Macrocyclic Tetramers—Structural Investigation of Peptide-Peptoid Hybrids
Abstract
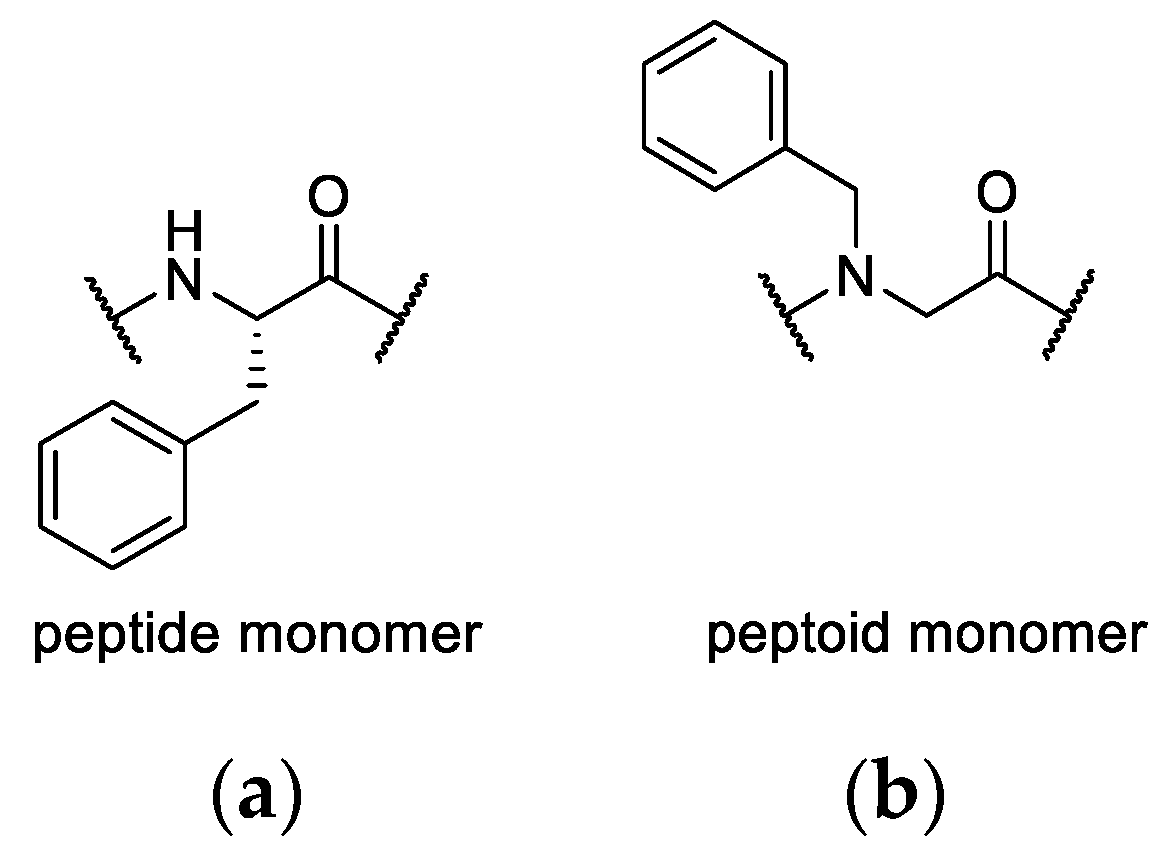
1. Introduction
2. Results and Discussion
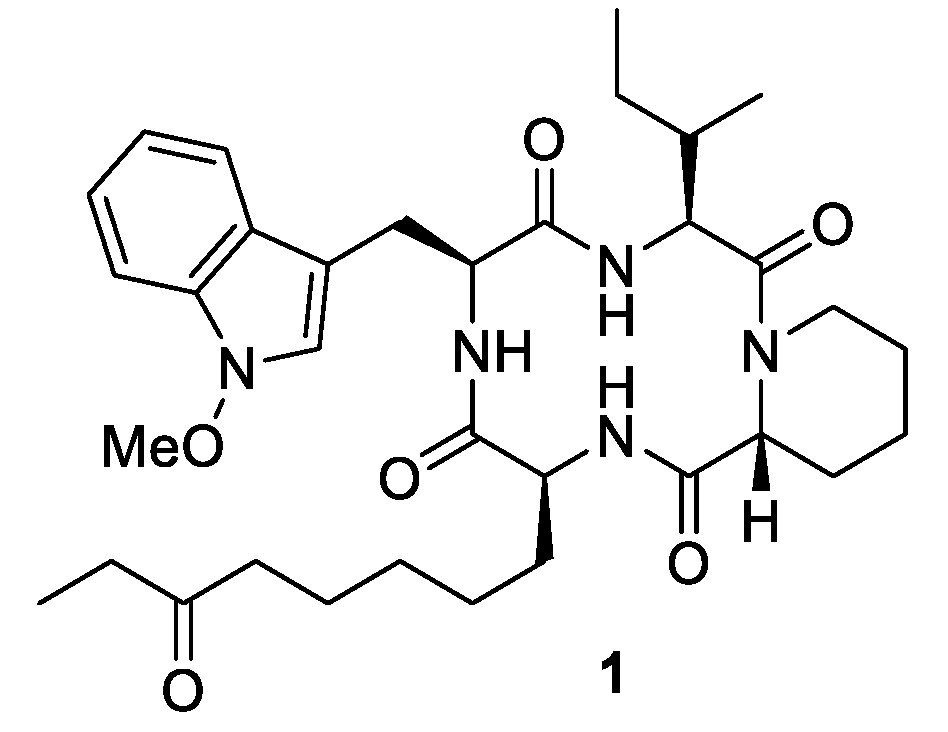
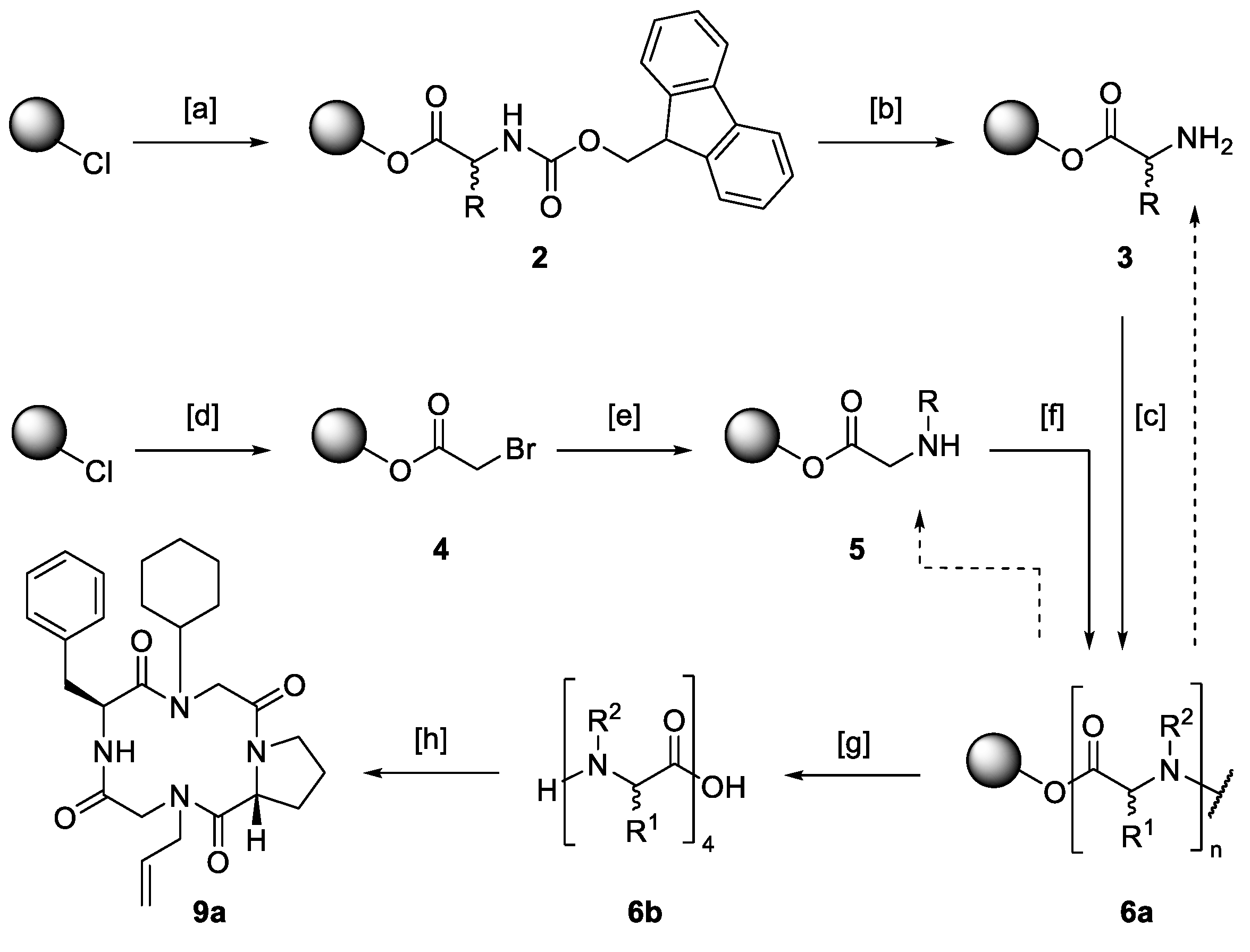
2.1. Synthesis of Macrocyclic Tetramers
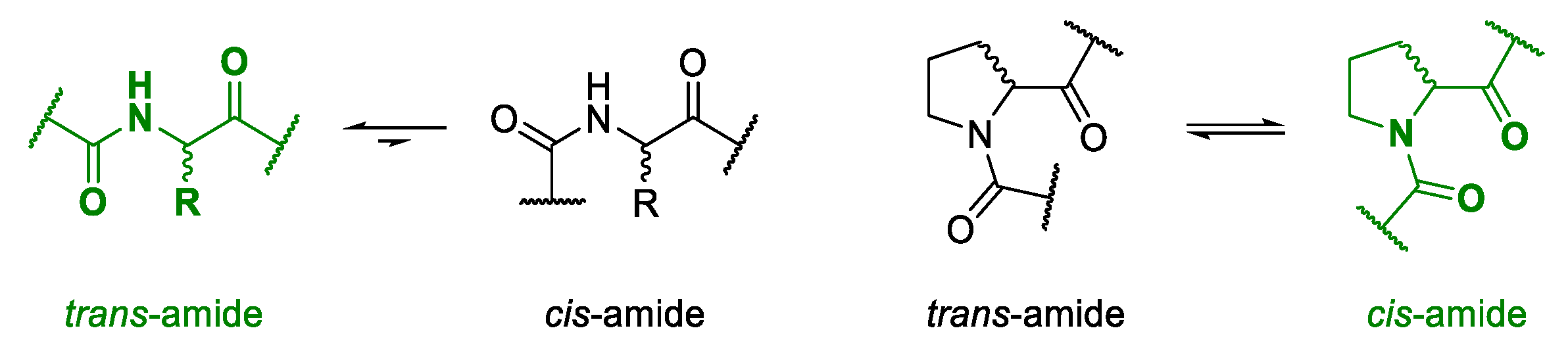
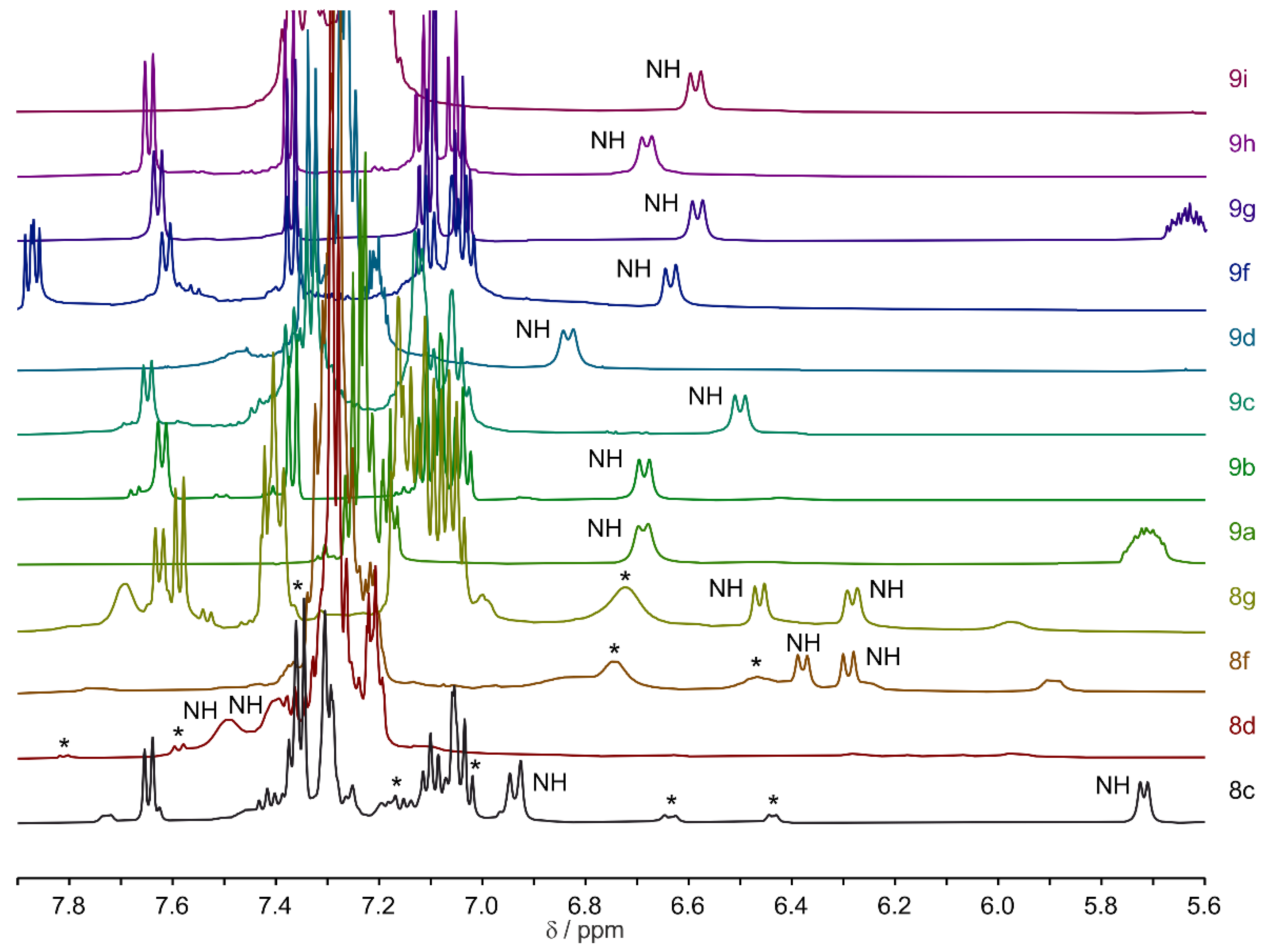
2.2. Multiconformational Equilibrium Detected by NMR
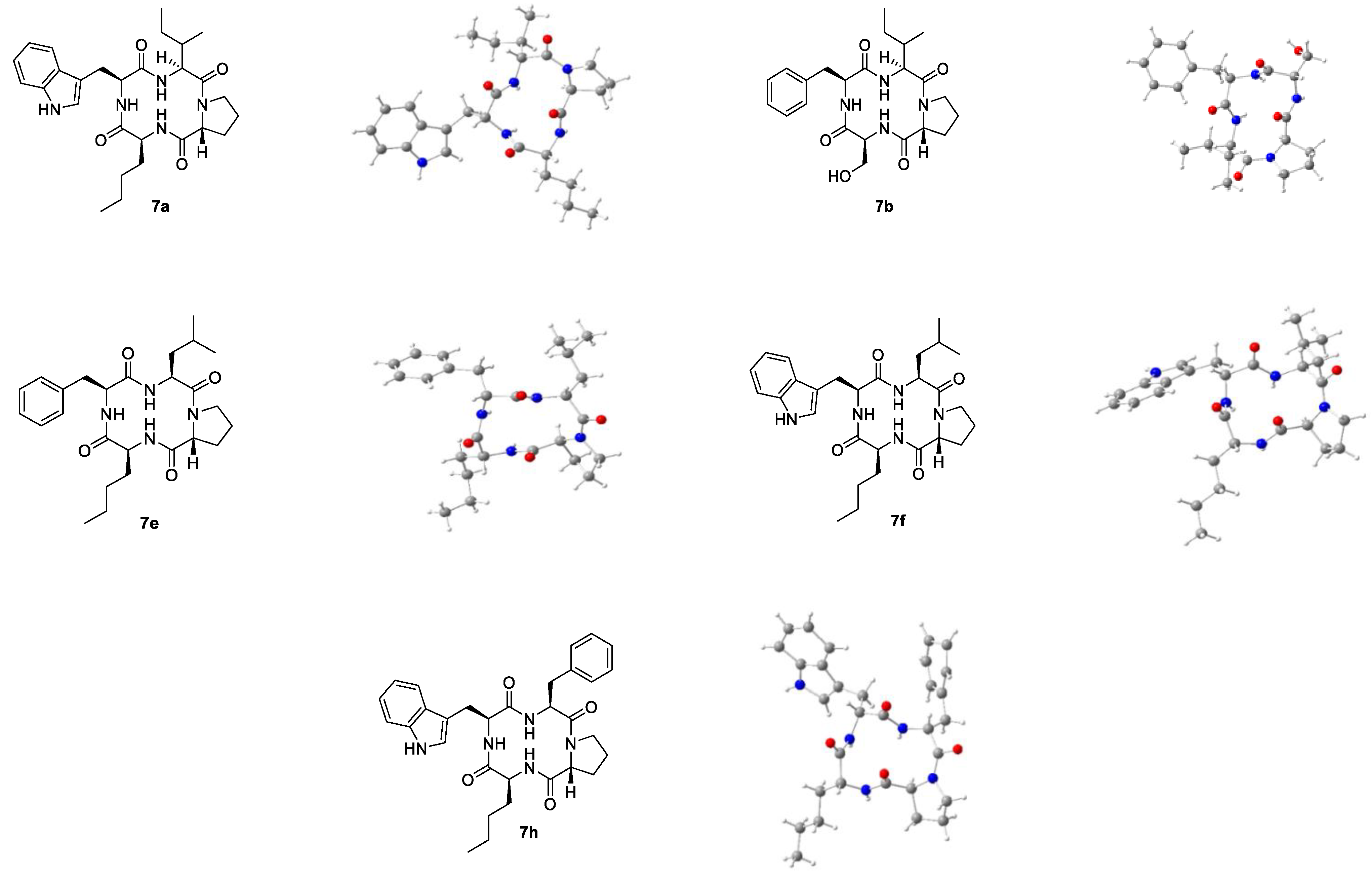
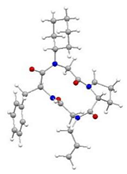
2.3. Spatial Structure in Solid-State
2.4. Spatial Structure in Solution State
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Beatriz, G.; Albericio, F. The pharmaceutical industry in 2019. An analysis of FDA drug approvals from the perspective of molecules. Molecules 2020, 25, 745. [Google Scholar]
- Lee, A.C.-L.; Harris, J.L.; Khanna, K.K.; Hong, J.-H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Ghosh, D.; Williams, O., III. Just how prevalent are peptide therapeutic products? A critical review. Int. J. Pharm. 2020, 587, 119491. [Google Scholar] [CrossRef]
- Drucker, D.J. Advances in oral peptide therapeutics. Nat. Rev. Drug Discov. 2019, 19, 277–289. [Google Scholar] [CrossRef]
- Zorzi, A.; Deyle, K.; Heinis, C. Cyclic peptide therapeutics: Past, present and future. Curr. Opin. Chem. Biol. 2017, 38, 24–29. [Google Scholar] [CrossRef]
- Choi, J.-S.; Joo, S.H. Recent trends in cyclic peptides as therapeutic agents and biochemical tools. Biomol. Ther. 2020, 28, 18. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.A.; Bourne, G.T.; Coughlan, J.; Kaiser, S.M.; Jacobs, C.M.; Jones, A.; Rühmann, A.; Turner, J.Y.; Smythe, M.L. Cyclic tetrapeptides via the ring contraction strategy: Chemical techniques useful for their identification. Org. Biomol. Chem. 2008, 6, 1386–1395. [Google Scholar] [CrossRef]
- Meutermans, W.D.; Bourne, G.T.; Golding, S.W.; Horton, D.A.; Campitelli, M.R.; Craik, D.; Scanlon, M.; Smythe, M.L. Difficult macrocyclizations: New strategies for synthesizing highly strained cyclic tetrapeptides. Org. Lett. 2003, 5, 2711–2714. [Google Scholar] [CrossRef]
- Sarojini, V.; Cameron, A.J.; Varnava, K.G.; Denny, W.A.; Sanjayan, G. Cyclic tetrapeptides from nature and design: A review of synthetic methodologies, structure, and function. Chem. Rev. 2019, 119, 10318–10359. [Google Scholar] [CrossRef]
- Davison, E.K.; Cameron, A.J.; Harris, P.W.; Brimble, M.A. Synthesis of endolides A and B: Naturally occurring N-methylated cyclic tetrapeptides. MedChemComm 2019, 10, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, M.J.; Brice-Tutt, A.C.; Coleman, J.S.; Simpson, G.G.; Wilson, L.L.; Eans, S.O.; Stacy, H.M.; Murray, T.F.; McLaughlin, J.P.; Aldrich, J.V. Design, Synthesis, and Characterization of the Macrocyclic Tetrapeptide cyclo [Pro-Sar-Phe-d-Phe]: A Mixed Opioid Receptor Agonist-Antagonist Following Oral Administration. ACS Chem. Neurosci. 2020, 11, 1324–1336. [Google Scholar] [CrossRef] [PubMed]
- Betancur, L.A.; Forero, A.M.; Romero-Otero, A.; Sepúlveda, L.Y.; Moreno-Sarmiento, N.C.; Castellanos, L.; Ramos, F.A. Cyclic tetrapeptides from the marine strain Streptomyces sp. PNM-161a with activity against rice and yam phytopathogens. J. Antibiot. 2019, 72, 744–751. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Kang, D.O.; Na, Y.J.; Yoon, S.; Choi, W.S.; Kang, K.W.; Chung, H.Y.; Jung, J.H.; Kim, H.S. Histone deacetylase inhibitor, apicidin, inhibits human ovarian cancer cell migration via class II histone deacetylase 4 silencing. Cancer Lett. 2012, 325, 189–199. [Google Scholar] [CrossRef]
- Colletti, S.L.; Myers, R.W.; Darkin-Rattray, S.J.; Schmatz, D.M.; Fisher, M.H.; Wyvratt, M.J.; Meinke, P.T. Design and synthesis of histone deacetylase inhibitors: The development of apicidin transition state analogs. Tetrahedron Lett. 2000, 41, 7837–7841. [Google Scholar] [CrossRef]
- Singh, S.B.; Zink, D.L.; Liesch, J.M.; Mosley, R.T.; Dombrowski, A.W.; Bills, G.F.; Darkin-Rattray, S.J.; Schmatz, D.M.; Goetz, M.A. Structure and chemistry of apicidins, a class of novel cyclic tetrapeptides without a terminal α-keto epoxide as inhibitors of histone deacetylase with potent antiprotozoal activities. J. Org. Chem. 2002, 67, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Risinger, A.L.; King, J.B.; Powell, D.R.; Cichewicz, R.H. A potent HDAC inhibitor, 1-alaninechlamydocin, from a Tolypocladium sp. induces G2/M cell cycle arrest and apoptosis in MIA PaCa-2 cells. J. Nat. Prod. 2014, 77, 1753–1757. [Google Scholar] [CrossRef]
- Furumai, R.; Komatsu, Y.; Nishino, N.; Khochbin, S.; Yoshida, M.; Horinouchi, S. Potent histone deacetylase inhibitors built from trichostatin A and cyclic tetrapeptide antibiotics including trapoxin. Proc. Natl. Acad. Sci. USA 2001, 98, 87–92. [Google Scholar] [CrossRef]
- Maubon, D.; Bougdour, A.; Wong, Y.-S.; Brenier-Pinchart, M.-P.; Curt, A.; Hakimi, M.-A.; Pelloux, H. Activity of the histone deacetylase inhibitor FR235222 on Toxoplasma gondii: Inhibition of stage conversion of the parasite cyst form and study of new derivative compounds. Antimicrob. Agents Chemother. 2010, 54, 4843–4850. [Google Scholar] [CrossRef]
- Sasamura, S.; Sakamoto, K.; Takagaki, S.; Yamada, T.; Takase, S.; Mori, H.; Fujii, T.; Hino, M.; Hashimoto, M. AS1387392, a novel immunosuppressive cyclic tetrapeptide compound with inhibitory activity against mammalian histone deacetylase. J. Antibiot. 2010, 63, 633–636. [Google Scholar] [CrossRef]
- Gu, W.; Cueto, M.; Jensen, P.R.; Fenical, W.; Silverman, R.B. Microsporins A and B: New histone deacetylase inhibitors from the marine-derived fungus Microsporum cf. gypseum and the solid-phase synthesis of microsporin A. Tetrahedron 2007, 63, 6535–6541. [Google Scholar] [CrossRef]
- Kawagishi, H.; Somoto, A.; Kuranari, J.; Kimura, A.; Chiba, S. A novel cyclotetrapeptide produced by Lactobacillus helveticus as a tyrosinase inhibitor. Tetrahedron Lett. 1993, 34, 3439–3440. [Google Scholar] [CrossRef]
- Saito, T.; Hirai, H.; Kim, Y.-J.; Kojima, Y.; Matsunaga, Y.; Nishida, H.; Sakakibara, T.; Suga, O.; Sujaku, T.; Kojima, N. CJ-15, 208, a novel kappa opioid receptor antagonist from a fungus, Ctenomyces serratus ATCC15502. J. Antibiot. Res. 2002, 55, 847–854. [Google Scholar] [CrossRef]
- Ross, N.C.; Reilley, K.J.; Murray, T.F.; Aldrich, J.V.; McLaughlin, J.P. Novel opioid cyclic tetrapeptides: Trp isomers of CJ-15,208 exhibit distinct opioid receptor agonism and short-acting κ opioid receptor antagonism. Br. J. Pharmacol. 2012, 165, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Weltrowska, G.; Nguyen, T.M.-D.; Chung, N.N.; Wood, J.; Ma, X.; Guo, J.; Wilkes, B.C.; Ge, Y.; Laferrière, A.; Coderre, T.J. A cyclic tetrapeptide (“cyclodal”) and its mirror-image isomer are both high-affinity μ opioid receptor antagonists. J. Med. Chem. 2016, 59, 9243–9254. [Google Scholar] [CrossRef] [PubMed]
- Frediansyah, A.; Straetener, J.; Brötz-Oesterhelt, H.; Gross, H. Massiliamide, a cyclic tetrapeptide with potent tyrosinase inhibitory properties from the Gram-negative bacterium Massilia albidiflava DSM 17472 T. J. Antibiot. Res. 2021, 74, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Mitova, M.; Popov, S.; De Rosa, S. Cyclic peptides from a Ruegeria strain of bacteria associated with the sponge Suberites domuncula. J. Nat. Prod. 2004, 67, 1178–1181. [Google Scholar] [CrossRef]
- Choub, V.; Maung, C.E.H.; Won, S.-J.; Moon, J.-H.; Kim, K.Y.; Han, Y.S.; Cho, J.-Y.; Ahn, Y.S. Antifungal Activity of Cyclic Tetrapeptide from Bacillus velezensis CE 100 against Plant Pathogen Colletotrichum gloeosporioides. Pathogens 2021, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, R.; Gautam, H. Toward the synthesis and biological screening of a cyclotetrapeptide from marine bacteria. Mar. Drugs 2011, 9, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Cha, R.; Rybak, M.J. Influence of protein binding under controlled conditions on the bactericidal activity of daptomycin in an in vitro pharmacodynamic model. J. Antimicrob. Chemother. 2004, 54, 259–262. [Google Scholar] [CrossRef]
- Chakraborty, S.; Tai, D.-F.; Lin, Y.-C.; Chiou, T.-W. Antitumor and antimicrobial activity of some cyclic tetrapeptides and tripeptides derived from marine bacteria. Mar. Drugs 2015, 13, 3029–3045. [Google Scholar] [CrossRef]
- Grauer, A.; König, B. Peptidomimetics—A versatile route to biologically active compounds. Eur. J. Org. Chem. 2009, 2009, 5099–5111. [Google Scholar] [CrossRef]
- Ko, E.; Liu, J.; Perez, L.M.; Lu, G.; Schaefer, A.; Burgess, K. Universal peptidomimetics. J. Am. Chem. Soc. 2011, 133, 462–477. [Google Scholar] [CrossRef]
- Zuckermann, R.N.; Kerr, J.M.; Kent, S.B.; Moos, W.H. Efficient method for the preparation of peptoids [oligo (N-substituted glycines)] by submonomer solid-phase synthesis. J. Am. Chem. Soc. 1992, 114, 10646–10647. [Google Scholar] [CrossRef]
- Sun, J.; Zuckermann, R.N. Peptoid polymers: A highly designable bioinspired material. ACS Nano 2013, 7, 4715–4732. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, R.N.; Kodadek, T. Peptoids as potential therapeutics. Curr. Opin. Mol. Ther. 2009, 11, 299–307. [Google Scholar] [PubMed]
- Dohm, M.T.; Kapoor, R.; Barron, A.E. Peptoids: Bio-inspired polymers as potential pharmaceuticals. Curr. Pharm. Des. 2011, 17, 2732–2747. [Google Scholar] [CrossRef]
- Yoo, B.; Kirshenbaum, K. Peptoid architectures: Elaboration, actuation, and application. Curr. Opin. Chem. Biol. 2008, 12, 714–721. [Google Scholar] [CrossRef]
- De Riccardis, F. The Challenge of Conformational Isomerism in Cyclic Peptoids. Eur. J. Org. Chem. 2020, 2020, 2981–2994. [Google Scholar] [CrossRef]
- Butterfoss, G.L.; Renfrew, P.D.; Kuhlman, B.; Kirshenbaum, K.; Bonneau, R. A preliminary survey of the peptoid folding landscape. J. Am. Chem. Soc. 2009, 131, 16798–16807. [Google Scholar] [CrossRef]
- Shin, S.B.; Yoo, B.; Todaro, L.J.; Kirshenbaum, K. Cyclic peptoids. J. Am. Chem. Soc. 2007, 129, 3218–3225. [Google Scholar] [CrossRef]
- Yoo, B.; Shin, S.B.Y.; Huang, M.L.; Kirshenbaum, K. Peptoid macrocycles: Making the rounds with peptidomimetic oligomers. Chem. Eur. J. 2010, 16, 5528–5537. [Google Scholar] [CrossRef]
- Vollrath, S.B.; Hu, C.; Bräse, S.; Kirshenbaum, K. Peptoid nanotubes: An oligomer macrocycle that reversibly sequesters water via single-crystal-to-single-crystal transformations. Chem. Commun. 2013, 49, 2317–2319. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.M.; Cobb, S.L. Recent advances in the synthesis of peptoid macrocycles. Chem. Eur. J. 2018, 24, 7560–7573. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, S.B.; Bräse, S.; Kirshenbaum, K. Twice tied tight: Enforcing conformational order in bicyclic peptoid oligomers. Chem. Sci. 2012, 3, 2726–2731. [Google Scholar] [CrossRef]
- Herlan, C.N.; Sommer, K.; Weis, P.; Nieger, M.; Bräse, S. Structural Diversity of Peptoids: Tube-Like Structures of Macrocycles. Molecules 2021, 26, 150. [Google Scholar] [CrossRef]
- D’Amato, A.; Pierri, G.; Tedesco, C.; Della Sala, G.; Izzo, I.; Costabile, C.; De Riccardis, F. Reverse turn and loop secondary structures in stereodefined cyclic peptoid scaffolds. J. Org. Chem. 2019, 84, 10911–10928. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, C.; Erra, L.; Izzo, I.; De Riccardis, F. Solid state assembly of cyclic α-peptoids. CrystEngComm 2014, 16, 3667–3687. [Google Scholar] [CrossRef]
- Biron, E.; Chatterjee, J.; Kessler, H. Optimized selective N-methylation of peptides on solid support. J. Pept. Sci. 2006, 12, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, J.; Gilon, C.; Hoffman, A.; Kessler, H. N-methylation of peptides: A new perspective in medicinal chemistry. Acc. Chem. Res. 2008, 41, 1331–1342. [Google Scholar] [CrossRef]
- Demmer, O.; Dijkgraaf, I.; Schottelius, M.; Wester, H.-J.; Kessler, H. Introduction of functional groups into peptides via N-alkylation. Org. Lett. 2008, 10, 2015–2018. [Google Scholar] [CrossRef]
- Titlestad, K.; Schwitters, B.; Sundholm, F. Cyclic tetra-and octapeptides of sarcosine in combination with alanine or glycine. Syntheses and conformation. Acta Chem. Scand. B 1977, 31, 641–661. [Google Scholar] [CrossRef][Green Version]
- Wessjohann, L.A.; Andrade, C.K.Z.; Vercillo, O.E.; Rivera, D.G. Macrocyclic peptoids: N-alkylated cyclopeptides and depsipeptides. Targets Heterocycl. Syst. 2006, 10, 24–53. [Google Scholar]
- Morimoto, J.; Kodadek, T. Synthesis of a large library of macrocyclic peptides containing multiple and diverse N-alkylated residues. Mol. Biosyst. 2015, 11, 2770–2779. [Google Scholar] [CrossRef]
- Olsen, C.A. Peptoid-peptide hybrid backbone architectures. ChemBioChem 2010, 11, 152–160. [Google Scholar] [CrossRef]
- Olsen, C.A.; Montero, A.; Leman, L.J.; Ghadiri, M.R. Macrocyclic peptoid–peptide hybrids as inhibitors of class I histone deacetylases. ACS Med. Chem. Lett. 2012, 3, 749–753. [Google Scholar] [CrossRef]
- Boehm, M.; Beaumont, K.; Jones, R.; Kalgutkar, A.S.; Zhang, L.; Atkinson, K.; Bai, G.; Brown, J.A.; Eng, H.; Goetz, G.H.; et al. Discovery of potent and orally bioavailable macrocyclic peptide-peptoid hybrid CXCR7 modulators. J. Med. Chem. 2017, 60, 9653–9663. [Google Scholar] [CrossRef]
- Greco, I.; Emborg, A.P.; Jana, B.; Molchanova, N.; Oddo, A.; Damborg, P.; Guardabassi, L.; Hansen, P.R. Characterization, mechanism of action and optimization of activity of a novel peptide-peptoid hybrid against bacterial pathogens involved in canine skin infections. Sci. Rep. 2019, 9, 3679. [Google Scholar] [CrossRef]
- Hansen, A.M.; Skovbakke, S.L.; Christensen, S.B.; Perez-Gassol, I.; Franzyk, H. Studies on acid stability and solid-phase block synthesis of peptide-peptoid hybrids: Ligands for formyl peptide receptors. Amino Acids 2019, 51, 205–218. [Google Scholar] [CrossRef]
- Frederiksen, N.; Hansen, P.R.; Björkling, F.; Franzyk, H. Peptide/peptoid hybrid oligomers: The Influence of hydrophobicity and relative side-chain length on antibacterial activity and cell Selectivity. Molecules 2019, 24, 4429. [Google Scholar] [CrossRef]
- Singh, S.B.; Zink, D.L.; Polishook, J.D.; Dombrowski, A.W.; Darkin-Rattray, S.J.; Schmatz, D.M.; Goetz, M.A. Apicidins: Novel cyclic tetrapeptides as coccidiostats and antimalarial agents from Fusarium pallidoroseum. Tetrahedron Lett. 1996, 37, 8077–8080. [Google Scholar] [CrossRef]
- Darkin-Rattray, S.J.; Gurnett, A.M.; Myers, R.W.; Dulski, P.M.; Crumley, T.M.; Allocco, J.J.; Cannova, C.; Meinke, P.T.; Colletti, S.L.; Bednarek, M.A. Apicidin: A novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA 1996, 93, 13143–13147. [Google Scholar] [CrossRef] [PubMed]
- Colletti, S.L.; Myers, R.W.; Darkin-Rattray, S.J.; Gurnett, A.M.; Dulski, P.M.; Galuska, S.; Allocco, J.J.; Ayer, M.B.; Li, C.; Lim, J. Broad spectrum antiprotozoal agents that inhibit histone deacetylase: Structure—Activity relationships of apicidin. Part 1. Bioorg. Med. Chem. Lett. 2001, 11, 107–111. [Google Scholar] [CrossRef]
- Meinke, P.T.; Colletti, S.L.; Ayer, M.B.; Darkin-Rattray, S.J.; Myers, R.W.; Schmatz, D.M.; Wyvratt, M.J.; Fisher, M.H. Synthesis of side chain modified apicidin derivatives: Potent mechanism-based histone deacetylase inhibitors. Tetrahedron Lett. 2000, 41, 7831–7835. [Google Scholar] [CrossRef]
- Grathwohl, C.; Wüthrich, K. NMR studies of the rates of proline cis–trans isomerization in oligopeptides. Biopolymers 1981, 20, 2623–2633. [Google Scholar] [CrossRef]
- Fischer, G. Chemical aspects of peptide bond isomerisation. Chem. Soc. Rev. 2000, 29, 119–127. [Google Scholar] [CrossRef]
- Schubert, M.; Labudde, D.; Oschkinat, H.; Schmieder, P. A software tool for the prediction of Xaa-Pro peptide bond conformations in proteins based on 13 C chemical shift statistics. J. Biomol. NMR 2002, 24, 149–154. [Google Scholar] [CrossRef]
- Mathieu, S.; Poteau, R.; Trinquier, G. Estimating the “steric clash” at cis peptide bonds. J. Phys. Chem. B 2008, 112, 7894–7902. [Google Scholar] [CrossRef] [PubMed]
- Scherer, G.; Kramer, M.L.; Schutkowski, M.; Reimer, U.; Fischer, G. Barriers to rotation of secondary amide peptide bonds. J. Am. Chem. Soc. 1998, 120, 5568–5574. [Google Scholar] [CrossRef]
- Wedemeyer, W.J.; Welker, E.; Scheraga, H.A. Proline cis-trans isomerization and protein folding. Biochemistry 2002, 41, 14637–14644. [Google Scholar] [CrossRef]
- Krieger, F.; Möglich, A.; Kiefhaber, T. Effect of proline and glycine residues on dynamics and barriers of loop formation in polypeptide chains. J. Am. Chem. Soc. 2005, 127, 3346–3352. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, K.A.; Sayyadi, N.; Luck, I.J.; Clegg, J.K.; Jolliffe, K.A. Synthesis of all-L cyclic tetrapeptides using pseudoprolines as removable turn inducers. Org. Lett. 2010, 12, 3136–3139. [Google Scholar] [CrossRef]
- Rodriguez, L.M.D.L.; Weidkamp, A.J.; Brimble, M.A. An update on new methods to synthesize cyclotetrapeptides. Org. Biomol. Chem. 2015, 13, 6906–6921. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Aldrich, J.V.; Kulkarni, S.S.; Senadheera, S.N.; Ross, N.C.; Reilley, K.J.; Eans, S.O.; Ganno, M.L.; Murray, T.F.; McLaughlin, J.P. Unexpected opioid activity profiles of analogs of the novel peptide kappa opioid receptor ligand CJ-15,208. ChemMedChem 2011, 6, 1739–1745. [Google Scholar] [CrossRef]
- El-Faham, A.; Albericio, F. Peptide coupling reagents, more than a letter soup. Chem. Rev. 2011, 111, 6557–6602. [Google Scholar] [CrossRef]
- Thakkar, A.; Trinh, T.B.; Pei, D. Global analysis of peptide cyclization efficiency. ACS Comb. Sci. 2013, 15, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.T.; Lam, H.Y.; Song, T.; Chen, G.; Li, X. Synthesis of constrained head-to-tail cyclic tetrapeptides by an imine-induced ring-closing/contraction strategy. Angew. Chem. Int. Ed. 2013, 52, 10212–10215. [Google Scholar] [CrossRef]
- Pedroso, E.; Grandas, A.; de las Heras, X.; Eritja, R.; Giralt, E. Diketopiperazine formation in solid phase peptide synthesis using p-alkoxybenzyl ester resins and Fmoc-amino acids. Tetrahedron Lett. 1986, 27, 743–746. [Google Scholar] [CrossRef]
- Gisin, B.F.; Merrifield, R. Carboxyl-catalyzed intramolecular aminolysis. Side reaction in solid-phase peptide synthesis. J. Am. Chem. Soc. 1972, 94, 3102–3106. [Google Scholar] [CrossRef]
- Kirshenbaum, K.; Barron, A.E.; Goldsmith, R.A.; Armand, P.; Bradley, E.K.; Truong, K.T.; Dill, K.A.; Cohen, F.E.; Zuckermann, R.N. Sequence-specific polypeptoids: A diverse family of heteropolymers with stable secondary structure. Proc. Natl. Acad. Sci. USA 1998, 95, 4303–4308. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, R.N. Peptoid origins. Pept. Sci. 2011, 96, 545–555. [Google Scholar] [CrossRef]
- Rich, D.H.; Kawai, M.; Jasensky, R.D. Conformational studies of cyclic tetrapeptides: Evidence for a bis γ-turn conformation for chlamydocin and Ala4-chlamydocin in nonpolar solvents. Int. J. Pept. Protein Res. 1983, 21, 35–42. [Google Scholar] [CrossRef]
- Merten, C.; Li, F.; Bravo-Rodriguez, K.; Sanchez-Garcia, E.; Xu, Y.; Sander, W. Solvent-induced conformational changes in cyclic peptides: A vibrational circular dichroism study. Phys. Chem. Chem. Phys. 2014, 16, 5627–5633. [Google Scholar] [CrossRef]
- Kranz, M.; Murray, P.J.; Taylor, S.; Upton, R.J.; Clegg, W.; Elsegood, M.R. Solution, solid phase and computational structures of apicidin and its backbone-reduced analogs. J. Pept. Sci. 2006, 12, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Kessler, H. Conformation and biological activity of cyclic peptides. Angew. Chem. Int. Ed. 1982, 21, 512–523. [Google Scholar] [CrossRef]
- Horne, W.S.; Olsen, C.A.; Beierle, J.M.; Montero, A.; Ghadiri, M.R. Probing the Bioactive Conformation of an Archetypal Natural Product HDAC Inhibitor with Conformationally Homogeneous Triazole-Modified Cyclic Tetrapeptides. Angew. Chem. Int. Ed. 2009, 48, 4718–4724. [Google Scholar] [CrossRef]
- Aberhart, D.J.; Cotting, J.-A.; Lin, H.-J. Separation by high-performance liquid chromatography of (3R)-and (3S)-β-leucine as diastereomeric derivatives. Anal. Biochem. 1985, 151, 88–91. [Google Scholar] [CrossRef]
- Soukup-Hein, R.J.; Schneiderheinze, J.; Mehelic, P.; Armstrong, D.W. LC and LC-MS separation of peptides on macrocyclic glycopeptide stationary phases: Diastereomeric series and large peptides. Chromatographia 2007, 66, 461–468. [Google Scholar] [CrossRef]
- Huang, Y.; Pan, L.; Zhao, L.; Mant, C.T.; Hodges, R.S.; Chen, Y. Structure-guided RP-HPLC chromatography of diastereomeric α-helical peptide analogs substituted with single amino acid stereoisomers. Biomed. Chromatogr. 2014, 28, 511–517. [Google Scholar] [CrossRef]
- Chruszcz, M.; Borek, D.; Domagalski, M.; Otwinowski, Z.; Minor, W. X-ray diffraction experiment: The last experiment in the structure elucidation process. Adv. Protein Chem. Struct. Biol. 2009, 77, 23–40. [Google Scholar]
- Garman, E.F. Developments in x-ray crystallographic structure determination of biological macromolecules. Science 2014, 343, 1102–1108. [Google Scholar] [CrossRef]
- Ramachandran, G.; Chandrasekaran, R.; Kopple, K.D. Variation of the NH–CαH coupling constant with dihedral angle in the NMR spectra of peptides. Biopolymers 1971, 10, 2113–2131. [Google Scholar] [CrossRef]
- Nakai, H.; Nagashima, K.; Itazaki, H. Structure of a new cyclotetrapeptide trapoxin A. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1991, 47, 1496–1499. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Balasubramani, S.G.; Chen, G.P.; Coriani, S.; Diedenhofen, M.; Frank, M.S.; Franzke, Y.J.; Furche, F.; Grotjahn, R.; Harding, M.E.; Hättig, C. TURBOMOLE: Modular program suite for ab initio quantum-chemical and condensed-matter simulations. Int. J. Chem. Phys. 2020, 152, 184107. [Google Scholar] [CrossRef]
- Gloge, T. Development of a Universal Alignment Medium for the Extraction of RDCs and Structure Elucidation with Tensorial Constraints. Ph.D. Thesis, Karlsruhe University of Technology, Karlsruhe, Germany, 2020. [Google Scholar]
- Enthart, A.; Freudenberger, J.C.; Furrer, J.; Kessler, H.; Luy, B. The CLIP/CLAP-HSQC: Pure absorptive spectra for the measurement of one-bond couplings. J. Magn. Reson. 2008, 192, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Tzvetkova, P.; Simova, S.; Luy, B. PE HSQC: A simple experiment for simultaneous and sign-sensitive measurement of (1JCH+ DCH) and (2JHH+ DHH) couplings. J. Magn. Reson. 2007, 186, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Vázquez, A. MSpin-RDC. A program for the use of residual dipolar couplings for structure elucidation of small molecules. Magn. Reson. Chem. 2012, 50 (Suppl. 1), S73–S79. [Google Scholar] [CrossRef] [PubMed]
- Culf, A.S.; Čuperlović-Culf, M.; Leger, D.A.; Decken, A. Small head-to-tail macrocyclic α-peptoids. Org. Lett. 2014, 16, 2780–2783. [Google Scholar] [CrossRef] [PubMed]
- Caumes, C.; Fernandes, C.; Roy, O.; Hjelmgaard, T.; Wenger, E.; Didierjean, C.; Taillefumier, C.; Faure, S. Cyclic α, β-tetrapeptoids: Sequence-dependent cyclization and conformational preference. Org. Lett. 2013, 15, 3626–3629. [Google Scholar] [CrossRef] [PubMed]
- Maulucci, N.; Izzo, I.; Bifulco, G.; Aliberti, A.; De Cola, C.; Comegna, D.; Gaeta, C.; Napolitano, A.; Pizza, C.; Tedesco, C.; et al. Synthesis, structures, and properties of nine-, twelve-, and eighteen-membered N-benzyloxyethyl cyclic α-peptoids. Chem. Commun. 2008, 33, 3927–3929. [Google Scholar] [CrossRef]
- Dale, J.; Titlestad, K. A common conformation for five cyclic tetrapeptides. J. Chem. Soc. D 1970, 1403–1404. [Google Scholar] [CrossRef]
- Mästle, W.; Link, U.; Witschel, W.; Thewalt, U.; Weber, T.; Rothe, M. Conformation and formation tendency of the cyclotetrapeptide cyclo (d-Pro-d-Pro-l-Pro-l-Pro): Experimental results and molecular modeling studies. Biopolymers 1991, 31, 735–744. [Google Scholar] [CrossRef]
- Seebach, D.; Bezençon, O.; Jaun, B.; Pietzonka, T.; Matthews, J.L.; Kühnle, F.N.; Schweizer, W.B. Further C-Alkylations of Cyclotetrapeptides via Lithium and Phosphazenium (P4) Enolates: Discovery of a New Conformation. Helv. Chim. Acta 1996, 79, 588–608. [Google Scholar] [CrossRef]
- Swepston, P.N.; Cordes, A.; Kuyper, L.; Meyer, W.L. Dihydrotentoxin: A cyclic tetrapeptide. Acta Crystallogr. B 1981, 37, 1139–1141. [Google Scholar] [CrossRef]
- Loiseau, N.; Gomis, J.M.; Santolini, J.; Delaforge, M.; André, F. Predicting the conformational states of cyclic tetrapeptides. Biopolymers 2003, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.C.; Karle, I.L. Crystal structure of the 1: 1 mixture of cyclic (L-Ala-L-Pro-L-Phe-L-Pro) and cyclic (L-Ala-L-Pro-D-Phe-L-Pro). Int. J. Pept. Protein Res. 1982, 20, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Crystallogr. B 2013, 69, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef] [PubMed]
- Huelsken, J.; Behrens, J. The Wnt signalling pathway. J. Cell Sci. 2002, 115, 3977–3978. [Google Scholar] [CrossRef]
- Hoffmeyer, K.; Raggioli, A.; Rudloff, S.; Anton, R.; Hierholzer, A.; Del Valle, I.; Hein, K.; Vogt, R.; Kemler, R. Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science 2012, 336, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Arenas, E. Emerging roles of Wnts in the adult nervous system. Nat. Rev. Neurosci. 2010, 11, 77–86. [Google Scholar] [CrossRef]
- Cisternas, P.; Henriquez, J.P.; Brandan, E.; Inestrosa, N.C. Wnt signaling in skeletal muscle dynamics: Myogenesis, neuromuscular synapse and fibrosis. Mol. Neurobiol. 2014, 49, 574–589. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xu, Y.; Cui, D. Wnt signaling pathway in schizophrenia. CNS Neurol. Disord. 2014, 13, 755–764. [Google Scholar] [CrossRef]
 | ||||
|---|---|---|---|---|
| Macrocycle | R1 | R2 | R3 | Yield |
| 7a |  | 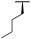 | 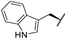 | 22% |
| 7b | 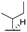 | 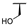 | 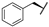 | 46% [a] |
| 7c | 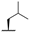 |  | 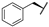 | 57% |
| 7d | 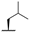 |  | 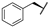 | 44% |
| 7e | 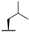 |  | 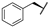 | 56% |
| 7f | 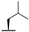 |  | 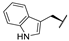 | 36% |
| 7g | 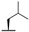 | 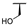 | 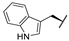 | 22% [a] |
| 7h | 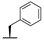 |  | 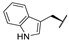 | 38% |
| 7i | 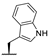 | 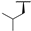 | 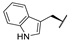 | 54% |
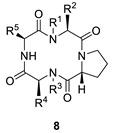 | ||||||
|---|---|---|---|---|---|---|
| Macrocycle | R1 | R2 | R3 | R4 | R5 | Yield |
| 8a |  | H | H |  | 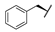 | 28% |
| 8b | 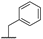 | H | H | 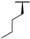 | 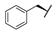 | 8.4% |
| 8c | 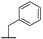 | H | H |  | 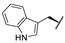 | 21% |
| 8d | H | 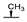 | 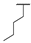 | H | 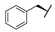 | 28% |
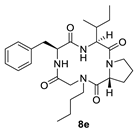
| 8e | H | 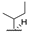 | 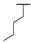 | H | 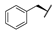 | 29% |
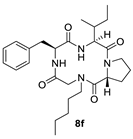
| 8f | H | 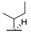 | 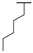 | H | 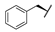 | 52% |
| 8g | H |  | 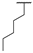 | H | 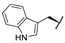 | 52% |
| 8h | H | 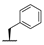 | 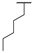 | H | 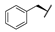 | 39% |
| 8i | H | 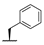 | 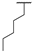 | H | 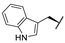 | 18% |
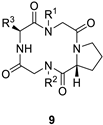 | ||||
|---|---|---|---|---|
| Macrocycle | R1 | R2 | R3 | Yield |
| 9a |  | 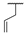 | 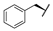 | 50% |
| 9b | 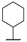 | 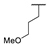 |  | 14% |
| 9c | 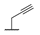 | 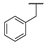 |  | 18% |
| 9d | 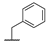 | 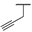 |  | 15% |
| 9e | 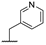 | 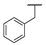 | 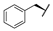 | 15% |
| 9f | 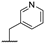 | 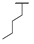 | 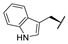 | 28% |
| 9g | 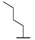 | 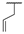 | 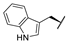 | 34% |
| 9h |  | 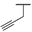 | 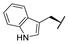 | 20% |
| 9i | 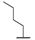 | 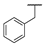 | 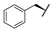 | 19% |
 | |||||
|---|---|---|---|---|---|
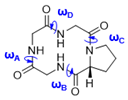
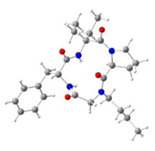
| Macrocycle | Structure | ωA | ωB | ωC | ωD |
| 7a | I | 155.7° | 171.1° | 5.2° | −167.6° |
| 7b | I | 159.0° | 171.5° | 6.5° | −172.7° |
| 7e | I | 157.3° | 170.1° | 12.1° | −173.3° |
| II | 158.1° | 169.5° | 6.5° | −172.4° | |
| III | 158.5° | 171.8° | 13.1° | −175.6° | |
| 7f | I | 160.4° | 167.9° | −3.7° | −172.6° |
| II | 160.8° | 167.4° | 13.6° | −174.4° | |
| III | 163.3° | 166.8° | 9.8° | −174.8 | |
| IV | 165.5° | 163.7° | 14.6° | −177.6° | |
| V | 165.3° | 163.9° | 10.9° | −177.0° | |
| 7h | I | 157.9° | 169.0° | 5.0° | −170.6° |
| II | 157.1° | 169.6° | −6.9° | −168.0° | |
| III | 153.1° | 169.6° | 13.5° | −172.7° | |
| IV | 155.3° | 171.0° | 3.1° | −165.6° | |
 | 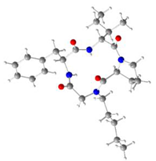 |  |  | ||||
|---|---|---|---|---|---|---|---|
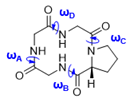 | |||||||
| Macrocycle | ωA | ωB | ωC | ωD | |||
| 8e | 155.2° | 169.4° | 12.2° | −173.0° | |||
| 8f | 155.3° | 170.7° | 13.5° | −172.4° | |||
 |  | ||||
|---|---|---|---|---|---|
| Macrocycle | ωA | ωB | ωC | ωD | |
| 9a | −179.3° | 14.8° | −177.1° | −11.2° | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herlan, C.N.; Sonnefeld, A.; Gloge, T.; Brückel, J.; Schlee, L.C.; Muhle-Goll, C.; Nieger, M.; Bräse, S. Macrocyclic Tetramers—Structural Investigation of Peptide-Peptoid Hybrids. Molecules 2021, 26, 4548. https://doi.org/10.3390/molecules26154548
Herlan CN, Sonnefeld A, Gloge T, Brückel J, Schlee LC, Muhle-Goll C, Nieger M, Bräse S. Macrocyclic Tetramers—Structural Investigation of Peptide-Peptoid Hybrids. Molecules. 2021; 26(15):4548. https://doi.org/10.3390/molecules26154548
Chicago/Turabian StyleHerlan, Claudine Nicole, Anna Sonnefeld, Thomas Gloge, Julian Brückel, Luisa Chiara Schlee, Claudia Muhle-Goll, Martin Nieger, and Stefan Bräse. 2021. "Macrocyclic Tetramers—Structural Investigation of Peptide-Peptoid Hybrids" Molecules 26, no. 15: 4548. https://doi.org/10.3390/molecules26154548
APA StyleHerlan, C. N., Sonnefeld, A., Gloge, T., Brückel, J., Schlee, L. C., Muhle-Goll, C., Nieger, M., & Bräse, S. (2021). Macrocyclic Tetramers—Structural Investigation of Peptide-Peptoid Hybrids. Molecules, 26(15), 4548. https://doi.org/10.3390/molecules26154548






