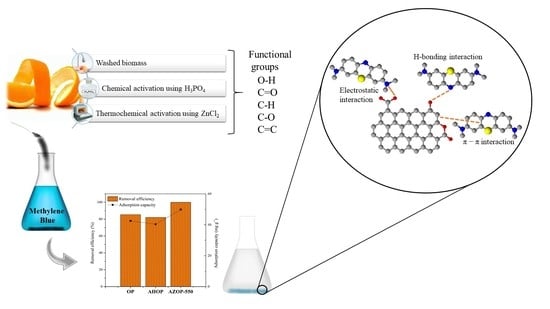
Experimental and Theoretical Insights on Methylene Blue Removal from Wastewater Using an Adsorbent Obtained from the Residues of the Orange Industry
Abstract
:1. Introduction
2. Methodology
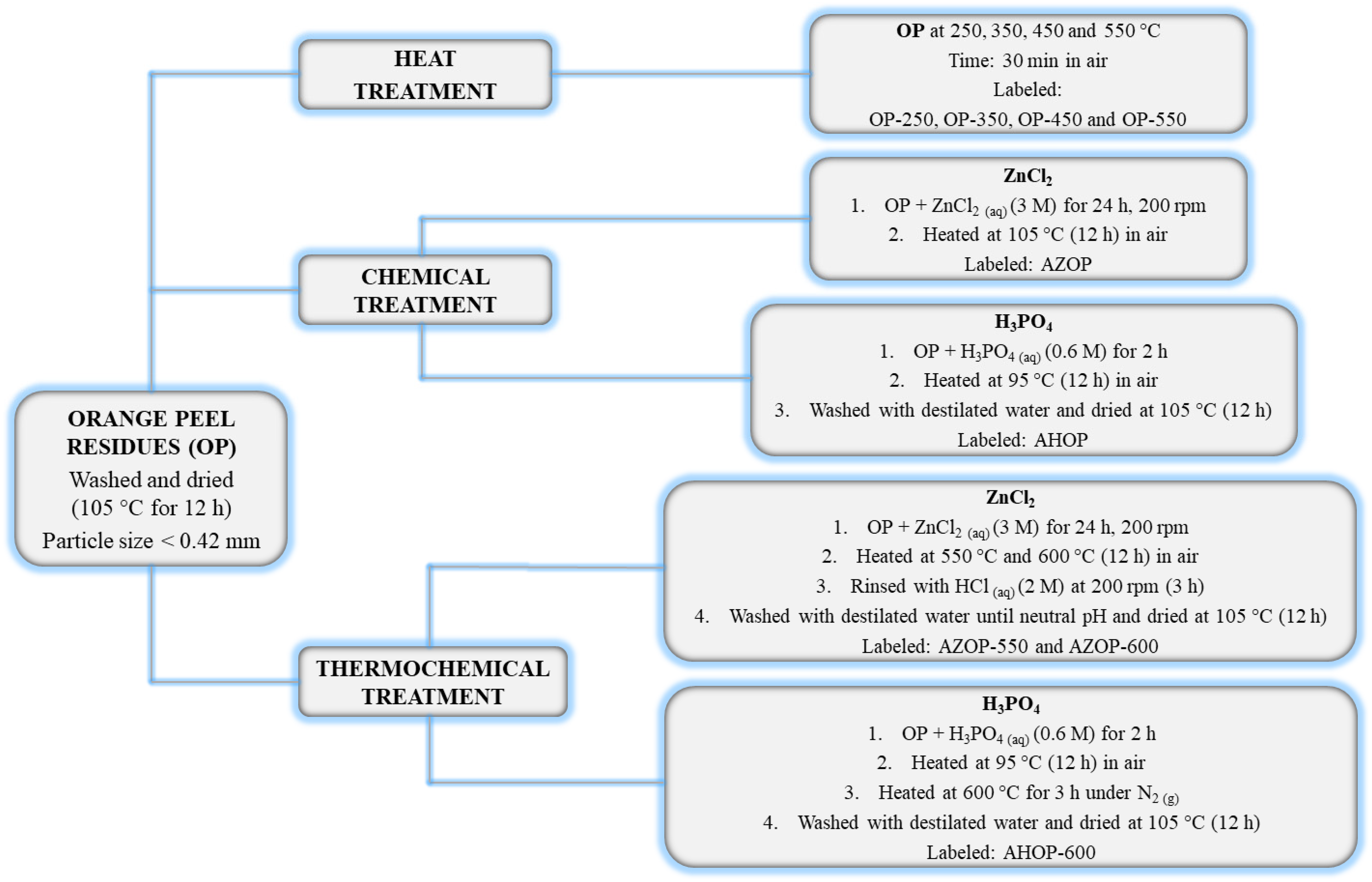
2.1. Materials and Transformation Methods
2.2. Physicochemical Characterization
2.3. Adsoption Tests
2.3.1. Batch Adsorption Studies
2.3.2. Adsorption Isotherms and Kinetics
2.4. DFT Calculations
3. Results and Discussion
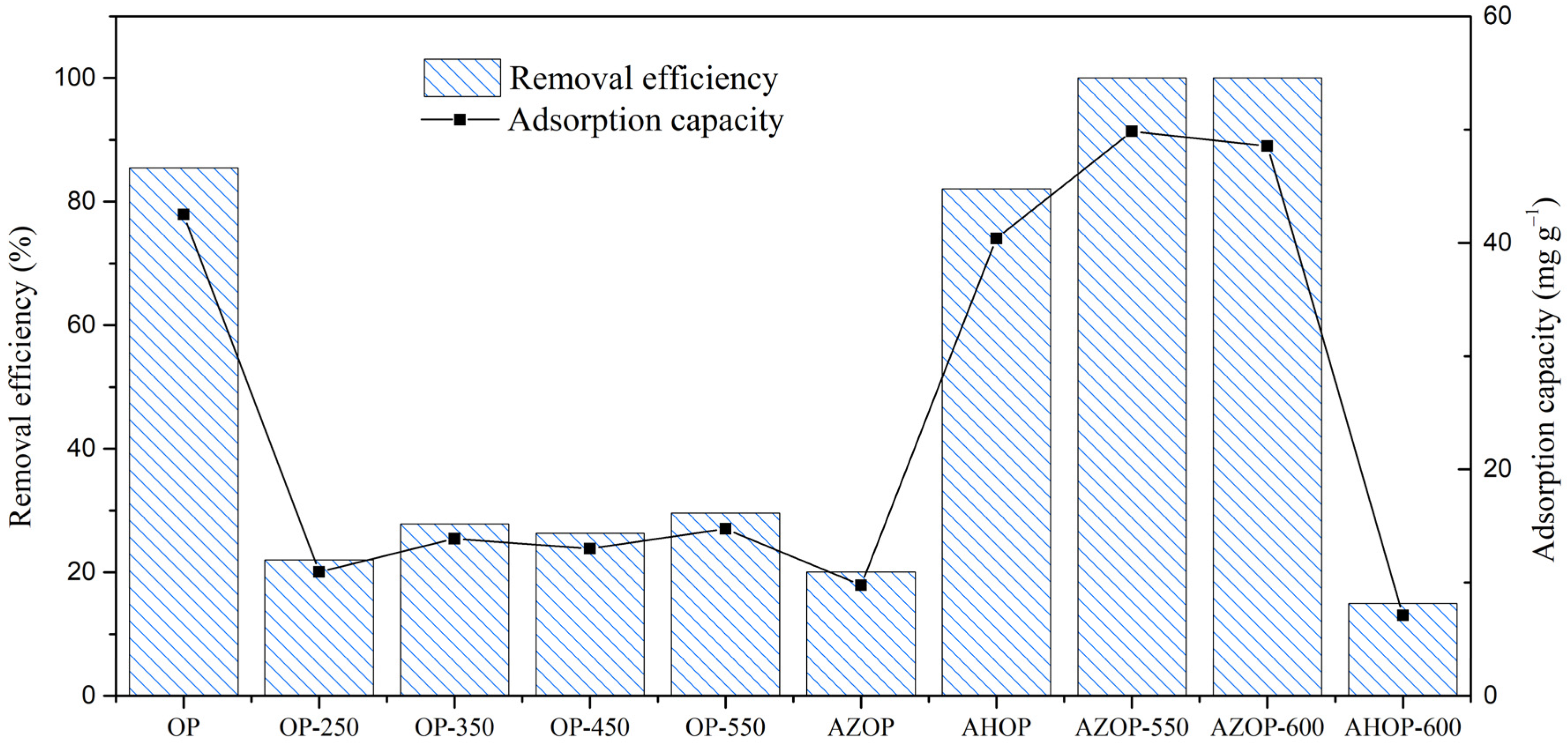
3.1. Performance of Adsorbent Material on MB Removal: Preliminary Tests
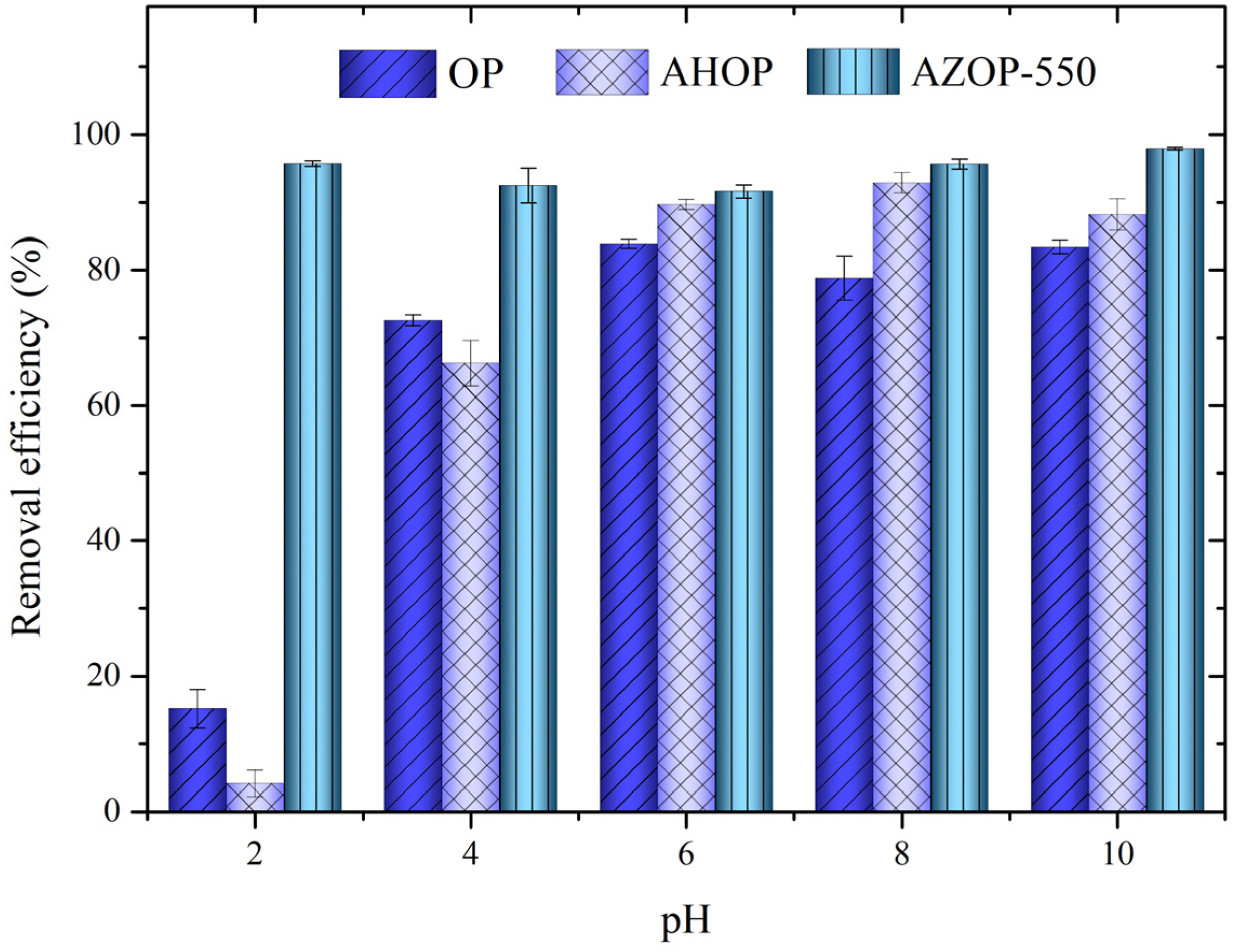
3.2. pH Effect on MB Adsorption
3.3. Adsorption Kinetics
3.4. Adsorption Isotherms
3.5. Adsorption Mechanism
3.6. Comparison with Other Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Qian, W.; Luo, X.; Wang, X.; Guo, M.; Li, B. Ecotoxicology and Environmental Safety Removal of methylene blue from aqueous solution by modi fi ed bamboo hydrochar. Ecotoxicol. Environ. Saf. 2018, 157, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xu, Z.; Li, Q.-S.; Chen, S.; An, S.-Q.; Zeng, Q.-F.; Zhu, H.-L. Treatment of simulated wastewater containing Reactive Red 195 by zero-valent iron/activated carbon combined with microwave discharge electrodeless lamp/sodium hypochlorite. J. Environ. Sci. 2010, 22, 512–518. [Google Scholar] [CrossRef]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Huan, Y. Acrylic acid grafted-multi-walled carbon nanotubes and their high-efficiency adsorption of methylene blue. J. Mater. Sci. 2019. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.C.; Juang, R.S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Ali, S.A.; Yaagoob, I.Y.; Mazumder, M.A.J.; Al-Muallem, H.A. Fast removal of methylene blue and Hg(II) from aqueous solution using a novel super-adsorbent containing residues of glycine and maleic acid. J. Hazard. Mater. 2019, 369, 642–654. [Google Scholar] [CrossRef]
- Yang, Z.; Chai, Y.; Zeng, L.; Gao, Z.; Zhang, J.; Ji, H. Effcient removal of copper ion from waste water using a stable chitosan gel material. Molecules 2019, 24, 4205. [Google Scholar] [CrossRef] [Green Version]
- Giraldo, S.; Ramirez, A.P.; Ulloa, M.; Flórez, E.; Acelas, N.Y. Dyes removal from water using low cost absorbents. J. Phys. Conf. Ser. 2017, 935. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, S.I.; Zohra, F.; Chaudhry, S.A. Nigella sativa seed based nanohybrid composite-Fe2O3–SnO2/ BC: A novel material for enhanced adsorptive removal of methylene blue from water. Environ. Res. 2019, 178, 108667. [Google Scholar] [CrossRef]
- Ramírez Muñoz, A.P.; Giraldo, S.; Flórez Yepes, E.; Acelas Soto, N.Y. Preparación de carbón activado a partir de residuos de palma de aceite y su aplicación para la remoción de colorantes. Rev. Colomb. Química 2017, 46, 33–41. [Google Scholar] [CrossRef]
- Benitez Monsalve, N. Con Cáscaras de Naranja, Quieren Mejorar la Industria y el Ambiente Colombiano. Available online: https://www.laopinion.com.co/economia/con-cascaras-de-naranja-quieren-mejorar-la-industria-y-el-ambiente-colombiano-108367#OP (accessed on 17 June 2021).
- Kebaili, M.; Djellali, S.; Radjai, M.; Drouiche, N.; Lounici, H. Valorization of orange industry residues to form a natural coagulant and adsorbent. J. Ind. Eng. Chem. 2018, 64, 292–299. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Zabihi, M.; Bastami, T.R.; Tahmasbi, M.; Ayati, A. Rapid removal of mercury ion (II) from aqueous solution by chemically activated eggplant hull adsorbent. J. Appl. Res. Water Wastewater 2016, 6, 236–240. [Google Scholar]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Giraldo, S.; Robles, I.; Ramirez, A.; Flórez, E.; Acelas, N. Mercury removal from wastewater using agroindustrial waste adsorbents. SN Appl. Sci. 2020, 2, 1029. [Google Scholar] [CrossRef]
- Danish, M.; Hashim, R.; Ibrahim, M.N.M.; Sulaiman, O. Effect of acidic activating agents on surface area and surface functional groups of activated carbons produced from Acacia mangium wood. J. Anal. Appl. Pyrolysis 2013, 104, 418–425. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Bendjamaa, I.; Bensid, T.; Meniai, A.H.; Derba, K. Effect of calcination on orange peels characteristics: Application of an industrial dye adsorption. Chem. Eng. Trans. 2014, 38, 361–366. [Google Scholar] [CrossRef]
- Boumediene, M.; Benaïssa, H.; George, B.; Molina, S.; Merlin, A. Characterization of two cellulosic waste materials (orange and almond peels) and their use for the removal of methylene blue from aqueous solutions. Maderas. Cienc. Tecnol. 2015, 17, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Andreas, A.; Reinaldo, J.; Tertira, K. A Study on The Adsorption Equilibrium and Kinetics of Methylene Blue onto Orange Peel Wastes as Biosorbents. In Proceedings of the 2016 2nd International Conference of Industrial, Mechanical, Electrical, and Chemical Engineering (ICIMECE), Yogyakarta, Indonesia, 6–7 October 2016; pp. 59–62. [Google Scholar]
- Salman, T.A.; Ali, M.I. Potential Application of Natural and Modified Orange Peel as an Eco-friendly Adsorbent for Methylene Blue Dye. Iraqi J. Sci. 2016, 57, 1–13. [Google Scholar]
- Fernandez, M.E.; Nunell, G.V.; Bonelli, P.R.; Cukierman, A.L. Activated carbon developed from orange peels: Batch and dynamic competitive adsorption of basic dyes. Ind. Crop. Prod. J. 2014, 62, 437–445. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Comparative study for adsorption of methylene blue dye on biochar derived from orange peel and banana biomass in aqueous solutions. Environ. Monit. Assess. 2019, 191, 735. [Google Scholar] [CrossRef] [PubMed]
- Bediako, J.K.; Lin, S.; Sarkar, A.K.; Zhao, Y.; Choi, J.; Song, M.; Cho, C.; Yun, Y. Evaluation of orange peel-derived activated carbons for treatment of dye-contaminated wastewater tailings. Environ. Sci. Pollut. Res. 2020, 27, 1053–1068. [Google Scholar] [CrossRef] [PubMed]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S.; Ferreira, M.E. Kinetics and equilibrium studies of methylene blue adsorption by spent coffee grounds. Desalination 2009, 249, 267–272. [Google Scholar] [CrossRef]
- Agarwal, S.; Tyagi, I.; Kumar, V.; Ghasemi, N.; Shahivand, M.; Ghasemi, M. Kinetics, equilibrium studies and thermodynamics of methylene blue adsorption on Ephedra strobilacea saw dust and modified using phosphoric acid and zinc chloride. J. Mol. Liq. 2016, 218, 208–218. [Google Scholar] [CrossRef]
- Achour, Y.; Khouili, M.; Abderrafia, H.; Melliani, S.; Laamari, M.R.; El Haddad, M. DFT Investigations and Experimental Studies for Competitive and Adsorptive Removal of Two Cationic Dyes onto an Eco-friendly Material from Aqueous Media. Int. J. Environ. Res. 2018, 12, 789–802. [Google Scholar] [CrossRef]
- Sellaoui, L.; Franco, D.; Ghalla, H.; Georgin, J.; Netto, M.S.; Luiz Dotto, G.; Bonilla-Petriciolet, A.; Belmabrouk, H.; Bajahzar, A. Insights of the adsorption mechanism of methylene blue on brazilian berries seeds: Experiments, phenomenological modelling and DFT calculations. Chem. Eng. J. 2020, 394, 125011. [Google Scholar] [CrossRef]
- Tovar, A.K.; Godínez, L.A.; Espejel, F.; Ramírez-Zamora, R.-M.; Robles, I. Optimization of the integral valorization process for orange peel waste using a design of experiments approach: Production of high-quality pectin and activated carbon. Waste Manag. 2019, 85, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Bakatula, E.N.; Richard, D.; Neculita, C.M.; Zagury, G.J. Determination of point of zero charge of natural organic materials. Environ. Sci. Pollut. Res. 2018, 25, 7823–7833. [Google Scholar] [CrossRef]
- Goertzen, S.L.; Thériault, K.D.; Oickle, A.M.; Tarasuk, A.C.; Andreas, H.A. Standardization of the Boehm titration. Part I. CO2 expulsion and endpoint determination. Carbon N. Y. 2010, 48, 1252–1261. [Google Scholar] [CrossRef]
- Ramirez, A.; Ocampo, R.; Giraldo, S.; Padilla, E.; Flórez, E.; Acelas, N. Removal of Cr (VI) from an aqueous solution using an activated carbon obtained from teakwood sawdust: Kinetics, equilibrium, and density functional theory calculations. J. Environ. Chem. Eng. 2020, 8, 103702. [Google Scholar] [CrossRef]
- Isah, U.A.; Abdulraheem, G.; Bala, S.; Muhammad, S.; Abdullahi, M. Kinetics, equilibrium and thermodynamics studies of C.I. Reactive Blue 19 dye adsorption on coconut shell based activated carbon. Int. Biodeterior. Biodegradation 2015, 102, 265–273. [Google Scholar] [CrossRef]
- Acelas, N.Y.; Martin, B.D.; López, D.; Jefferson, B. Selective removal of phosphate from wastewater using hydrated metal oxides dispersed within anionic exchange media. Chemosphere 2015, 119, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Tareq, R.; Akter, N.; Azam, S. Chapter 10—Biochars and Biochar Composites: Low-Cost Adsorbents for Environmental Remediation. Biochar Biomass Waste 2019, 169–210. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Temkin, M.J.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta Physicochim. URSS 1940, 12, 217–222. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H.; et al. Gaussian 09; Revision A.02; University of Cincinnati Libraries: Wallingford, CT, USA, 2009. [Google Scholar]
- Keith, T.A.; Frisch, M.J. Inclusion of Explicit Solvent Molecules in a Self-Consistent-Reaction Field Model of Solvation. In Modeling the Hydrogen Bond; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1994; Volume 569, pp. 22–35. ISBN 9780841229815. [Google Scholar]
- Li, Z.; Hanafy, H.; Zhang, L.; Sellaoui, L.; Schadeck, M.; Oliveira, M.L.S.; Seliem, M.K.; Luiz, G.; Bonilla-petriciolet, A. Adsorption of congo red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions: Experiments, characterization and physical interpretations. Chem. Eng. J. 2020, 388, 124263. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Removal of methylene blue dye from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 66, 154–163. [Google Scholar] [CrossRef]
- Gayathri, K.; Palanisamy, N. Methylene blue adsorption onto an eco-friendly modified polyacrylamide/graphite composites: Investigation of kinetics, equilibrium, and thermodynamic studies. Sep. Sci. Technol. 2020, 55, 1–12. [Google Scholar] [CrossRef]
- Thi, H.T.; Le, A.H.; Huu, T.P.; Dinhd, T.N.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Adsorption isotherms and kinetic modeling of methylene blue dye onto a carbonaceous hydrochar adsorbent derived from coffee husk waste. Sci. Total Environ. 2020, 725, 138325. [Google Scholar] [CrossRef]
- Guimarães Gusmão, K.A.; Alves Gurgel, L.V.; Sacramento Melo, T.M.; Frédéric Gil, L. Adsorption studies of methylene blue and gentian violet on sugarcane bagasse modified with EDTA dianhydride (EDTAD) in aqueous solutions: Kinetic and equilibrium aspects. J. Environ. Manag. 2013, 118, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar-rabago, J.J.; Leyva-ramos, R.; Rivera-utrilla, J.; Ocampo-perez, R.; Cerino-cordova, F.J. Biosorption mechanism of Methylene Blue from aqueous solution onto White Pine (Pinus durangensis) sawdust: Effect of operating conditions. Sustain. Environ. Res. 2017, 27, 32–40. [Google Scholar] [CrossRef]
- Rodrigues Sousa, H.; Santos Silva, L.; Abreu Sousa, P.A.; Magalhães Sousa, R.R.; Gardênnia Fonseca, M.; Anteveli Osajima, J.; Silva-Filho, E.C. Evaluation of methylene blue removal by plasma activated palygorskites. J. Mater. Res. Technol. 2019, 8, 5432–5442. [Google Scholar] [CrossRef]
- Boumediene, M.; Benaïssa, H.; George, B.; Molina, S.; Merlin, A. Effects of pH and ionic strength on methylene blue removal from synthetic aqueous solutions by sorption onto orange peel and desorption study. J. Mater. Environ. Sci. 2018, 9, 1700–1711. [Google Scholar] [CrossRef]
- Guediri, A.; Bouguettoucha, A.; Chebli, D.; Chafai, N.; Amrane, A. Molecular dynamic simulation and DFT computational studies on the adsorption performances of methylene blue in aqueous solutions by orange peel-modified phosphoric acid. J. Mol. Struct. J. 2020, 1202, 1–14. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, W.; Yin, Y.; Chen, Z.; Qiu, R.; Simonnot, M.-O.; Wang, X. Adsorption-reduction removal of Cr(VI) by tobacco petiole pyrolytic biochar: Batch experiment, kinetic and mechanism studies. Bioresour. Technol. 2018, 268, 149–157. [Google Scholar] [CrossRef]
- Nguyen, H.; You, S.; Hosseini-bandegharaei, A. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Li, B.; Lv, J.; Guo, J.; Fu, S.; Guo, M.; Yang, P. The polyaminocarboxylated modifed hydrochar for efficient capturing methylene blue and Cu (II) from water. Bioresour. Technol. 2019, 275, 360–367. [Google Scholar] [CrossRef]
- Wu, Z.; Zhong, H.; Yuan, X.; Wang, H.; Wang, L.; Chen, X.; Zeng, G.; Wu, Y. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res. 2014, 67, 330–344. [Google Scholar] [CrossRef] [PubMed]



| Material | Surface Area BET (m2 g−1) | Pore Volume (cm3 g−1) |
|---|---|---|
| OP | 3.098 | 0.0013 |
| AHOP | 7.0142 | 0.0030 |
| AZOP-550 | 1078.56 | 0.5205 |
| Material | Carboxylic Group (mmol g−1) | Lactone Group (mmol g−1) | Phenol Group (mmol g−1) | Total Acid Sites (mmol g−1) | Total Basic Sites (mmol g−1) | pH (PZC) |
|---|---|---|---|---|---|---|
| OP | 0.650 | 0.925 | 0.200 | 1.774 | 0.000 | 3.5 ± 0.08 |
| AHOP | 0.925 | 1.325 | - | 2.250 | 0.125 | 3.4 ± 0.11 |
| AZOP-550 | 0.775 | 0.150 | 0.050 | 0.975 | 0.225 | 6.3 ± 0.03 |
| Material | OP | AHOP | AZOP-550 |
|---|---|---|---|
| Langmuir | |||
| Qm (mg g−1) | 192.31 | 277.78 | 232.56 |
| KL (L mg−1) | 0.03 | 0.02 | 0.54 |
| RL | 0.40–0.11 | 0.48–0.16 | 0.4–0.01 |
| R2 | 0.97 | 0.98 | 1.00 |
| Freundlich | |||
| KF (mg g−1) | 14.17 | 16.24 | 83.60 |
| n | 1.86 | 1.87 | 3.03 |
| R2 | 0.85 | 0.98 | 0.73 |
| Temkin | |||
| KT (L g−1) | 0.31 | 4.39 | 9.12 |
| b (kJ mol−1) | 0.06 | 0.04 | 0.06 |
| R2 | 0.96 | 0.98 | 0.89 |
| Dubinin–Radushkevich | |||
| KDR (mol2 kJ−2) | 20.39 | 18.83 | 0.31 |
| qs (mg g−1) | 134 | 166 | 206 |
| E (KJ mol−1) | 0.16 | 0.16 | 1.28 |
| R2 | 0.98 | 0.86 | 0.94 |
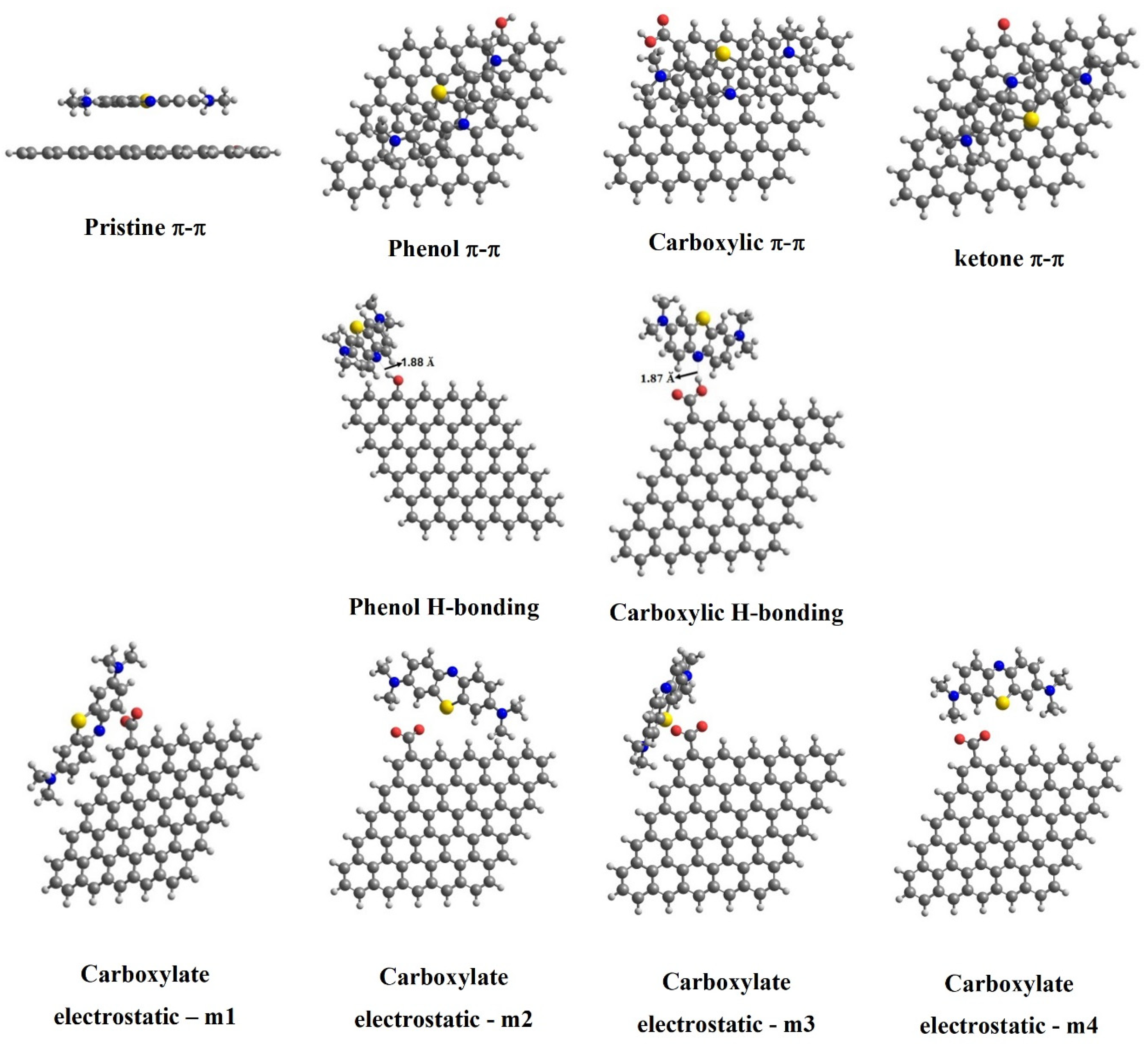
| Interactions | Pristine | Functional Groups | |||
|---|---|---|---|---|---|
| Phenol (–OH) | Carboxylate (–COO−) | Carboxylic (–COOH) | Ketone (–CO) | ||
| Adsorption Energy (kcal mol−1) | |||||
| (π–π) | 0.20 | −0.50 | −0.25 | 1.61 | |
| (H–bonding) | −5.76 | −6.44 | |||
| Electrostatic | −3.60 (m1) −8.46 (m2) −8.08 (m3) −7.95 (m4) | ||||
| Material | Treatment | pH | Qm (mg g−1) | Reference |
|---|---|---|---|---|
| OP | Washed biomass | pH natural (4.5) | 192.31 | This study |
| AZOP-550 | Thermochemical activation using ZnCl2 (550 °C) | pH natural (7.5) | 232.56 | This study |
| AHOP | Chemical activation using H3PO4 | pH natural (3.7) | 277.78 | This study |
| ZnCl2-AC | Thermochemical activation using ZnCl2 (800 °C) | 7–8 | 281.52 | [24] |
| OP | Washed biomass | 4 | 14.16 | [21] |
| SOP | Chemical activation using NaOH | 9 | 18.28 | [21] |
| OP | Washed biomass | pH natural (4.2) | 218 | [19] |
| OP-H3PO4 | Chemical activation using H3PO4 | pH natural (6.2) | 307.63 | [51] |
| COP 400 °C | Calcination a 400 °C | 4.98 | 14.85 | [18] |
| OP-ZnCl2 | Chemical activation using ZnCl2 | 9 | 7.57 | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraldo, S.; Robles, I.; Godínez, L.A.; Acelas, N.; Flórez, E. Experimental and Theoretical Insights on Methylene Blue Removal from Wastewater Using an Adsorbent Obtained from the Residues of the Orange Industry. Molecules 2021, 26, 4555. https://doi.org/10.3390/molecules26154555
Giraldo S, Robles I, Godínez LA, Acelas N, Flórez E. Experimental and Theoretical Insights on Methylene Blue Removal from Wastewater Using an Adsorbent Obtained from the Residues of the Orange Industry. Molecules. 2021; 26(15):4555. https://doi.org/10.3390/molecules26154555
Chicago/Turabian StyleGiraldo, Stephanie, Irma Robles, Luis A. Godínez, Nancy Acelas, and Elizabeth Flórez. 2021. "Experimental and Theoretical Insights on Methylene Blue Removal from Wastewater Using an Adsorbent Obtained from the Residues of the Orange Industry" Molecules 26, no. 15: 4555. https://doi.org/10.3390/molecules26154555
APA StyleGiraldo, S., Robles, I., Godínez, L. A., Acelas, N., & Flórez, E. (2021). Experimental and Theoretical Insights on Methylene Blue Removal from Wastewater Using an Adsorbent Obtained from the Residues of the Orange Industry. Molecules, 26(15), 4555. https://doi.org/10.3390/molecules26154555