Controlled (Co)Polymerization of Methacrylates Using a Novel Symmetrical Trithiocarbonate RAFT Agent Bearing Diphenylmethyl Groups
Abstract
:1. Introduction
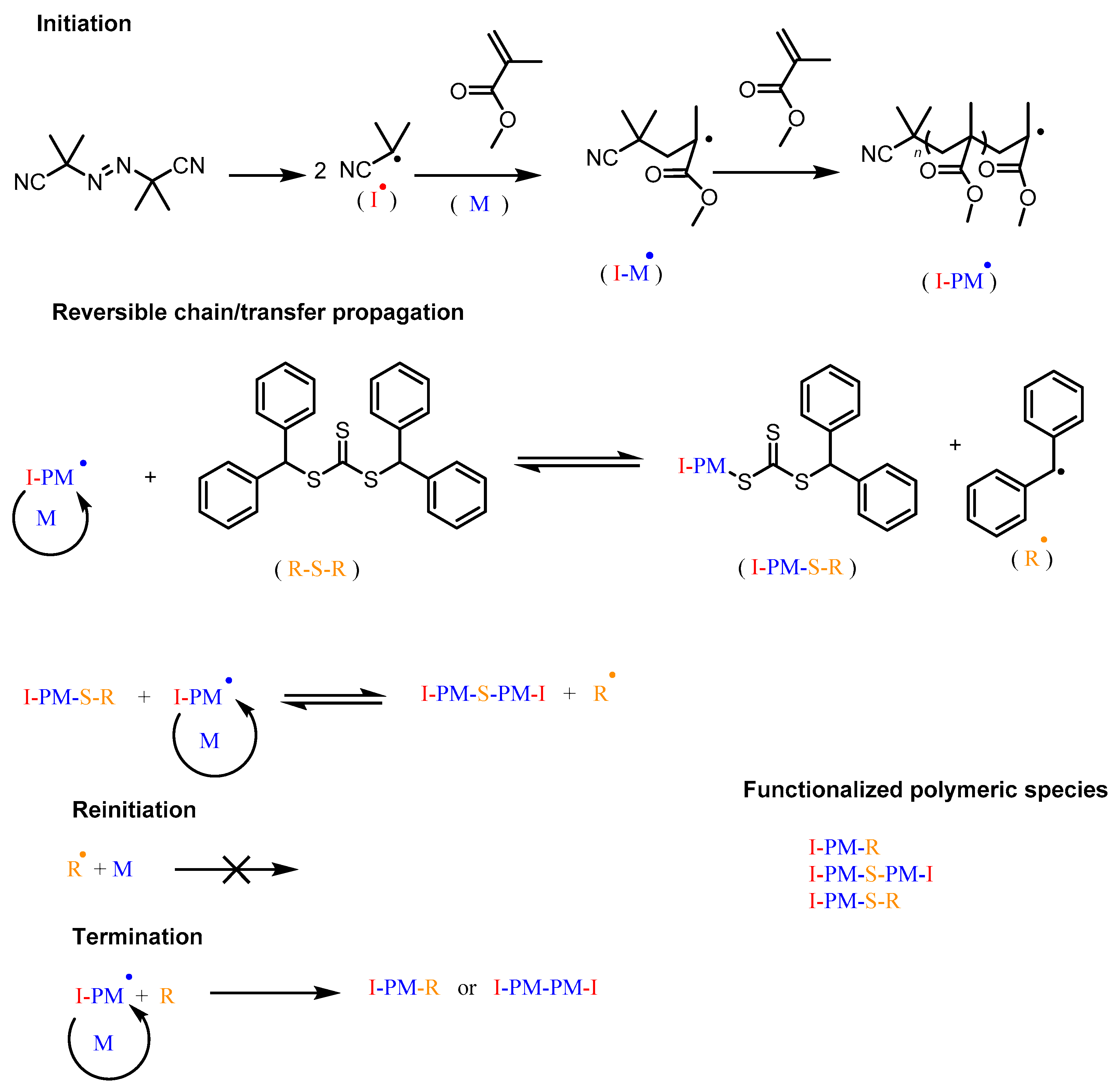
2. Results and Discussion
2.1. Chain Transfer Agents
2.2. Methyl Methacrylate
2.3. Glycidyl Methacrylate and Ethyl Methacrylate
2.4. Styrene and Butyl Acrylate
2.5. Block Copolymerizations
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chiefari, J.; Chong, Y.K.; Ercole, K.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition-Fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Keddie, D.J. A guide to the synthesis of block copolymers using reversible-addition fragmentation chain transfer (RAFT) polymerization. Chem. Soc. Rev. 2014, 43, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Barner-Kowollik, C.; Davis, T.P.; Stenzel, M.H. Synthesis of star polymers using RAFT polymerization: What is possible? Aust. J. Chem. 2006, 59, 719–727. [Google Scholar] [CrossRef]
- Zheng, Q.; Pan, C.-Y. Preparation and characterization of dendrimer-star PNIPAAM using dithiobenzoate-terminated PPI dendrimer via RAFT polymerization. Eur. Polym. J. 2006, 42, 807–814. [Google Scholar] [CrossRef]
- Liu, J.; Cui, K.; Zhao, Q.L.; Huang, J.; Jiang, T.; Ma, Z. New ABA tri-block copolymers of poly(tert-butylacrylate)-b-poly(2,2,2-trifluoroethyl acrylate)-b-poly(tert-butylacrylate): Synthesis, self-assembly and fabrication of their porous films, spheres, and fibers. Eur. Polym. J. 2019, 113, 52–59. [Google Scholar] [CrossRef]
- Smith, A.E.; Xu, X.; Kirkland-York, S.E.; Savin, D.A.; McCormick, C.L. “Schizophrenic” self-assembly of block copolymers synthesized via aqueous RAFT polymerization: From micelles to vesicles. Macromolecules 2010, 43, 1210–1217. [Google Scholar] [CrossRef]
- Zhang, W.; D’Agosto, F.; Dugas, P.-Y.; Rieger, J.; Charleux, B. RAFT-mediated one-pot aqueous emulsion polymerization of methyl methacrylate in presence of poly(methacrylic acid-co-poly(ethylene oxide) methacrylate) trithiocarbonate macromolecular chain transfer agent. Polymer 2013, 54, 2011–2019. [Google Scholar] [CrossRef]
- Jaramillo-Soto, G.; García-Morán, P.R.; Enríquez-Medrano, F.J.; Maldonado-Textle, H.; Albores-Velasco, M.E.; Guerrero-Santos, R.; Vivaldo-Lima, E. Effect of stabilizer concentration and controller structure and composition on polymerization rate and molecular Wright development in RAFT polymerization of styrene in supercritical carbon dioxide. Polymer 2009, 50, 5024–5030. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization-A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Discovery of the RAFT Process and Its Impact on Radical Polymerization. Macromolecules 2020, 53, 495–497. [Google Scholar] [CrossRef] [Green Version]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the RAFT process a third update. Aust. J. Chem. 2012, 65, 985–1076. [Google Scholar] [CrossRef]
- Jenkins, A.D.; Jones, R.G.; Moad, G. Terminology for reversible-deactivation radical polymerization previously called ‘controlled’ radical or ‘living’ radical polymerization (IUPAC recommendations 2010). Pure Appl. Chem. 2010, 82, 483–491. [Google Scholar] [CrossRef]
- Ballard, N.; Asua, J.M. Can We Push Rapid Reversible Deactivation Radical Polymerizations toward Immortality? ACS Macro Lett. 2020, 9, 190–196. [Google Scholar] [CrossRef]
- Goto, A.; Sato, K.; Tsujii, Y.; Fukuda, T.; Moad, G.; Rizzardo, E.; Thang, S.H. Mechanism and kinetics of RAFT-based living radical polymerization of styrene and methyl methacrylate. Macromolecules 2001, 34, 402–408. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Buback, M.; Charleux, B.; Coote, M.L.; Drache, M.; Fukuda, T.; Goto, A.; Klumperman, B.; Lowe, A.B.; Mcleary, J.B.; et al. Mechanism and kinetics of dithiobenzoate-mediated RAFT polymerization. I. The current situation. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5809–5831. [Google Scholar] [CrossRef]
- Thomas, D.B.; Convertine, A.J.; Hester, R.D.; Lowe, A.B.; McCormick, C. Hydrolytic susceptibility of dithioester chain transfer agents and implications in aqueous RAFT polymerizations. Macromolecules 2004, 37, 1735–1741. [Google Scholar] [CrossRef]
- Sahnoun, M.; Charreyre, M.T.; Veron, L.; Delair, T.; D’Agosto, F. Synthetic and characterization aspects of dimethylaminoethyl methacrylate reversible addition fragmentation chain transfer (RAFT) polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3551–3565. [Google Scholar] [CrossRef]
- Summers, G.J.; Mdletshe, T.S.; Summers, C.A. RAFT polymerization of styrene mediated by naphthyl-functionalized trithiocarbonate RAFT agents. Polym. Bull. 2019, 77, 3831–3851. [Google Scholar] [CrossRef]
- Gardiner, J.; Martinez-Botella, I.; Tsanaktsidis, J.; Moad, G. Dithiocarbamate RAFT agents with broad applicability-the 3,5-dimethyl-1H-pyrazole-1-carbodithioates. Polym. Chem. 2016, 7, 481–492. [Google Scholar] [CrossRef]
- Ma’Radzi, A.H.; Sugihara, S.; Toida, T.; Maeda, Y. Synthesis of polyvinyl alcohol stereoblock copolymer via the combination of living cationic polymerization and RAFT/MADIX polymerization using xanthate with vinyl ether moiety. Polymer 2014, 55, 5332–5345. [Google Scholar] [CrossRef] [Green Version]
- Benvenuta-Tapia, J.J.; Vivaldo-Lima, E.; Tenorio-López, J.A.; Vargas-Hernández, M.A.; Vázquez-Torres, H. Kinetic analysis of the RAFT copolymerization of styrene and maleic anhydride by differential scanning calorimetry. Thermochim. Acta 2018, 667, 93–101. [Google Scholar] [CrossRef]
- Sato, T.; Ishida, Y.; Kameyama, A. RAFT homopolymerization of vinylbenzyl chloride with benzyl ethyl trithiocarbonate and synthesis of block copolymers from poly(VBC) macro-RAFT agent and N-isopropylacrylamide. Polym. J. 2014, 46, 239–242. [Google Scholar] [CrossRef]
- Keddie, D.J.; Guerrero-Sanchez, C.; Moad, G. The reactivity of N-vinylcarbazole in RAFT polymerization: Trithiocarbonates deliver optimal control for the synthesis of homopolymers and block copolymers. Polym. Chem. 2013, 4, 3591–3601. [Google Scholar] [CrossRef]
- Mayadunne, R.T.A.; Rizzardo, E.; Chiefari, J.; Krstina, J.; Moad, G.; Postma, A.; Thang, S.H. Living Polymers by the Use of Trithiocarbonates as Reversible Addition-Fragmentation Chain Transfer (RAFT) Agents: ABA Triblock Copolymers by Radical Polymerization in Two Steps. Macromolecules 2000, 33, 243–245. [Google Scholar] [CrossRef]
- Chong, Y.K.; Krstina, J.; Le, T.P.T.; Moad, G.; Postma, A.; Rizzardo, E.; Thang, S.H. Thiocarbonylthio compounds [S=C(Ph)S-R] in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT polymerization). Role of the free-radical leaving group (R). Macromolecules 2003, 36, 2256–2272. [Google Scholar] [CrossRef]
- Perevyazko, I.Y.; Vollrath, A.; Pietsch, C.; Schubert, S.; Pavlov, G.M.; Schubert, U.S. Nanoprecipitation of poly(methyl methacrylate)-based nanoparticles: Effect of the molar mass and polymer behavior. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2906–2913. [Google Scholar] [CrossRef]
- Li, C.; He, J.; Zhou, Y.; Gu, Y.; Yang, Y. Radical-Induced Oxidation of RAFT Agents-A Kinetic Study. Polymer 2011, 49, 1351–1360. [Google Scholar] [CrossRef]
- Qu, T.; Wang, A.; Yuan, J.; Shi, J.; Gao, Q. Preparation and characterization of thermo-responsive amphiphilic triblock copolymer and its self-assembled micelle for controlled drug release. Colloids Surf. B Biointerfaces 2009, 72, 94–100. [Google Scholar] [CrossRef]
- Ma, J.; Yang, C.; Luo, J.; Lu, M. Reversible Addition-Fragmentation Chain Transfer Polymerization of Methyl Methacrylate in Microemulsion: The Influence of Reaction Conditions on Polymerization. J. Macromol. Sci. Part A Pure Appl. Chem. 2012, 49, 321–329. [Google Scholar] [CrossRef]
- Lai, J.T.; Filla, D.; Shea, R. Functional polymers from novel carboxyl-terminated trithiocarbonates as highly efficient RAFT agents. Macromolecules 2002, 35, 6754–6756. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Terpugova, P.S.; Plutalova, A.V.; Garina, E.S.; Sivtsov, E.V. Pseudoliving radical polymerization of methyl methacrylate in the presence of S,S’-Bis(methyl-2-isobutyrate) trithiocarbonate. Polym. Sci. Ser. B 2010, 52, 119–128. [Google Scholar] [CrossRef]
- Favier, A.; Charreyre, M.-T. Experimental requirements for an efficient control of free-radical polymerizations via the Reversible Addition Fragmentation Chain Transfer (RAFT) Process. Macromol. Rapid Commun. 2006, 27, 653–692. [Google Scholar] [CrossRef]
- Willcock, H.; O’Reilly, R.K. End group removal and modification of RAFT polymers. Polym. Chem. 2010, 1, 149–157. [Google Scholar] [CrossRef]
- Steinhauer, W.; Hoogenboom, R.; Keul, H.; Moeller, M. Copolymerization of 2-hydroxyethyl acrylate and 2-methoxyethyl acrylate via RAFT: Kinetics and thermoresponsive properties. Macromolecules 2010, 43, 7041–7047. [Google Scholar] [CrossRef]
- Karahan, O.; Aydin, K.; Edizer, S.; Odabasi, N.; Avci, D. Development of reactive methacrylates based on glycidyl methacrylate. J. Polym. Sci. Part A Polym. Chem. 2013, 48, 3787–3796. [Google Scholar] [CrossRef]
- Boyer, C.; Liu, J.; Wong, L.; Tippett, M.; Bulmus, V.; Davis, T.P. Stability and Utility of Pyridyl Disulfide Functionality in RAFT and Conventional Radical Polymerizations. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7207–7224. [Google Scholar] [CrossRef]
- Bae, S.K.; Lee, S.Y.; Hong, S.C. Thiol-terminated polystyrene through the reversible addition-fragmentation chain transfer technique for the preparation of gold nanoparticles and their application in organic memory devices. React. Funct. Polym. 2011, 71, 187–194. [Google Scholar] [CrossRef]
- Bivigou-Koumba, A.M.; Görnitz, E.; Laschewsky, A.; Müller-Buschbaum, P.; Papadakis, C.M. Thermoresponsive amphiphilic symmetrical triblock copolymers with a hydrophilic middle block made of poly(N-isopropylacrylamide): Synthesis, self-organization, and hydrogel formation. Colloid Polym. Sci. 2010, 288, 499–517. [Google Scholar] [CrossRef]
- Dietrich, S.M.; Smith, A.E. Facile conversion of RAFT polymers into hydroxyl functional polymers: A detailed investigation of variable monomer and RAFT agent combinations. Polym. Chem. 2010, 1, 634–644. [Google Scholar] [CrossRef]

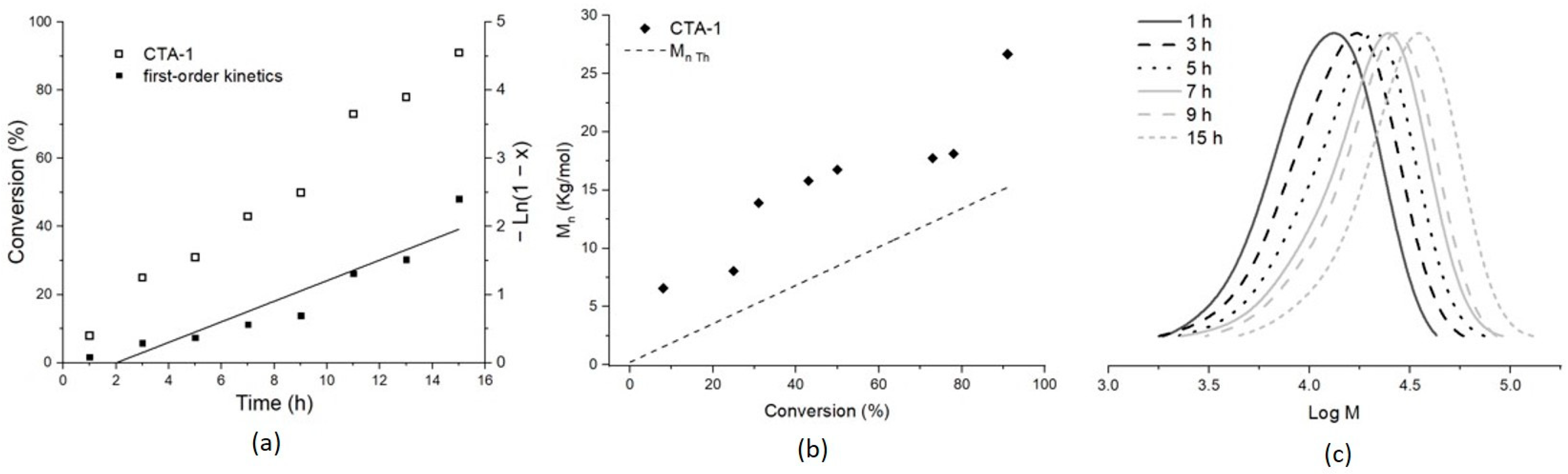
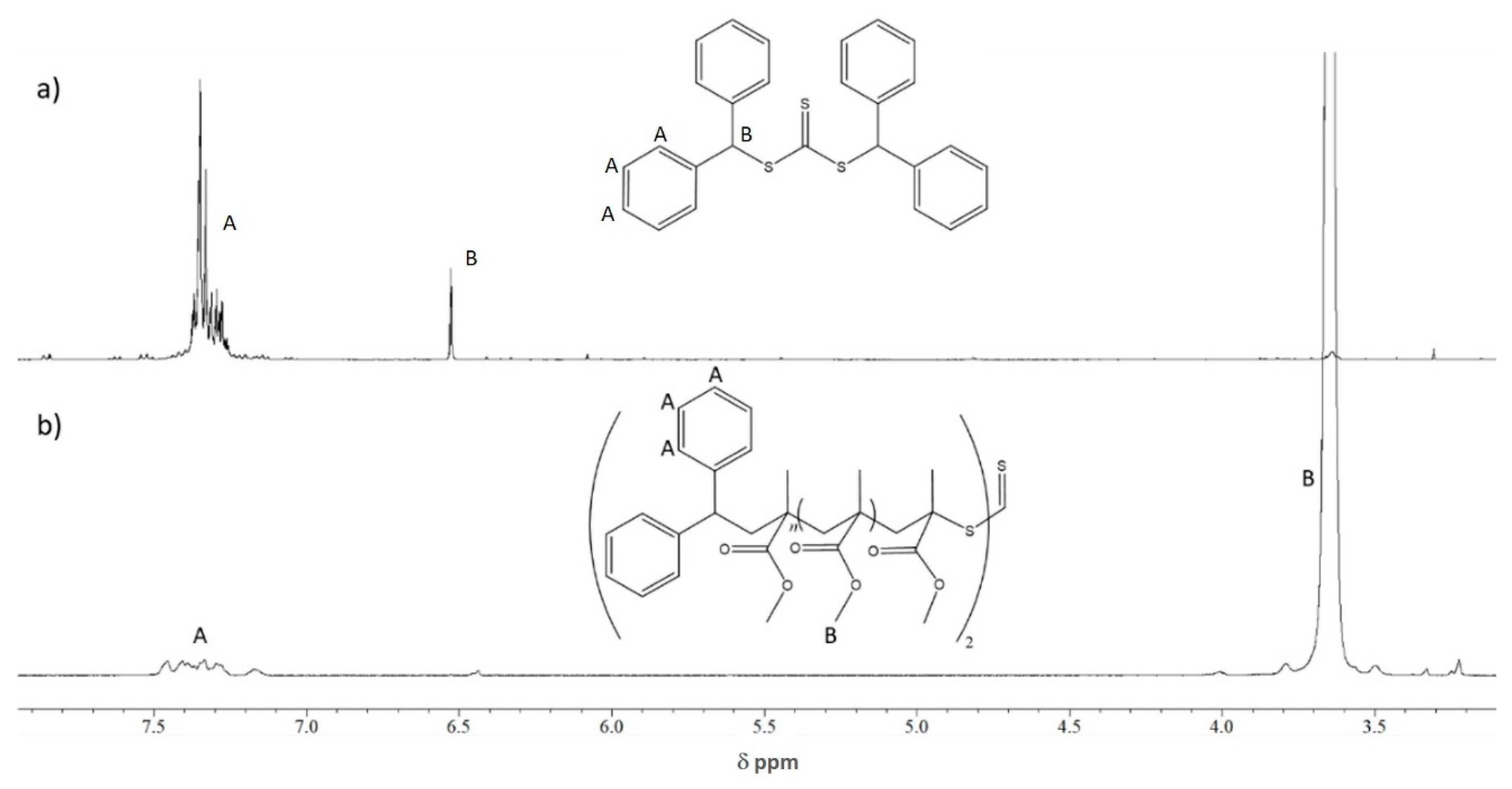
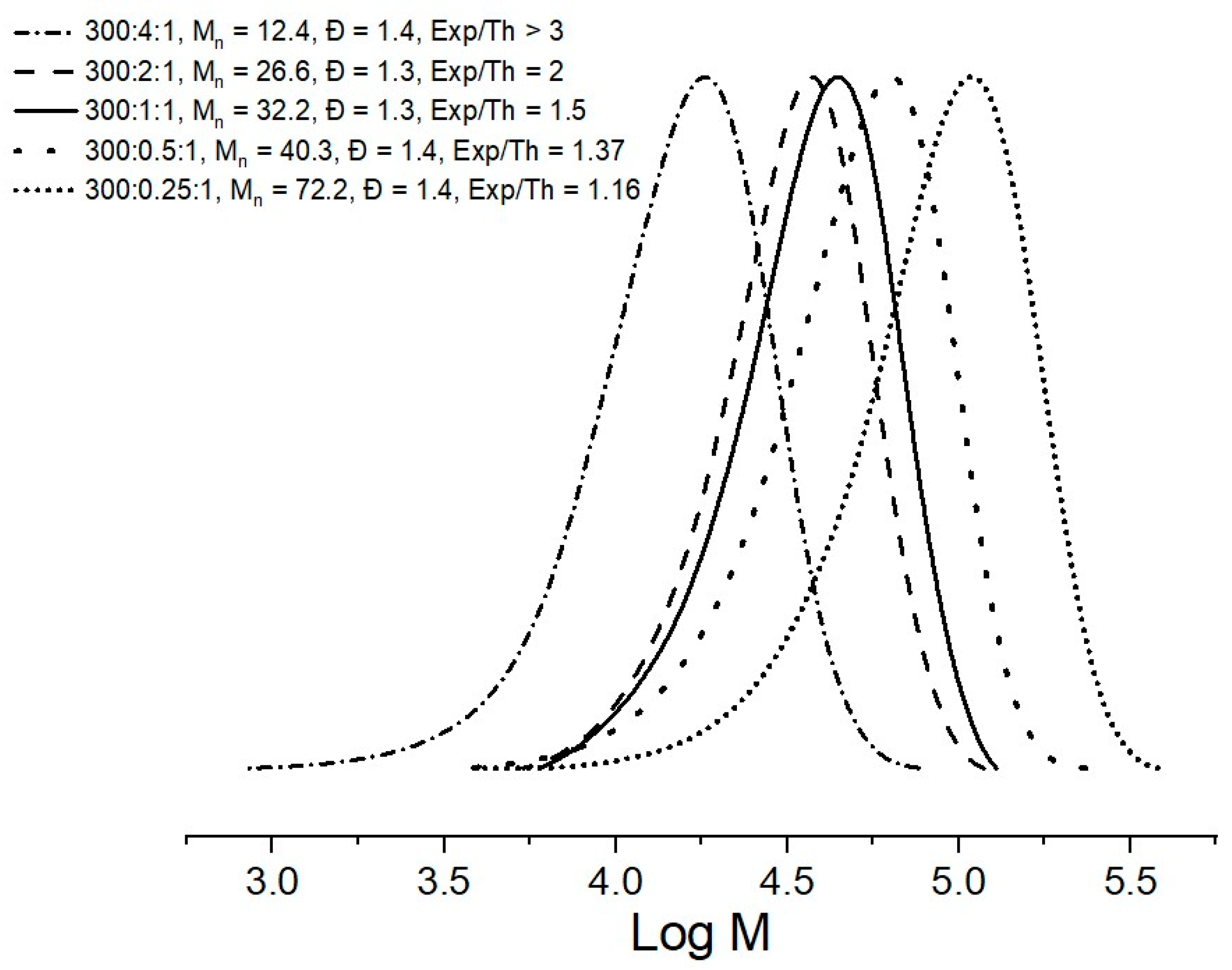
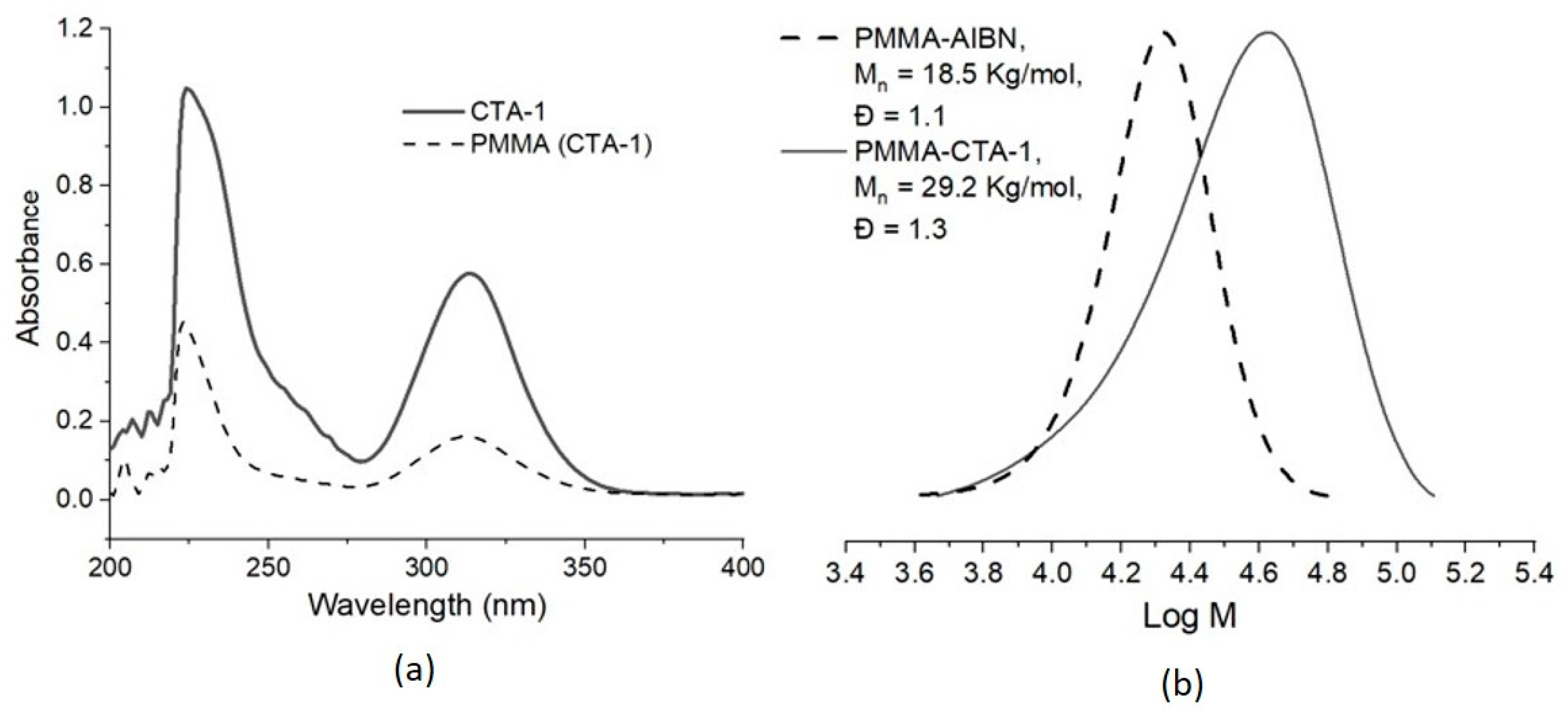

| Entry | CTA | Monomer | Molar Ratio [Monomer]/[CTA]/ [AIBN] | A Conversion (%) | BMn,Th (Kg/mol) | C Mn,SEC (Kg/mol) | Ð |
|---|---|---|---|---|---|---|---|
| 1 | CTA-1 | MMA | 300:2:1 | 91 | 13.9 | 26.6 | 1.3 |
| 2 | CTA-2 | MMA | 300:2:1 | 82 | 12.4 | 189.7 | 2.4 |
| 3 | CTA-1 | MMA | 300:2:0.66 | 70 | 10.7 | 25.8 | 1.3 |
| 4 | CTA-1 | MMA | 300:2:0.33 | 54 | 8.3 | 21.7 | 1.3 |
| 5 | CTA-1 | GMA | 300:2:1 | 96 | 14.6 | 45.1 | 1.9 |
| 6 | CTA-2 | GMA | 300:2:1 | >95 | D n.d. | D n.d. | D n.d. |
| 7 | CTA-1 | EMA | 300:2:1 | 79 | 13.5 | 30.5 | 1.3 |
| 8 | CTA-2 | EMA | 300:2:1 | >95 | 15.4 | 189.3 | 1.9 |
| Entry | CTA | Monomer | A Conversion (%) | B Mn,Th (Kg/mol) | C Mn,SEC (Kg/mol) | Ð |
|---|---|---|---|---|---|---|
| 9 | CTA-1 | St | 3 | 0.7 | 0.4 | 1.1 |
| 10 | CTA-2 | St | 51 | 8.1 | 3.6 | 1.3 |
| 11 | CTA-1 | BuA | 3 | 0.7 | 0.3 | 1.1 |
| 12 | CTA-2 | BuA | 95 | 18.4 | 21.3 | 1.2 |
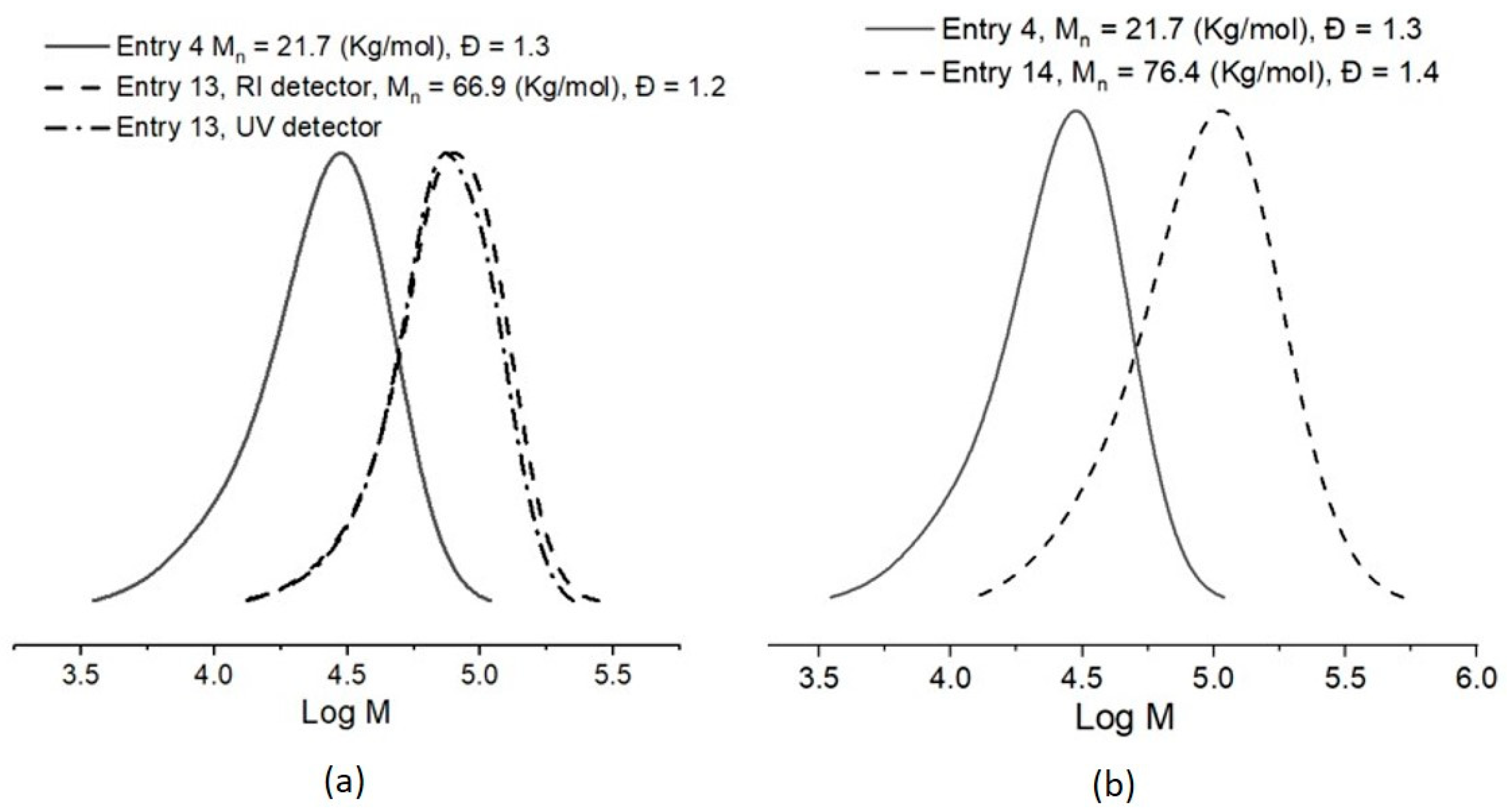
| Entry | Macro-CTA | Mn,SEC (Kg/mol), Ð | Monomer | T (°C), t (h) | Molar Ratio [Monomer]/ [macro-CTA]/ [AIBN] | A Conversion (%) | B Mn,Th (Kg/mol) | C Mn,SEC (Kg/mol) | Ð |
|---|---|---|---|---|---|---|---|---|---|
| 13 | Entry 4 | 21.7, 1.3 | St | 60, 15 | 608:1:1.3 | 65 | 63.9 | 66.9 | 1.2 |
| 14 | Entry 4 | 21.7, 1.3 | BuA | 60, 15 | 509:1:1.3 | 96 | 84.1 | 76.4 | 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robles Grana, A.L.; Maldonado-Textle, H.; Torres-Lubián, J.R.; St Thomas, C.; Díaz de León, R.; Olivares-Romero, J.L.; Valencia, L.; Enríquez-Medrano, F.J. Controlled (Co)Polymerization of Methacrylates Using a Novel Symmetrical Trithiocarbonate RAFT Agent Bearing Diphenylmethyl Groups. Molecules 2021, 26, 4618. https://doi.org/10.3390/molecules26154618
Robles Grana AL, Maldonado-Textle H, Torres-Lubián JR, St Thomas C, Díaz de León R, Olivares-Romero JL, Valencia L, Enríquez-Medrano FJ. Controlled (Co)Polymerization of Methacrylates Using a Novel Symmetrical Trithiocarbonate RAFT Agent Bearing Diphenylmethyl Groups. Molecules. 2021; 26(15):4618. https://doi.org/10.3390/molecules26154618
Chicago/Turabian StyleRobles Grana, Alvaro Leonel, Hortensia Maldonado-Textle, José Román Torres-Lubián, Claude St Thomas, Ramón Díaz de León, José Luis Olivares-Romero, Luis Valencia, and Francisco Javier Enríquez-Medrano. 2021. "Controlled (Co)Polymerization of Methacrylates Using a Novel Symmetrical Trithiocarbonate RAFT Agent Bearing Diphenylmethyl Groups" Molecules 26, no. 15: 4618. https://doi.org/10.3390/molecules26154618
APA StyleRobles Grana, A. L., Maldonado-Textle, H., Torres-Lubián, J. R., St Thomas, C., Díaz de León, R., Olivares-Romero, J. L., Valencia, L., & Enríquez-Medrano, F. J. (2021). Controlled (Co)Polymerization of Methacrylates Using a Novel Symmetrical Trithiocarbonate RAFT Agent Bearing Diphenylmethyl Groups. Molecules, 26(15), 4618. https://doi.org/10.3390/molecules26154618









