Thermal Preparation and Application of a Novel Silicon Fertilizer Using Talc and Calcium Carbonate as Starting Materials
Abstract
:1. Introduction
2. Results and Discussion
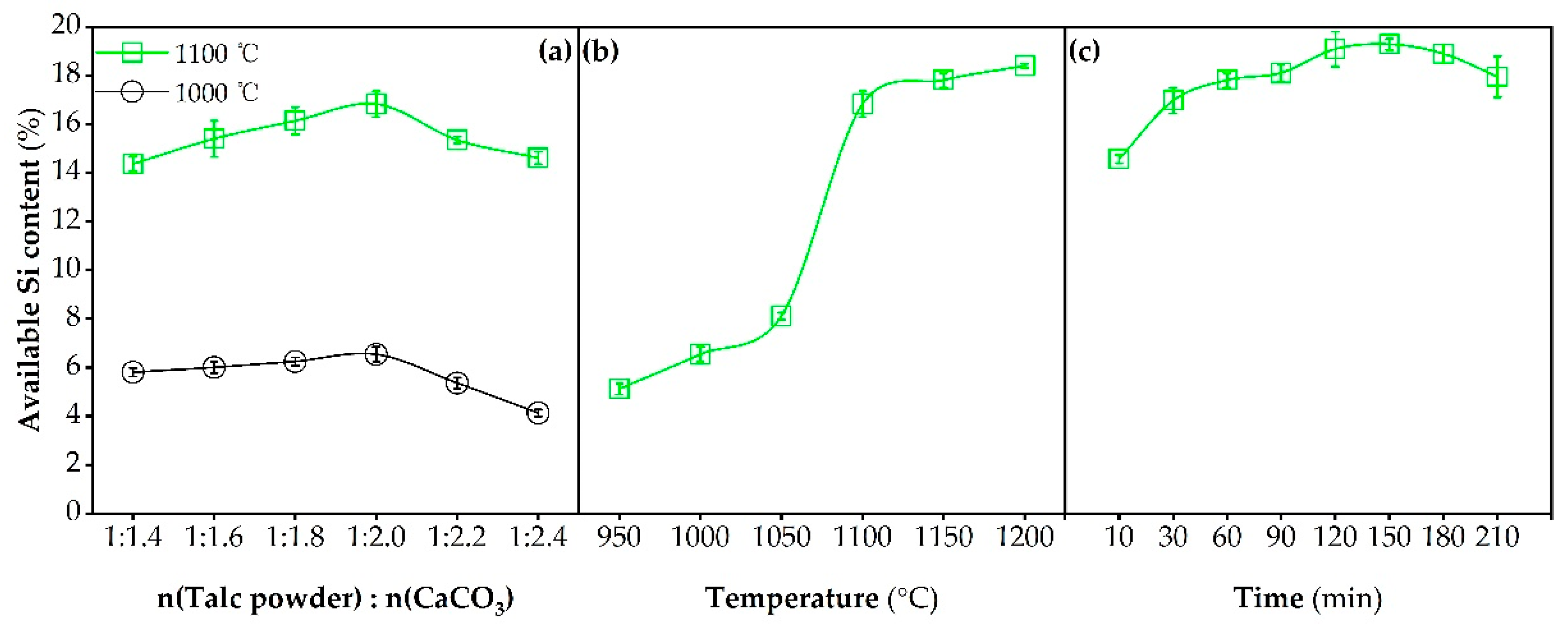
2.1. Effects of Process Conditions on Available Si Contents of Prepared Fertilizers
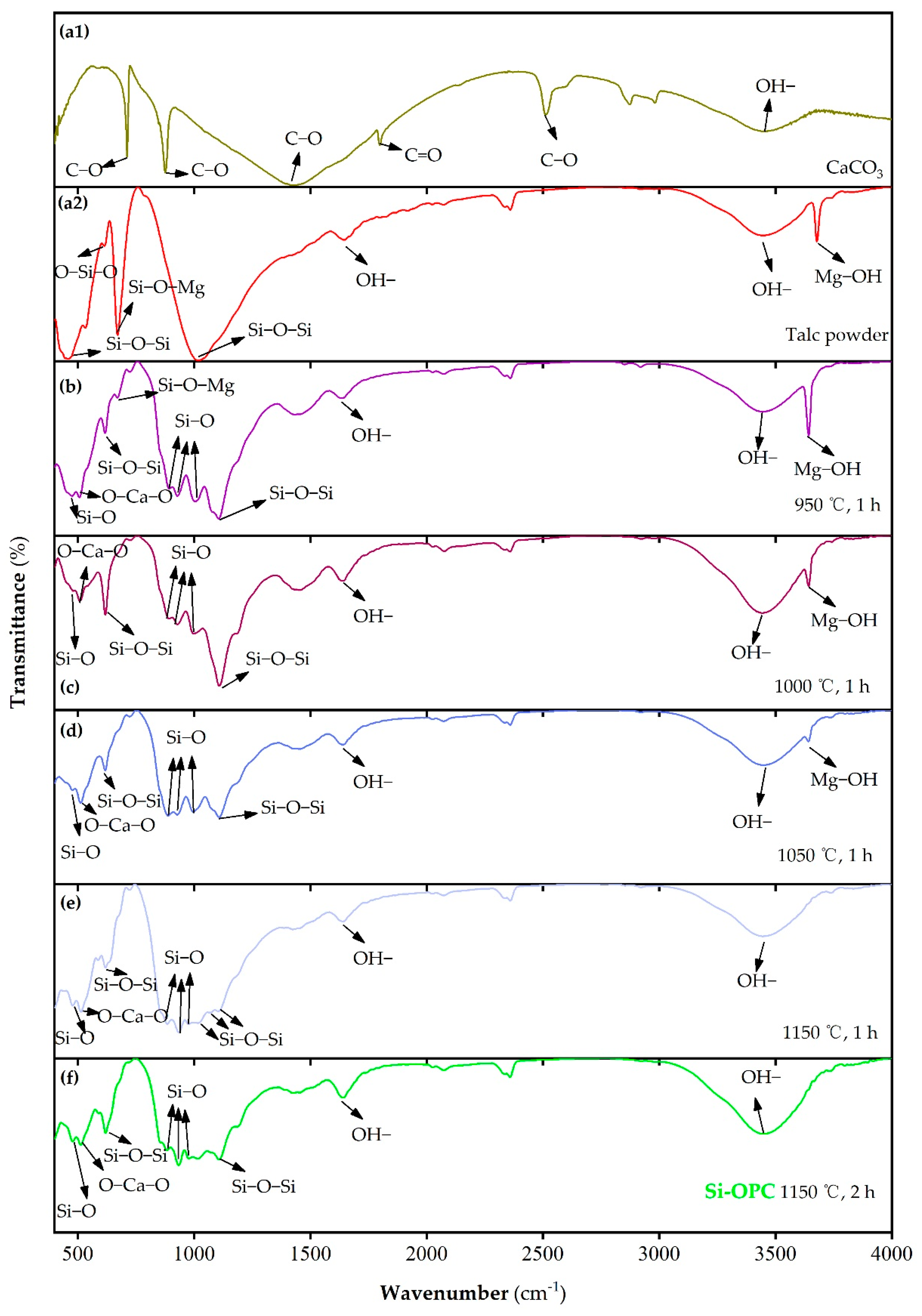
2.2. Calcination Temperature-Dependent Microstructure Evolution of Si Fertilizers
2.3. Dissolution Property and Physicochemical Characterization of Si-OPC Fertilizer
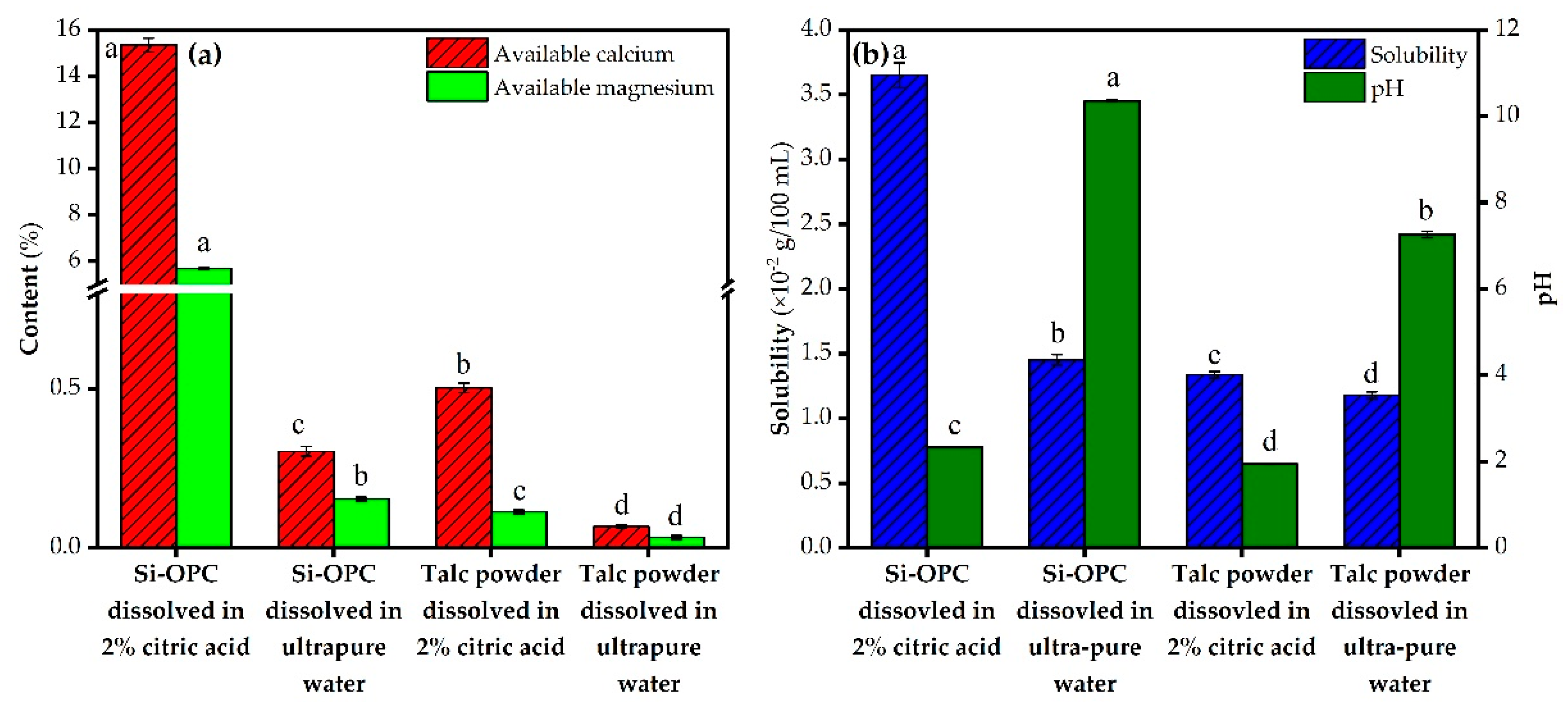
2.3.1. Available Metallic Element Content and Solubility
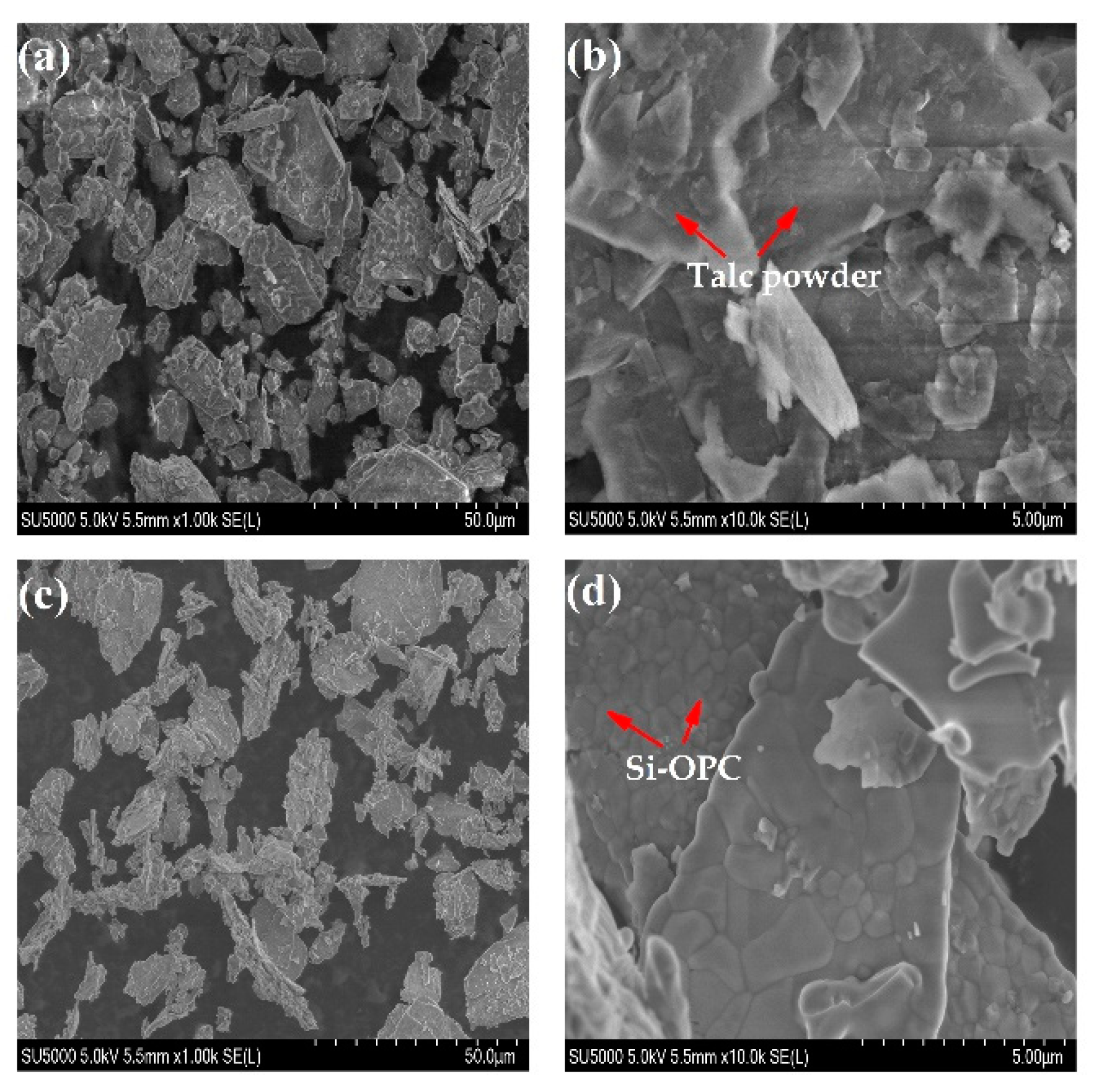
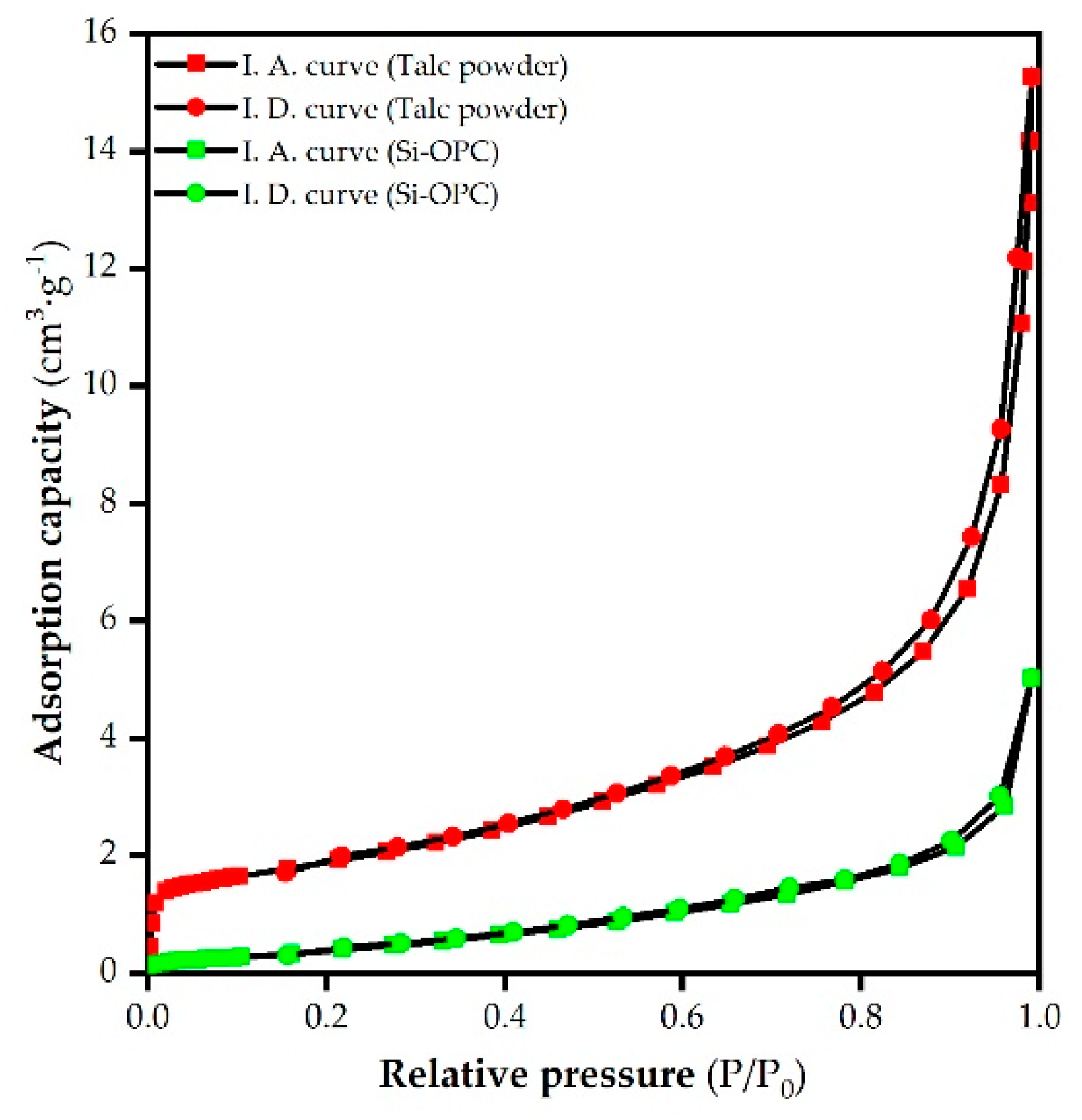
2.3.2. Surface Morphology and Properties
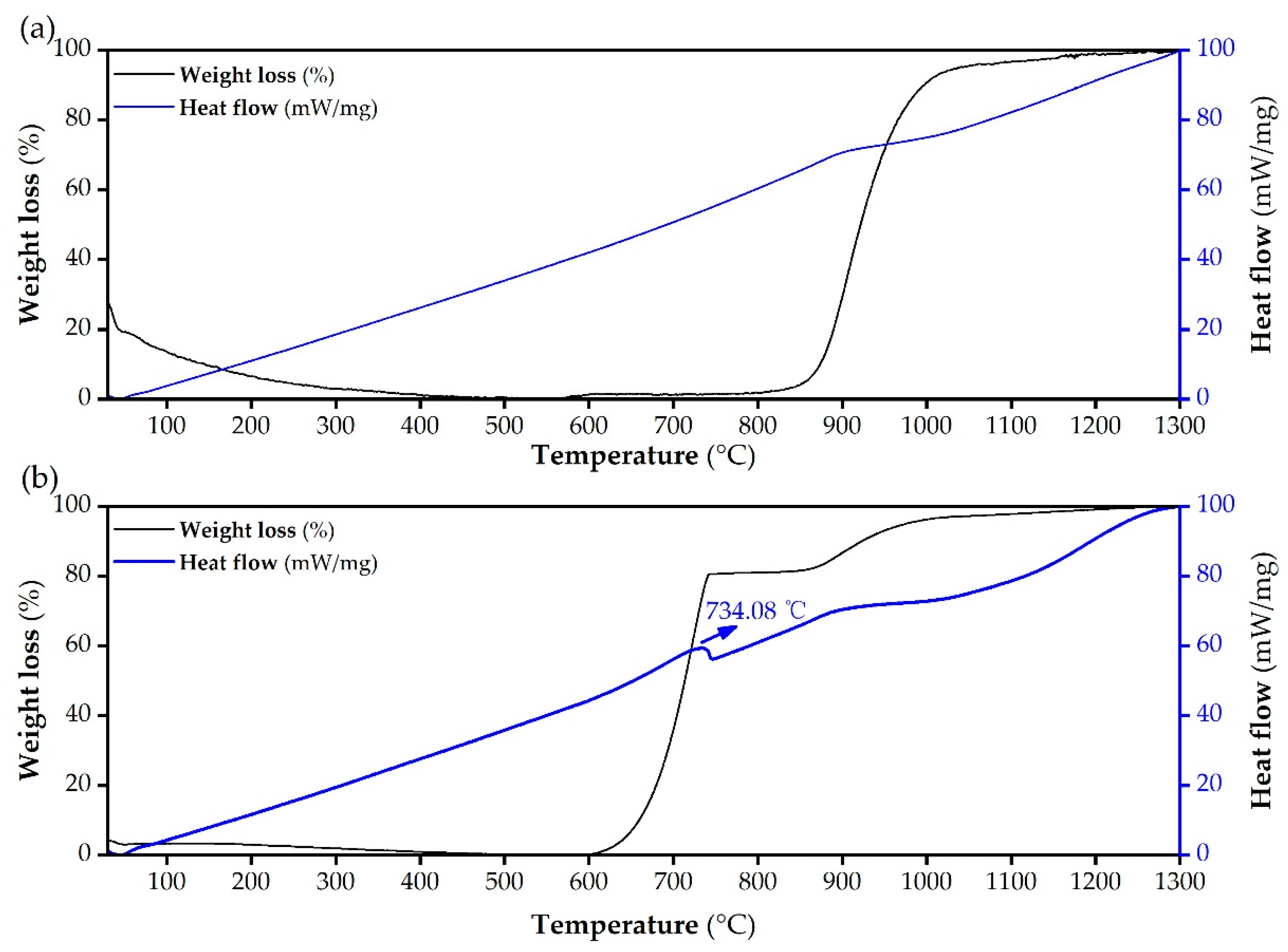
2.3.3. Thermogravimetry–Differential Scanning Calorimetry (TG-DSC) Analysis
2.4. Effects of Silicon Fertilizer on Soil Environment and Growth of Pak Choi
3. Materials and Methods
3.1. Materials
3.2. Preparation of Si Fertilizers
3.3. Release Performance and Solubility Tests
3.4. Composition and Microstructural Characterization
3.5. Pot Experiments
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yan, G.C.; Nikolic, M.; Ye, M.-J.; Xiao, Z.-X.; Liang, Y.-C. Silicon acquisition and accumulation in plant and its significance for agriculture. J. Integr. Agric. 2018, 17, 2138–2150. [Google Scholar] [CrossRef]
- Liang, Y.C.; Nikolic, M.; Bélanger, R.; Gong, H.J.; Song, A. Silicon in Agriculture from Theory to Practice, 1st ed.; Springer: Berlin, Germany, 2015; pp. 1–235. [Google Scholar]
- Yin, L.; Wang, S.; Tanaka, K.; Fujihara, S.; Itai, A.; Den, X.; Zhang, S. Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant Cell Environ. 2015, 39, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-X.; Gong, H.-J.; Yin, J.-L. Role of Silicon in Mediating Salt Tolerance in Plants: A Review. Plants 2019, 8, 147. [Google Scholar] [CrossRef] [Green Version]
- Richmond, K.E.; Sussman, M. Got silicon? The non-essential beneficial plant nutrient. Curr. Opin. Plant Biol. 2003, 6, 268–272. [Google Scholar] [CrossRef]
- Ning, D.F.; Liang, Y.C.; Liu, Z.D.; Xiao, J.F.; Duan, A.W. Impacts of Steel-Slag-Based Silicate Fertilizer on Soil Acidity and Silicon Availability and Metals-Immobilization in a Paddy Soil. PLoS ONE 2016, 11, e0168163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A Review of Silicon in Soils and Plants and Its Role in US Agriculture. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef] [Green Version]
- Desplanques, V.; Cary, L.; Mouret, J.-C.; Trolard, F.; Bourrié, G.; Grauby, O.; Meunier, J.-D. Silicon transfers in a rice field in Camargue (France). J. Geochem. Explor. 2006, 88, 190–193. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.-D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2011, 32, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.L.; Li, C.H.; Liang, Y.C. Chapter 21 agricultural utilization of silicon in China. In SIlicon in Agriculture, 1st ed.; Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 343–358. [Google Scholar]
- Machado, R.; Serralheiro, R. Soil salinity: Effect on vegetable crop growth. management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.H.; Zhou, Y.R.; Ma, X.; Wang, X.K.; Tong, W.S.; Luan, X.L.; Chu, P.K. Preparation and effectiveness of slow-release silicon fertilizer by sintering with iron ore tailings. Environ. Prog. Sustain. Energy 2017, 37, 1011–1019. [Google Scholar] [CrossRef]
- Artyszak, A.; Gozdowski, D.; Kucińska, K. Impact of Foliar Fertilization on the Content of Silicon and Macronutrients in Sugar Beet. Plants 2019, 8, 136. [Google Scholar] [CrossRef] [Green Version]
- Michot, L.J.; Villieras, F.; Francois, M.; Yvon, J.; Dred, R.L.; Cases, J.M. The Structural Microscopic Hydrophilicity of Talc. Langmuir 1994, 10, 3765–3773. [Google Scholar] [CrossRef]
- Sani, H.A.; Ahmad, M.B.; Saleh, T.A. Synthesis of zinc oxide/talc nanocomposite for enhanced lead adsorption from aqueous solutions. RSC Adv. 2016, 6, 108819–108827. [Google Scholar] [CrossRef]
- Kalantari, K.; Ahmad, M.B.; Masoumi, H.R.; Shameli, K.; Basri, M.; Khandanlou, R. Rapid Adsorption of Heavy Metals by Fe3O4/Talc Nanocomposite and Optimization Study Using Response Surface Methodology. Int. J. Mol. Sci. 2014, 15, 12913–12927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darajeh, N.; Masoumi, H.R.F.; Kalantari, K.; Ahmad, M.B.; Shameli, K.; Basri, M.; Khandanlou, R. Optimization of process parameters for rapid adsorption of Pb(II), Ni(II), and Cu(II) by magnetic/talc nanocomposite using wavelet neural network. Res. Chem. Intermed. 2015, 42, 1977–1987. [Google Scholar] [CrossRef]
- Moradihamedani, P.; Kalantari, K.; Abdullah, A.H.; Morad, N.A. High efficient removal of lead(II) and nickel(II) from aqueous solution by novel polysulfone/Fe3O4–talc nanocomposite mixed matrix membrane. Desalination Water Treat. 2016, 57, 28900–28909. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Kowalkowski, T.; Tutu, H.; Cukrowska, E.M.; Buszewski, B. Adsorption performance of talc for uranium removal from aqueous solution. Chem. Eng. J. 2011, 171, 1185–1193. [Google Scholar] [CrossRef]
- Ma, J.F.; Takahashi, E. Soil, Fertilizer, and Plant Silicon Research in Japan, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2002; pp. 1–281. [Google Scholar]
- Şener, S.; Özyılmaz, A. Adsorption of naphthalene onto sonicated talc from aqueous solutions. Ultrason. Sonochem. 2010, 17, 932–938. [Google Scholar] [CrossRef]
- Mathur, N.K.; Jakhar, S.R.; Mathur, R. Calcium carbonate and derived products. Int. J. Sci. Res. 2016, 5, 2128–2133. [Google Scholar]
- Duan, W.J. Study on preparation of silicon fertilizer using wollastonite. Master’s Thesis, South China University of Technology, Guangzhou, China, 2015. [Google Scholar]
- Liu, X.W.; Liu, X.X.; Hu, Y.H. Investigation of the thermal decomposition of talc. Clay Clay Min. 2014, 62, 137–144. [Google Scholar] [CrossRef]
- Li, X.Y.; Yu, W.M.; Zhao, B.Q. Effect of sintering temperature of potassium feldspar-limestone/dolomite on composition and microstructure of silicate fertilizers. Int. J. Ceram. Eng. Sci. 2019, 1, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Reddy, A.A.; Tulyaganov, D.U.; Goel, A.; Kapoor, S.; Pascual, M.J.; Ferreira, J.M.F. Sintering and devitrification of glass-powder compacts in the akermanite–gehlenite system. J. Mater. Sci. 2013, 48, 4128–4136. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.H.; Wang, X.K.; Zhou, Y.R.; Tong, W.S.; Luan, X.L.; Ma, X.; Zhou, F.S.; Chu, P.K.; Zhao, P.D. K2MgSi3O8 in Slow-Release Mineral Fertilizer Prepared by Sintering of By-Product of Red Mud-Based Flocculant. Environ. Eng. Sci. 2018, 35, 829–835. [Google Scholar] [CrossRef]
- Weeks, W.F. A Thermochemical Study of Equilibrium Relations during Metamorphism of Siliceous Carbonate Rocks. J. Geol. 1956, 64, 245–270. [Google Scholar] [CrossRef]
- Choudhary, R.; Koppala, S.; Swamiappan, S. Bioactivity studies of calcium magnesium silicate prepared from eggshell waste by sol–gel combustion synthesis. J. Asian Ceram. Soc. 2015, 3, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Stuart, B.H.; Thomas, P.S.; Barrett, M.; Head, K. Modelling clay materials used in artworks: An infrared spectroscopic investigation. Herit. Sci. 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Tang, Y.J.; Zhang, J.H. Surface modification of CaCO3 nanoparticles with silane coupling agent for improvement of the interfacial compatibility with styrene-butadiene rubber (SBR) latex. Chalcogenide Lett. 2013, 10, 131–141. [Google Scholar]
- Ma, X.; Ma, H.W.; Yang, J. Sintering Preparation and Release Properties of K2MgSi3O8 Slow-Release Fertilizer Using Biotite Acid-Leaching Residues as Silicon Source. Ind. Eng. Chem. Res. 2016, 55, 10926–10931. [Google Scholar] [CrossRef]
- Sharafabadi, A.K.; Abdellahi, M.; Kazemi, A.; Khandan, A.; Ozada, N. A novel and economical route for synthesizing akermanite (Ca2MgSi2O7) nano-bioceramic. Mater. Sci. Eng. C 2017, 71, 1072–1078. [Google Scholar] [CrossRef]
- Park, J.H.; Min, D.J.; Song, H.S. FT-IR spectroscopic study on structure of CaO-SiO2 and CaO-SiO2-CaF2 slags. ISIJ Int. 2002, 42, 344–351. [Google Scholar] [CrossRef]
- Kalinkina, E.V.; Kalinkin, A.M.; Forsling, W.; Makarov, V.N. Sorption of atmospheric carbon dioxide and structural changes of Ca and Mg silicate minerals during grinding. Int. J. Miner. Process. 2001, 61, 289–299. [Google Scholar] [CrossRef]
- Mangrich, A.S.; Tessaro, L.C.; Anjos, A.D.; Wypych, F.; Soares, J.F. A slow-release K+ fertilizer from residues of the brazilian oil-shale industry: Synthesis of kalsilite-type structures. Environ. Geol. 2001, 40, 1030–1036. [Google Scholar]
- Wang, J.; Somasundaran, P. Study of galactomannose interaction with solids using AFM, IR and allied techniques. J. Colloid Interface Sci. 2007, 309, 373–383. [Google Scholar] [CrossRef]
- Xu, C.B.; Li, X.M.; Zhang, L.H. The Effect of Calcium Chloride on Growth, Photosynthesis, and Antioxidant Responses of Zoysia japonica under Drought Conditions. PLoS ONE 2013, 8, e68214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.Q.; Zhang, F.S.; Li, X.X. Magnesium Fertilization Improves Crop Yield in Most Production Systems: A Meta-Analysis. Front. Plant Sci. 2020, 10, 1727. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.F.; Li, X.Y.; Wu, Q.S.; Liu, Y.F.; Lyu, Y.N. Evolution of mineral phases and microstructure of high efficiency Si–Ca–K–Mg fertilizer prepared by water-insoluble K-feldspar. J. Sol Gel Sci. Technol. 2020, 94, 3–10. [Google Scholar] [CrossRef]
- Xia, D.H.; Ren, L.; Chen, L.Z. Study of Ca-Mg-S-Si Fertilizer Produced by Magnesium Slag. Adv. Mater. Res. 2011, 347–353, 3166–3170. [Google Scholar] [CrossRef]
- Al-Fariss, T.F.; El-Aleem, F.A.A.; Arafat, Y.; El-Nagdy, K.A.; El-Midany, A.A. Low solubility of calcined phosphate: Surface area reduction or chemical composition change? Part. Sci. Technol. 2014, 32, 80–85. [Google Scholar] [CrossRef]
- Ayed, F.B.; Bouaziz, J.; Bouzouita, K. Pressureless sintering of fluorapatite under oxygen atmosphere. J. Eur. Ceram. Soc. 2000, 20, 1069–1076. [Google Scholar] [CrossRef]
- Wang, W.; Liu, P.; Zhang, M.; Hu, J.; Xing, F. The Pore Structure of Phosphoaluminate Cement. Open J. Compos. Mater. 2012, 2, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Bloise, A.; Catalano, M.; Barrese, E.; Gualtieri, A.F.; Gandolfi, N.B.; Capella, S.; Belluso, E. TG/DSC study of the thermal behaviour of hazardous mineral fibres. J. Therm. Anal. Calorim. 2015, 123, 2225–2239. [Google Scholar] [CrossRef] [Green Version]
- Rashid, R.A.; Shamsudin, R.; Hamid, M.A.A.; Jalar, A. Low temperature production of wollastonite from limestone and silica sand through solid-state reaction. J. Asian Ceram. Soc. 2014, 2, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.H.; Chu, C.B.; Wei, H.W.; Zhang, L.M.; Ahmad, Z.; Wu, S.H.; Xie, B. Ameliorative effects of silicon fertilizer on soil bacterial community and pakchoi (Brassica chinensis L.) grown on soil contaminated with multiple heavy metals. Environ. Pollut. 2020, 267, 115411. [Google Scholar] [CrossRef] [PubMed]
- Jett, L.W. Growing Chinese Cabbages in West Virginia, 1st ed.; West Virginia University Commercial Horticulture Extension: Morgantown, WV, USA, 2015; pp. 1–3. [Google Scholar]
- Artyszak, A. Effect of Silicon Fertilization on Crop Yield Quantity and Quality—A Literature Review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Murad, M.A.; Khan, A.L.; Muneer, S. Silicon in Horticultural Crops: Cross-talk, Signaling, and Tolerance Mechanism under Salinity Stress. Plants 2020, 9, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazelton, P.; Murphy, B.L. Interpreting Soil Test Results, 1st ed.; CSIRO Publishing: Clayton, Australia, 2007; pp. 1–169. [Google Scholar]
- Rabhi, M.; Farhat, N.; Msilini, N.; Rajhi, H.; Smaoui, A.; Abdelly, C.; Lachaâl, M.; Karray-Bouraoui, N. Physiological responses of Carthamus tinctorius to CaCl2 salinity under Mg-sufficient and Mg-deficient conditions. Flora 2018, 246–247, 96–101. [Google Scholar] [CrossRef]
- Liu, T.-W.; Wu, F.-H.; Wang, W.-H.; Chen, J.; Li, Z.-J.; Dong, X.J.; Patton, J.; Pei, Z.-M.; Zheng, H.-L.; Rennenberg, H. Effects of calcium on seed germination, seedling growth and photosynthesis of six forest tree species under simulated acid rain. Tree Physiol. 2011, 31, 402–413. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, F. Synthesis of a novel slow-release potassium fertilizer from modified Pidgeon magnesium slag by potassium carbonate. J. Air Waste Manag. Assoc. 2016, 66, 758–767. [Google Scholar] [CrossRef]
- Santos, W.O.; Mattiello, E.M.; Vergutz, L.; Costa, R.F. Production and evaluation of potassium fertilizers from silicate rock. J. Plant Nutr. Soil Sci. 2016, 179, 547–556. [Google Scholar] [CrossRef] [Green Version]
- França, A.; Schultz, J.; Borges, R.; Wypych, F.; Mangrich, A. Rice Husk Ash as Raw Material for the Synthesis of Silicon and Potassium Slow-Release Fertilizer. J. Braz. Chem. Soc. 2017, 28, 2211–2217. [Google Scholar] [CrossRef]
- Rao, B.; Gao, L.K.; Dai, H.X.; Hong, Z.; Xie, H.Y. An Efficient and Sustainable Approach for Preparing Silicon Fertilizer by Using Crystalline Silica from Ore. JOM 2019, 71, 3915–3922. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, P.; Zhu, T.; Li, Q.; Cao, J. The Characteristics of Soil C, N, and P Stoichiometric Ratios as Affected by Geological Background in a Karst Graben Area, Southwest China. Forests 2019, 10, 601. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, J.; Zheng, J.; Lin, H.; Chen, G.; Wang, C.; Chhuon, K.; Wei, Z.; Jin, C.; Zhang, X. Thermal Preparation and Application of a Novel Silicon Fertilizer Using Talc and Calcium Carbonate as Starting Materials. Molecules 2021, 26, 4493. https://doi.org/10.3390/molecules26154493
Wang Y, Zhang J, Zheng J, Lin H, Chen G, Wang C, Chhuon K, Wei Z, Jin C, Zhang X. Thermal Preparation and Application of a Novel Silicon Fertilizer Using Talc and Calcium Carbonate as Starting Materials. Molecules. 2021; 26(15):4493. https://doi.org/10.3390/molecules26154493
Chicago/Turabian StyleWang, Yian, Jie Zhang, Junjian Zheng, Hua Lin, Gongning Chen, Chao Wang, Kong Chhuon, Zhonghua Wei, Chengfenghe Jin, and Xuehong Zhang. 2021. "Thermal Preparation and Application of a Novel Silicon Fertilizer Using Talc and Calcium Carbonate as Starting Materials" Molecules 26, no. 15: 4493. https://doi.org/10.3390/molecules26154493
APA StyleWang, Y., Zhang, J., Zheng, J., Lin, H., Chen, G., Wang, C., Chhuon, K., Wei, Z., Jin, C., & Zhang, X. (2021). Thermal Preparation and Application of a Novel Silicon Fertilizer Using Talc and Calcium Carbonate as Starting Materials. Molecules, 26(15), 4493. https://doi.org/10.3390/molecules26154493





