 (m), 3.76 (m) |
| 19 | Glutamine | 2.12 (m), 2.44 (m), 3.70 (m) |
| 20 | Glycerol | 3.54 (dd), 3.62 (dd) |
| 21 | Glutathione | 2.54 (m); 2.97 (dd) |
| 22 | Glycine | 3.51 (s) |
| 23 | 3-hydroxybutyrate | 1.20 (d); 2.400 (m); 2.430 (m) |
| 24 | Hypoxanthine | 8.17 (s), 8.20 (s) |
| 25 | IMP | 8.53 (s), 8.21 (s) |
| 26 | Inosine | 3.88 (m), 3.83 (m), 4.28 (m), 4.44 (m), 6.03 (d), 6.12 (d), 8.18 (s), 8.28 (s) |
| 27 | Isoleucine | 0.92 (t); 0.99 (d); 1.23 (m); 3.64 (d) |
| 28 | leucine | 0.94 (d), 0.96 (d), 3.72 (m) |
| 29 | Lactate | 1.32 (d), 4.11 (q) |
| 30 | Malate | 4.29 (dd) |
| 31 | Methionine | 2.14 (m), 2.66 (dd), 3.78 (m) |
| 32 | Niacinamide | 7.60 (m), 8.71 (dd), 8.94 (m) |
| 33 | Phenylalanine | 7.32 (m), 7.37 (m), 7.42 (m) |
| 34 | Valine | 0.99 (d), 1.02 (d), 2.28 (m) |
| 35 | Phosphocholine | 3.20 (s), 4.17 (m) |
| 36 | Pyruvate | 7.67 (s) |
| 37 | Uracil | 5.83 (d) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, M.T.; Samar, M.; Shami, A.A.; Mumtaz, M.W.; Mukhtar, H.; Tahir, A.; Shahzad-ul-Hussan, S.; Chaudhary, S.U.; Kaka, U. 1H-NMR-Based Metabolomics: An Integrated Approach for the Detection of the Adulteration in Chicken, Chevon, Beef and Donkey Meat. Molecules 2021, 26, 4643. https://doi.org/10.3390/molecules26154643
Akhtar MT, Samar M, Shami AA, Mumtaz MW, Mukhtar H, Tahir A, Shahzad-ul-Hussan S, Chaudhary SU, Kaka U. 1H-NMR-Based Metabolomics: An Integrated Approach for the Detection of the Adulteration in Chicken, Chevon, Beef and Donkey Meat. Molecules. 2021; 26(15):4643. https://doi.org/10.3390/molecules26154643
Chicago/Turabian StyleAkhtar, Muhammad Tayyab, Muneeba Samar, Anam Amin Shami, Muhammad Waseem Mumtaz, Hamid Mukhtar, Amna Tahir, Syed Shahzad-ul-Hussan, Safee Ullah Chaudhary, and Ubedullah Kaka. 2021. "1H-NMR-Based Metabolomics: An Integrated Approach for the Detection of the Adulteration in Chicken, Chevon, Beef and Donkey Meat" Molecules 26, no. 15: 4643. https://doi.org/10.3390/molecules26154643
APA StyleAkhtar, M. T., Samar, M., Shami, A. A., Mumtaz, M. W., Mukhtar, H., Tahir, A., Shahzad-ul-Hussan, S., Chaudhary, S. U., & Kaka, U. (2021). 1H-NMR-Based Metabolomics: An Integrated Approach for the Detection of the Adulteration in Chicken, Chevon, Beef and Donkey Meat. Molecules, 26(15), 4643. https://doi.org/10.3390/molecules26154643








