Bioactive Diterpenes from the Brazilian Native Plant (Moquiniastrum pulchrum) and Their Application in Weed Control
Abstract
1. Introduction
2. Results and Discussion
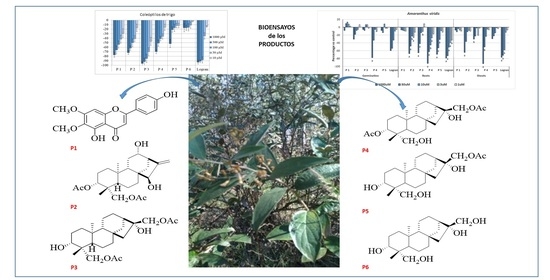
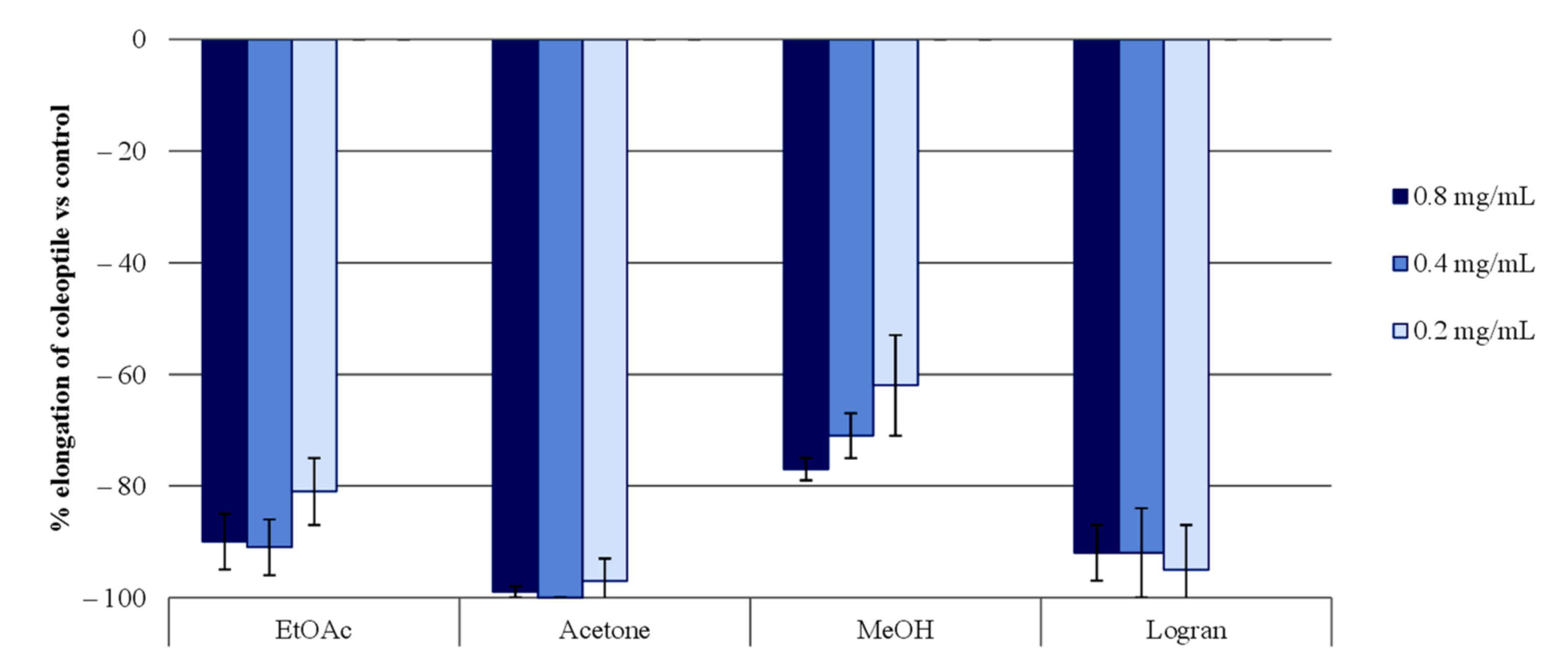
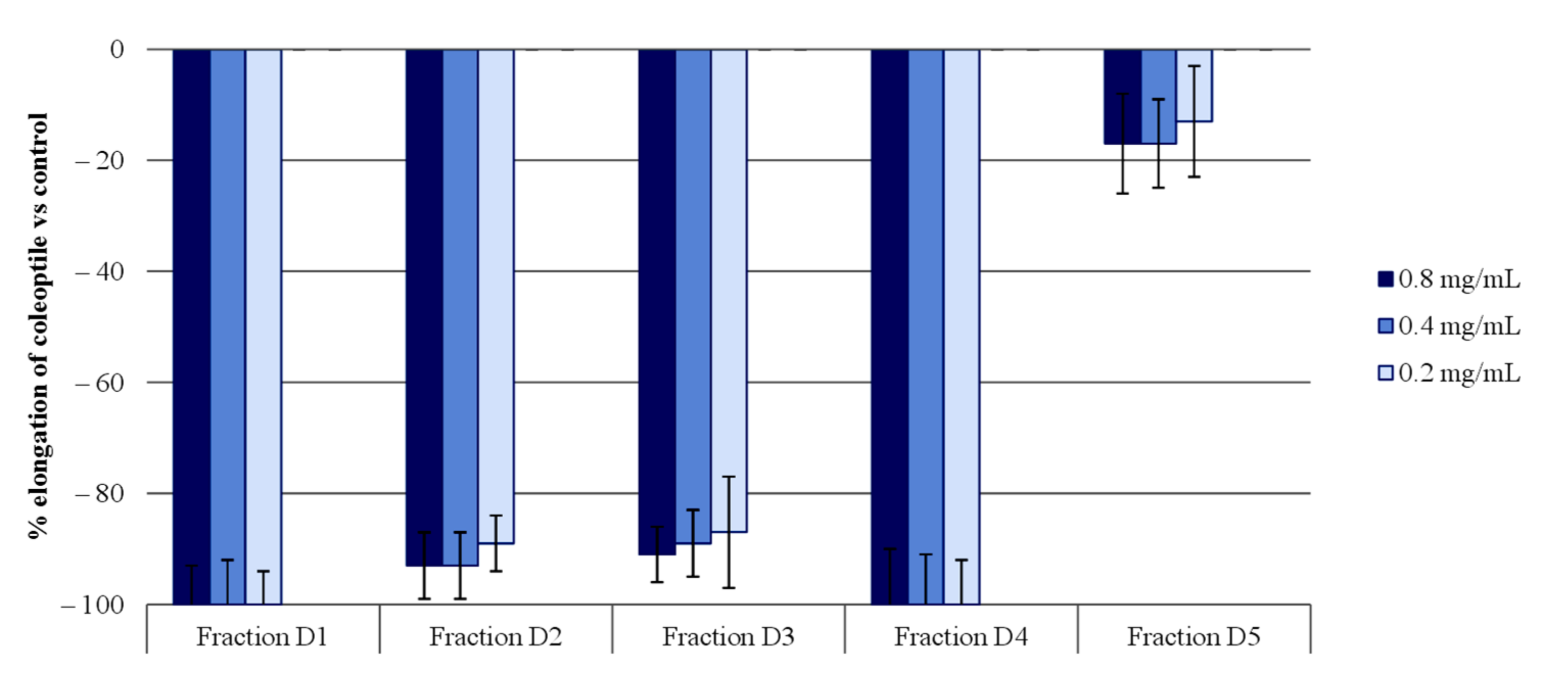
2.1. Extract Separation Process
2.2. Isolation of Phytotoxic Compounds
2.3. Wheat Coleoptile Bioassays on Isolated Compounds
2.4. Phytotoxic Bioassay on the Most Active Compounds
3. Material and Methods
3.1. General Experimental Procedures
3.2. Organic Solvents
3.3. Preparation of Extracts and Isolation of Their Compounds
3.4. Spectroscopic Data of the New Compounds
3.5. Coleoptiles Bioassay
3.6. Phytotoxic Bioassay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Myers, N.; Mittermier, A.R.; Mittermier, C.G.; Fonseca, G.A.B. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Klink, C.A.; Machado, R.B. Conservation of the Brazilian Cerrado. Conserv. Biol. 2005, 19, 707–713. [Google Scholar] [CrossRef]
- Malhado, A.C.M.; Pires, G.F.; Costa, M.H. Cerrado Conservation is Essential to Protect the Amazon Rainforest. Ambio 2010, 39, 580–584. [Google Scholar] [CrossRef]
- Strassburg, B.B.N.; Brooks, T.; Feltran-Barbieri, R.; Iribarrem, A.; Crouzeilles, R.; Loyola, R.; Latawiec, A.E.; De Scaramuzza, C.A.M.; Oliveira Filho, F.J.B.; Scarano, F.R. Moment of truth for the Cerrado hotspot. Nat. Ecol. Evol. 2017, 1, 1–3. [Google Scholar] [CrossRef]
- Novaes, P.; Molinillo, J.M.G.; Varela, R.M.; Macías, F.A. Ecological phytochemistry of Cerrado (Brazilian savanna) plants. Phytochem. Rev. 2013, 12, 839–855. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy; Kozlowski, T.T., Ed.; Academic Press: New York, NY, USA, 1984; ISBN 9781483267845. [Google Scholar]
- Weir, T.L.; Park, S.-W.; Vivanco, J.M. Biochemical and physiological mechanisms mediated by allelochemicals. Curr. Opin. Plant. Biol. 2004, 7, 472–479. [Google Scholar] [CrossRef]
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Uddin, M.R.; Park, S.U.; Dayan, F.E.; Pyon, J.Y. Herbicidal activity of formulated sorgoleone, a natural product of sorghum root exudate. Pest. Manag. Sci. 2014, 70, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Tranel, P.J.; Wright, T.R. Resistance of weeds to ALS-inhibiting herbicides: What have we learned? Weed Sci. 2002, 50, 700–712. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E.; Rimando, A.M.; Schrader, K.K.; Aliotta, G.; Oliva, A.; Romagni, J.G. Invited Paper: Chemicals from nature for weed management. Weed Sci. 2002, 50, 138–151. [Google Scholar] [CrossRef]
- Cala, A.; Salcedo, J.R.; Torres, A.; Varela, R.M.; Molinillo, J.M.G.; Macias, F.A. A Study on the Phytotoxic Potential of the Seasoning Herb Marjoram (Origanum majorana L.) Leaves. Molecules 2021, 26, 3356. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Gandara, F.; Torres, A.; Fernández-Ponce, M.T.; Molinillo, J.M.G.; Casas, L.; Mantell, C.; Varela, R.M.; Martinez de la Ossa, J.E.; Macias, F.A. Selective Fractionation and Isolation of Allelopathic Compounds from Helianthus annuus L. Leaves by Means of High-Pressure Techniques. J. Supercrit. Fluids 2019, 143, 32–41. [Google Scholar] [CrossRef]
- El Marsni, Z.; Torres, A.; Varela, R.M.; Molinillo, J.M.G.; Casas, L.; Mantell, C.; Martinez de la Ossa, E.J.; Macias, F.A. Isolation of bioactive compounds from sunflower leaves (Helianthus annuus L.) extracted with supercritical carbon dioxide. J. Agric. Food Chem. 2015, 63, 6410–6421. [Google Scholar] [CrossRef] [PubMed]
- Rial, C.; Garcia, B.F.; Varela, R.M.; Torres, A.; Molinillo, J.M.G.; Macias, F.A. The Joint Action of Sesquiterpene Lactones from Leaves as an Explanation for the Activity of Cynara cardunculus. J. Agric. Food Chem. 2016, 64, 6416–6424. [Google Scholar] [CrossRef]
- Zappi, D.C.; Ranzato Filardi, F.L.; Leitman, P.; Souza, V.C.; Walter, B.M.T.; Pirani, J.R.; Morim, M.P.; Queiroz, L.P.; Cavalcanti, T.B.; Mansano, V.F. Growing knowledge: An overview of Seed Plant diversity in Brazil. Rodriguesia 2015, 66, 1085–1113. [Google Scholar] [CrossRef]
- Sobral, M.; Stehmann, J.R. An analysis of new angiosperm species discoveries in Brazil (1990–2006). Taxon 2009, 58, 227–232. [Google Scholar] [CrossRef]
- Nakajima, J.N.; Junqueira, T.V.; Freitas, F.S.; Teles, A.M. Comparative analysis of red lists of the Brazilian flora: Asteraceae. Rodriguesia 2012, 63, 39–54. [Google Scholar] [CrossRef]
- Sancho, G.; Funk, V.A.; Roque, N. Moquiniastrum (Gochnatieae, Asteraceae): Disentangling the paraphyletic Gochnatia. Phytotaxa 2013, 147, 26–34. [Google Scholar] [CrossRef]
- Gonçalves, V.M.; Moreira, A.S.; Oliveira, P.D. Genus Moquiniastrum (Asteraceae): Overview of Chemical and Bioactivity Studies. Curr. Bioact. Compd. 2019, 15, 377–398. [Google Scholar] [CrossRef]
- Silva, L.B.; Strapasson, R.L.B.; Riva, D.; Salvador, M.J.; Stefanello, M.E.A. Triterpenes from the flowers of Gochnatia polymorpha subsp. floccosa. Rev. Bras. Farmacogn. 2011, 21, 556–559. [Google Scholar] [CrossRef]
- Lucarini, R.; Tozatti, M.G.; Silva, M.L.A.; Gimenez, V.M.M.; Pauletti, P.M.; Groppo, M.; Turatti, I.C.C.; Cunha, W.R.; Martins, C.H.G. Antibacterial and anti-inflammatory activities of an extract, fractions, and compounds isolated from Gochnatia pulchra aerial parts. Braz. J. Med. Biol. Res. 2015, 48, 822–830. [Google Scholar] [CrossRef]
- Duke, S.O.; Abbas, H.K. Natural products with potential use as herbicides. In Allelopathy. Organisms, Processes, and Applications; Inderjit Dakshini, K.M.M., Einhellig, F.A., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 348–362. ISBN 9780841230613. [Google Scholar]
- Dayan, F.E.; Duke, S.O. Natural Compounds as Next-Generation Herbicides. Plant. Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowska, A. Allelochemicals as bioherbicides-present and perspectives. In Herbicides-Current Research and Case Studies in Use; Price, A.J., Kelton, J.A., Eds.; Books on Demand: Norderstedt, Germany, 2013; pp. 517–542. ISBN 9789535111122. [Google Scholar]
- Macias, F.A.; Oliveros-Bastidas, A.; Marín, D.; Carrera, C.; Chinchilla, N.; Molinillo, J.M.G. Plant biocommunicators: Their phytotoxicity, degradation studies and potential use as herbicide models. Phytochem. Rev. 2007, 7, 179–194. [Google Scholar] [CrossRef]
- Almeida, A.M.; Fonseca, C.R.; Prado, P.I.; Almeida-Neto, M.; Diniz, S.; Kubota, U.; Braun, M.R.; Raimundo, R.L.G.; Anjos, L.A.; Mendonça, T.G.; et al. Diversidade e ocorrência de Asteraceae em Cerrados de São Paulo. Biota Neotrop. 2005, 5, 1–17. [Google Scholar] [CrossRef][Green Version]
- Cutler, S.J.; Hoagland, R.E.; Cutler, H.G. Evaluation of selected pharmaceuticals. In Allelopathy in Ecological Agriculture and Forestry; Narwal, S.S., Hoagland, R.E., Dilday, R.H., Reigosa Roger, M.J., Eds.; Springer Science & Business Media: Berlin, Germany, 2000; pp. 129–137. ISBN 9789401141734. [Google Scholar]
- Mitscher, L.A.; Rao, G.S.R.; Veysoglu, T.; Drake, S.; Haas, T. Isolation and identification of trachyloban-19-oic and kaur-16-en-19-oic acids as antimicrobial agents from the prairie sunflowers, Helianthus annuus. J. Nat. Prod. 1983, 46, 745–746. [Google Scholar] [CrossRef]
- Picman, A.; Schneider, E.; Gershenzon, J. Antifugal activities of sunflower terpenoids. Biochemistry 1990, 18, 325–328. [Google Scholar] [CrossRef]
- Liu, Y.; Ho, D.K.; Cassady, J.M.; Cook, V.M.; Baird, W.M. Isolation of potential cáncer chemopreventive agents from Eriodictyon califorcum. J. Nat. Prod. 1992, 55, 357–363. [Google Scholar] [CrossRef]
- Sacilotto, A.C.B.C.; Vichnewski, W.; Herz, W. Ent-kaurene diterpenes from Gochnatia polymorpha var. polymorpha. Phytochemistry 1997, 44, 659–661. [Google Scholar] [CrossRef]
- Qui, Y.K.; Kang, T.G.; Dou, D.Q.; Liang, L.; Dong, F. Three novel compounds from the leaves of Smallanthus sonchifoius. J. Asian Nat. Prod. Res. 2008, 10, 1109–1115. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q.; Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Li, C.-H.; Luo, S.-H.; Li, S.-H.; Gao, J.-M. New antifeedant grayanane diterpenoids from the flowers of Pieris formosa. Molecules 2017, 22, 1431. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.P.; Nepomuceno, M.P.; Varela, R.M.; Torres, A.; Molinillo, J.M.G.; Alves, P.L.C.A.; Macías, F.A. Phytotoxicity Study on Bidens Sulphurea Sch. Bip. as a Preliminary Approach for Weed Control. J. Agric. Food Chem. 2017, 65, 5161–5172. [Google Scholar] [CrossRef] [PubMed]
- Macias, F.A.; Castellano, D.; Molinillo, J.M.G. Search for a Standard Phytotoxic Bioassay for Allelochemicals. Selection of Standard Target Species. J. Agric. Food Chem. 2000, 48, 2512–2521. [Google Scholar] [CrossRef] [PubMed]
- Castellano, D.; Macías, F.A.; Castellano, M.; Cambronero, R.M. FITOMED (Automated System for Measurement of Variable Lengths). Spain Patent P9901565, 15 June 2001. [Google Scholar]




| 4 a,c | 5 b,c | ||||
|---|---|---|---|---|---|
| Position | δH [J in Hz] | δC | Position | δH [J in Hz] | δC |
| 1a | 1.87 (1H, dd, J = 13; 3, 5 Hz) | 36.8 | 1a | 1.90 (1H, dd, J = 13; 3, 5 Hz) | 36.5 |
| 1b | 1.63 (1H, ddd, J = 13; 13; 3, 5 Hz) | 1b | 1.61 (1H, ddd, J = 13; 13; 4, 5 Hz) | ||
| 2 | 1.77 (2H, m) | 23.5 | 2a | 1.81 (1H, m) | 27.0 |
| 3 | 4.59 (1H, dd, J = 11; 6 Hz) | 82.8 | 2b | 1.69 (1H, m) | |
| 4 | - | 42.5 | 3 | 3.30 (1H, dd, J = 11; 5 Hz) | 79.7 |
| 5 | 0.98 (1H, da J = 13 Hz) | 55.8 | 4 | - | 42.2 |
| 6a | 1.68 (1H, m) | 20.5 | 5 | 0.89 (1H, da, J = 13 Hz) | 55.4 |
| 6b | 1.35 (1H, m) | 6a | 1.69 (1H, m) | 20.2 | |
| 7a | 1.65 (1H, m) | 41.8 | 6b | 1.40 (1H, m) | |
| 7b | 1.47 (1H, m) | 7a | 1.64 (1H, m) | 42.0 | |
| 8 | - | 44.5 | 7b | 1.48 (1H, m) | |
| 9 | 0.99 (1H, m) | 56.2 | 8 | - | 44.3 |
| 10 | - | 38.7 | 9 | 1.03 (1H, m) | 56.6 |
| 11 | 1.53 (1H, m) | 18.4 | 10 | - | 38.6 |
| 12 | 1.52 (2H, m) | 26.0 | 11 | 1.58 (2H, m) | 18.0 |
| 13 | 2.04 (1H, m) | 45.9 | 12 | 1.56 (2H, m) | 25.8 |
| 14a | 1.83 (1H, d, J = 13 Hz) | 38.2 | 13 | 2.08 (1H, ta, J = 3, 5 Hz) | 45.4 38.2 |
| 14b | 0.96 (1H, dd, J = 13; 5 Hz) | 14a | 1.85 (1H, d, J = 13 Hz) | 38.2 | |
| 15a | 1.57 (1H, d, J = 15 Hz) | 52.7 | 14b | 0.89 (1H, dd, J = 13; 5 Hz) | |
| 15b | 1.48 (1H, d, J = 15 Hz) | 15a | 1.62 (1H, d, J = 15 Hz) | 52.4 | |
| 6 | - | 79.8 | 15b | 1.44 (1H, d, J = 15 Hz) | |
| 17 | 4.20 (2H, sa) | 68.4 | 16 | - | 79.3 |
| 18 | 1.04 (3H, s) | 22.4 | 17a | 4.25 (1H, sa) | 68.0 |
| 19a | 4.12 (1H, d, J = 12 Hz) | 63.8 | 17b | 4.16 (1H, sa) | |
| 19b | 3.36 (1H, t, J = 11 Hz) | 18 | 1.19 (3H, s) | 22.0 | |
| 20 | 0.98 (3H, s) | 18.1 | 19a | 4.07 (1H, d, J = 11, 5 Hz) | 63.7 |
| Ac | 2.09 (3H, s) 2.07 (3H, s) | 21.4 20.9 | 19b | 3.36 (1H, d, J = 11, 5 Hz) | |
| Ac | 2.07 (3H, s) | 20.9 | 20 | 1.05 (3H, s) | 17.3 |
| C=O | - | 171.2 169.8 | Ac | 2.06 (3H, s) | 19.4 |
| C=O | - | 169.8 | C=O | - | 171.0 |
| Compound | IC50 [µg mL−1] | r2 | clogP |
|---|---|---|---|
| 1 | 56.79 | 0.925 | 2.247 |
| 2 | 76.29 | 0.927 | 2.801 |
| 3 | 11.33 | 0.998 | 3.155 |
| 4 | 276 | 0.919 | 3.773 |
| 5 | 1187 | 0.985 | 2.865 |
| 6 | - | - | 1.969 |
| Logran® | 39.45 | 0.950 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vela, F.; Anese, S.; Varela, R.M.; Torres, A.; Molinillo, J.M.G.; Macías, F.A. Bioactive Diterpenes from the Brazilian Native Plant (Moquiniastrum pulchrum) and Their Application in Weed Control. Molecules 2021, 26, 4632. https://doi.org/10.3390/molecules26154632
Vela F, Anese S, Varela RM, Torres A, Molinillo JMG, Macías FA. Bioactive Diterpenes from the Brazilian Native Plant (Moquiniastrum pulchrum) and Their Application in Weed Control. Molecules. 2021; 26(15):4632. https://doi.org/10.3390/molecules26154632
Chicago/Turabian StyleVela, Fátima, Simoni Anese, Rosa M. Varela, Ascensión Torres, José M. G. Molinillo, and Francisco A. Macías. 2021. "Bioactive Diterpenes from the Brazilian Native Plant (Moquiniastrum pulchrum) and Their Application in Weed Control" Molecules 26, no. 15: 4632. https://doi.org/10.3390/molecules26154632
APA StyleVela, F., Anese, S., Varela, R. M., Torres, A., Molinillo, J. M. G., & Macías, F. A. (2021). Bioactive Diterpenes from the Brazilian Native Plant (Moquiniastrum pulchrum) and Their Application in Weed Control. Molecules, 26(15), 4632. https://doi.org/10.3390/molecules26154632