Synthesis, Characterization, and Catalytic Application of Palladium Complexes Containing Indolyl-NNN-Type Ligands
Abstract
1. Introduction
2. Results and Discussion
2.1. Syntheses and Characterization of Ligand Precursors and Palladium Compounds
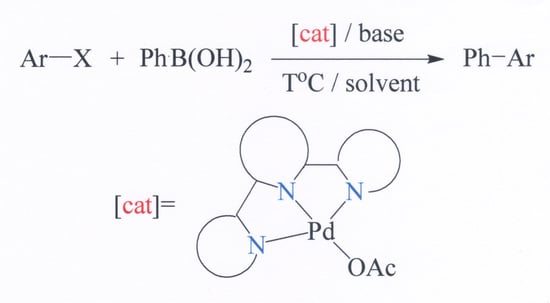
2.2. Catalytic Studies
3. Materials and Methods
3.1. Preparations
3.2. Crystal Structure Data
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, Y.-F.; Shi, Z.-J. Upgrading Cross-Coupling Reactions for Biaryl Syntheses. Acc. Chem. Res. 2019, 52, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Korch, K.M.; Watson, D.A. Cross-Coupling of Heteroatomic Electrophiles. Chem. Rev. 2019, 119, 8192–8228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Szostak, M. Palladium-catalyzed cross-couplings by C–O bond activation. Catal. Sci. Technol. 2020, 10, 5702–5739. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Negishi, E.-I. Magical Power of Transition Metals: Past, Present, and Future (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6738–6764. [Google Scholar] [CrossRef]
- Selander, N.; Szabó, K.J. Catalysis by Palladium Pincer Complexes. Chem. Rev. 2011, 111, 2048–2076. [Google Scholar] [CrossRef] [PubMed]
- González-Sebastián, L.; Morales-Morales, D. Cross-coupling reactions catalysed by palladium pincer complexes. A review of recent advances. J. Organomet. Chem. 2019, 893, 39–51. [Google Scholar] [CrossRef]
- Peng, K.-F.; Chen, Y.; Chen, C.-T. Synthesis and catalytic application of magnesium complexes bearing pendant indolyl ligands. Dalton Trans. 2015, 44, 9610–9619. [Google Scholar] [CrossRef]
- Chen, C.-T.; Lin, D.-H.; Peng, K.-F. Magnesium Pyrazolyl-Indolyl Complexes as Catalysts for Ring-Opening Polymerization of L-Lactide. Polymers 2015, 7, 1954–1964. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Yajima, T.; Takani, M.; Yamauchi, O. Metal complexes involving indole rings: Structures and effects of metal–indole interactions. Coord. Chem. Rev. 2009, 253, 479–492. [Google Scholar] [CrossRef]
- Martinez-De-Leon, C.G.; Rodriguez-Alvarez, A.; Flores-Parra, A.; Grevy, J.-M. Palladium(II) complexes of hemilabile NNS and NNSe iminophosphorane ligands: Synthesis, characterization, and reactivity. Inorg. Chim. Acta 2019, 495, 118945–118957. [Google Scholar] [CrossRef]
- Cravotto, G.; Demartin, F.; Palmisano, G.; Penoni, A.; Radice, T.; Tollari, S. Novel cyclometallated Pd(II) and Pt(II) complexes with indole derivatives and their use as catalysts in Heck reaction. J. Organomet. Chem. 2005, 690, 2017–2026. [Google Scholar] [CrossRef]
- Wu, K.-M.; Huang, C.-A.; Peng, K.-F.; Chen, C.-T. Palladacycles bearing pendant benzamidinate ligands as catalysts for the Suzuki and Heck coupling reactions. Tetrahedron 2005, 61, 9679–9687. [Google Scholar] [CrossRef]
- Decken, A.; Gossage, R.A.; Yadav, R.N. Oxazoline chemistry. Part VIII. Synthesis and characterization of a new class of pincer ligands derived from the 2-(o-anilinyl)-2-oxazoline skeleton—Applications to the synthesis of group X transition metal catalysts. Can. J. Chem. 2005, 83, 1185–1189. [Google Scholar] [CrossRef]
- Mazet, C.; Gade, L.H. A Bis(oxazolinyl)pyrrole as a New Monoanionic Tridentate Supporting Ligand: Synthesis of a Highly Active Palladium Catalyst for Suzuki-Type C-C Coupling. Organometallics 2001, 20, 4144–4146. [Google Scholar] [CrossRef]
- Chen, C.-T.; Chan, Y.-S.; Tzeng, Y.-R.; Chen, M.-T. Palladacyclic complexes bearing CNN-type ligands as catalysts in the Heck reaction. Dalton Trans. 2004, 2691–2696. [Google Scholar] [CrossRef]
- Peng, K.-F.; Chen, C.-T. Synthesis, Structural Studies, and Catalytic Application of Palladium Complexes Containing Anilido-Oxazolinate Ligands. Eur. J. Inorg. Chem. 2011, 2011, 5182–5195. [Google Scholar] [CrossRef]
- Chen, M.-T.; Wu, K.-M.; Chen, C.-T. C–H Bond Activation of Palladium Complexes That Feature Pendant Benzamidinate Ligands and Their Catalytic Behaviours. Eur. J. Inorg. Chem. 2012, 720–726. [Google Scholar] [CrossRef]
- Shinde, V.N.; Bhuvanesh, N.; Kumar, A.; Joshi, H. Design and Syntheses of Palladium Complexes of NNN/CNN Pincer Ligands: Catalyst for Cross Dehydrogenative Coupling Reaction of Heteroarenes. Organometallics 2020, 39, 324–333. [Google Scholar] [CrossRef]
- Wang, W.-C.; Peng, K.-F.; Chen, M.-T.; Chen, C.-T. Palladacycles bearing tridentate CNS-type benzamidinate ligands as catalysts for cross-coupling reactions. Dalton Trans. 2012, 41, 3022–3029. [Google Scholar] [CrossRef][Green Version]
- Chen, M.-T.; Huang, C.-A.; Chen, C.-T. Synthesis, Characterization, and Catalytic Applications of Palladacyclic Complexes Bearing C,N,S-Donor Ligands. Eur. J. Inorg. Chem. 2006, 2006, 4642–4648. [Google Scholar] [CrossRef]
- Lemster, T.; Pindur, U.; Lenglet, G.; Depauw, S.; Dassi, C.; David-Cordonnier, M.-H. Photochemical electrocyclisation of 3-vinylindoles to pyrido[2,3-a]-, pyrido[4,3-a]- and thieno[2,3-a]-carbazoles: Design, synthesis, DNA binding and antitumor cell cytotoxicity. Eur. J. Med. Chem. 2009, 44, 3235–3252. [Google Scholar] [CrossRef]
- Pagano, N.; Maksimoska, J.; Bregman, H.; Williams, D.S.; Webster, R.D.; Xue, F.; Meggers, E. Ruthenium half-sandwich complexes as protein kinase inhibitors: Derivatization of the pyridocarbazole pharmacophore ligand. Org. Biomol. Chem. 2007, 5, 1218–1227. [Google Scholar] [CrossRef]
- Zhao, D.; Hughes, D.L.; Bender, D.R.; DeMarco, A.M.; Reider, P.J. Regioselective Fischer indole route to 3-unsubstituted indoles. J. Org. Chem. 1991, 56, 3001–3006. [Google Scholar] [CrossRef]
- Chu, W.-K.; Lo, L.T.-L.; Yiu, S.-M.; Ko, C.-C. Synthesis, characterization and photophysical study of a series of neutral isocyano rhodium(I) complexes with pyridylindolide ligands. J. Organomet. Chem. 2011, 696, 3223–3230. [Google Scholar] [CrossRef]
- Poriel, C.; Lachia, M.; Wilson, C.; Davies, J.R.; Moody, C.J. Oxidative Rearrangement of Indoles: A New Approach to the EFHG-Tetracyclic Core of Diazonamide A. J. Org. Chem. 2007, 72, 2978–2987. [Google Scholar] [CrossRef]
- Maiden, T.M.M.; Swanson, S.; Procopiou, P.A.; Harrity, J.P.A. Synthesis of Functionalized Pyridines via a Regioselective Oxazoline Promoted C−H Amidation Reaction. Org. Lett. 2016, 18, 3434–3437. [Google Scholar] [CrossRef]
- Wang, Y.; Hämäläinen, A.; Tois, J.; Franzén, R. Preparation of indole-phosphine oxazoline (IndPHOX) ligands and their application in allylic alkylation. Tetrahedron Asymmetry 2010, 21, 2376–2384. [Google Scholar] [CrossRef]
- Bruker, SAINT v7.34A.; Bruker AXS Inc.: Madison, WI, USA, 2005.
- Sheldrick, G.M. SADABS-2012/1. Bruker Area Detector Absorption Correction Program; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Burnett, M.N.; Johnson, C.K. ORTEPIII; Report ORNL-6895; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1996. [Google Scholar]





| NE1-Pd-NE2 a | Ref. | Pd-Nindolyl | Ref. | Pd-Noxazolinyl | Ref. |
| (5-membered ring) | 1.941(6) | 3 | 2.050(3) | 3 | |
| 78.0(2) | 3 | 1.923(3) | 5 | 2.144(3) | [13] |
| 79.33(11) | 5 | 2.032(3) to 2.040(4) | [11] | 2.0254(14) | [14] |
| 81.14(12) | [12] | 2.012(3) | [12] | 2.011(3) to 2.052(8) | [15] |
| 79.89(7), 80.06(9) | [16] | 1.972(2) to 2.055(4) | [17] | ||
| 81.78(8) | [18] | ||||
| 81.37(11) | [13] | Pd-Npyridinyl | Ref. | Pd-OOAc | Ref. |
| 81.39(12) | [19] | 2.043(4), 2.055(5) | 3 | 2.045(4) | 3 |
| 81.76(6) | [14] | 2.087(3) | 5 | 2.048(2) | 5 |
| 2.021(3) | [12] | 2.035(2) | [12] | ||
| (6-membered ring) | 2.1244(17) | [16] | 2.0558(18) | [16] | |
| 90.8(8) | 3 | 2.113(2) | [18] | 2.0545(16) | [18] |
| 89.01(11) | 5 | 2.119(3) | [13] | 2.064(3) to 2.045(2) | [13] |
| 2.021(4) | [17] | ||||
| 2.0412(18), 2.054(3) | [20] | ||||
| 2.036(2) | [21] |
| Entry | Catalyst | Aryl Halide | Base | Solvent | [Pd] (mol%) | T/°C | t/h | Conversion (%) b |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 4-bromoacetophenone | Cs2CO3 | DMSO | 1 | 70 | 1 | 83 |
| 2 | 2 | 4-bromoacetophenone | Cs2CO3 | DMA | 1 | 70 | 1 | 74 |
| 3 | 1 | 4-bromoacetophenone | K2CO3 | Toluene | 1 | 70 | 1 | trace |
| 4 | 2 | 4-bromoacetophenone | K2CO3 | Toluene | 1 | 70 | 1 | 93(78) |
| 5 | 3 | 4-bromoacetophenone | K2CO3 | Toluene | 1 | 70 | 1 | 98(95) |
| 6 | 4 | 4-bromoacetophenone | K2CO3 | Toluene | 1 | 70 | 1 | 94 |
| 7 | 5 | 4-bromoacetophenone | K2CO3 | Toluene | 1 | 70 | 1 | 67 |
| 8 | 3 | 4-bromoacetophenone | Cs2CO3 | Toluene | 1 | 70 | 1 | 32 |
| 9 | 3 | 4-bromoacetophenone | K3PO4 | Toluene | 1 | 70 | 1 | 61 |
| 10 | 3 | 4-bromoacetophenone | K2CO3 | DMF | 1 | 70 | 1 | 50 |
| 11 | 3 | 4-bromoacetophenone | K3PO4 | DMF | 1 | 70 | 1 | 5 |
| 12 | 3 | 4-bromoacetophenone | K2CO3 | THF | 1 | 70 | 1 | 4 |
| 13 | 3 | 4-bromoacetophenone | K2CO3 | EtOH | 1 | 70 | 1 | trace |
| 14 | 3 | 4-bromoacetophenone | K2CO3 | DMA | 1 | 70 | 1 | 38 |
| 15 | 3 | 4-bromoacetophenone | K2CO3 | Toluene | 0.5 | 70 | 1 | 96(83) |
| 16 | 4 | 4-bromoacetophenone | K2CO3 | Toluene | 0.5 | 70 | 1 | 53 |
| 17 | 3 | 4-bromoacetophenone | K2CO3 | Toluene | 0.5 | 70 | 0.5 | 94(82) |
| 18 | 3 | 4-bromoacetophenone | K2CO3 | Toluene | 1 | room temp. | 1 | 87 |
| 19 | 4 | 4-bromoacetophenone | K2CO3 | Toluene | 1 | room temp. | 1 | 3 |
| 20 | 3 | 4-bromoanisole | K2CO3 | Toluene | 1 | 70 | 1 | trace |
| 21 | 3 | 4-bromoanisole | K2CO3 | Toluene | 1 | 70 | 2 | 11 |
| 22 | 3 | 4-chloroacetophenone | K2CO3 | Toluene | 1 | 70 | 1 | trace |
| 23 | 3 | 4-chloroacetophenone | K2CO3 | Toluene | 1 | 70 | 2 | trace |
| 3 | 5 | |
|---|---|---|
| Formula | C20H15N3O2Pd | C20H19N3O3Pd |
| Fw | 435.75 | 455.78 |
| T, K | 150(2) | 150(2) |
| Crystal system | Triclinic | Monoclinic |
| Space group | P-1 | P21/n |
| a, Å | 8.0571(10) | 12.0182(14) |
| b, Å | 9.7364(14) | 12.9950(16) |
| c, Å | 10.8370(16) | 12.8255(16) |
| α | 89.045(5) | 90 |
| β | 85.676(4) | 117.027(4) |
| γ | 76.663(4) | 90 |
| V, Å3 | 824.8(2) | 1784.3(4) |
| Z | 2 | 4 |
| ρcalc, Mg/m3 | 1.754 | 1.697 |
| μ(Mo Kα), mm−1 | 1.145 | 1.067 |
| Reflections collected | 22,618 | 32,093 |
| No. of parameters | 235 | 245 |
| R1 a | 0.0483 | 0.0318 |
| wR2 a | 0.137 | 0.0864 |
| GoF b | 1.008 | 1.182 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, P.-C.; Yang, C.-W.; Wu, W.-K.; Chen, C.-T. Synthesis, Characterization, and Catalytic Application of Palladium Complexes Containing Indolyl-NNN-Type Ligands. Molecules 2021, 26, 4426. https://doi.org/10.3390/molecules26154426
Lo P-C, Yang C-W, Wu W-K, Chen C-T. Synthesis, Characterization, and Catalytic Application of Palladium Complexes Containing Indolyl-NNN-Type Ligands. Molecules. 2021; 26(15):4426. https://doi.org/10.3390/molecules26154426
Chicago/Turabian StyleLo, Pang-Chia, Chun-Wei Yang, Wen-Kai Wu, and Chi-Tien Chen. 2021. "Synthesis, Characterization, and Catalytic Application of Palladium Complexes Containing Indolyl-NNN-Type Ligands" Molecules 26, no. 15: 4426. https://doi.org/10.3390/molecules26154426
APA StyleLo, P.-C., Yang, C.-W., Wu, W.-K., & Chen, C.-T. (2021). Synthesis, Characterization, and Catalytic Application of Palladium Complexes Containing Indolyl-NNN-Type Ligands. Molecules, 26(15), 4426. https://doi.org/10.3390/molecules26154426