Induced Nematic Phase of New Synthesized Laterally Fluorinated Azo/Ester Derivatives
Abstract
1. Introduction
2. Experimental
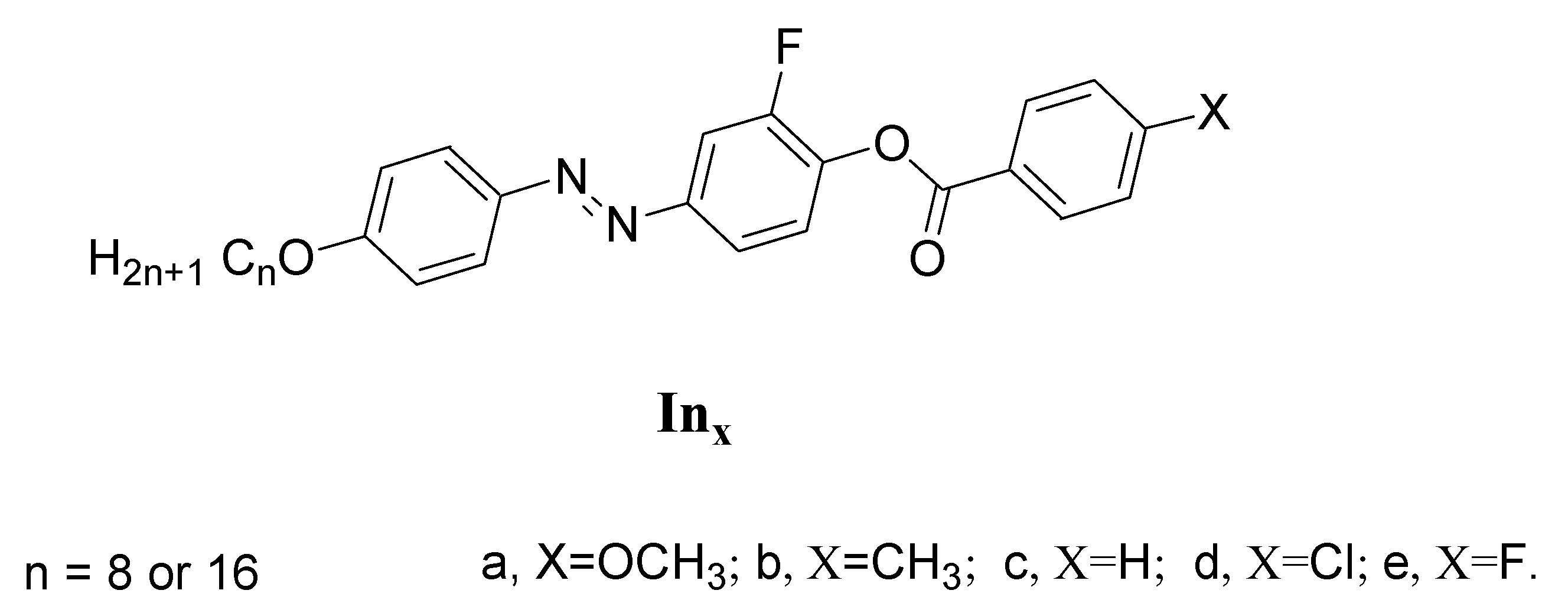
Synthesis of 2-Fluoro-4-((4-(alkyloxy)phenyl)diazenyl)phenyl 4-Substitutedbenzoate Inx
3. Results and Discussion
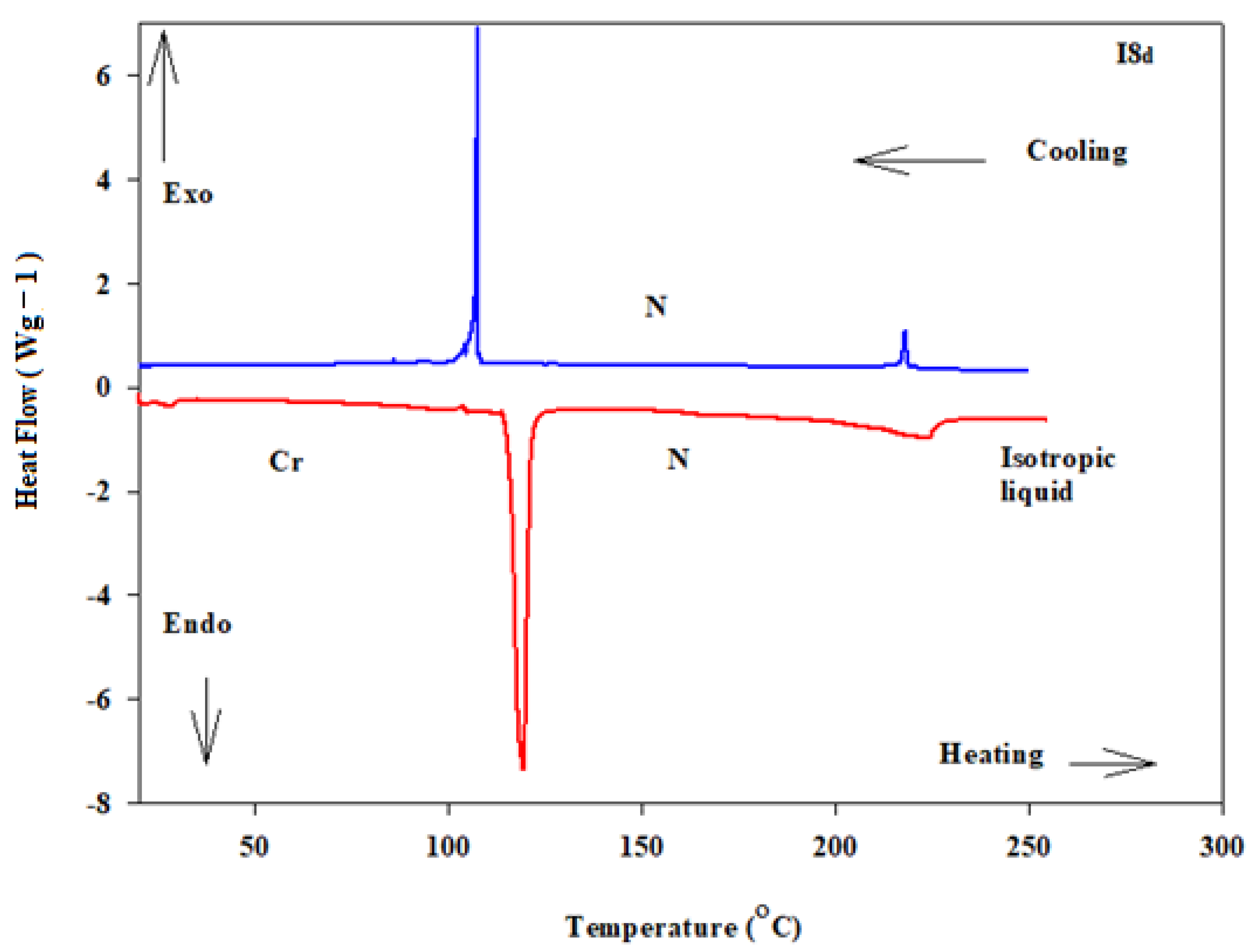
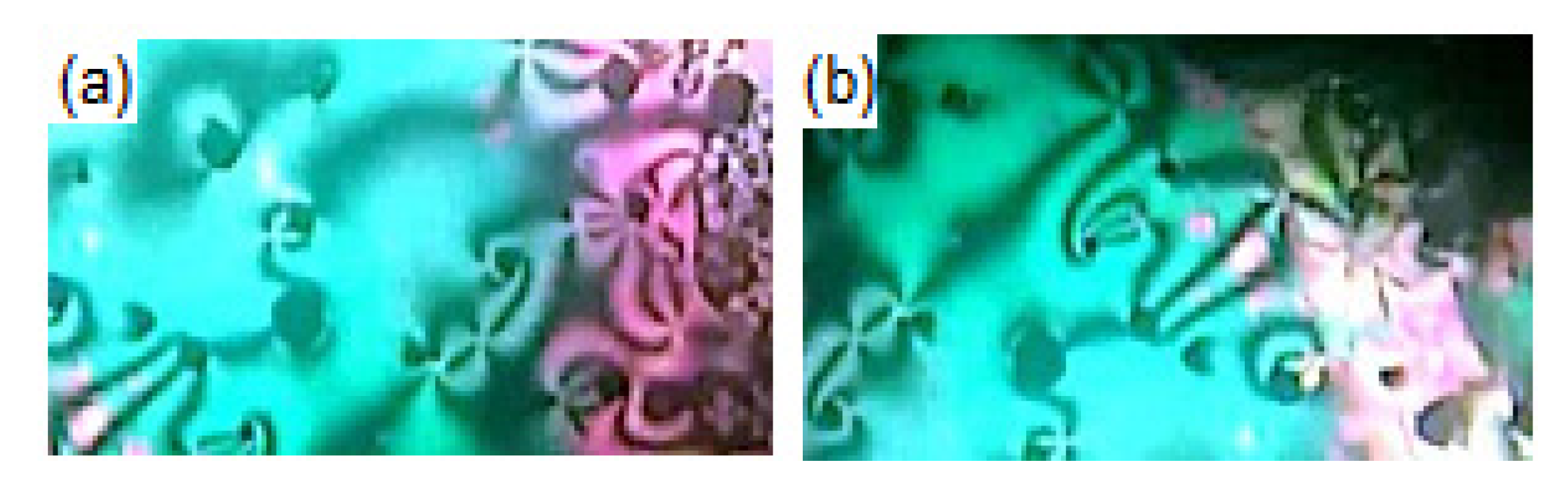
3.1. Mesomorphic and Optical Properties
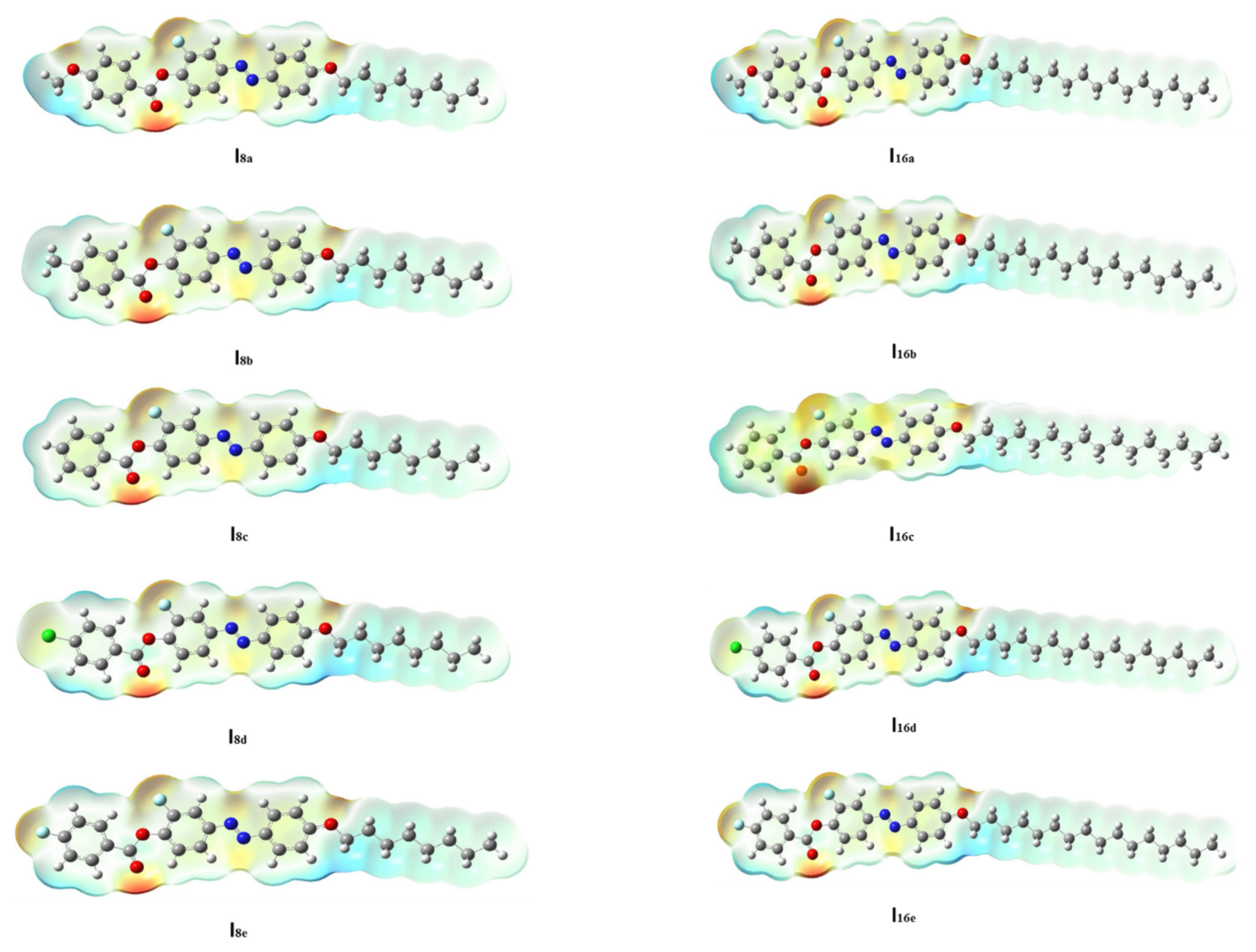
3.2. Computational Calculations
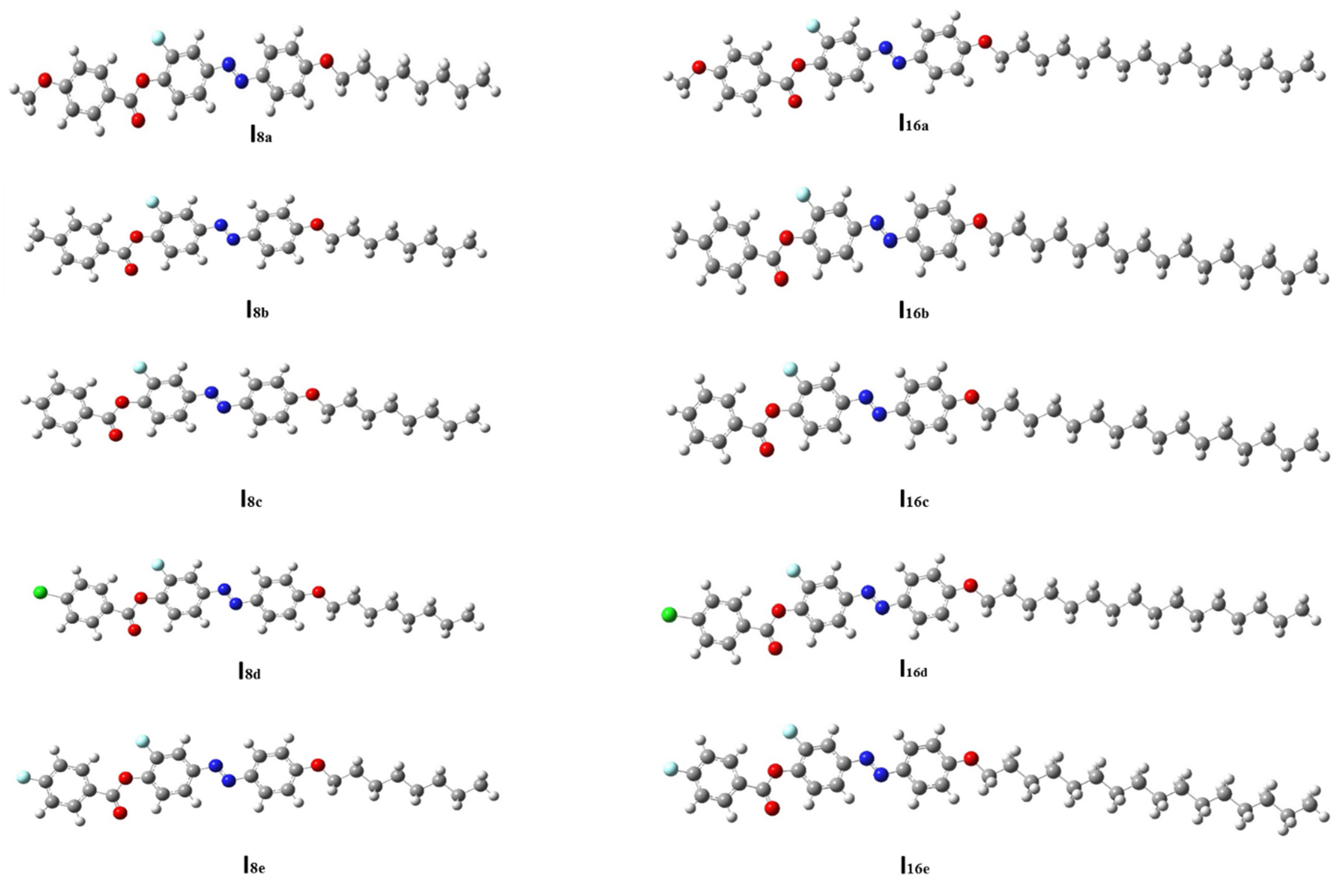
3.2.1. Thermal and Geometrical Parameters
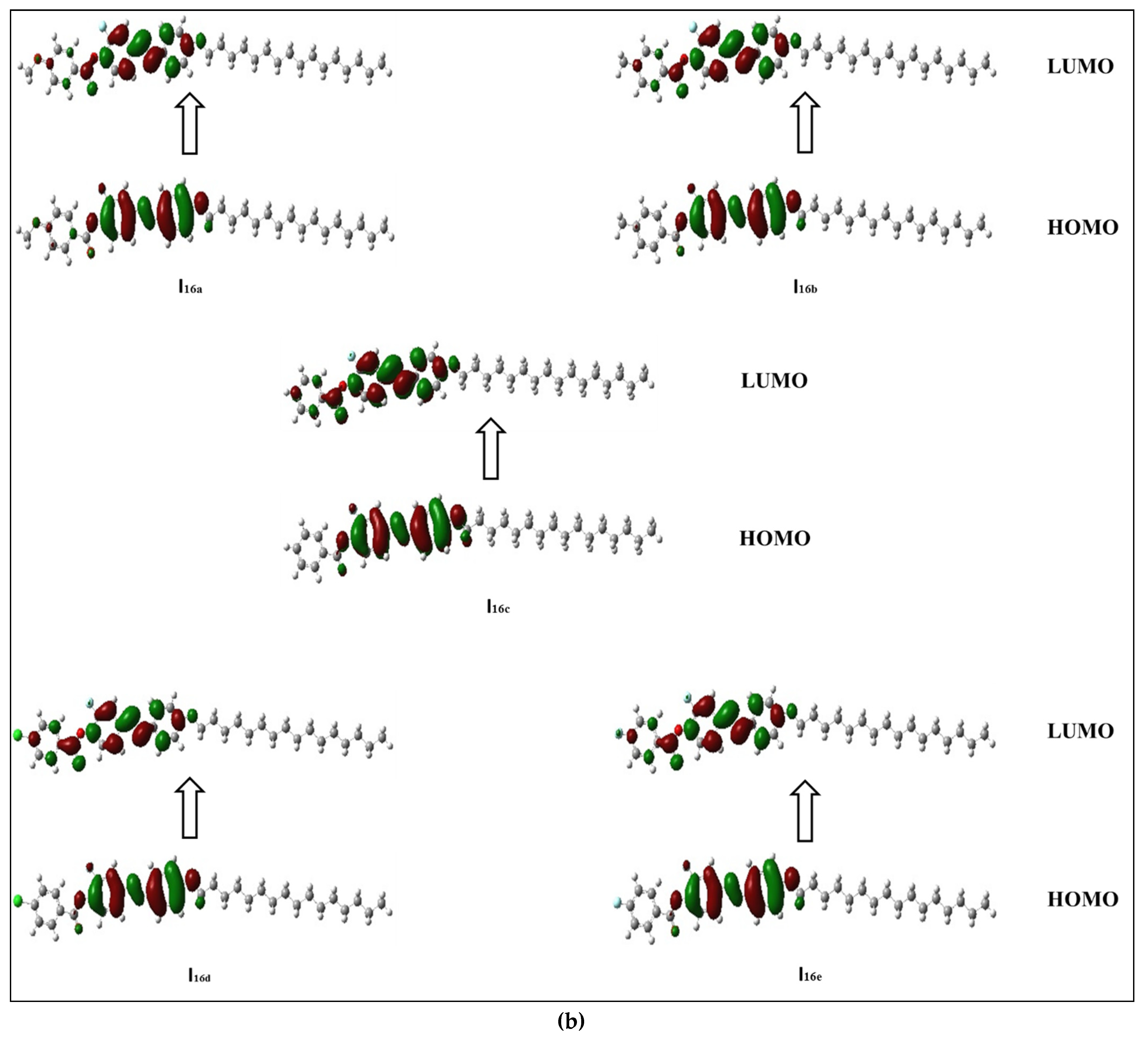
3.2.2. Frontier Molecular Orbitals (FMOs)
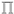 -electrons, and thereby decreases the ∆E of the FMOs.
-electrons, and thereby decreases the ∆E of the FMOs. 3.2.3. Molecular Electrostatic Potential (MEP)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikeda, T.; Wu, Y. Photoinduced alignment behavior of polymer liquid crystals containing azobenzene moieties in the side chain. Pure Appl. Chem. 1999, 71, 2131–2136. [Google Scholar] [CrossRef][Green Version]
- Eich, M.; Wendorff, J.H.; Reck, B.; Ringsdorf, H. Reversible digital and holographic optical storage in polymeric liquid crystals. Die Makromol. Chem. Rapid Commun. 1987, 8, 59–63. [Google Scholar] [CrossRef]
- Eich, M.; Wendorff, J.H. Erasable holograms in polymeric liquid crystals. Die Makromol. Chem. Rapid Commun. 1987, 8, 467–471. [Google Scholar] [CrossRef]
- Anderle, K.; Birenheide, R.; Werner, M.J.A.; Wendorff, J.H. Molecular addressing? Studies on light-induced reorientation in liquid-crystalline side chain polymers. Liq. Cryst. 1991, 9, 691–699. [Google Scholar] [CrossRef]
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional Liquid-Crystalline Assemblies: Self-Organized Soft Materials. Angew. Chem. Int. Ed. 2006, 45, 38–68. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Mamiya, J.-I.; Yu, Y. Photomechanics of Liquid-Crystalline Elastomers and Other Polymers. Angew. Chem. Int. Ed. 2007, 46, 506–528. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, K. Photoalignment of Liquid-Crystal Systems. Chem. Rev. 2000, 100, 1847–1874. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T. Photomodulation of liquid crystal orientations for photonic applications. J. Mater. Chem. 2003, 13, 2037–2057. [Google Scholar] [CrossRef]
- Beharry, A.A.; Woolley, G.A. ChemInform Abstract: Azobenzene Photoswitches for Biomolecules. Chem. Soc. Rev. 2011, 42, 4422–4437. [Google Scholar] [CrossRef]
- Tanaka, D.; Ishiguro, H.; Shimizu, Y.; Uchida, K. Thermal and photoinduced liquid crystalline phase transitions with a rod–disc alternative change in the molecular shape. J. Mater. Chem. 2012, 22, 25065–25071. [Google Scholar] [CrossRef]
- Alaasar, M.; Poppe, S.; Tschierske, C. Photoresponsive halogen bonded polycatenar liquid crystals. J. Mol. Liq. 2019, 277, 233–240. [Google Scholar] [CrossRef]
- Iftime, M.-M.; Cozan, V.; Airinei, A.; Varganici, C.; Ailiesei, G.; Timpu, D.; Sava, I. Asymmetric azomethine amines with azobenzene moieties—liquid crystalline and optical properties. Liq. Cryst. 2019, 46, 1584–1594. [Google Scholar] [CrossRef]
- Wang, M.; Han, Y.; Guo, L.-X.; Lin, B.-P.; Yang, H. Photocontrol of helix handedness in curled liquid crystal elastomers. Liq. Cryst. 2018, 46, 1231–1240. [Google Scholar] [CrossRef]
- Gomha, S.; Ahmed, H.; Shaban, M.; Abolibda, T.; Khushaim, M.; Alharbi, K. Synthesis, Optical Characterizations and Solar Energy Applications of New Schiff Base Materials. Materials 2021, 14, 3718. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lu, H.; Zhang, X.; Zhang, Q.; Xu, M.; Zhu, J.; Zhang, G.; Ding, Y.; Qiu, L. Tuning helical twisting power and photoisomerisation kinetics of axially chiral cyclic azobenzene dopants in cholesteric liquid crystals. Liq. Cryst. 2019, 46, 2181–2189. [Google Scholar] [CrossRef]
- Yu, Y.-B.; He, W.-L.; Jiang, Z.-M.; Yu, Z.-F.; Ren, L.; Lu, Y.; Yang, Z.; Cao, H.; Wang, D. The effects of azo-oxadiazole-based bent-shaped molecules on the temperature range and the light-responsive performance of blue phase liquid crystal. Liq. Cryst. 2019, 46, 1024–1034. [Google Scholar] [CrossRef]
- Abberley, J.P.; Storey, J.; Imrie, C.T. Structure-property relationships in azobenzene-based twist-bend nematogens. Liq. Cryst. 2019, 46, 2102–2114. [Google Scholar] [CrossRef]
- Aya, S.; Salamon, P.; Paterson, D.A.; Storey, J.M.; Imrie, C.T.; Araoka, F.; Jákli, A.; Buka, Á. Fast-and-giant photorheological effect in a liquid crystal dimer. Adv. Mater. Interfaces 2019, 6, 1802032. [Google Scholar] [CrossRef]
- Paterson, D.; Walker, R.; Abberley, J.P.; Forestier, J.; Harrison, W.T.A.; Storey, J.; Pociecha, D.; Gorecka, E.; Imrie, C. Azobenzene-based liquid crystal dimers and the twist-bend nematic phase. Liq. Cryst. 2017, 44, 2060–2078. [Google Scholar] [CrossRef]
- Paterson, D.; Xiang, J.; Singh, G.; Walker, R.; Agra-Kooijman, D.M.; Martinez-Felipe, A.; Gao, M.; Storey, J.; Kumar, S.; Lavrentovich, O.D.; et al. Reversible Isothermal Twist–Bend Nematic–Nematic Phase Transition Driven by the Photoisomerization of an Azobenzene-Based Nonsymmetric Liquid Crystal Dimer. J. Am. Chem. Soc. 2016, 138, 5283–5289. [Google Scholar] [CrossRef]
- Altowyan, A.; Ahmed, H.; Gomha, S.; Mostafa, A. Optical and Thermal Investigations of New Schiff Base/Ester Systems in Pure and Mixed States. Polymers 2021, 13, 1687. [Google Scholar] [CrossRef]
- Khushaim, M.S.; Alalawy, H.H.; Naoum, M.M.; Ahmed, H.A. Experimental and computational simulations of nematogenic liquid crystals based on cinnamic acid in pure and mixed state. Liq. Cryst. 2021. [Google Scholar] [CrossRef]
- Naoum, M.M.; Metwally, N.H.; Eltawab, M.M.A.; Ahmed, H.A. Polarity and steric effect of the lateral substituent on the mesophase behaviour of some newly prepared liquid crystals. Liq. Cryst. 2015, 42, 1351–1369. [Google Scholar] [CrossRef]
- Ahmed, H.; Saad, G. Mesophase behaviour of laterally di-fluoro-substituted four-ring compounds. Liq. Cryst. 2015, 42, 1765–1772. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Ahmed, H.A. Effect of the Relative Orientation of the Two Fluoro-Substituents on the Mesophase Behavior of Phenylazophenyl Benzoates. Mol. Cryst. Liq. Cryst. 2012, 562, 43–65. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Ahmed, H.A. Liquid crystalline behaviour of model compounds di-laterally substituted with different polar groups. Liq. Cryst. 2011, 38, 511–519. [Google Scholar] [CrossRef]
- Naoum, M.; Ahmed, H. Effect of dipole moment and conformation on the mesophase behavior of di-laterally substituted phenylazophenyl benzoate liquid crystals. Thermochim. Acta 2011, 521, 202–210. [Google Scholar] [CrossRef]
- Naoum, M.M.; Mohammady, S.Z.; Ahmed, H.A. Lateral protrusion and mesophase behaviour in pure and mixed states of model compounds of the type 4-(4′-substituted phenylazo)-2-(or 3-)methyl phenyl-4′-alkoxy benzoates. Liq. Cryst. 2010, 37, 1245–1257. [Google Scholar] [CrossRef]
- Dave, J.S.; Menon, M. Azomesogens with a heterocyclic moiety. Bull. Mater. Sci. 2000, 23, 237–238. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.; Alshabanah, L.; Gomha, S.; Ahmed, H. Synthesis, Thermal and Optical Characterizations of New Lateral Organic Systems. Crystals 2021, 11, 551. [Google Scholar] [CrossRef]
- Alamro, F.S.; Gomha, S.M.; Shaban, M. Optical investigations and photoactive solar energy applications of new synthesized Schiff base liquid crystal derivatives. Sci. Rep. 2021, 11, 15046. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; An, Z.; Wang, W.; Chen, X.; Chen, P. Lateral substituent effects on UV stability of high-birefringence liquid crystals with the diaryl-diacetylene core: DFT/TD-DFT study. Liq. Cryst. 2017, 44, 1515–1524. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; El-Sayed, T.H.; Alnoman, R.B. Schiff base/ester liquid crystals with different lateral substituents: Mesophase behaviour and DFT calculations. Liq. Cryst. 2019, 46. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; Saad, G.R. Mesophase stability of new Schiff base ester liquid crystals with different polar substituents. Liq. Cryst. 2018, 45, 1324–1332. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Mansour, E.; Hagar, M. Mesomorphic study and DFT simulation of calamitic Schiff base liquid crystals with electronically different terminal groups and their binary mixtures. Liq. Cryst. 2020, 47, 2292–2304. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddadd, O. DFT Calculations and Mesophase Study of Coumarin Esters and Its Azoesters. Crystal 2018, 8, 359. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Saad, G. Synthesis and mesophase behaviour of Schiff base/ester 4-(arylideneamino)phenyl-4″-alkoxy benzoates and their binary mixtures. J. Mol. Liq. 2019, 273, 266–273. [Google Scholar] [CrossRef]
- Paterson, D.A.; Gao, M.; Kim, Y.-K.; Jamali, A.; Finley, K.L.; Robles-Hernández, B.; Diez-Berart, S.; Salud, J.; de la Fuente, M.R.; Timimi, B.A. Understanding the twist-bend nematic phase: The characterisation of 1-(4-cyanobiphenyl-4′-yloxy)-6-(4-cyanobiphenyl-4′-yl) hexane (CB6OCB) and comparison with CB7CB. Soft Matter 2016, 12, 6827–6840. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.D.; Deb, R.; Chakraborty, N.; Mohiuddin, G.; Nath, R.K.; Nandiraju, V.R. Cholesterol-based dimeric liquid crystals: Synthesis, mesomorphic behaviour of frustrated phases and DFT study. Liq. Cryst. 2013, 40, 468–481. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Mondal, S.; Sinha, R.K. Synthesis and characterization of novel cholesterol based mesogenic compounds using ‘click’ chemistry. New J. Chem. 2010, 34, 1255–1260. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Senior, S.; El-Atawy, M.A.; El-Sadany, S.K.; Hamed, E.A. DFT calculations of 2,4,6-trinitrophenylbenzoate derivatives: Structure, ground state properties and spectral properties. J. Mol. Struct. 2011, 1006, 303–311. [Google Scholar] [CrossRef]
- Omar, A.Z.; Mahmoud, M.N.; El-Sadany, S.K.; Hamed, E.A.; El-Atawy, M.A. A combined experimental and DFT investigation of mono azo thiobarbituric acid based chalcone disperse dyes. Dyes Pigments 2021, 185, 108887. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Hegazi, A.H.; Al Khalaf, M.; Amer, A. The structure elucidation of the isomeric mixture of 3-[L-threo-2,3,4-tri -hydroxy-1-(phenyl-hydrazono)butyl] quinoxalin-2(1H)-one in dimethyl sulfoxide solution revisited: Experimental and theoretical study. Struct. Chem. 2020, 31, 1065–1072. [Google Scholar] [CrossRef]
- Cruickshank, E.; Salamończyk, M.; Pociecha, D.; Strachan, G.J.; Storey, J.; Wang, C.; Feng, J.; Zhu, C.; Gorecka, E.; Imrie, C.T. Sulfur-linked cyanobiphenyl-based liquid crystal dimers and the twist-bend nematic phase. Liq. Cryst. 2019, 46, 1595–1609. [Google Scholar] [CrossRef]
- Hird, M. Fluorinated liquid crystals—Properties and applications. Chem. Soc. Rev. 2007, 36, 2070–2095. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Chen, B.; Tang, H.; Shi, G.; Xu, S. Synthesis and physical properties of laterally fluorinated liquid crystals containing 1,3,2-dioxaborinane and cyclohexyl units. Liq. Cryst. 2004, 31, 1151–1158. [Google Scholar] [CrossRef]
- Matharu, A.S.; Cowling, S.J.; Wright, G. Laterally fluorinated liquid crystals containing the 2,2′bithiophene moiety. Liq. Cryst. 2007, 34, 489–506. [Google Scholar] [CrossRef]
- El-Atawy, M.; Alhaddad, O.A.; Ahmed, H.A. Experimental and geometrical structure characterizations of new synthesized laterally fluorinated nematogenic system. Liq. Cryst. 2021. [Google Scholar] [CrossRef]
- Saccone, M.; Kuntze, K.; Ahmed, Z.; Siiskonen, A.; Giese, M.; Priimagi, A. Ortho-Fluorination of azophenols increases the mesophase stability of photoresponsive hydrogen-bonded liquid crystals. J. Mater. Chem. C 2018, 6, 9958–9963. [Google Scholar] [CrossRef]
- Altowyan, A.S.; Mostafa, A.M.; Ahmed, H.A. Effect of liquid media and laser energy on the preparation of Ag nanoparticles and their nanocomposites with Au nanoparticles via laser ablation for optoelectronic applications. Optik 2021, 241, 167217. [Google Scholar] [CrossRef]
- Mishra, R.; Hazarika, J.; Hazarika, A.; Gogoi, B.; Dubey, R.; Bhattacharjee, D.; Singh, K.N.; Alapati, P.R. Dielectric properties of a strongly polar nematic liquid crystal compound doped with gold nanoparticles. Liq. Cryst. 2018, 45, 1661–1671. [Google Scholar] [CrossRef]
- Zaki, A.A.; Ahmed, H.; Hagar, M. Impact of fluorine orientation on the optical properties of difluorophenylazophenyl benzoates liquid crystal. Mater. Chem. Phys. 2018, 216, 316–324. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.A.; Alshabanah, L.A.; Ahmed, H.A.; Gomha, S.M.; Abolibda, T.Z.; Shaban, M. Synthesis, Mesomorphic and Electrical Investigations of New Furan Liquid Crystal Derivatives. Front. Chem. 2021. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; Alnoman, R.B.; Jaremko, M.; Emwas, A.-H.; Sioud, S.; Abu Al-Ola, K.A. New Liquid Crystal Assemblies Based on Cyano-Hydrogen Bonding Interactions. Front. Chem. 2021, 9, 9. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.; Alshabanah, L.; Ahmed, H.; Alalawy, H.; Al Alwani, M. Synthesis, Mesomorphic and Computational Characterizations of Nematogenic Schiff Base Derivatives in Pure and Mixed State. Molecules 2021, 26, 2038. [Google Scholar] [CrossRef]
- Sultan, A.M.; Fahmi, A.A.; Saad, G.R.; Naoum, M.M. Effect of orientation of extra fused benzene ring and lateral methyl substituent on the mesophase behaviour of three-ring azo/ester molecules. Liq. Cryst. 2019, 46, 2269–2280. [Google Scholar] [CrossRef]
- Ester, D.F.; Williams, V.E. Exploiting molecular symmetry reduction to enrich liquid crystal phase diversity. Liq. Cryst. 2019, 46, 1505–1516. [Google Scholar] [CrossRef]
- Walker, R.; Majewska, M.; Pociecha, D.; Makal, A.; Storey, J.M.; Gorecka, E.; Imrie, C.T. Twist-Bend Nematic Glasses: The Synthesis and Characterisation of Pyrene-based Nonsymmetric Dimers. ChemPhysChem 2021, 22, 461–470. [Google Scholar] [CrossRef]
- Ahmed, H.A.; El-Atawy, M.A. Synthesis, Mesomorphic and Geometrical approaches of New non-symmetrical System based on central Naphthalene moiety. Liq. Cryst. 2021. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Naoum, M.M.; Al-Zahrani, S.A.; Ahmed, H.A. New nitro-laterally substituted azomethine derivatives; Synthesis, mesomorphic and computational characterizations. Molecules 2021, 26, 1927. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, S.A.; Ahmed, H.A.; El-Atawy, M.A.; Abu Al-Ola, K.A.; Omar, A.Z. Synthetic, Mesomorphic, and DFT Investigations of New Nematogenic Polar Naphthyl Benzoate Ester Derivatives. Materials 2021, 14, 2587. [Google Scholar] [CrossRef]
- Gray, G.W. Molecular Structure and the Properties of Liquid Crystals; Academic Press: London, UK, 1962. [Google Scholar]
- Imrie, C.T.; Taylor, L. The preparation and properties of low molar mass liquid crystals possessing lateral alkyl chains. Liq. Cryst. 1989, 6, 1–10. [Google Scholar] [CrossRef]
- Chan, T.-N.; Lu, Z.; Yam, W.; Yeap, G.-Y.; Imrie, C.T. Non-symmetric liquid crystal dimers containing an isoflavone moiety. Liq. Cryst. 2012, 39, 393–402. [Google Scholar] [CrossRef]
- Henderson, P.; Niemeyer, O.; Imrie, C. Methylene-linked liquid crystal dimers. Liq. Cryst. 2001, 28, 463–472. [Google Scholar] [CrossRef]
- Lee, H.-C.; Lu, Z.; Henderson, P.A.; Achard, M.F.; Mahmood, W.A.K.; Yeap, G.Y.; Imrie, C.T. Cholesteryl-based liquid crystal dimers containing a sulfur–sulfur link in the flexible spacer. Liq. Cryst. 2012, 39, 259–268. [Google Scholar] [CrossRef]
- Imrie, C.T.; Karasz, F.E.; Attard, G.S. Comparison of the mesogenic properties of monomeric, dimeric, and side-chain polymeric liquid crystals. Macromolecules 1993, 26, 545–550. [Google Scholar] [CrossRef]
- Donaldson, T.; Staesche, H.; Lu, Z.; Henderson, P.; Achard, M.; Imrie, C. Symmetric and non-symmetric chiral liquid crystal dimers. Liq. Cryst. 2010, 37, 1097–1110. [Google Scholar] [CrossRef]
- Henderson, P.; Imrie, C.T. Methylene-linked liquid crystal dimers and the twist-bend nematic phase. Liq. Cryst. 2011, 38, 1407–1414. [Google Scholar] [CrossRef]
- Alamro, F.S.; Ahmed, H.A.; Gomha, S.M.; Shaban, M. Synthesis, mesomorphic and solar energy characterizations of new non- symmetrical Schiff base Systems. Front. Chem. 2021. [Google Scholar] [CrossRef]
- Popoola, S.A.; Almohammedi, A.R.; Haruna, K. Spectroscopic and DFT evaluation of the positional effect of amino group on the properties of aminobenzenesulphonic acid: Solvents interactions. Chem. Pap. 2021, 75, 2775–2789. [Google Scholar] [CrossRef]
- Davies, M. Some Electrical and Optical Aspects of Molecular Behaviour: The Commonwealth and International Library: Chemistry Division; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Sıdır, I.; Sıdır, Y.G.; Kumalar, M.; Taşal, E. Ab initio Hartree–Fock and density functional theory investigations on the conformational stability, molecular structure and vibrational spectra of 7-acetoxy-6-(2,3-dibromopropyl)-4,8-dimethylcoumarin molecule. J. Mol. Struct. 2010, 964, 134–151. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.A.; Alshabanah, L.A.; Ahmed, H.A.; El-Atawy, M.A. Synthesis, Optical and DFT Characterizations of Laterally Fluorinated Phenyl Cinnamate Liquid Crystal Non-Symmetric System. Symmetry 2021, 13, 1145. [Google Scholar] [CrossRef]
- Alamro, F.S.; Ahmed, H.A.; Popoola, S.A.; Altaleb, H.A.; Abu Al-Ola, K.A.; Gomha, S.M. Effect of the Relative Positions of Di-Laterally Substituted Schiff Base Derivatives: Phase Transition and Computational Investigations. Crystals 2021, 11, 870. [Google Scholar] [CrossRef]
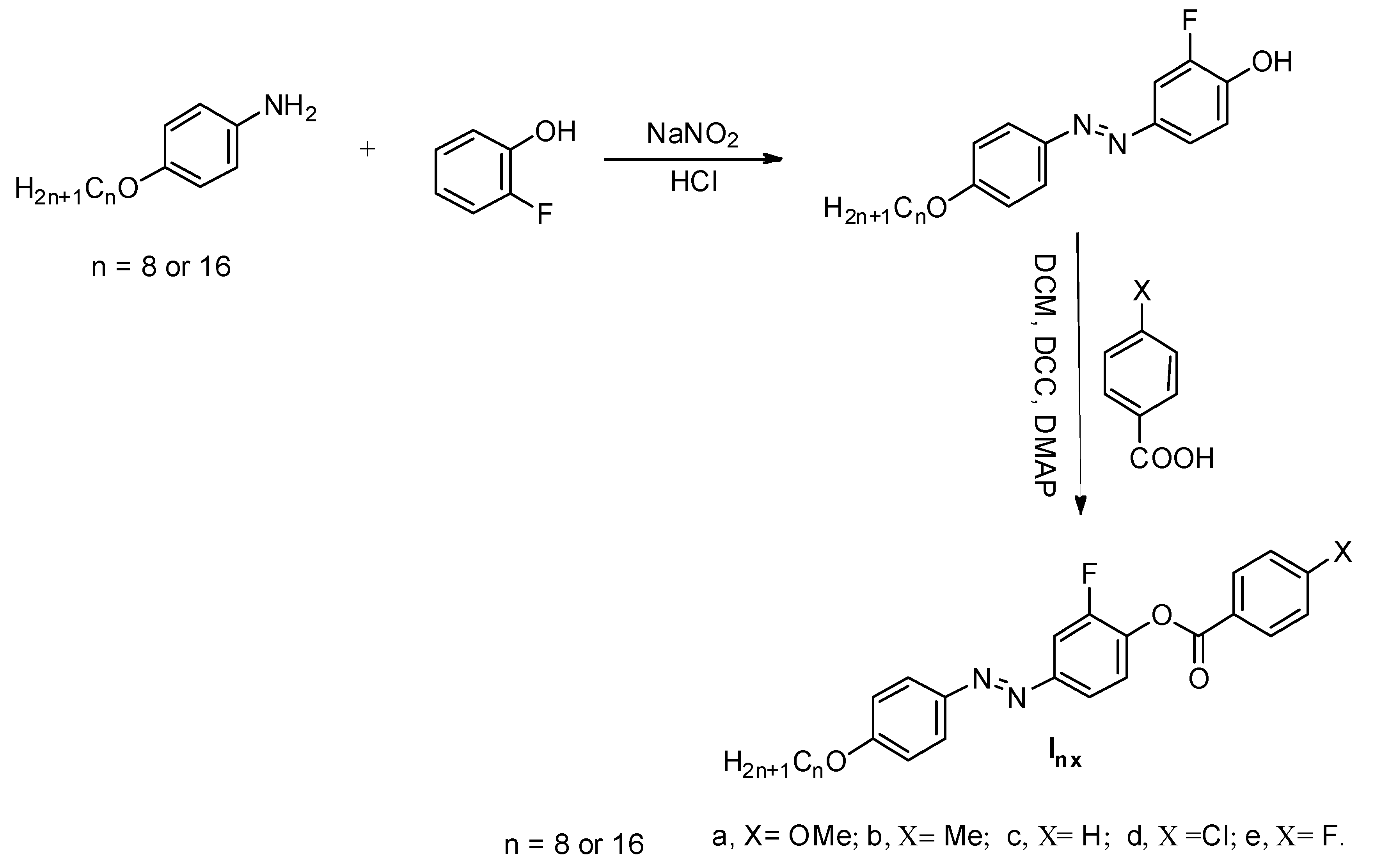
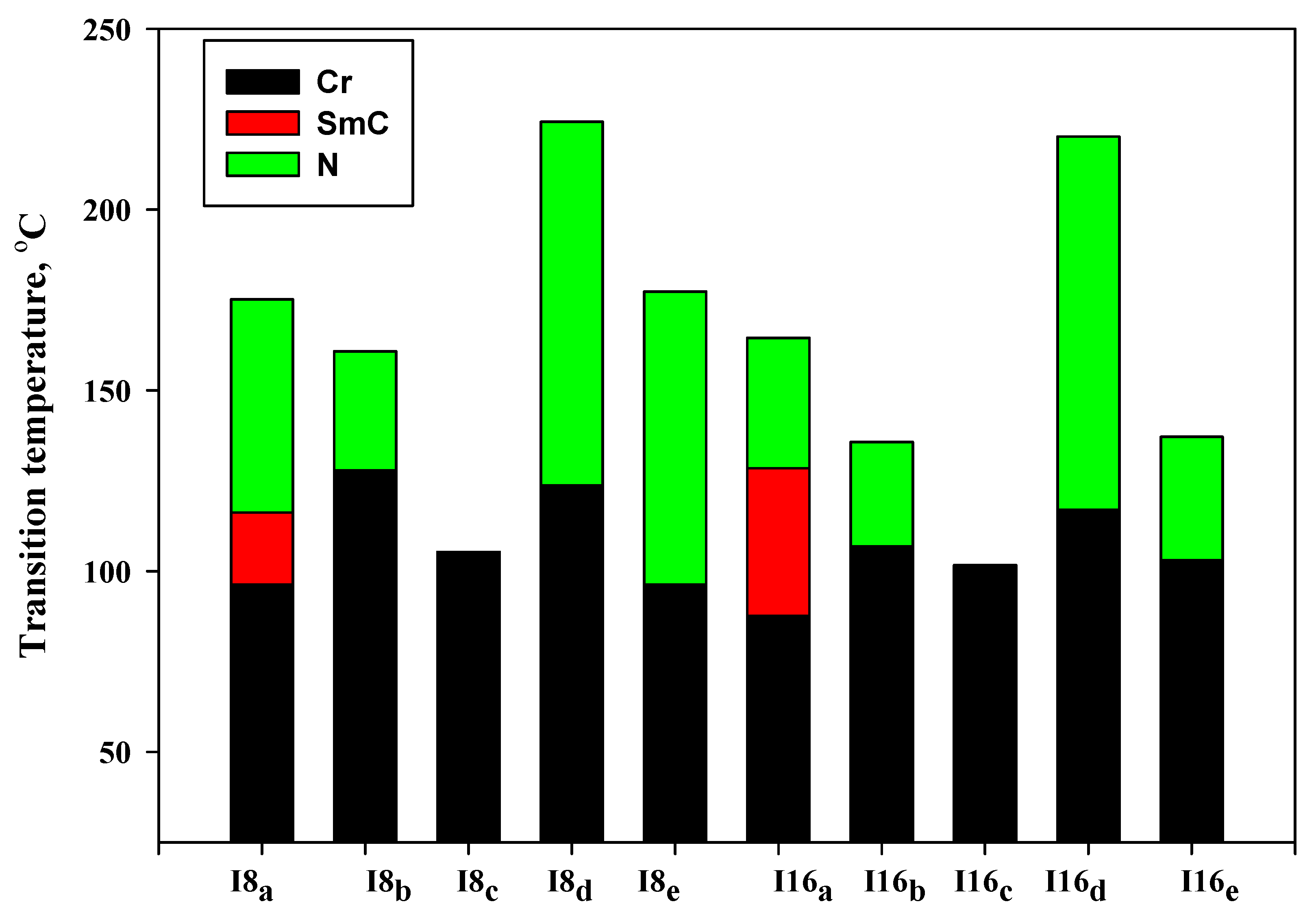

| Comp | X | TCr-I | ΔHCr-I | TCr-SmC | ΔHCr-SmC | TCr-N | ΔHCr-N | TSmC-N | ΔHSmC-N | TN-I | ΔHN-I | ΔS/R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I8a | -OCH3 | - | - | 96.3 | 45.15 | - | - | 116.2 | 2.80 | 175.2 | 1.94 | 0.52 |
| I8b | -CH3 | - | - | - | - | 127.9 | 51.76 | - | - | 160.8 | 2.13 | 0.59 |
| I8c | -H | 105.3 | 57.64 | - | - | - | - | - | - | - | - | - |
| I8d | -Cl | - | - | - | - | 123.7 | 46.70 | - | - | 224.3 | 2.09 | 0.51 |
| I8e | -F | - | - | - | - | 96.3 | 39.38 | - | - | 177.3 | 1.23 | 0.33 |
| I16a | -OCH3 | - | - | 87.6 | 48.78 | - | - | 128.5 | 2.90 | 164.5 | 1.48 | 0.41 |
| I16b | -CH3 | - | - | - | - | 106.9 | 44.6 | - | - | 135.7 | 1.62 | 0.48 |
| I16c | -H | 101.6 | 59.87 | - | - | - | - | - | - | - | - | - |
| I16d | -Cl | - | - | - | - | 116.9 | 46.32 | - | - | 220.2 | 1.87 | 0.46 |
| I16e | -F | - | - | - | - | 103.1 | 42.30 | - | - | 137.1 | 1.36 | 0.40 |
| Compound | ZPE (Kcal/Mol) | Thermal Energy (Kcal/Mol) | Enthalpy (Kcal/Mol) | Gibbs Free Energy (Kcal/Mol) | Entropy (Cal mol·k) |
|---|---|---|---|---|---|
| I8a | 339.931 | 360.875 | 361.467 | 295.216 | 222.206 |
| I8b | 336.987 | 358.034 | 358.627 | 290.124 | 229.761 |
| I8c | 319.853 | 339.674 | 340.266 | 275.311 | 217.862 |
| I8d | 313.578 | 334.225 | 334.818 | 267.740 | 224.983 |
| I8e | 314.447 | 334.832 | 335.424 | 269.207 | 222.091 |
| I16a | 483.249 | 511.573 | 512.165 | 426.784 | 286.371 |
| I16b | 480.291 | 507.554 | 508.147 | 425.184 | 278.260 |
| I16c | 463.181 | 489.788 | 490.381 | 408.976 | 273.032 |
| I16d | 456.900 | 484.336 | 484.928 | 401.334 | 280.376 |
| I16e | 457.772 | 484.943 | 485.536 | 402.825 | 277.414 |
| Compound | Total Energy (Ha) | EHOMO (ev) | EluMO (ev) | ∆E (ev) | Dipole Moment (D) | IE (ev) | EA (ev) | Polarizability Bohr3 |
|---|---|---|---|---|---|---|---|---|
| I8a | −1595.223 | −6.043 | −2.515 | 3.528 | 2.6252 | 6.043 | 2.515 | 424.26 |
| I8b | −1520.026 | −6.074 | −2.547 | 3.527 | 1.8676 | 6.074 | 2.547 | 414.42 |
| I8c | −1480.739 | −6.108 | −2.586 | 3.522 | 2.6458 | 6.108 | 2.586 | 396.84 |
| I8d | −1940.352 | −6.194 | −2.701 | 3.493 | 5.4948 | 6.194 | 2.701 | 414.23 |
| I8e | −1579.994 | −6.175 | −2.668 | 3.507 | 5.0740 | 6.175 | 2.668 | 397.89 |
| I16a | −1909.479 | −6.042 | −2.515 | 3.527 | 2.6315 | 6.042 | 2.515 | 519.56 |
| I16b | −1834.285 | −6.074 | −2.547 | 3.527 | 1.9022 | 6.074 | 2.547 | 509.61 |
| I16c | −1794.997 | −6.107 | −2.586 | 3.521 | 2.6949 | 6.107 | 2.586 | 492.04 |
| I16d | −2254.610 | −6.193 | −2.701 | 3.492 | 5.5510 | 6.193 | 2.701 | 509.50 |
| I16e | −1894.251 | −6.174 | −2.667 | 3.507 | 5.1293 | 6.174 | 2.667 | 493.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamro, F.S.; Ahmed, H.A.; El-Atawy, M.A.; Al-Zahrani, S.A.; Omar, A.Z. Induced Nematic Phase of New Synthesized Laterally Fluorinated Azo/Ester Derivatives. Molecules 2021, 26, 4546. https://doi.org/10.3390/molecules26154546
Alamro FS, Ahmed HA, El-Atawy MA, Al-Zahrani SA, Omar AZ. Induced Nematic Phase of New Synthesized Laterally Fluorinated Azo/Ester Derivatives. Molecules. 2021; 26(15):4546. https://doi.org/10.3390/molecules26154546
Chicago/Turabian StyleAlamro, Fowzia S., Hoda A. Ahmed, Mohamed A. El-Atawy, Salma A. Al-Zahrani, and Alaa Z. Omar. 2021. "Induced Nematic Phase of New Synthesized Laterally Fluorinated Azo/Ester Derivatives" Molecules 26, no. 15: 4546. https://doi.org/10.3390/molecules26154546
APA StyleAlamro, F. S., Ahmed, H. A., El-Atawy, M. A., Al-Zahrani, S. A., & Omar, A. Z. (2021). Induced Nematic Phase of New Synthesized Laterally Fluorinated Azo/Ester Derivatives. Molecules, 26(15), 4546. https://doi.org/10.3390/molecules26154546







