Thiourea Derivatives, Simple in Structure but Efficient Enzyme Inhibitors and Mercury Sensors
Abstract
:1. Introduction
2. Results and Discussion
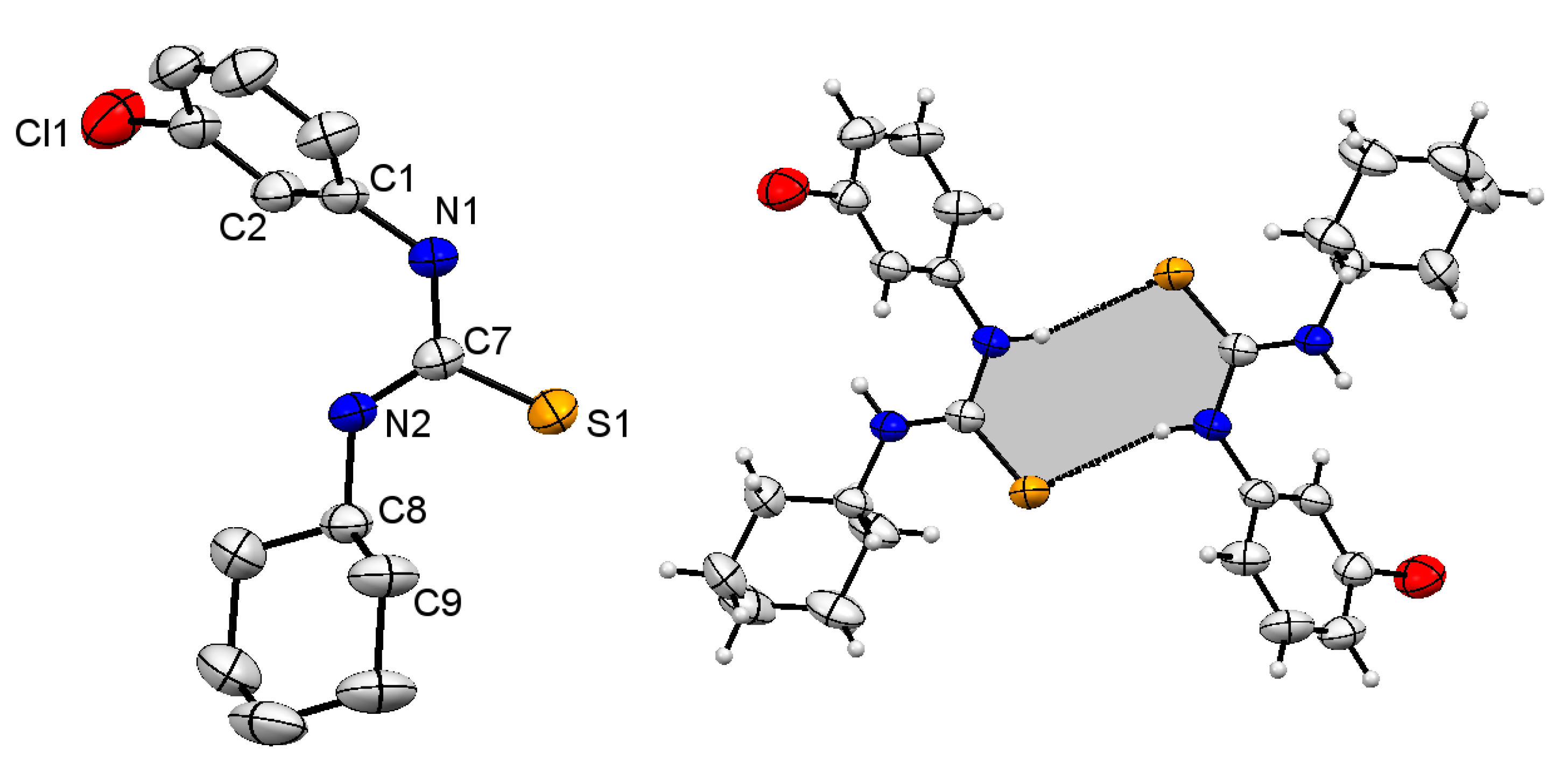
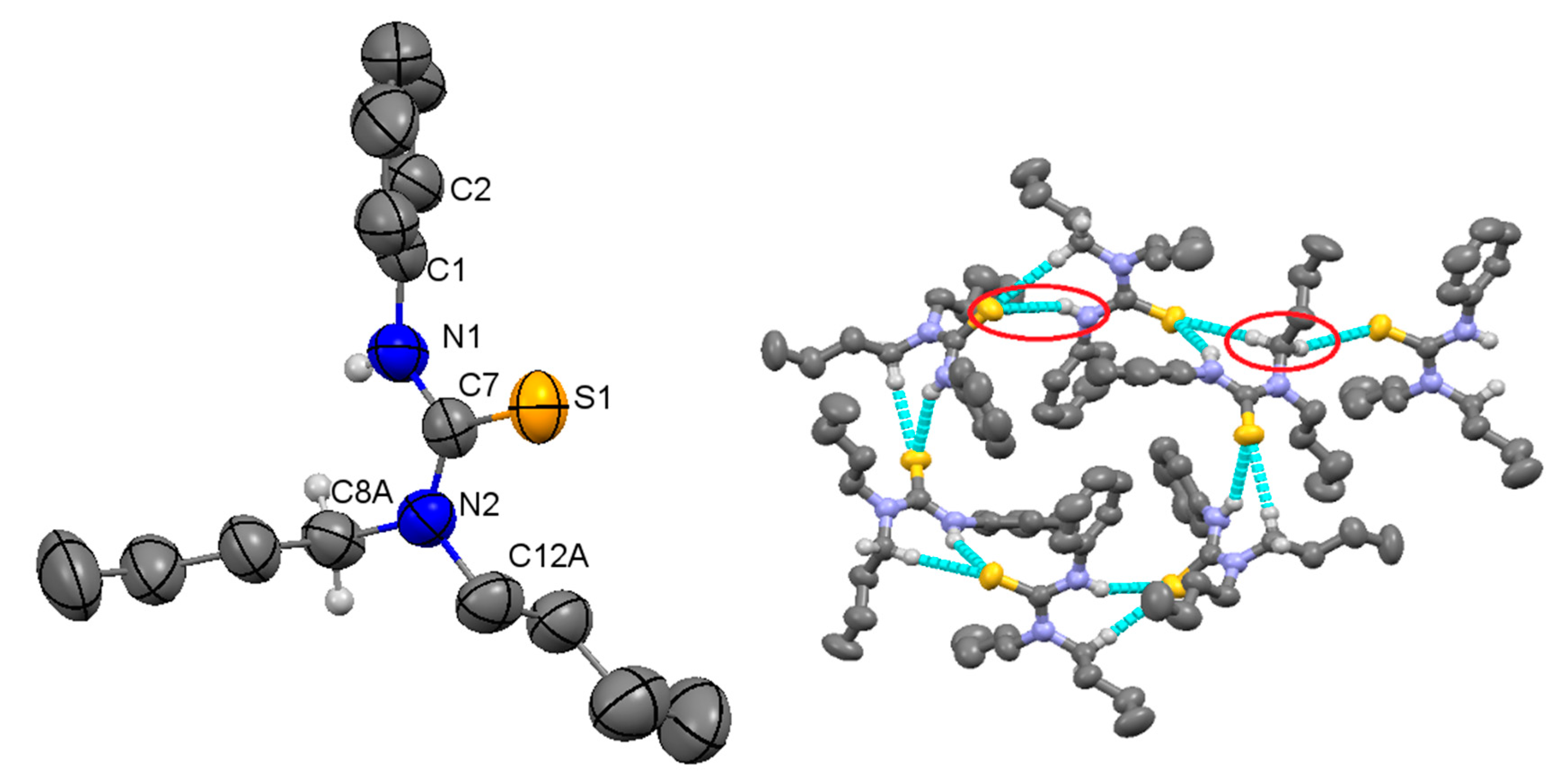
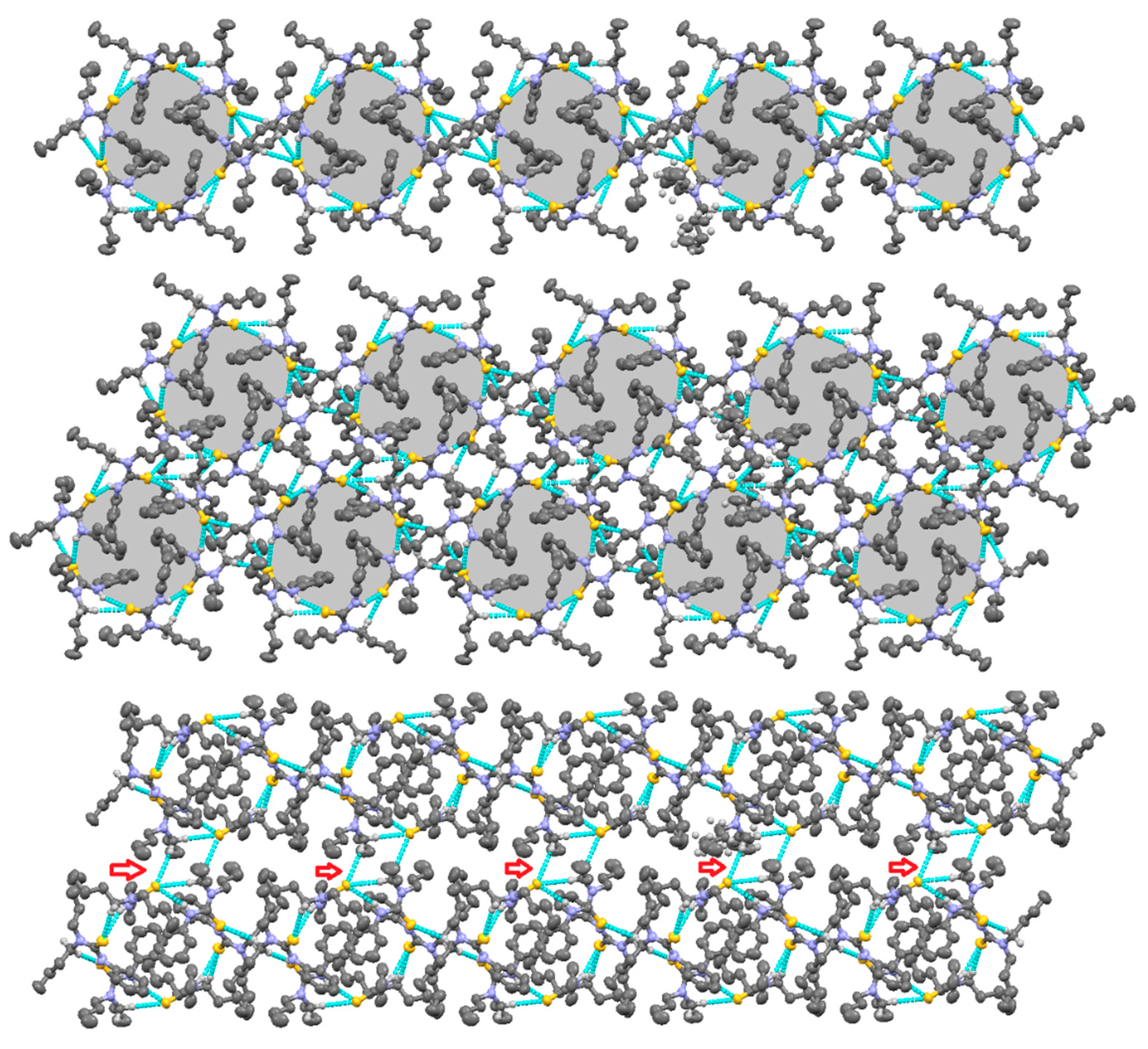
2.1. Structural Description of Compound 3
2.2. Structural Description of Compound 4
2.3. Enzyme Inhibition Studies
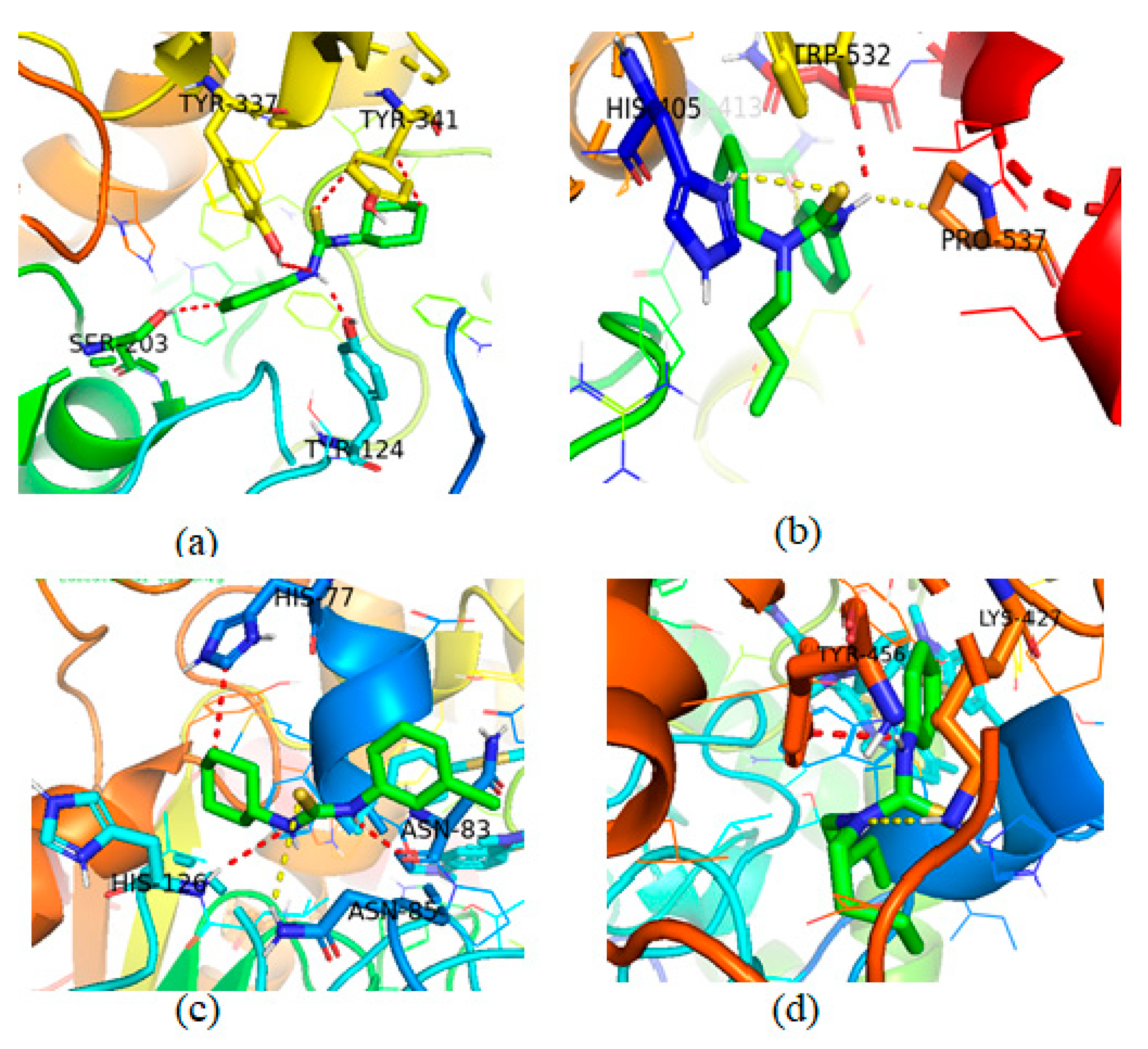
Molecular Docking Studies of Compounds 3 and 4
2.4. Fluorescence and UV-Visible Characteristics of the Compounds 1–3, 5 and 6
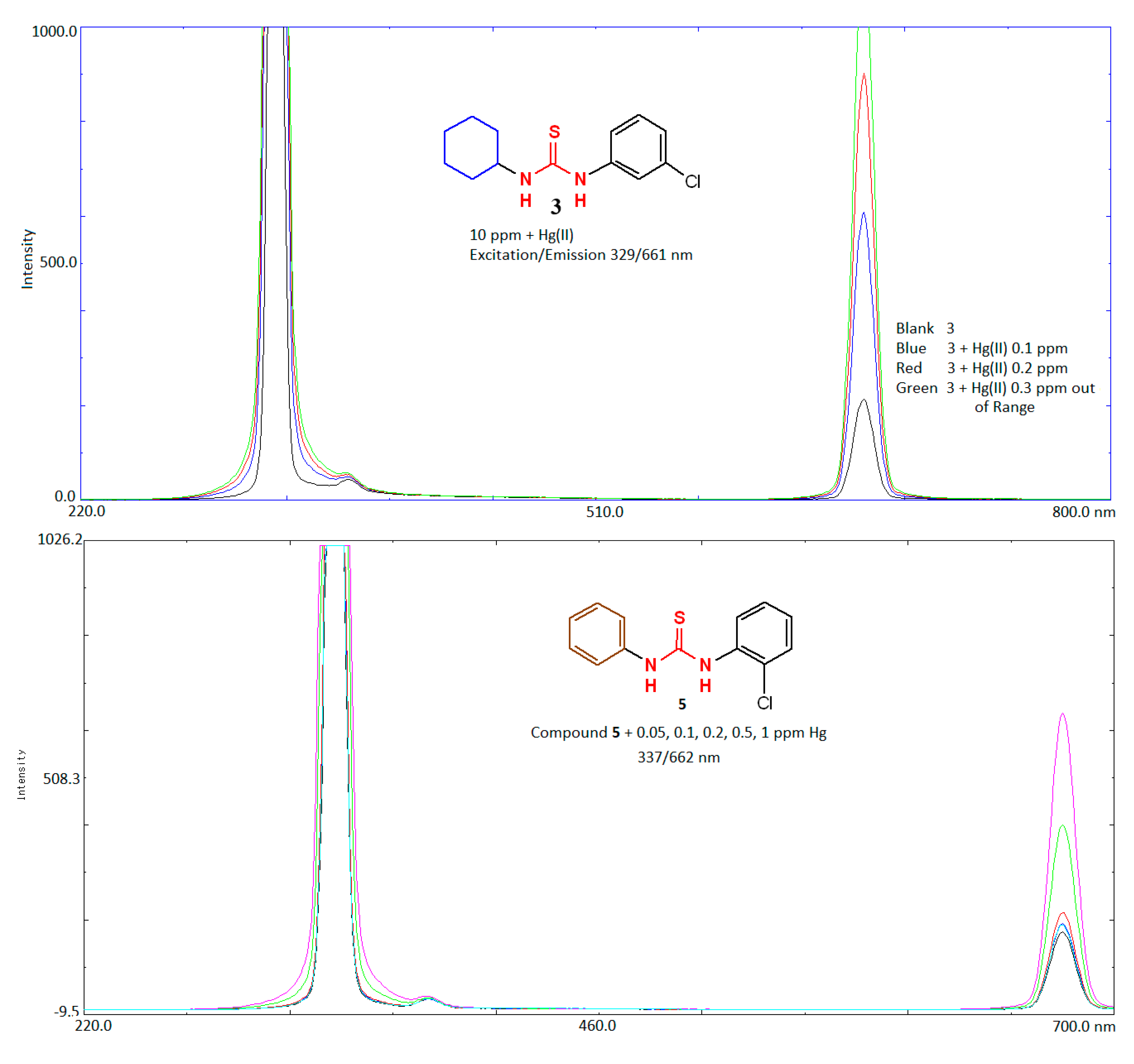
2.5. Application of Compounds 1–3, 5, 6 as Sensing Probe for Determination of Mercury
3. Materials and Methods
3.1. General Consideration
3.2. Anticholinesterase Evaluation
3.3. Molecular Docking Study
3.4. Fluorescence Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hegde, S.; Joshi, S.; Mukherjee, T.; Kapoor, S. Photochemical synthesis of gold nanoparticles in N, N′-dimethylformamide via thiourea-derivatized polyoxometalate. Res. Chem. Intermed. 2014, 40, 1125–1133. [Google Scholar] [CrossRef]
- Zhou, W.; Peng, K.; Tao, F.-M. Theoretical mechanism for the oxidation of thiourea by hydrogen peroxide in gas state. J. Mol. Struct. THEOCHEM 2007, 821, 116–124. [Google Scholar] [CrossRef]
- Bai, W.; Ji, J.; Huang, Q.; Wei, W. Synthesis and evaluation of new thiourea derivatives as antitumor and antiangiogenic agents. Tetrahedron Lett. 2020, 61, 152366. [Google Scholar] [CrossRef]
- Khan, E.; Khan, S.; Gul, Z.; Muhammad, M. Medicinal importance, coordination chemistry with selected metals (cu, Ag, au) and Chemosensing of Thiourea derivatives. A review. Crit. Rev. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Bregović, V.B.; Basarić, N.; Mlinarić-Majerski, K. Anion binding with urea and thiourea derivatives. Coord. Chem. Rev. 2015, 295, 80–124. [Google Scholar] [CrossRef]
- Thanigaimalai, P.; Lee, K.-C.; Sharma, V.K.; Joo, C.; Cho, W.-J.; Roh, E.; Kim, Y.; Jung, S.-H. Structural requirement of phenylthiourea analogs for their inhibitory activity of melanogenesis and tyrosinase. Bioorganic Med. Chem. Lett. 2011, 21, 6824–6828. [Google Scholar] [CrossRef]
- Taylor, E.C.; Ravindranathan, R. Reaction of Anthranilonitrile and N-Methylanthranilonitrile with Phenyl Isocyanate and Phenyl Isothiocyanate1. J. Org. Chem. 1962, 27, 2622–2627. [Google Scholar] [CrossRef]
- Maddani, M.R.; Prabhu, K.R. A concise synthesis of substituted thiourea derivatives in aqueous medium. J. Org. Chem. 2010, 75, 2327–2332. [Google Scholar] [CrossRef]
- Binzet, G.; Kavak, G.; Külcü, N.; Özbey, S.; Flörke, U.; Arslan, H. Synthesis and characterization of novel thiourea derivatives and their nickel and copper complexes. J. Chem. 2013, 2013, 536562. [Google Scholar] [CrossRef] [Green Version]
- Molter, A.; Rust, J.; Lehmann, C.W.; Deepa, G.; Chiba, P.; Mohr, F. Synthesis, structures and anti-malaria activity of some gold (I) phosphine complexes containing seleno-and thiosemicarbazonato ligands. Dalton Trans. 2011, 40, 9810–9820. [Google Scholar] [CrossRef]
- Meng, G.; Wang, M.; Dong, M.; Zheng, A.; Shi, J.; De Clercq, E.; Pannecouque, C.; Balzarini, J. Synthesis and Anti Hiv-1 Reverse Transcriptase Evaluation of a Series of N-Mono Substituted Thio-urea Derivatives. Int. J. AIDS Res. 2015, 2, 19–27. [Google Scholar]
- Dong, Y.; Venkatachalam, T.; Narla, R.K.; Trieu, V.N.; Sudbeck, E.A.; Uckun, F.M. Antioxidant function of phenethyl-5-bromo-pyridyl thiourea compounds with potent anti-HIV activity. Bioorganic Med. Chem. Lett. 2000, 10, 87–90. [Google Scholar] [CrossRef]
- Kang, I.-J.; Wang, L.-W.; Lee, C.-C.; Lee, Y.-C.; Chao, Y.-S.; Hsu, T.-A.; Chern, J.-H. Design, synthesis, and anti-HCV activity of thiourea compounds. Bioorganic Med. Chem. Lett. 2009, 19, 1950–1955. [Google Scholar] [CrossRef]
- Khatri, N.; Lather, V.; Madan, A. Diverse classification models for anti-hepatitis C virus activity of thiourea derivatives. Chemom. Intell. Lab. Syst. 2015, 140, 13–21. [Google Scholar] [CrossRef]
- Shakeel, A.; Altaf, A.A.; Qureshi, A.M.; Badshah, A. Thiourea derivatives in drug design and medicinal chemistry: A short review. J. Drug Des. Med. Chem. 2016, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Vedavathi, P.; Sudhamani, H.; Raju, C.N. Synthesis and antimicrobial activity of new urea and thiourea derivatives of (2′-(1H-tetrazol-5-yl) biphenyl-4-yl) methanamine. Res. Chem. Intermed. 2017, 43, 3251–3263. [Google Scholar] [CrossRef]
- Rehman, T.U.; Khan, I.U.; Riaz, S. Novel substituted 3-phenyl 1-(4-(5-bromopyridin-3-yl)-6-phenylpyrimidin-2-yl)-thiourea compounds as key small organic molecules for the potential treatment of type II diabetes mellitus: In vitro studies against yeast α-glucosidase. Med. Chem. Res. 2017, 26, 1098–1106. [Google Scholar] [CrossRef]
- Darvesh, S.; Hopkins, D.A.; Geula, C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003, 4, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Mattes, C.; Bradley, R.; Slaughter, E.; Browne, S. Cocaine and butyrylcholinesterase (BChE): Determination of enzymatic parameters. Life Sci. 1996, 58, PL257–PL261. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, J.; Sheng, R.; Dong, X.; He, Q.; Yang, B.; Hu, Y. Synthesis and biological evaluation of novel flavonoid derivatives as dual binding acetylcholinesterase inhibitors. J. Enzym. Inhib. Med. Chem. 2009, 24, 372–380. [Google Scholar] [CrossRef]
- Zelík, P.; Lukešová, A.; Voloshko, L.N.; Štys, D.; Kopecký, J. Screening for acetylcholinesterase inhibitory activity in cyanobacteria of the genus Nostoc. J. Enzym. Inhib. Med. Chem. 2009, 24, 531–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, R.M.; Potkin, S.G.; Enz, A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int. J. Neuropsychopharmacol. 2006, 9, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.U.; Bibi, M.; Altaf, A.A.; Tahir, M.N.; Ullah, F.; Zia Ur, R.; Khan, E. Zn, Cd and Hg complexes with unsymmetric thiourea derivatives; syntheses, free radical scavenging and enzyme inhibition essay. J. Mol. Struct. 2020, 1211, 128096. [Google Scholar] [CrossRef]
- Shade, C.W.; Hudson, R.J.M. Determination of MeHg in Environmental Sample Matrices Using Hg−Thiourea Complex Ion Chromatography with On-line Cold Vapor Generation and Atomic Fluorescence Spectrometric Detection. Environ. Sci. Technol. 2005, 39, 4974–4982. [Google Scholar] [CrossRef] [PubMed]
- An, F.-Q.; Wang, Y.; Xue, X.-Y.; Hu, T.-P.; Gao, J.-F.; Gao, B.-J. Design and application of thiourea modified D301 resin for the effective removal of toxic heavy metal ions. Chem. Eng. Res. Des. 2018, 130, 78–86. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahmed, J.; Asiri, A.M. Thiourea sensor development based on hydrothermally prepared CMO nanoparticles for environmental safety. Biosens. Bioelectron. 2018, 99, 586–592. [Google Scholar] [CrossRef]
- El-Korashy, S.A.; Elwakeel, K.Z.; El-Hafeiz, A.A. Fabrication of bentonite/thiourea-formaldehyde composite material for Pb(II), Mn(VII) and Cr(VI) sorption: A combined basic study and industrial application. J. Clean. Prod. 2016, 137, 40–50. [Google Scholar] [CrossRef]
- Loto, C.; Loto, R.; Popoola, A. Corrosion inhibition of thiourea and thiadiazole derivatives: A review. J. Mater. Environ. Sci. 2012, 3, 885–894. [Google Scholar]
- El-Liethy, M.A.; Elwakeel, K.Z.; Ahmed, M.S. Comparison study of Ag(I) and Au(III) loaded on magnetic thiourea-formaldehyde as disinfectants for water pathogenic microorganism’s deactivation. J. Environ. Chem. Eng. 2018, 6, 4380–4390. [Google Scholar] [CrossRef]
- Yusof, M.S.M.; Jusoh, R.t.H.; Khairul, W.M.; Yamin, B.M. Synthesis and characterisation a series of N-(3,4-dichlorophenyl)-N′-(2,3 and 4-methylbenzoyl)thiourea derivatives. J. Mol. Struct. 2010, 975, 280–284. [Google Scholar] [CrossRef]
- Saxe, S.R.; Wekstein, M.W.; Kryscio, R.J.; Henry, R.G.; Cornett, C.R.; Snowdon, D.A.; Grant, F.T.; Schmitt, F.A.; Donegan, S.J.; Wekstein, D.R.; et al. Alzheimer’s disease, dental amalgam and mercury. J. Am. Dent. Assoc. 1999, 130, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Bakulski, K.M.; Seo, Y.A.; Hickman, R.C.; Brandt, D.; Vadari, H.S.; Hu, H.; KyunPark, S. Heavy metals exposure and Alzheimer’s disease and related dementias. J. Alzheimer’s Dis. 2020, 76, 1215–1242. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, C.; Yan, M.; Ge, S.; Yu, J. A novel fluorescence probe based on p-acid-Br and its application in thiourea detection. RSC Adv. 2016, 6, 45001–45008. [Google Scholar] [CrossRef]
- Kumar, V.; Kaushik, M.P.; Srivastava, A.K.; Pratap, A.; Thiruvenkatam, V.; Row, T.N.G. Thiourea based novel chromogenic sensor for selective detection of fluoride and cyanide anions in organic and aqueous media. Anal. Chim. Acta 2010, 663, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ngah, F.A.A.; Zakariah, E.I.; Hassan, N.I.; Yamin, B.; Sapari, S.; Hasbullah, S.A. Synthesis of thiourea derivatives and binding behavior towards the mercury ion. Malays. J. Anal. Sci. 2017, 21, 1226–1234. [Google Scholar]
- Mishra, J.; Kaur, H.; Ganguli, A.K.; Kaur, N. Fluorescent chemosensor based on urea/thiourea moiety for sensing of Hg (II) ions in an aqueous medium with high sensitivity and selectivity: A comparative account on effect of molecular architecture on chemosensing. J. Mol. Struct. 2018, 1161, 34–43. [Google Scholar] [CrossRef]
- Gan, S.-F.; Wan, J.-P.; Pan, Y.-J.; Sun, C.-R. Highly efficient and catalyst-free synthesis of substituted thioureas in water. Mol. Divers. 2011, 15, 809–815. [Google Scholar] [CrossRef]
- Shetty, P. Corrosion inhibition behaviour of thiourea derivatives in acid media against mild steel deterioration: An overview. Surf. Eng. Appl. Electrochem. 2017, 53, 587–591. [Google Scholar] [CrossRef]
- Owen, J.S.; Hendricks, M.P.; Campos, M.P.; Cleveland, G.T.; Jen-La PLANTE, I.; Hamachi, L.S. Methods of Producing Metal Sulfides, Metal Selenides, and Metal Sulfides/Selenides Having Controlled Architectures Using Kinetic Control. U.S. Patent 10,767,112, 8 September 2020. [Google Scholar]
- Wang, R.; Yang, W.-j.; Yue, L.; Pan, W.; Zeng, H.-y. DDQ-Promoted C–S Bond Formation: Synthesis of 2-Aminobenzothiazole Derivatives under Transition-Metal-, Ligand-, and Base-Free Conditions. Synlett 2012, 23, 1643–1648. [Google Scholar] [CrossRef]
- Mital, A.; Murugesan, D.; Kaiser, M.; Yeates, C.; Gilbert, I.H. Discovery and optimisation studies of antimalarial phenotypic hits. Eur. J. Med. Chem. 2015, 103, 530–538. [Google Scholar] [CrossRef] [Green Version]
- Štrukil, V. Mechanochemical synthesis of thioureas, ureas and guanidines. Beilstein J. Org. Chem. 2017, 13, 1828–1849. [Google Scholar] [CrossRef] [Green Version]
- Altaf, A.A.; Shahzad, A.; Gul, Z.; Khan, S.A.; Badshah, A.; Tahir, M.N.; Zafar, Z.I.; Khan, E. Synthesis, crystal structure, and DFT calculations of 1, 3-diisobutyl thiourea. J. Chem. 2015, 2015, 913435. [Google Scholar] [CrossRef] [Green Version]
- Haribabu, J.; Subhashree, G.R.; Saranya, S.; Gomathi, K.; Karvembu, R.; Gayathri, D. Synthesis, crystal structure, and in vitro and in silico molecular docking of novel acyl thiourea derivatives. J. Mol. Struct. 2015, 1094, 281–291. [Google Scholar] [CrossRef]
- Saeed, A.; Zaib, S.; Ashraf, S.; Iftikhar, J.; Muddassar, M.; Zhang, K.Y.; Iqbal, J. Synthesis, cholinesterase inhibition and molecular modelling studies of coumarin linked thiourea derivatives. Bioorganic Chem. 2015, 63, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Czaplińska, B.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Slodek, A.; Korzec, M.; Musiol, R. Theoretical and Experimental Investigations of Large Stokes Shift Fluorophores Based on a Quinoline Scaffold. Molecules 2020, 25, 2488. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, S.; Wang, S.; Hu, R.; Feng, J.; Li, Y.; Qian, Y. Novel fluorescent probes based on intramolecular charge-and proton-transfer compounds. Pure Appl. Chem. 2013, 85, 1465–1478. [Google Scholar] [CrossRef]
- Khan, U.A.; Badshah, A.; Tahir, M.N.; Khan, E. Gold (I), silver (I) and copper (I) complexes of 2, 4, 6-trimethylphenyl-3-benzoylthiourea; synthesis and biological applications. Polyhedron 2020, 181, 114485. [Google Scholar] [CrossRef]
- Khan, E.; Khan, A.; Gul, Z.; Ullah, F.; Tahir, M.N.; Khalid, M.; Asif, H.M.; Asim, S.; Braga, A.A.C. Molecular salts of terephthalic acids with 2-aminopyridine and 2-aminothiazole derivatives as potential antioxidant agents; Base-Acid-Base type architectures. J. Mol. Struct. 2020, 1200, 127126. [Google Scholar] [CrossRef]
- Gul, Z.; Din, N.U.; Khan, E.; Ullah, F.; Tahir, M.N. Synthesis, molecular structure, anti-microbial, anti-oxidant and enzyme inhibition activities of 2-amino-6-methylbenzothiazole and its Cu (II) and Ag (I) complexes. J. Mol. Struct. 2020, 1199, 126956. [Google Scholar] [CrossRef]
- Khan, E.; Ahmad, T.; Gul, Z.; Ullah, F.; Tahir, M.N.; Noor, A. Methyl-substituted 2-aminothiazole--based cobalt (II) and silver (I) complexes: Synthesis, X-ray structures, and biological activities. Turk. J. Chem. 2019, 43, 857–868. [Google Scholar] [CrossRef]
- Dingova, D.; Leroy, J.; Check, A.; Garaj, V.; Krejci, E.; Hrabovska, A. Optimal detection of cholinesterase activity in biological samples: Modifications to the standard Ellman’s assay. Anal. Biochem. 2014, 462, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Ahmad, S.; Khan, E.; Shahzad, A.; Ali, M.; Tahir, M.N.; Shaheen, F.; Ahmad, M. Isolation, crystal structure determination and cholinesterase inhibitory potential of isotalatizidine hydrate from Delphinium denudatum. Pharm. Biol. 2017, 55, 680–686. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Ahmad, H.; Khan, H.U.; Shahzad, A.; Khan, E.; Ali Shah, S.A.; Ali, M.; Wadud, A.; Ghufran, M.; Naz, H.; et al. Crystal structure, phytochemical study and enzyme inhibition activity of Ajaconine and Delectinine. J. Mol. Struct. 2016, 1123, 441–448. [Google Scholar] [CrossRef]
- Qiao, L.; Huang, J.; Hu, W.; Zhang, Y.; Guo, J.; Cao, W.; Miao, K.; Qin, B.; Song, J. Synthesis, characterization, and in vitro evaluation and in silico molecular docking of thiourea derivatives incorporating 4-(trifluoromethyl) phenyl moiety. J. Mol. Struct. 2017, 1139, 149–159. [Google Scholar] [CrossRef]
- Chigurupati, S.; Selvaraj, M.; Mani, V.; Selvarajan, K.K.; Mohammad, J.I.; Kaveti, B.; Bera, H.; Palanimuthu, V.R.; Teh, L.K.; Salleh, M.Z. Identification of novel acetylcholinesterase inhibitors: Indolopyrazoline derivatives and molecular docking studies. Bioorganic Chem. 2016, 67, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, F.; Zengin Kurt, B.; Gazioglu, I.; Basile, L.; Dag, A.; Cappello, V.; Ginex, T.; Kucukislamoglu, M.; Guccione, S. Design, synthesis and docking study of novel coumarin ligands as potential selective acetylcholinesterase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 285–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
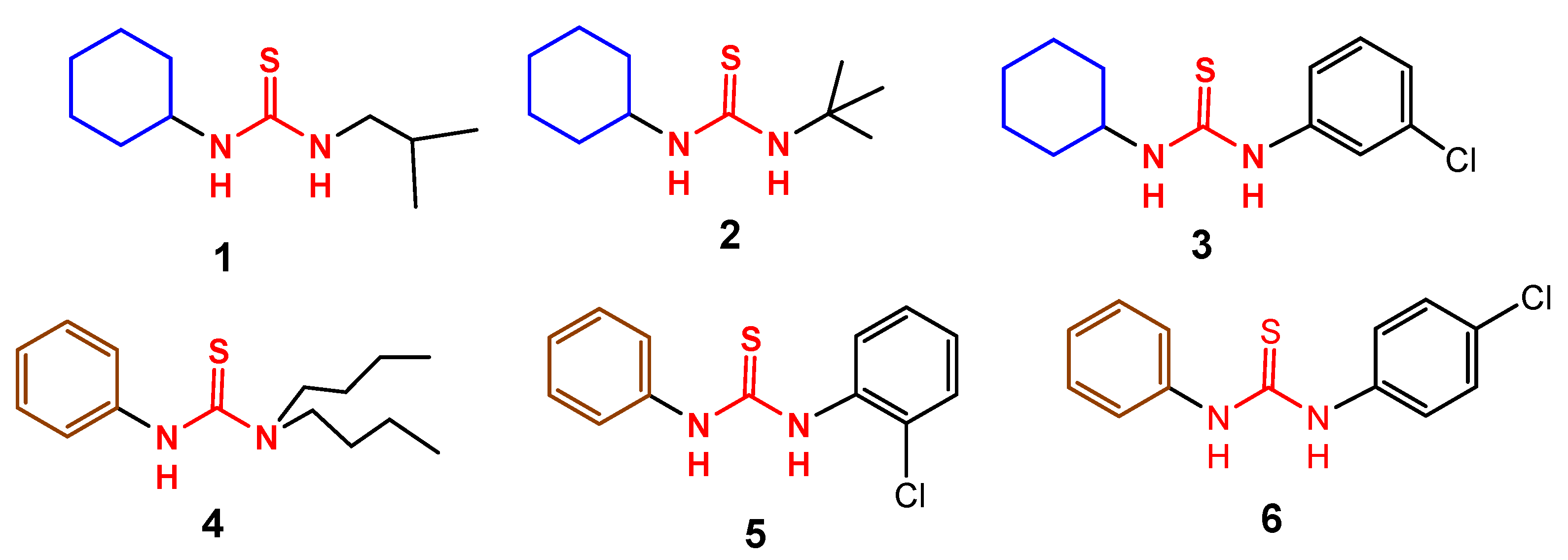
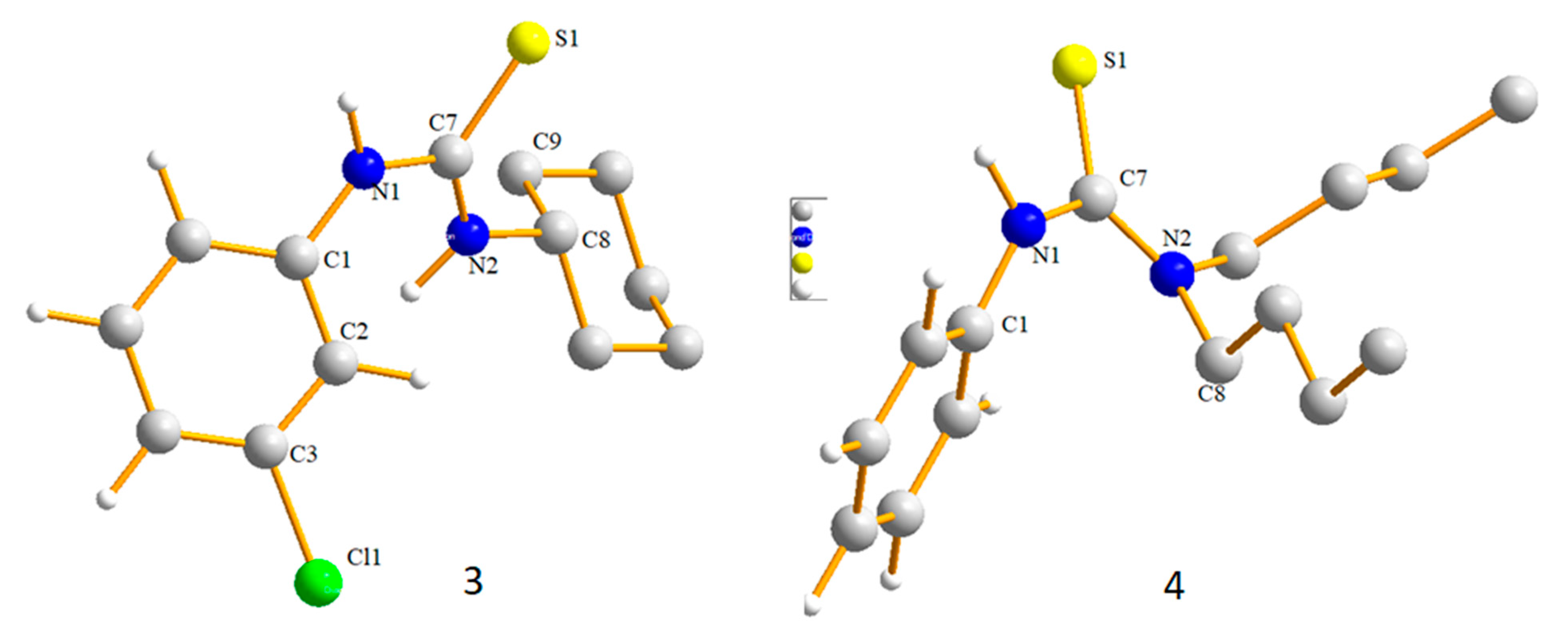

| Compound 3 | Compound 4 | |
|---|---|---|
| CCDC No * | 2050647 | 2050648 |
| Chemical formula | C13H17ClN2S | C15H24N2S |
| Mr, | 268.79 | 264.42 |
| Temperature (K) | 296 | 296 |
| Crystal system, space group | Monoclinic, P21/n | Trigonal, R3:H |
| a, b, c (Å) | 5.5080 (4), 22.1068 (17), 11.4404 (8) | 25.550 (2), 25.550 (2), 13.1763(11), |
| β (°) | 99.967 (4) | 90.00 |
| V (Å3) | 1372.01 (17) | 7448.8(13) |
| Z | 4 | 18 |
| Radiation type | Mo Kα | Mo Kα |
| µ (mm−1) | 0.41 | 0.18 |
| Crystal size (mm) | 0.40 × 0.32 × 0.26 | 0.44 × 0.38 × 0.36 |
| Diffractometer | Bruker Kappa APEXII CCD | |
| Absorption correction | Multi-scan | Multi-scan |
| Tmin, Tmax | 0.815, 0.925 | 0.875, 0.955 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 9399, 3283, 2340 | 12267, 3614, 1852 |
| Rint, | 0.048 | 0.074 |
| (sin θ/λ) max (Å−1) | 0.660 | 0.641 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.048, 0.135, 1.02 | 0.056, 0.168, 1.02 |
| No. of reflections | 3283 | 3614 |
| No. of parameters/restrains | 154/0 | 188/214 |
| H-atom treatment | H-atom parameters constrained | |
| Δρmax, Δρmin (e Å−3) | 0.34, −0.32 | 0.18, −0.16 |
| Compound | Atoms | Exp. | Cal. | Atoms | Exp. | Cal. |
|---|---|---|---|---|---|---|
| 3 | Bond length | Bond angle | ||||
| S1-C7 | 1.695(19) | 1.730 | C7-N1-C1 | 127.52(17) | 128.41 | |
| N1-C7 | 1.351(2) | 1.383 | C7-N2-C8 | 124.61(16) | 124.81 | |
| N1-C1 | 1.423(2) | 1.434 | N2-C7-N1 | 117.68(17) | 117.29 | |
| N2-C7 | 1.326(2) | 1.342 | N2-C7-S1 | 123.18(15) | 123.53 | |
| N2-C8 | 1.464(2) | 1.483 | N1-C7-S1 | 119.14(15) | 118.73 | |
| 4 | S1-C7 | 1.686(2) | 1.731 | C7-N1-C1 | 127.84(19) | 128.23 |
| Ni-C7 | 1.352(3) | 1.390 | N2-C7-N1 | 115.86(18) | 116.10 | |
| N1-C1 | 1.426(3) | 1.421 | N2-C7-S1 | 123.03(18) | 124.34 | |
| N2-C7 | 1.344(3) | 1.351 | C7-N2-C8B | 123.6(17) | 123.59 | |
| Compound | D—H···A | D—H | H···A | D···A | D—H···A | Symmetry Codes |
|---|---|---|---|---|---|---|
| 3 | N1—H1···S1 | 0.86 | 2.53 | 3.343 (18) | 157.4 | −x, −y, −z + 2 |
| C2—H2···Cl1 | 0.93 | 2.96 | 3.878 (2) | 167.6 | −x, −y, −z + 1 | |
| C9—H9B···S1 | 0.97 | 2.91 | 3.483 (3) | 118.9 | - | |
| 4 | N1—H1···S1 | 0.86 | 2.70 | 3.484 (2) | 151.5 | x − y + 2/3, x + 1/3, −z + 4/3 |
| C8A—H8A ··S1 | 0.97 | 2.96 | 3.840 (12) | 151.5 | −x + y − 1/3, −x + 1/3, z + 1/3 | |
| C8A—H8B ··S1 | 0.97 | 2.75 | 3.711 (11) | 172.2 | x − y + 2/3, x + 1/3, −z + 4/3 | |
| C8B—H8C ··S1 | 0.97 | 2.95 | 3.84 (4) | 152.3 | −x + y − 1/3, −x + 1/3, z + 1/3 | |
| C8B—H8D ··S1 | 0.97 | 2.62 | 3.53 (4) | 155.3 | x − y + 2/3, x + 1/3, −z + 4/3 | |
| C9B—H9C ··N1 | 0.97 | 2.65 | 3.179 (14) | 114.8 | - | |
| C12B—H12D ··S1 | 0.97 | 2.56 | 3.00 (10) | 107.6 | - |
| No | Conc. (µg/mL) | %AChE Inhibition | IC50 (µg/mL) | %BChE Inhibition | IC50 (µg/mL) |
|---|---|---|---|---|---|
| 1 | 1000 | 69.47 ± 0.22 | 89 | 74.4 ± 0.68 | 84 |
| 500 | 63.94 ± 0.45 | 66.2 ± 0.73 | |||
| 250 | 57.61 ± 1.70 | 61.0 ± 0.33 | |||
| 125 | 53.64 ± 0.16 | 56.4 ± 0.63 | |||
| 62.5 | 46.52 ± 0.38 | 46.9 ± 0.42 | |||
| 2 | 1000 | 63.34 ± 0.98 | 300 | 65.17 ± 0.72 | 185 |
| 500 | 56.32 ± 1.06 | 57.85 ± 0.97 | |||
| 250 | 48.05 ± 0.75 | 51.37 ± 1.65 | |||
| 125 | 44.70 ± 1.25 | 46.73 ± 0.78 | |||
| 62.5 | 38.74 ± 0.68 | 41.34 ± 1.01 | |||
| 3 | 1000 | 73.39. ± 0.60 | 50 | 76.7 ± 0.66 | 60 |
| 500 | 67.39 ±0.49 | 71.3 ± 1.11 | |||
| 250 | 61.36 ± 0.49 | 65.5 ± 1.04 | |||
| 125 | 57.34 ± 0.55 | 57.2 ± 0.57 | |||
| 62.5 | 51.90 ± 1.16 | 49.9 ± 0.65 | |||
| 4 | 1000 | 93.58 ± 1.12 | 58 | 98.00 ± 0.00 | 63 |
| 500 | 85.40 ± 0.20 | 94.40 ± 0.50 | |||
| 250 | 77.85 ± 2.26 | 89.80 ± 1.50 | |||
| 125 | 71.80 ± 1.50 | 77.40 ± 1.70 | |||
| 62.5 | 47.90 ± 0.47 | 53.50 ± 0.90 | |||
| 31.5 | 39.01 ± 0.88 | 47.30 ± 0.70 | |||
| 5 | 1000 | 66.79 ± 0.63 | 345 | 71.62 ± 0.74 | 310 |
| 500 | 59.67 ± 0.61 | 63.86 ± 0.60 | |||
| 250 | 41.69 ± 0.77 | 44.48 ± 0.64 | |||
| 125 | 35.54 ± 0.50 | 37.54 ± 0.50 | |||
| 62.5 | 29.00 ± 0.30 | 31.74 ± 0.61 | |||
| 6 | 1000 | 69.58 ± 1.12 | 285 | 71.33 ± 0.49 | 265 |
| 500 | 61.65 ± 1.34 | 63.03 ± 0.23 | |||
| 250 | 47.90 ± 0.96 | 49.00 ± 0.58 | |||
| 125 | 39.03 ± 0.48 | 42.67 ± 0.89 | |||
| 62.5 | 31.90 ± 0.48 | 33.00 ± 1.15 | |||
| Galantamine | 1000 | 83.19 ± 0.73 | 15 | 89.64 ± 0.62 | 15 |
| 500 | 77.54 ± 0.91 | 81.83 ± 0.37 | |||
| 250 | 71.93 ± 0.14 | 74.29 ± 0.73 | |||
| 125 | 65.72 ± 0.49 | 67.92 ± 0.98 | |||
| 62.5 | 61.67 ± 0.94 | 64.93 ± 0.87 |
| Compound | Concentration (µg mL−1) | Scan Range (nm) | λex (nm) | λem (nm) | Mode of Sensitivity | Fluorescence Intensity |
|---|---|---|---|---|---|---|
| 1 | 10 | 350–700 | 312 | 613 | High | 276.483 |
| 2 | 220–750 | 304.0 | 328.0 | High | 16.189 | |
| 3 | 220–650 | 329 | 661 | Low | 212.958 | |
| 5 | 220–600 | 337 | 662 | Low | 180.234 | |
| 6 | 220–600 | 311 | 624 | Low | 275.143 |
| Compound | Concentration (ppm) | λex/λem(nm) Fluorescence Intensity | Concentration of Hg(II) ppm | Fluorescence Intensity |
|---|---|---|---|---|
| 3 | 10 | 329/661 FI = 212.958 Curve:e | 0.1 | 608.132 |
| 0.2 | 901.543 | |||
| 0.3 | 1015.790 | |||
| 1 | 10 | 312/613 FI = 276.483 Curve:e | 0.1 | 460.580 |
| 0.2 | 646.740 | |||
| 0.3 | 876.296 | |||
| 0.5 | 1015.790 | |||
| 2 | 0.01 | 264/284 FI = 16.189 Curve:e | 0.01 | 60.193 |
| 0.02 | 81.624 | |||
| 0.03 | 117.136 | |||
| 0.04 | 139.836 | |||
| 5 | 5.0 | 337/662 FI = 180 Curve:e | 0.05 | 197 |
| 0.1 | 220 | |||
| 0.2 | 400 | |||
| 0.5 | 550 | |||
| 6 | 10 | 311/624 FI = 275 Curve:e | 0.05 | No interaction |
| 0.1 | ||||
| 0.2 | ||||
| 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, F.U.; Bibi, M.; Khan, E.; Shah, A.B.; Muhammad, M.; Tahir, M.N.; Shahzad, A.; Ullah, F.; Zahoor, M.; Alamery, S.; et al. Thiourea Derivatives, Simple in Structure but Efficient Enzyme Inhibitors and Mercury Sensors. Molecules 2021, 26, 4506. https://doi.org/10.3390/molecules26154506
Rahman FU, Bibi M, Khan E, Shah AB, Muhammad M, Tahir MN, Shahzad A, Ullah F, Zahoor M, Alamery S, et al. Thiourea Derivatives, Simple in Structure but Efficient Enzyme Inhibitors and Mercury Sensors. Molecules. 2021; 26(15):4506. https://doi.org/10.3390/molecules26154506
Chicago/Turabian StyleRahman, Faizan Ur, Maryam Bibi, Ezzat Khan, Abdul Bari Shah, Mian Muhammad, Muhammad Nawaz Tahir, Adnan Shahzad, Farhat Ullah, Muhammad Zahoor, Salman Alamery, and et al. 2021. "Thiourea Derivatives, Simple in Structure but Efficient Enzyme Inhibitors and Mercury Sensors" Molecules 26, no. 15: 4506. https://doi.org/10.3390/molecules26154506
APA StyleRahman, F. U., Bibi, M., Khan, E., Shah, A. B., Muhammad, M., Tahir, M. N., Shahzad, A., Ullah, F., Zahoor, M., Alamery, S., & Batiha, G. E.-S. (2021). Thiourea Derivatives, Simple in Structure but Efficient Enzyme Inhibitors and Mercury Sensors. Molecules, 26(15), 4506. https://doi.org/10.3390/molecules26154506










