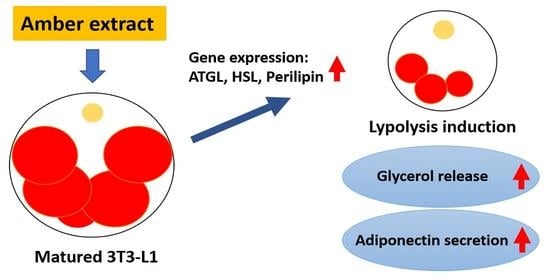
Amber Extract Reduces Lipid Content in Mature 3T3-L1 Adipocytes by Activating the Lipolysis Pathway
Abstract
1. Introduction
2. Results
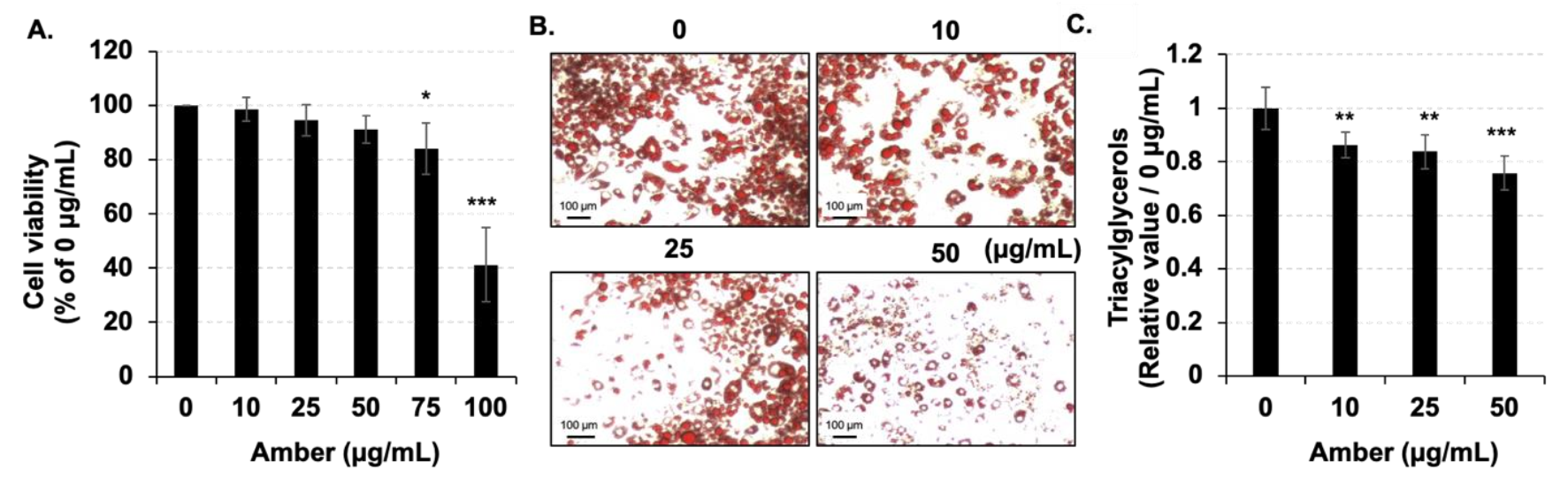
2.1. Amber Reduced the Accumulation of Lipid Droplets in Mature 3T3-L1 Cells
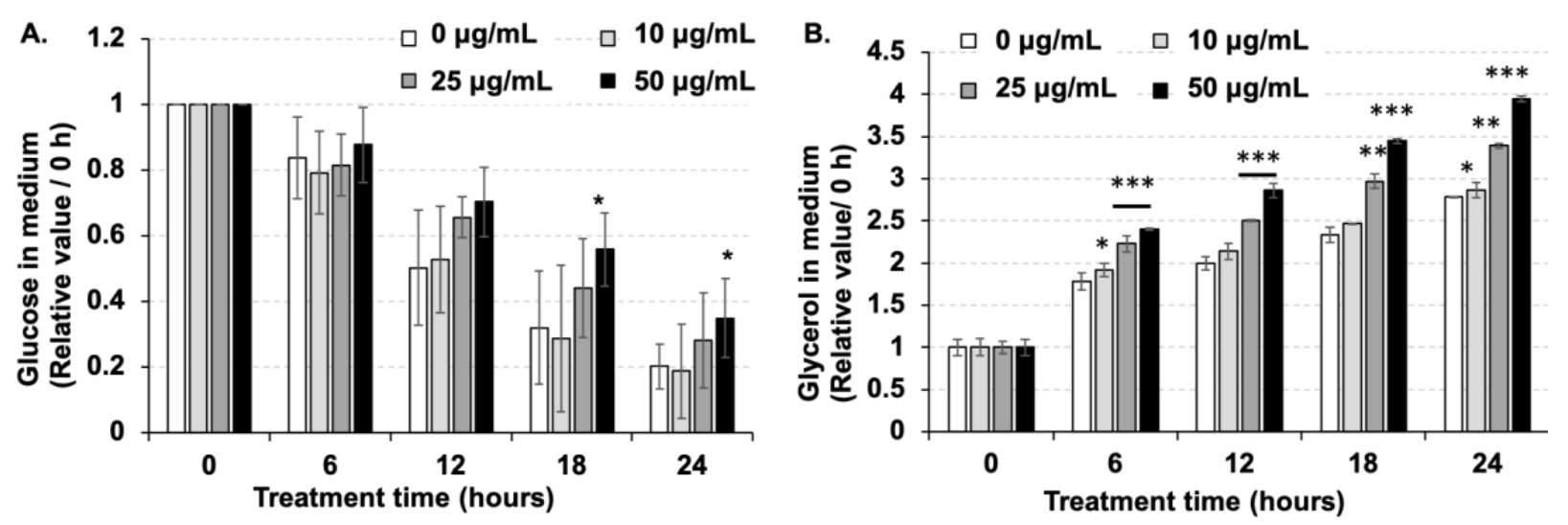
2.2. Amber Inhibited Glucose Uptake and Promoted Glycerol Release in Mature 3T3-L1 Cells
2.3. Treatment with Amber Extract Altered the Expression of Factors Involved in Lipolysis
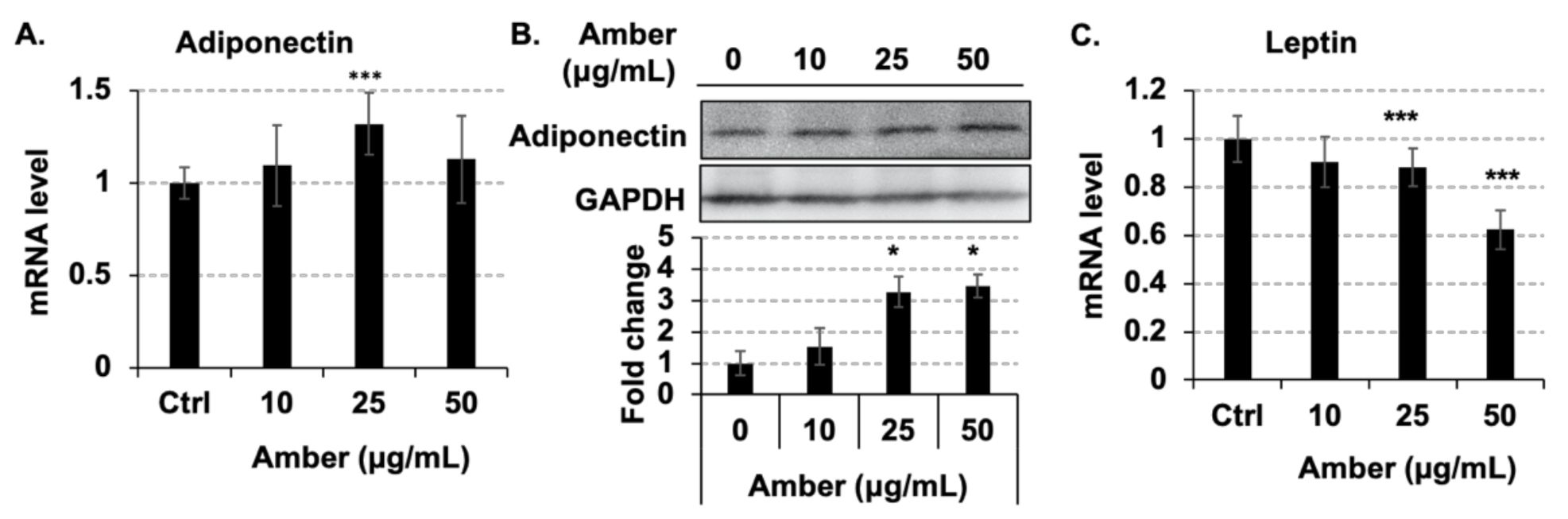
2.4. Administration of Amber Altered the Secretion of Adipocytokines
3. Discussion
4. Materials and Methods
4.1. Amber Extract
4.2. Cell Culture
4.3. Cytotoxicity Test
4.4. Oil Red O Assay
4.5. Glucose Uptake
4.6. Glycerol Release
4.7. Real-Time Polymerase Chain Reaction (RT-PCR)
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- González-Castejón, M.; Rodriguez-Casado, A. Dietary phytochemicals and their potential effects on obesity: A review. Pharmacol. Res. 2011, 64, 438–455. [Google Scholar] [CrossRef]
- He, F.E.; Nowson, C.A.; Lucas, M.; MacGregor, G.A. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: Meta-analysis of cohort studies. J. Hum. Hypertens. 2007, 21, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Miyoshi, N.; Isemura, M. Health-promoting effects of green tea. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012, 88, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.; Qi, X.; Qianyong, Z.; Xiaoli, P.; Jundong, Z.; Mantian, M. Flavonoids, flavonoid subclasses and breast cancer risk: A meta-analysis of epidemiologic studies. PLoS ONE 2013, 8, e54318. [Google Scholar] [CrossRef] [PubMed]
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; Willett, W.; Hu, F.B.; Sun, Q.; van Dam, R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012, 95, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Otto, A.; Krumbiegel, G.; Simoneit, B.R.T. The natural product biomarkers in succinite, glessite and stantienite ambers from Bitterfeld, Germany. Rev. Palaeobot. Palynol. 2006, 140, 27–49. [Google Scholar] [CrossRef]
- Luo, Y.N.; Zhou, S.Q.; Haeiwa, H.; Takeda, R.; Okazaki, K.; Sekita, M.; Yamamoto, T.; Yamano, M.; Sakamoto, K. Role of amber extract in protecting SHSY5Y cells against amyloid β1-42-induced neurotoxicity. Biomed. Pharmacother. 2021, 141, 111804. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, S.Q.; Takeda, R.; Okazaki, K.; Sekita, M.; Sakamoto, K. Anti-inflammatory activities of amber extract in lipopolysaccharide-induced RAW 264.7 macrophages. Biomed. Pharmacother. 2021, 141, 111854. [Google Scholar] [CrossRef]
- Martin, S.; Okano, S.; Kistler, C.; Fernandez-Rojo, M.A.; Hill, M.M.; Parton, R.G. Spatiotemporal regulation of early lipolytic signaling in adipocytes. J. Biol. Chem. 2009, 284, 32097–32107. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Strauss, J.G.; Haemmerle, G.; Schoiswohl, G.; Birner-Gruenberger, R.; Riederer, M.; Lass, A.; Neuberger, G.; Eisenhaber, F.; Hermetter, A.; et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef]
- Haemmerle, G.; Zimmermann, R.; Hayn, M.; Theussl, C.; Waeg, G.; Wagner, E.; Sattler, W.; Magin, T.M.; Wagner, E.F.; Zechner, R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 2002, 277, 4806–4815. [Google Scholar] [CrossRef] [PubMed]
- Senior, J.R.; Isselbacher, K.J. Demonstration of an intestinal monoglyceride lipase: An enzyme with a possible role in the intracellular completion of fat digestion. J. Clin. Invest. 1963, 42, 187–195. [Google Scholar] [CrossRef]
- Sztalryd, C.; Xu, G.; Dorward, H.; Tansey, J.T.; Contreras, J.A.; Kimmel, A.R.; Londos, C. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J. Cell. Biol. 2003, 161, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- McDonough, P.M.; Ingermanson, R.S.; Loy, P.A.; Koon, E.D.; Whittaker, R.; Laris, C.A.; Hilton, J.M.; Nicoll, J.B.; Buehrer, B.M.; Price, J.H. Quantification of hormone sensitive lipase phosphorylation and colocalization with lipid droplets in murine 3T3L1 and human subcutaneous adipocytes via automated digital microscopy and high-content analysis. Assay Drug Dev. Technol. 2011, 9, 262–280. [Google Scholar] [CrossRef]
- Ekmekci, H.; Ekmekci, O.B. The role of adiponectin in atherosclerosis and thrombosis. Clin. Appl. Thromb. Hemost. 2006, 12, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Duncan, B.B.; Schmidt, M.I.; Pankow, J.S.; Bang, H.; Couper, D.; Ballantyne, C.M.; Hoogeveen, R.C.; Heiss, G. Adiponectin and the development of type 2 diabetes: The atherosclerosis risk in communities study. Diabetes 2004, 53, 2473–2478. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed]
- Houseknecht, K.L.; Baile, C.A.; Matteri, R.L.; Spurlock, M.E. The biology of leptin: A review. J. Anim. Sci. 1998, 76, 1405–1420. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Pierce, K.A.; Jedrychowski, M.P.; Garrity, R.; Winther, S.; Vidoni, S.; Yoneshiro, T.; Spinelli, J.B.; Lu, G.Z.; Kazak, L.; et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 2018, 560, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Ives, S.J.; Zaleski, K.S.; Slocum, C.; Escudero, D.; Sheridan, C.; Legesse, S.; Vidal, K.; Lagalwar, S.; Reynolds, T.H. The effect of succinic acid on the metabolic profile in high-fat diet-induced obesity and insulin resistance. Physiol. Rep. 2020, 8, e14630. [Google Scholar] [CrossRef]
- Xiao, N.; Yang, L.L.; Yang, Y.L.; Liu, L.W.; Li, J.; Liu, B.L.; Liu, K.; Qi, L.W.; Li, P. Ginsenoside Rg5 inhibits succinate-associated lipolysis in adipose tissue and prevents muscle insulin resistance. Front. Pharmacol. 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a potential anti-obesity target: A review of pharmacological treatment and natural products. Diabetes Metab. Syndr. Obes. 2021, 14, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Brasaemle, D.L.; Levin, D.M.; Adler-Wailes, D.C.; Londos, C. The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim. Biophys. Acta. 2000, 1483, 251–262. [Google Scholar] [CrossRef]
- Miyoshi, H.; Souza, S.C.; Zhang, H.H.; Strissel, K.J.; Christoffolete, M.A.; Kovsan, J.; Rudich, A.; Kraemer, F.B.; Bianco, A.C.; Obin, M.S.; et al. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J. Biol. Chem. 2006, 281, 15837–15844. [Google Scholar] [CrossRef]
- Anthonsen, M.W.; Rönnstrand, L.; Wernstedt, C.; Degerman, E.; Holm, C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J. Biol. Chem. 1998, 273, 215–221. [Google Scholar] [CrossRef]
- Su, C.L.; Sztalryd, C.; Contreras, J.A.; Holm, C.; Kimmel, A.R.; Londos, C. Mutational analysis of the hormone-sensitive lipase translocation reaction in adipocytes. J. Biol. Chem. 2003, 278, 43615–43619. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Kim, J.Y.; Wall, E.; Laplante, M.; Azzara, A.; Trujillo, M.E.; Hofmann, S.M.; Schraw, T.; Durand, J.L.; Li, H.; Li, G.Y.; et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 2007, 117, 2621–2637. [Google Scholar] [CrossRef]
- Paracchini, V.; Pedotti, P.; Taioli, E. Genetics of leptin and obesity: A HuGE review. Am. J. Epidemiol. 2005, 162, 101–114. [Google Scholar] [CrossRef]
- Ruud, J.; Brüning, J.C. Metabolism: Light on leptin link to lipolysis. Nature 2015, 527, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Y.; Choi, J.H. Korean Curcuma longa L. induces lipolysis and regulates leptin in adipocyte cells and rats. Nutr. Res. Pract. 2016, 10, 487–493. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Landin, K.; Stigendal, L.; Eriksson, E.; Krotkiewski, M.; Risberg, B.; Tengborn, L.; Smith, U. Abdominal obesity is associated with an impaired fibrinolytic activity and elevated plasminogen activator inhibitor-1. Metabolism 1990, 39, 1044–1048. [Google Scholar] [CrossRef]
- Hoo, R.L.C.; Chow, W.S.; Yau, M.H.; Xu, A.; Tso, A.W.K.; Tse, H.F.; Fong, C.H.Y.; Tam, S.; Chan, L.; Lam, K.S.L. Adiponectin mediates the suppressive effect of rosiglitazone on plasminogen activator inhibitor-1 production. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2777–2782. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Pessin, J.E. Insulin regulation of glucose uptake: A complex interplay of intracellular signalling pathways. Diabetologia 2002, 45, 1475–1483. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sogo, E.; Zhou, S.; Haeiwa, H.; Takeda, R.; Okazaki, K.; Sekita, M.; Yamamoto, T.; Yamano, M.; Sakamoto, K. Amber Extract Reduces Lipid Content in Mature 3T3-L1 Adipocytes by Activating the Lipolysis Pathway. Molecules 2021, 26, 4630. https://doi.org/10.3390/molecules26154630
Sogo E, Zhou S, Haeiwa H, Takeda R, Okazaki K, Sekita M, Yamamoto T, Yamano M, Sakamoto K. Amber Extract Reduces Lipid Content in Mature 3T3-L1 Adipocytes by Activating the Lipolysis Pathway. Molecules. 2021; 26(15):4630. https://doi.org/10.3390/molecules26154630
Chicago/Turabian StyleSogo, Erica, Siqi Zhou, Haruna Haeiwa, Reiko Takeda, Kazuma Okazaki, Marie Sekita, Takuya Yamamoto, Mikio Yamano, and Kazuichi Sakamoto. 2021. "Amber Extract Reduces Lipid Content in Mature 3T3-L1 Adipocytes by Activating the Lipolysis Pathway" Molecules 26, no. 15: 4630. https://doi.org/10.3390/molecules26154630
APA StyleSogo, E., Zhou, S., Haeiwa, H., Takeda, R., Okazaki, K., Sekita, M., Yamamoto, T., Yamano, M., & Sakamoto, K. (2021). Amber Extract Reduces Lipid Content in Mature 3T3-L1 Adipocytes by Activating the Lipolysis Pathway. Molecules, 26(15), 4630. https://doi.org/10.3390/molecules26154630