Natural Products from Madagascar, Socio-Cultural Usage, and Potential Applications in Advanced Biomedicine: A Concise Review
Abstract
1. Introduction
2. Some Common Madagascan Natural Products
2.1. Vonenina
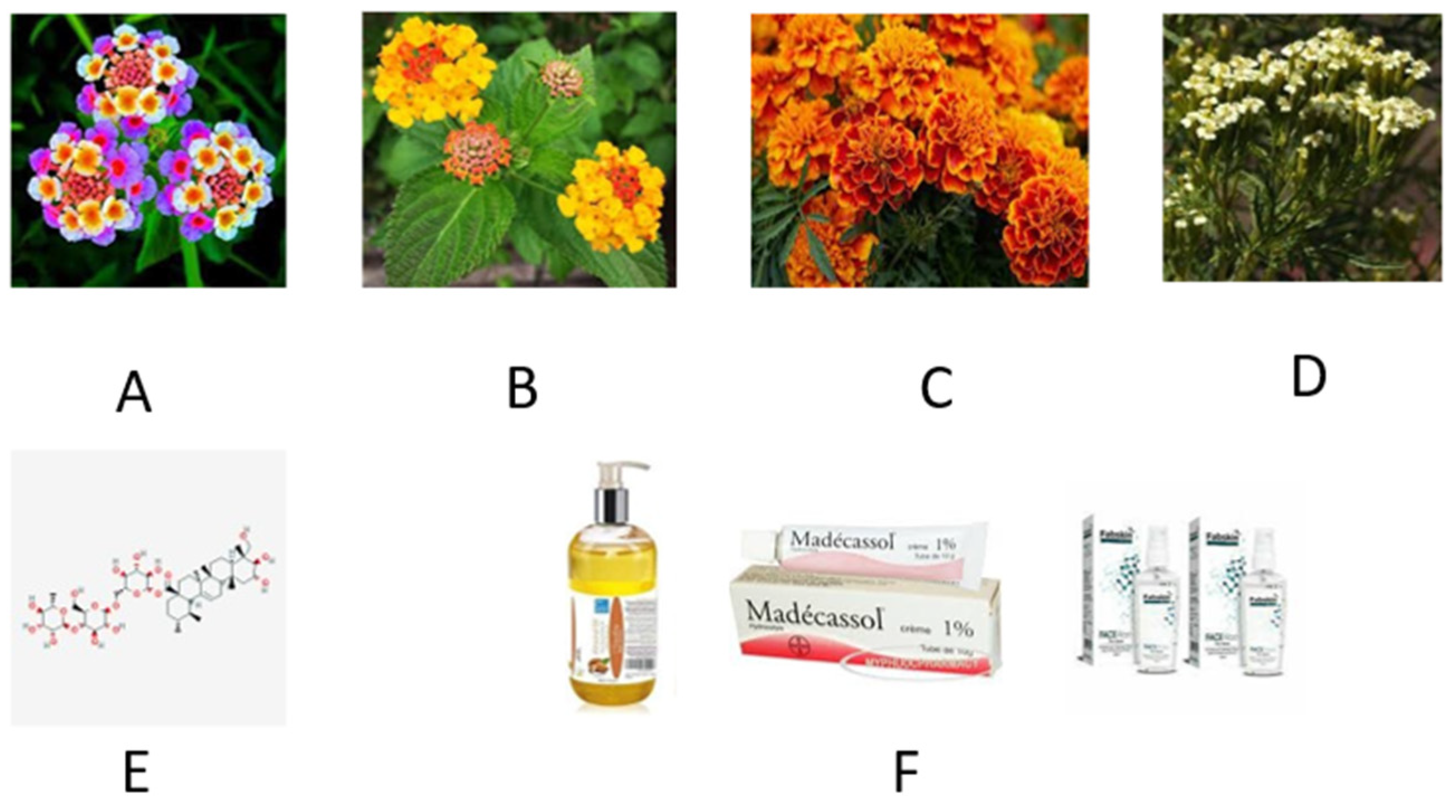
2.2. Lantana and Marigold
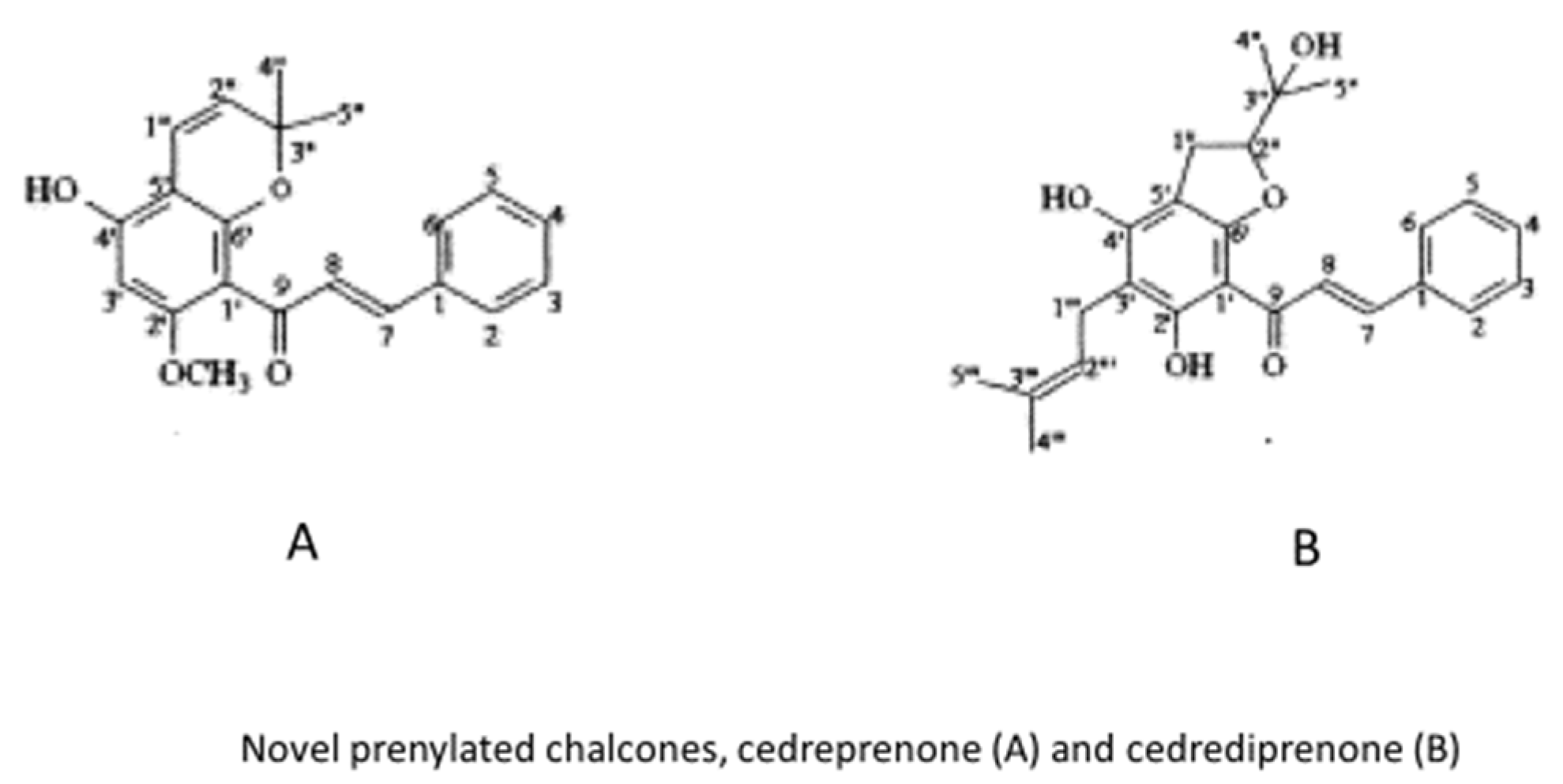
2.3. Katrafay
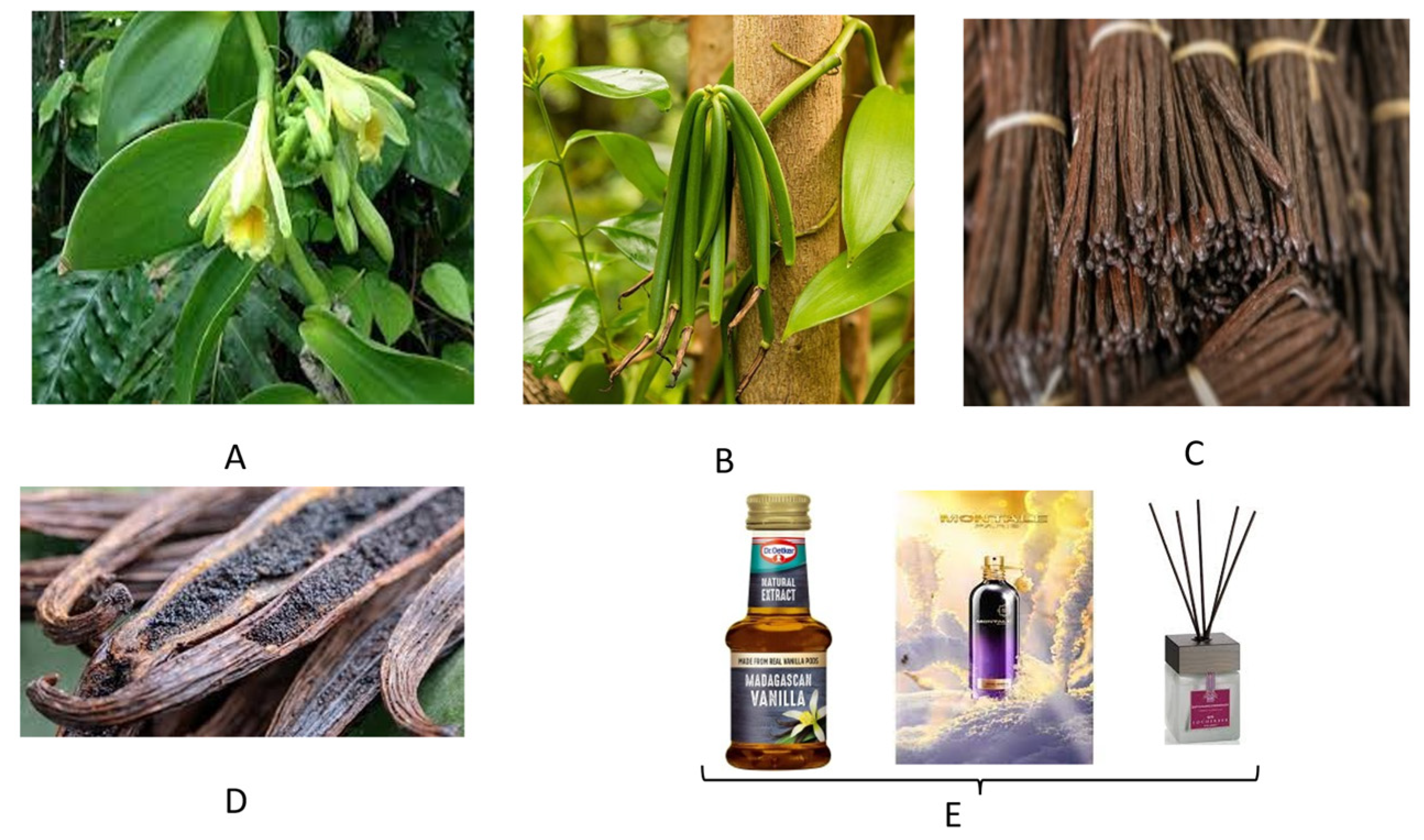
3. Vanilla and Masonjoany
3.1. Vanilla
3.2. Masonjoany
4. Nanomaterials in Advanced Biomedicine: Therapies of the Future?
Liposomes, Cat-Anionic Vesicles, and Nanotubes
5. Conclusive Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Clusius, C. Genus XII of the Pernicious Mushrooms. 1601. Available online: https://books.google.com.hk/books?hl=zh-CN&lr=&id=ZEm5-F2tmhUC&oi=fnd&pg=PA1&dq=Rariorum+plantarum+historia&ots=KyhGY2GC2s&sig=-IECn6VTHZ3YKQyDEsVw0lMHufM&redir_esc=y&hl=zh-CN&sourceid=cndr#v=onepage&q&f=false (accessed on 23 June 2021).
- Linnaeus, C. Rariorum Plantarum Historia. Flora Svecica [suecica] Exhibens Plantas per Regnum Sueciae Crescentes Systematice cum Differentiis Specierum, Synonymis Autorum, No-Minibus Incolarum, Solo Locorum, usu Pharmacopæorum; Laurentii Salvii: Stockholm, Sweden, 1745. (In Latin) [Google Scholar]
- Zimmerman, A.D.; Parfitt, B.D. Lophophora williamsii. In Flora of North America Editorial Committee; Oxford University Press: Oxford, UK; New York, NY, USA, 1993; pp. 242–262. [Google Scholar]
- Cox, L.R. Thoughts on the classification of the Gasteropoda. J. Molluscan Stud. 1960, 33, 239–261. [Google Scholar]
- Piperno, D.R.; Ranere, A.J.; Holst, I.; Iriarte, J.; Dickau, R. Starch grain and phytolith evidence for early ninth millennium B.P. maize from the Central Balsas River Valley, Mexico. Proc. Natl. Acad. Sci. USA 2009, 106, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Verheye, I.W.H. Plant Breeding and Genetics; Soils, Plant Growth and Crop Production Volume; Eolss Publishers: Paris, France, 2010; pp. 1859–2024. [Google Scholar]
- Aiello, C.; Berardi, V.; Ricci, F.; Risuleo, G. Biological properties of a methanolic extract of neem oil, a natural oil from the seeds of the Neem Tree (Azadirachta indica var. A. Juss). In Nuts & Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: London, UK; Burlington, MA, USA; San Diego, CA, USA, 2011; Chapter 96; pp. 813–821. ISBN 978-0-12-375688-6. [Google Scholar]
- Risuleo, G. Biological properties of a partially purified component of Neem oil: An updated and revised work. In Nuts and Seeds in Health and Disease Prevention, 2nd ed.; Preedy, W., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 6; pp. 67–72. [Google Scholar] [CrossRef]
- La Mesa, C.; Corbo, A.; Gkouvi, A.; Risuleo, G. Bio-Active Principles from the Animal and Plant Kingdom: A Review. In Advance Research in Organic and Inorganic Chemistry (AROIC); Distributed under Creative Commons CC-BY 4.0; Corpus Publishers: Glenroy, Australia, 2020. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Boiteau, P.; Allorge-Boiteau, L. Plantes Médicinales de Madagascar: Cinquante-Huit Plantes Médicinales Utilisées sur le Marche de Tananarive (Zoma) à Madagascar; ACCT et Èdition Khartala: Paris, France, 1993; ISBN 2-86537-407-6. [Google Scholar]
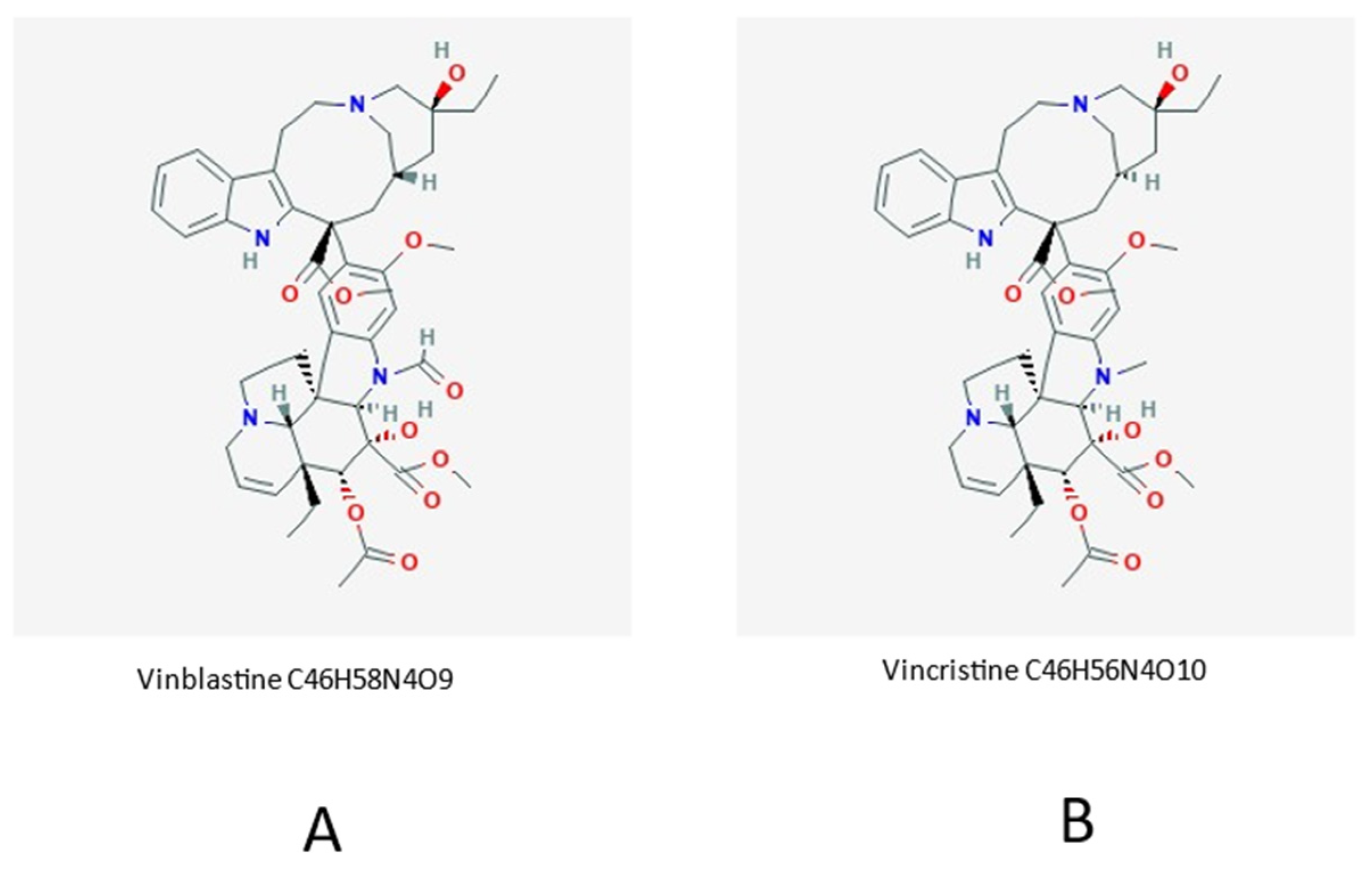
- Van Der Heijden, R.; Jacobs, D.I.; Snoeijer, W.; Hallard, D.; Verpoorte, R. The Catharanthus alkaloids: Pharmacognosy and biotechnology. Curr. Med. Chem. 2004, 11, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Keglevich, P.; Hazai, L.; Kalaus, G.; Szántay, C. Modifications on the basic skeletons of vinblastineee and vincristine. Molecules 2012, 17, 5893–5914, PMC 6268133. [Google Scholar] [CrossRef] [PubMed]
- Sears, J.E.; Boger, D.L. Total synthesis of vinblastineee, related natural products, and key analogues and development of inspired methodology suitable for the systematic study of their structure-function properties. Acc. Chem. Res. 2015, 48, 653–662, PMC 4363169. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.W. Taxonomy of Lantana sect Lantana (Verbenaceae): II Taxonomic revision. J. Bot. Res. Inst. Tex. 2012, 6, 403–441. [Google Scholar]
- Ramaroson-Raonizafinimanana, B.; Ramanoelina, P.A.R.; Rasoarahona, J.R.E.; Gaydou, E.M. Chemical Compositions of Aerial Part of Tagetes minuta L: Chemotype Essential Oils from Madagascar. J. Essent. Oil Res. 2009, 21, 390–392. [Google Scholar] [CrossRef]
- Hadfield, R.A.; Vlahovic, T.C.; Khan, M.T. The Use of Marigold Therapy for Podiatric Skin Conditions. Foot Ankle J. 2008, 1, 1–8. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Petitjean, A.M.; Ratsimamanga-Urverg, S.; Rakoto Ratsimamanga, A. Medicinal plants used to treat malaria in Madagascar. J. Ethnopharmacol. 1992, 37, 117–127. [Google Scholar] [CrossRef]
- Gupta, P.; Vasudeva, N. In vitro antiplasmodial and antimicrobial potential of Tagetes erecta roots. Pharm. Biol. 2010, 48, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Novy, J.W. Medicinal plants of the eastern region of Madagascar. J. Ethnopharmacol. 1997, 55, 119–126. [Google Scholar] [CrossRef]
- Koorbanally, N.A.; Randrianarivelojosia, M.; Mulholland, D.A.; Quarles van Ufford, L.; van den Berg, A.J.J. Chalcones from the seed of Cedrelopsis grevei (Ptaeroxylaceae). Phytochemistry 2003, 62, 1225–1229. [Google Scholar] [CrossRef]
- Mingorance, C.; Andriantsitohaina, R.; Alvarez de Sotomayor, M. Cedrelopsis grevei improves endothelial vasodilatation in aged rats through an increase of NO participation. J. Ethnopharmacol. 2008, 17, 76–83. [Google Scholar] [CrossRef]
- Rakotobe, M.; Menut, C.; Sahondra, H.; Andrianoelisoa, H.S.; Rahajanirina, V.; Collas de Chatelperrond, P.; Roger, E.; Danthu, P. The Bark Essential Oil Composition and Chemotaxonomical Appraisal of Cedrelopsis grevei H. Baillon from Madagascar. Nat. Prod. Commun. 2008, 3, 1–6. [Google Scholar]
- Gobley, N.T. Recherches sur le principe odorant de la vanilla (Research on the fragrant substance of vanilla). J. Pharm. Chim. Ser. 1858, 34, 401–405. [Google Scholar]
- Raharivelomanana, P.; Bianchini, J.P.; Ramanoelina, A.R.P.; Rasoharahona, J.R.E.; Chatel, F.; Faure, R. Structures of cadinane- and guaiane-type sesquiterpenoids from Enterospermum madagascariensis (Baill.) Homolle. Magn. Reson. Chem. 2005, 43, 1049–1052. [Google Scholar] [CrossRef]
- Muzzalupo, R.; Nicoletta, F.P.; Trombino, S.; Cassano, R.; Iemma, F.; Picci, N. A new crown ether as vesicular carrier for 5-fluoruracil: Synthesis, characterization and drug delivery evaluation. Colloids Surf. B Biointerfaces 2007, 58, 197–202. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Leroux, J.-C.J. Organogels and their use in drug delivery—A review. J. Controlled Release 2008, 125, 179–192. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Lonez, C.; Vandenbranden, M.; Ruysschaert, J.M. Cationic liposomal lipids: From gene carriers to cell signaling. Prog. Lipid Res. 2008, 47, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Patri, A.K.; Simanek, E. Biological applications of dendrimers. Mol. Pharm. 2012, 5, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Mok, C.; Ko, S.; Liang, J.; Recharla, N. Nanotechnological approaches to enhance the bioavailability and therapeutic efficacy of green tea polyphenols. J. Funct. Foods 2017, 34, 139–151. [Google Scholar] [CrossRef]
- Patri, A.K.; Kukowska-Latallo, J.F.; Baker, J.R., Jr. Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Deliv. Rev. 2005, 14, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Self-Peng, F.; Zhang, W.; Qiu, F. Self-assembling Peptides in Current Nanomedicine: Versatile Nanomaterials for Drug Delivery. Curr. Med. Chem. 2020, 27, 4855–4881. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A. Graphene: Safe or toxic? The two faces of the medal. Angew. Chem. Int. 2013, 52, 4986–4997. [Google Scholar] [CrossRef]
- Risuleo, G.; La Mesa, C. Nanoparticles and molecular delivery: State of the art and future perspectives. In Nutraceuticals in Veterinary Medicine; Springer: Cham, Switzerland, 2019; pp. 737–747. [Google Scholar] [CrossRef]
- La Mesa, C.; Risuleo, G. Colloid Stability Influences on the Biological Organization and Functions. Colloid Science. Karakus, S., Ed.; Available online: https://www.intechopen.com/online-first/ (accessed on 23 June 2021). [CrossRef]
- La Mesa, C.; Risuleo, G. Surfactant Mixtures: Performances vs. Aggregation States. In Surfactants and Detergents; Dutta, A., Ed.; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-78984-661-4. [Google Scholar] [CrossRef]
- Piccioni, F.; Borioni, A.; Delfini, M.; Del Giudice, M.R.; Mustazza, C.; Rodomonte, A.; Risuleo, G. Metabolic alterations in cultured mouse fibroblasts induced by an inhibitor of the tyrosine kinase receptors Fibroblast Growth Factor Receptor 1. Anal. Biochem. 2007, 367, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Letizia, C.; Andreozzi, P.; Scipioni, A. Protein Binding onto Surfactant-Based Synthetic Vesicles. J. Phys. Chem. B 2007, 111, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.; Mitchell, D.J.; Ninham, B.W.J. Theory of self-assembly of hydrocar-bon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Berardi, V.; Aiello, C.; Bonincontro, A.; Risuleo, G. Alterations of the plasma membrane caused by murine polyomavirus proliferation: An electrorotation study. J. Membr. Biol. 2009, 229, 19–25. [Google Scholar] [CrossRef]
- Cosimati, R.; Milardi, G.L.; Bombelli, C.; Bonincontro, A.; Bordi, F.; Mancini, G.; Risuleo, G. Interactions of DMPC and DMPC/gemini liposomes with the cell membrane investigated by electrorotation. Biochim. Biophys. Acta 2013, 1828, 352–356. [Google Scholar] [CrossRef]
- Stefanutti, E.; Papacci, F.; Sennato, S.; Bombelli, C.; Viola, I.; Bonincontro, A.; Bordi, F.; Mancini, G.; Gigli, G.; Risuleo, G. Cationic liposomes formulated with DMPC and a gemini surfactant traverse the cell membrane without causing a significant bio-damage. Biochim. Biophys. Acta 2014, 1838, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Bonincontro, A.; Risuleo, G. Electrorotation: A Spectroscopic Imaging Approach to Study the Alterations of the Cytoplasmic Membrane. Adv. J. Mol. Imaging 2015, 5, 1–15. [Google Scholar] [CrossRef]
- Kuo, J.H.; Jan, M.S.; Chang, C.H. Cytotoxicity characterization of catanionic vesicles in RAW 264.7 murine macrophage-like cells. Colloids Surf. B Biointerfaces 2005, 41, 189–196. [Google Scholar] [CrossRef]
- Vlachy, N.; Touraud, D.; Heilmann, J.; Kunz, W. Determining the cytotoxicity of catanionic surfactant mixtures on HeLa cells. Colloids Surf. B Biointerfaces 2009, 70, 278–280. [Google Scholar] [CrossRef]
- Aiello, C.; Andreozzi, P.; La Mesa, C.; Risuleo, G. Biological activity of SDS-CTAB cat-anionic vesicles in cultured cells and assessment of their cytotoxicity ending in apoptosis. J. Coll. Surf. B Biointerfaces 2010, 78, 149–154. [Google Scholar] [CrossRef]
- Lozano, N.; Perez, L.; Pons, R.; Pinazo, A. Diacyl glycerol arginine-based surfactants: Biological and physicochemical properties of catanionic formulations. Amino Acids 2011, 40, 721–729. [Google Scholar] [CrossRef]
- Russo, L.; Berardi, V.; Tardani, F.; La Mesa, C.; Risuleo, G. Delivery of RNA and its intracellular translation into protein mediated by SDS-CTAB vesicles: Potential use in nanobiotechnology. Biomed Res. Int. 2013, 734596. [Google Scholar] [CrossRef]
- Pucci, C.; Scipioni, A.; La Mesa, C. Albumin binding onto synthetic vesicles. Soft Matter 2012, 8, 9669–9675. [Google Scholar] [CrossRef]
- Prato, M.; Kostarelos, K.; Bianco, A. Functionalized carbon nanotubes in drug design and discovery. Acc. Chem. Res. 2008, 41, 60–68. [Google Scholar] [CrossRef]
- Ji, D.K.; Ménard-Moyon, C.; Bianco, A. Physically triggered nanosystems based on two-dimensional materials for cancer theranostics. Adv. Drug Deliv. Rev. 2019, 138, 211–232. [Google Scholar] [CrossRef]
- Venkatesan, J.; Pallela, R.; Kim, S.K. Applications of carbon nanomaterials in bone tissue engineering. J. Biomed. Nanotecnol. 2014, 10, 3105–3123. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Behnam, B.; Sahebkar, A. Biomedical applications of carbon-nanomaterials: Drug and gene delivery potentials. J. Cell Physiol. 2018, 234, 298–319. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Firestein, K.L.; Golberg, D.V. Fabrication and application of BN nanoparticles, nanosheets and their nanohybrids. Nanoscale 2018, 10, 17477–17493. [Google Scholar] [CrossRef]
- Holt, B.D.; Shawky, J.H.; Dahl, K.N.; Davidson, L.A.; Islam, M.F. Developing Xenopus embryos recover by compacting and expelling single wall carbon nanotubes. J. Appl. Toxicol. 2016, 36, 579–585. [Google Scholar] [CrossRef]
- Risuleo, G.; La Mesa, C. Dispersability of carbon nanotubes in biopolymer-based fluids and their potential biotechnological applications. Trends Nanotechnol. Mater. Sci. 2016, 1, 1–7. [Google Scholar]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon nanotubes: Evaluation of toxicity at biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, E.N.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. Carbon Dots as Nanotherapeutics for Biomedical Application. Curr. Pharm. Des. 2020, 26, 2207–2221. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.H.K.; Chan, K.K.; Tjin, S.C.; Yong, K.T. Carbon Allotrope-Based Optical Fibers for Environmental and Biological Sensing: A Review. Sensors 2020, 5, 2040–2046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muzi, L.; Ménard-Moyon, C.; Russier, J.; Ang, W.H.; Pastorin, G.; Risuleo, G.; Bianco, A. A Comparative study on the anticancer efficacy of two types of Functionalized Multi-walled carbon nanotubes filled with a cisplatin prodrug. Nanoscale 2015, 7, 5383–5394. [Google Scholar] [CrossRef] [PubMed]
- Muzi, L.; Cadarsi, S.; Mouchet, F.; Pinelli, E.; Janowska, I.; Russier, J.; Ménard-Moyon, C.; Risuleo, G.; Soula, B.; Galibert, A.-M.; et al. Examining the impact of few-layer graphene using cellular and amphibian models. 2D Matter 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Muzi, L.; Tardani, F.; La Mesa, C.; Bonincontro, A.; Bianco, A.; Risuleo, G. Interactions and effects of BSA-functionalized single-walled carbon nanotubes on different cell lines. Nanotechnology 2016, 15, 155704. [Google Scholar] [CrossRef] [PubMed]
- Zanni, E.; De Bellis, G.; Bracciale, M.P.; Broggi, A.; Santarelli, M.L.; Sarto, M.S.; Palleschi, C.; Uccelletti, D. Graphite nanoplatelets and Caenorhabditis elegans: Insights from an in vivo model. Nano Lett. 2012, 12, 2740–2744. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Dong, S.; Petersen, E.J. Biological uptake and depuration of radio-labeled graphene by Daphnia magna. Environ. Sci. Technol. 2013, 47, 12524–12531. [Google Scholar] [CrossRef]
- Pretti, C.; Oliva, M.; Di Pietro, R.; Monni, G.; Cevasco, G.; Chiellini, F.; Pomelli, C.; Chiappe, C. Ecotoxicity of pristine graphene to marine organisms. Ecotoxicol. Environ. Saf. 2014, 101, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, F.; Landois, P.; Datsyuk, V.; Puech, P.; Pinelli, E.; Flahaut, E.; Gauthier, L. International amphibian micronucleus standardized procedure (ISO 21427-1) for in vivo evaluation of double-walled carbon nanotubes toxicity and genotoxicity in water. Environ. Toxicol. 2011, 26, 136–145. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesa, C.L.; Ranalison, O.; Randriantseheno, L.N.; Risuleo, G. Natural Products from Madagascar, Socio-Cultural Usage, and Potential Applications in Advanced Biomedicine: A Concise Review. Molecules 2021, 26, 4507. https://doi.org/10.3390/molecules26154507
Mesa CL, Ranalison O, Randriantseheno LN, Risuleo G. Natural Products from Madagascar, Socio-Cultural Usage, and Potential Applications in Advanced Biomedicine: A Concise Review. Molecules. 2021; 26(15):4507. https://doi.org/10.3390/molecules26154507
Chicago/Turabian StyleMesa, Camillo La, Oliarinony Ranalison, Lovasoa N. Randriantseheno, and Gianfranco Risuleo. 2021. "Natural Products from Madagascar, Socio-Cultural Usage, and Potential Applications in Advanced Biomedicine: A Concise Review" Molecules 26, no. 15: 4507. https://doi.org/10.3390/molecules26154507
APA StyleMesa, C. L., Ranalison, O., Randriantseheno, L. N., & Risuleo, G. (2021). Natural Products from Madagascar, Socio-Cultural Usage, and Potential Applications in Advanced Biomedicine: A Concise Review. Molecules, 26(15), 4507. https://doi.org/10.3390/molecules26154507







