Complexation of Cyclodextrins with Benzoic Acid in Water-Organic Solvents: A Solvation-Thermodynamic Approach
Abstract
:1. Introduction
- -
- NMR data for the complexation of benzoic acid with β-cyclodextrin in D2O-DMSO-d6 and in D2O-EtOH, the stability constants of [BA⊂β-CD] molecular complexes calculated from NMR results;
- -
- Changes in the Gibbs energy of benzoic acid resolvation at the transfer from water to water–dimethylsulfoxide solvents obtained by a method of interfacial distribution of the substance between two immiscible phases;
- -
- Quantum chemical calculations of the interaction energy between β-CD and BA and the structure characteristics of the benzoic acid complex with β-cyclodextrin in vacuum and in water, methanol and DMSO.
2. Results
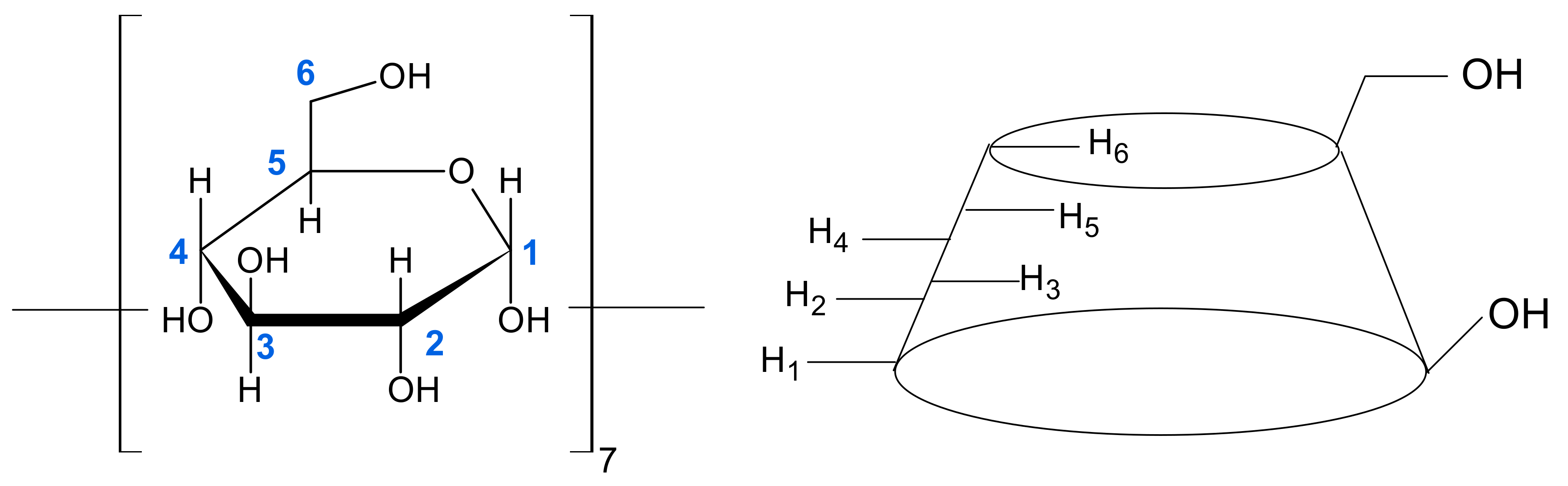
2.1. 1H NMR Studies of Inclusion Complex Formation of Benzoic Acid with β-Cyclodextrin in D2O, D2O-DMSO-d6 and D2O-EtOH
2.2. Quantum Calculation Results
2.2.1. Geometry Optimization and Properties of β-CD and BA
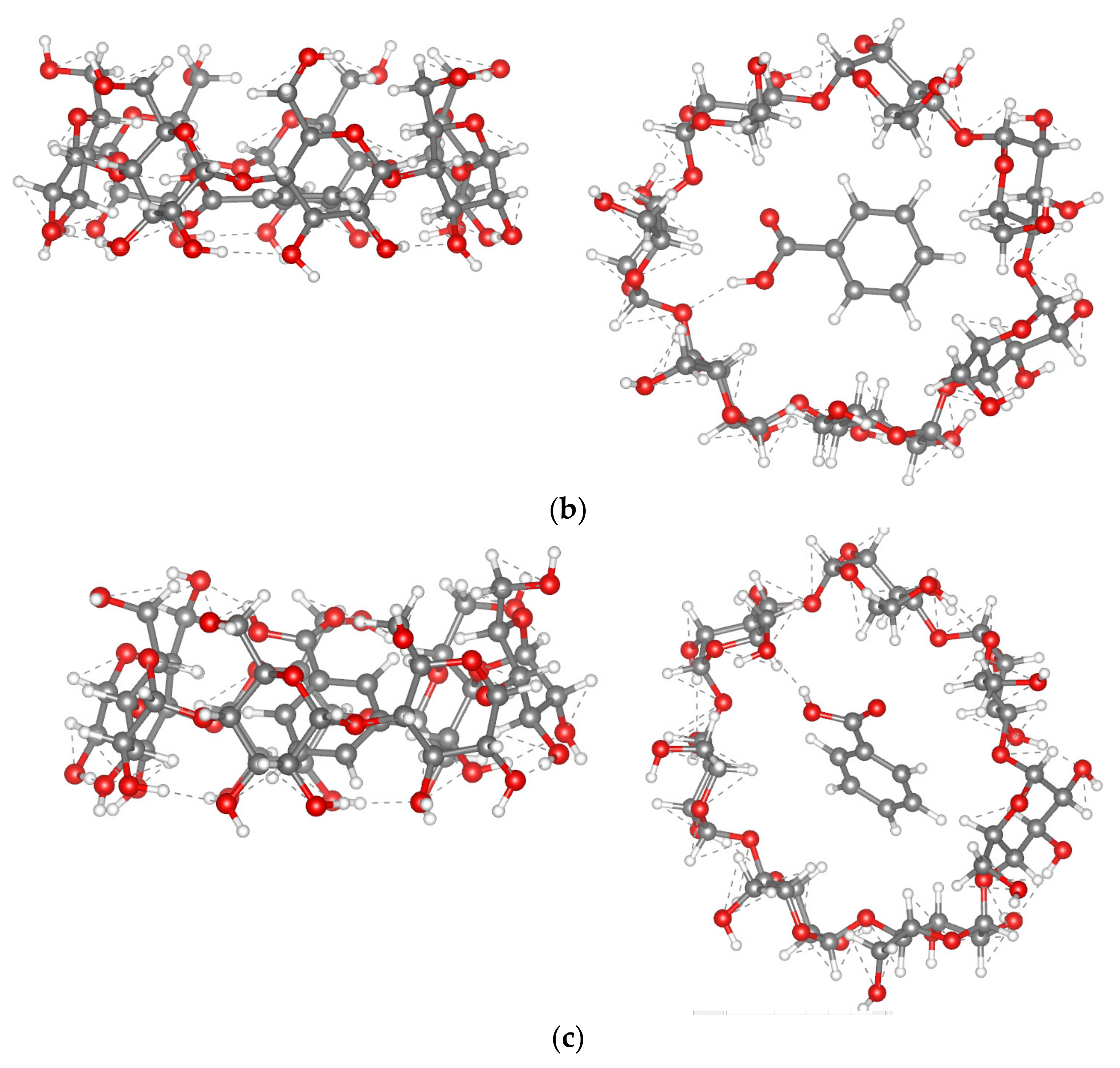
2.2.2. [BA⊂β-CD] Complex Formation
2.3. Interfacial Distribution-Study of Reagents Solvation State
3. Discussion
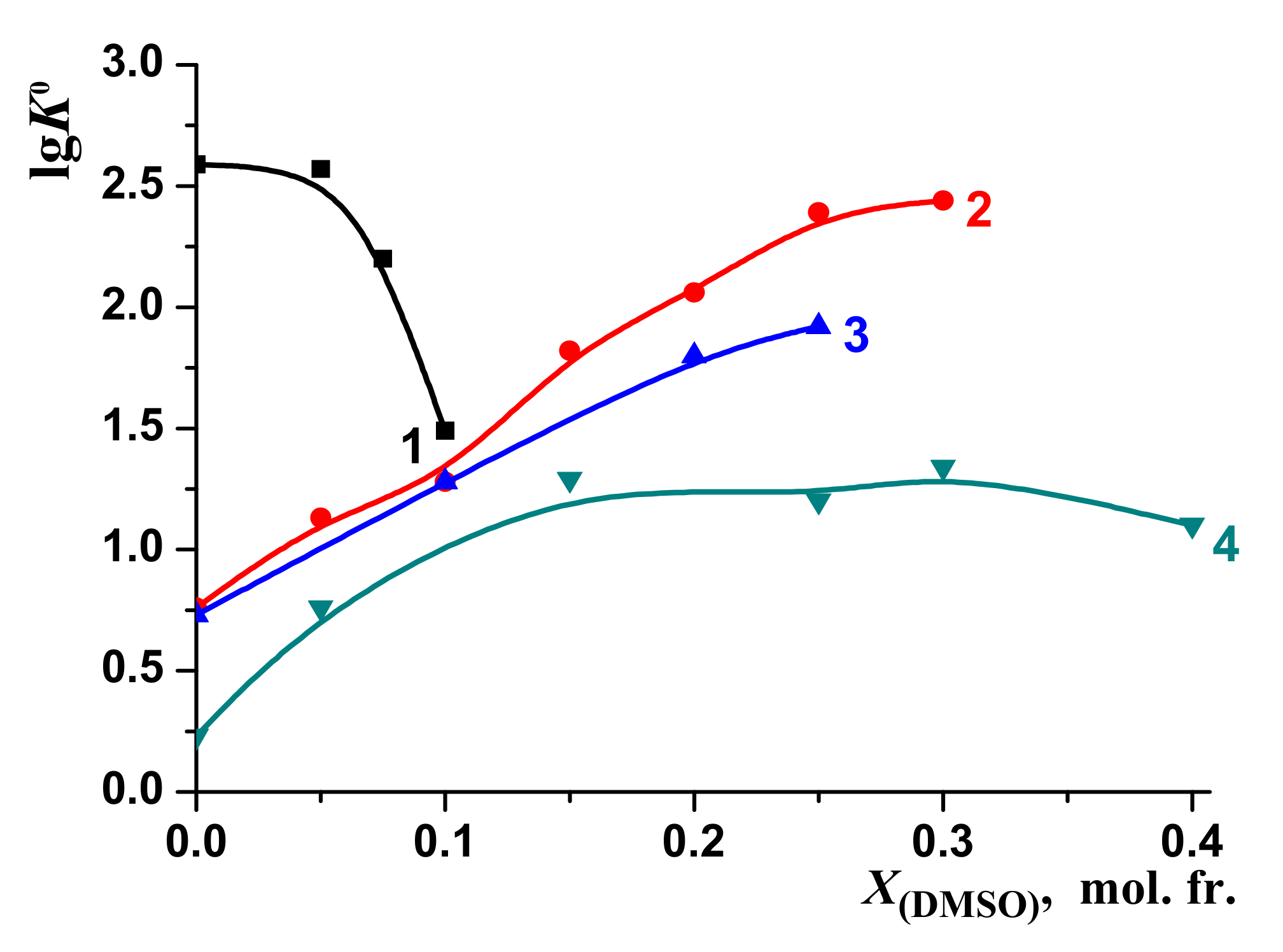
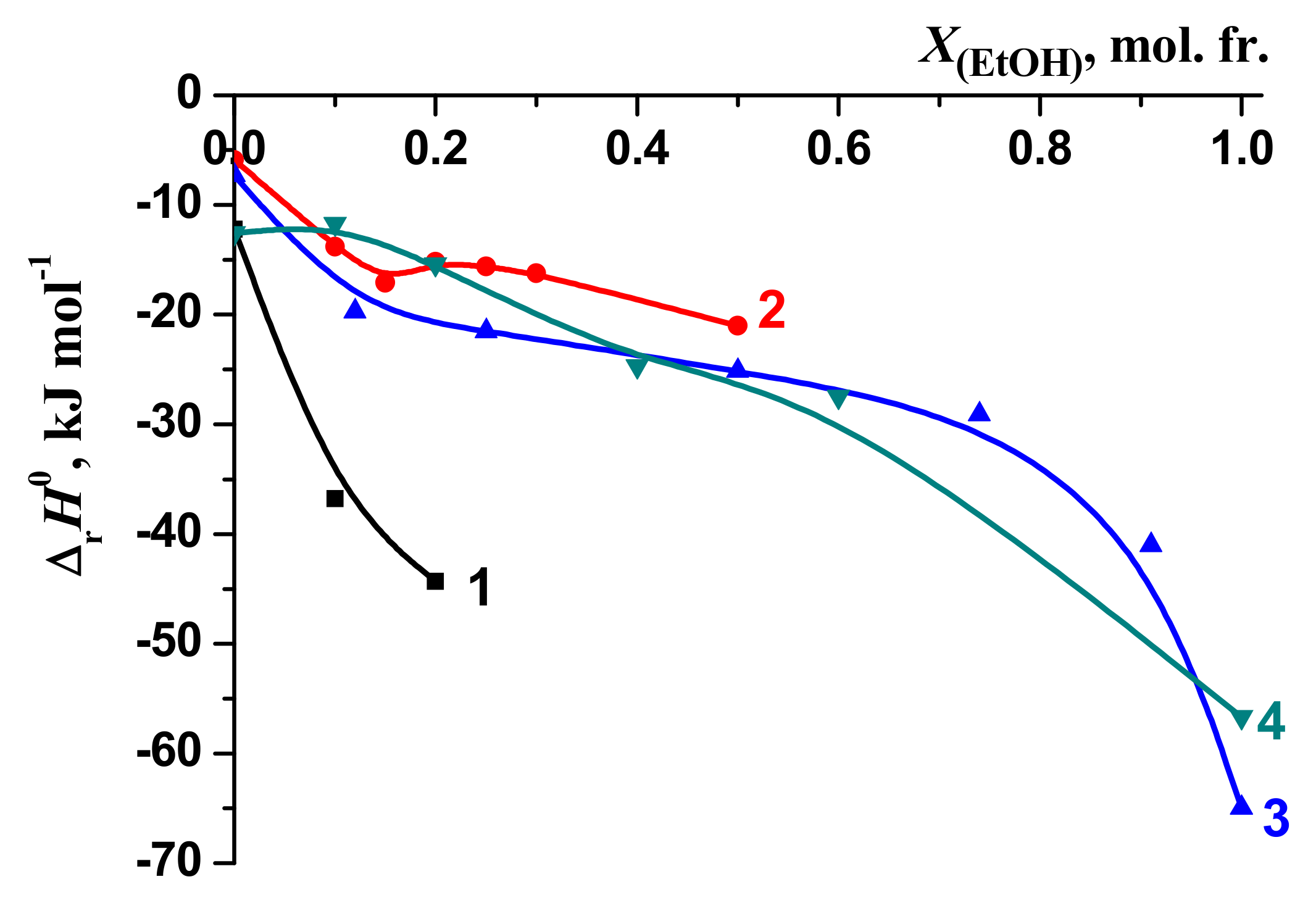
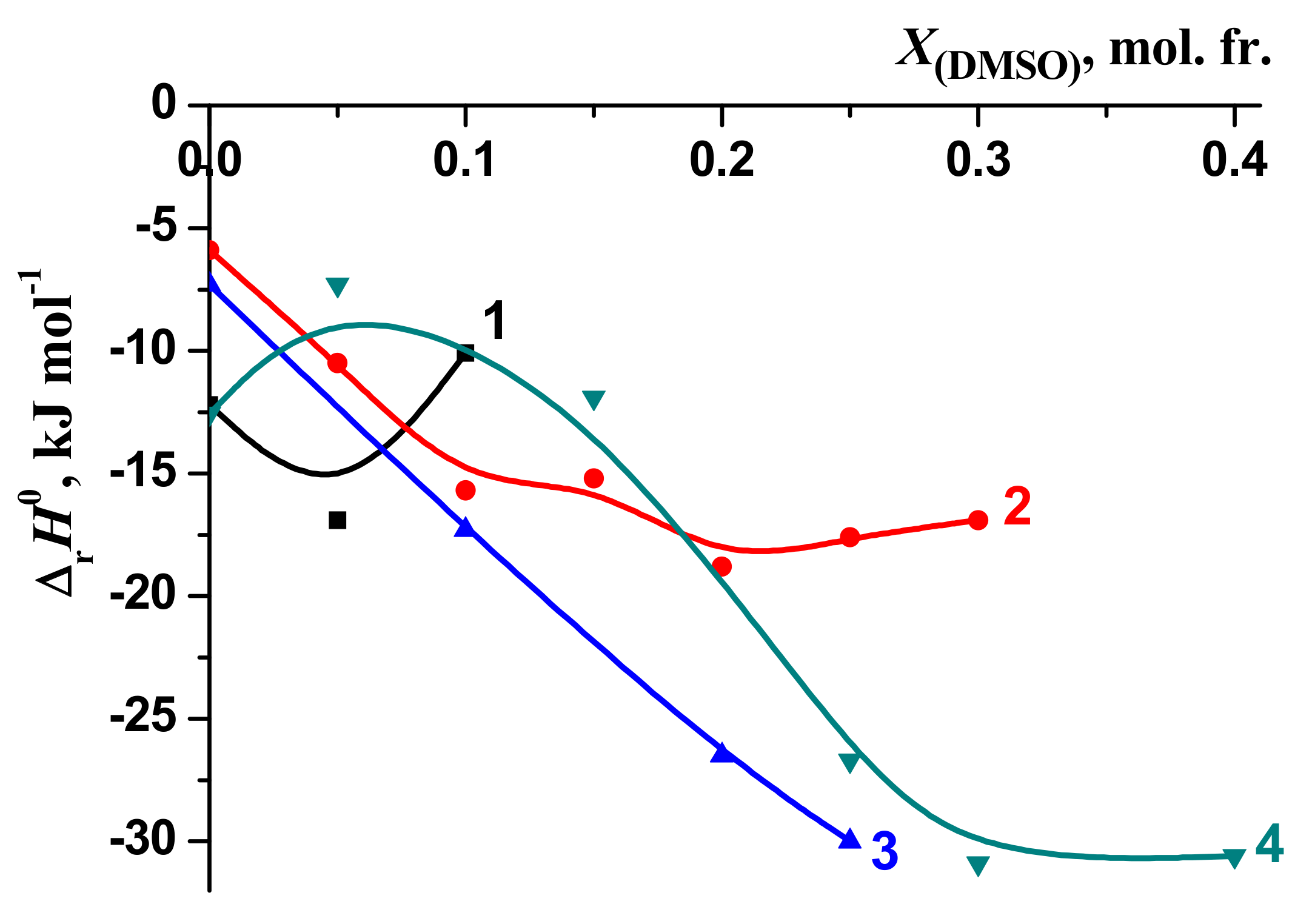
3.1. Thermodynamic Parameters of the [BA⊂β-CD] Complexes Formation in H2O-DMSO and H2O-EtOH Solvents
| X, mol. fr. | lgK0 | –ΔrH0 kJ mol–1 | –ΔrG0 kJ mol–1 | –TΔrS0 kJ mol–1 | Experimental Method | Reference |
|---|---|---|---|---|---|---|
| 0.00 | 2.4 ± 0.10 (2) | 12.2 ± 0.5 | 13.7 ± 0.6 | −1.5 ± 0.6 | Isothermal titration calorimetry (pH 3.6) | [45] |
| 1.85 ± 0.16 (3) | - | 10.6 ± 0.8 | - | UV–Vis spectroscopy (pH 3.6) | [45] | |
| 1.94 (1) | - | 11.3 | - | Solubility method (pH 2.9) | [37] | |
| 2.60 ± 0.10 (2) | 13.4 ± 0.4 | 14.7 ± 0.7 | −1.3 ± 0.8 | Isothermal titration calorimetry (acidic medium) | [21] | |
| 2.50 (5) | 22.3 ± 0.8 | 14.4 | 7.9 | Circular dichroism spectroscopy | [49] | |
| 2.50 (4) | - | 14.2 | - | 1H NMR (acidic medium) | [12] | |
| 2.59 (4) | - | 14.8 | - | 1H NMR | This work | |
| 2.40 ± 0.2 (6) | - | 13.5 ± 1.1 | - | Densitometry (acidic medium) | [12] | |
| 2.10 ± 0.4 (2) | 32 ± 2.7 | 12 ± 0.5 | 20 | Isothermal titration calorimetry | [36] | |
| H2O-DMSO | ||||||
| 0.05 | 1.67 ± 0.2 (2) | 16.8 ± 0.3 * | 9.5 ± 0.36 | 7.3 ± 0.47 * | Isothermal titration calorimetry (pH 3.6) | [45] |
| 1.78 ± 0.2 (3) | - | 10.2 ± 0.2 | - | UV–Vis spectroscopy (pH 3.6) | [45] | |
| 1.94 ± 0.45 (2) | 16.9 ± 1.4 | 11.07 | 5.2 | Isothermal titration calorimetry (pH 1.65) | [45] | |
| 2.57 (4) | - | 14.7 | - | 1H NMR | This work | |
| 0.075 | 2.2 (4) | - | - | - | 1H NMR | This work |
| 0.10 | 1.86 ± 0.41 (2) | 10.9 ± 0.9 * | 10.9 ± 0.9 | 0.3 ± 1.3 * | Isothermal titration calorimetry (pH 3.6) | [45] |
| 1.86 ± 0.41 (3) | - | 10.9 ± 0.9 | - | UV–Vis spectroscopy (pH 3.6) | [45] | |
| 1.85 ± 0.32 (2) | 10.1 ± 0.2 | 10.6 ± 0.9 | –0.5 | Isothermal titration calorimetry (pH 1.65) | [45] | |
| 1.49 (4) | - | 8.5 | - | 1H NMR | This work | |
| H2O-EtOH | ||||||
| 0.05 | 2.47 (4) | - | - | - | 1H NMR | This work |
| 0.075 | 2.40 (4) | - | - | - | 1H NMR | This work |
| 0.10 | 1.90 ± 0.10 (2) | 36.8 ± 0.2 | 10.8 ± 0.6 | 26.0 ± 0.7 | Isothermal titration calorimetry (pH 3.6) | [50] |
| 2.29 (4) | - | - | - | 1H NMR | This work | |
| 0.20 | 0.70 ± 0.10 (2) | 44.3 ± 0.6 | 3.9 ± 0.6 | 40.4 ± 0.8 | Isothermal titration calorimetry (pH 3.6) | [50] |
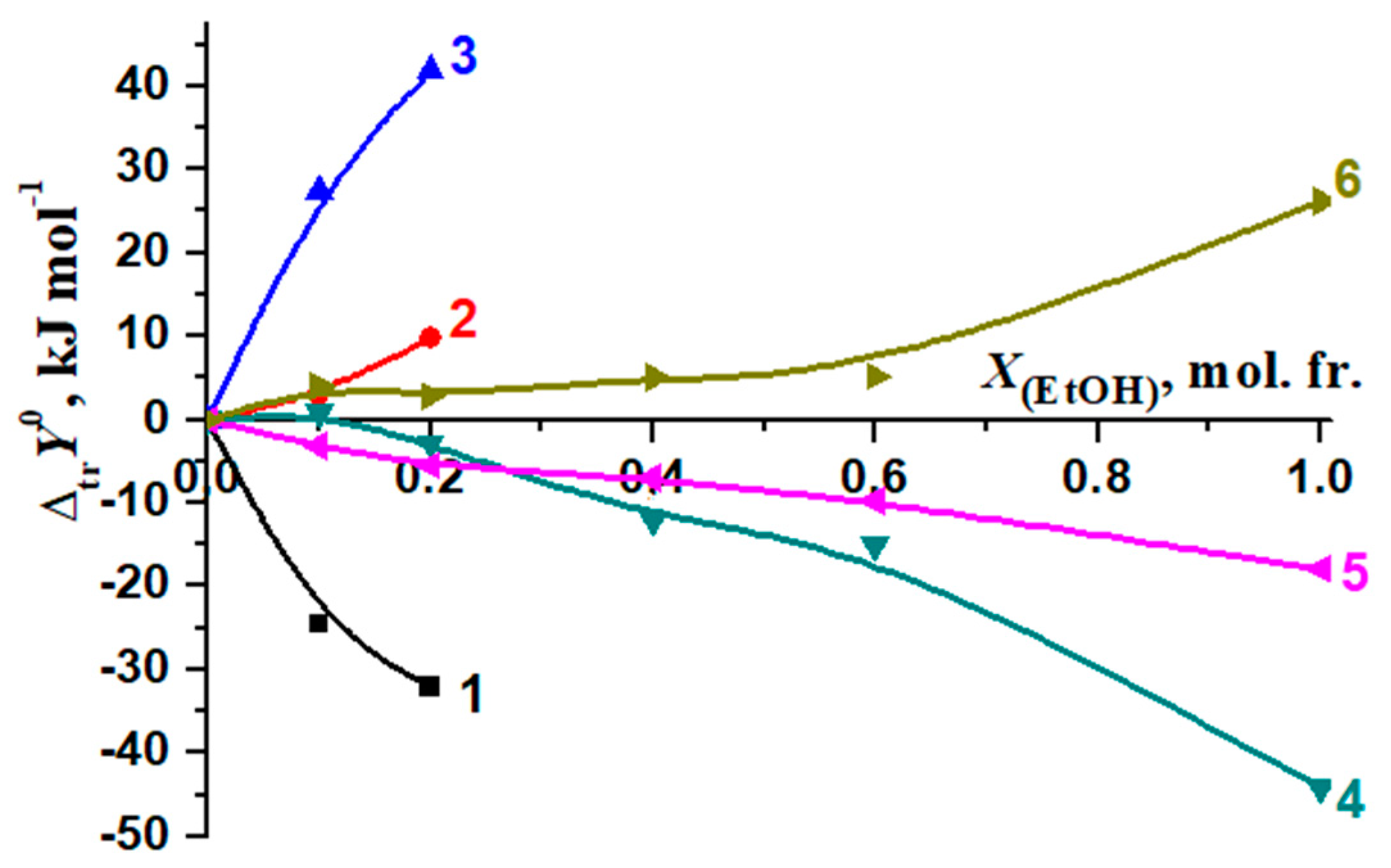
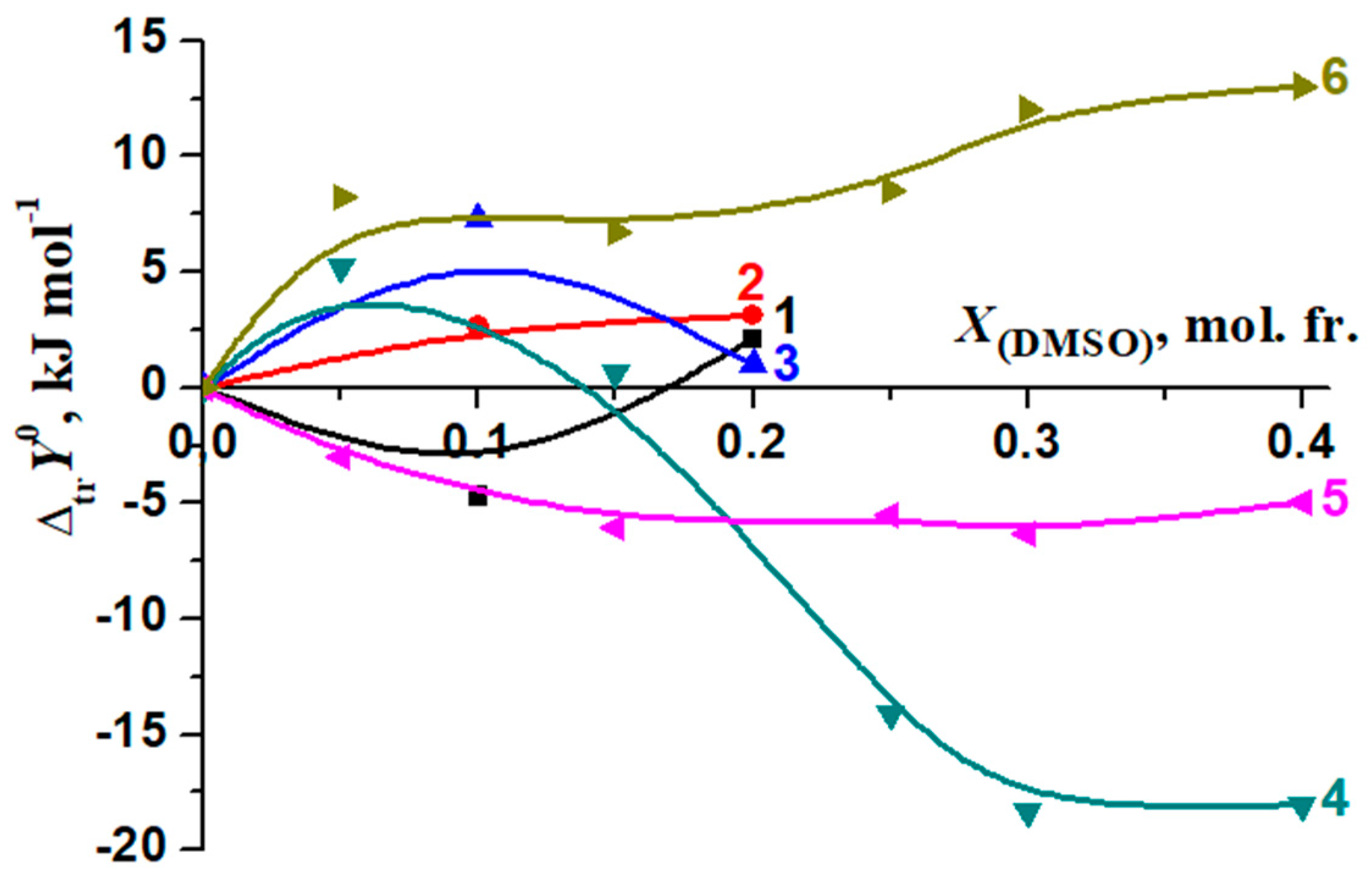
3.2. Solvation/Thermodynamic Approach
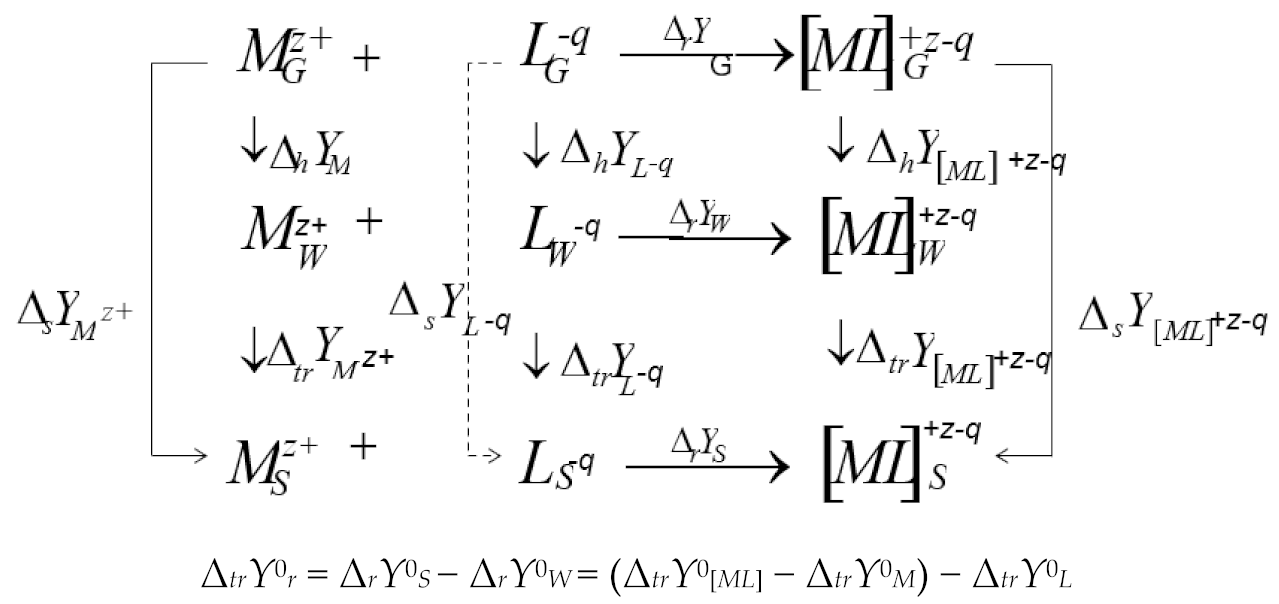
3.3. The Thermodynamic Characteristics of the Molecular Complexation and Solvation of the Reagents: A Solvation-Thermodynamic Analysis
4. Materials and Methods
4.1. Materials
- -
- Benzoic acid (C6H5COOH) (“chemically pure” grade), used without additional purification. The purity of reagents was declared by the manufacturer >99% by weight.
- -
- β-cyclodextrin (C42H70O35) from Sigma-Aldrich Saint (Louis, MO, USA) with a CD content of ≥99% was used without additional purification;
- -
- Dimethylsulfoxide (C2H6OS) was purified by distillation according to the method [75] before use. The DMSO content was 99.4 wt.%. The residual water content in the organic solvents used was taken into account when preparing the solutions.
- -
- Deuterated water and dimethylsulphoxide (atomic fraction of deuterium more than 99.9%) were purchased from Sigma-Aldrich USA. Ethyl alcohol (C2H5OH) (96% by vol.) was purified by distillation at the atmospheric pressure.
4.2. Methods
4.2.1. NMR Spectroscopy
4.2.2. Models and Computational Methods
4.2.3. Interfacial Distribution-Study of Reagents Solvation State
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Koifman, O.I.; Ganyushkina, V.V.; Malyasova, A.S. Scientific Schools of Ivanovo Chemtech: Through the Prism of History; Ivanovo State University of Chemical Technology: Ivanovo, Russia, 2020; p. 496. (In Russian) [Google Scholar]
- Shormanov, V.A.; Sharnin, V.A. In the monograph Achievements and problems of solvation theory. In Structural and ThermoDynamic Aspects; Kutepov, A.M., Ed.; Nauka: Moscow, Russia, 1998; pp. 173–207. (In Russian) [Google Scholar]
- Sharnin, V.A.; Usacheva, T.R.; Kuzmina, I.A.; Gamow, G.A.; Alexandriiskii, V.V. Complexation in Non-Aqueous Media: A Solvation Approach to Describing the Role of a Solvent; Sharnina, V.A., Ed.; URSS: Moscow, Russia, 2019; p. 304. (In Russian) [Google Scholar]
- Sharnin, V.A.; Aleksandriysky, V.V.; Dushina, S.V.; Gamov, G.A. Stability of H-complexes of nicotinamide nitrogen heteroatom with water and ethanol in mixed solvents by 13C NMR probing. Magn. Reson. Chem. 2013, 51, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Sharnin, V.A.; Dushina, S.V.; Zevakin, M.A.; Gushchina, A.S.; Grazhdan, K.V. Potentiometric and calorimetric study on stability of nicotinamid complexes of silver(I) and copper(II) in aqueous ethanol and dimethylsulfoxide. Inorg. Chim. Acta 2009, 362, 437–442. [Google Scholar] [CrossRef]
- Sharnin, V.A.; Kuzmina, I.A. Regularities of changes in thermodynamic characteristics of reactions of complexation and solvation of reagents in the formation of metal coronates in binary mixtures of non-aqueous solvents. J. Gen. Chem. 2013, 83, 375–378. [Google Scholar] [CrossRef]
- Kuzmina, I.A.; Volkova, M.A.; Kuzmina, K.I.; Usacheva, T.R.; Sharnin, V.A.; Arena, G. Effect of reactant solvation on the stability of complexes of silver(I) with 18-crown-6 in ethanol-dimethyl sulfoxide mixtures. J. Mol. Liq. 2019, 276, 78–82. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Thi, L.P.; Terekhova, I.V.; Kumeev, R.S.; Sharnin, V.A. Thermodynamics of molecular complexation of glycyl–glycyl–glycine with cryptand [2.2.2] in water–dimethylsulfoxide solvent at 298.15 K. J. Therm. Anal. Calorim. 2016, 126, 307–314. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Thi, L.P.; Terekhova, I.V.; Kumeev, R.S.; Sharnin, V.A. Application of isothermal titration calorimetry for evaluation of water-acetone and water-dimethylsulfoxide solvents influence on the molecular complex formation between 18-crown-6 and triglycine at 298.15K. J. Therm. Anal. Calorim. 2015, 121, 975–981. [Google Scholar] [CrossRef]
- Salvatierra, D.; Jaime, C.; Virgili, A.; Sanchez-Ferrando, F. Determination of the inclusion geometry for the β-cyclodextrin/benzoic acid complex by NMR and molecular modeling. J. Org. Chem. 1996, 61, 9578–9581. [Google Scholar] [CrossRef]
- Aree, T.; Chaichit, N. Crystal structure of β-cyclodextrin-benzoic acid inclusion complex. Carbohydr. Res. 2003, 338, 439–446. [Google Scholar] [CrossRef]
- Terekhova, I.V.; Koźbiał, M.; Kumeev, R.S.; Gierycz, P. Complex formation of native and hydroxypropylated cyclodextrins with benzoic acid in aqueous solution: Volumetric and 1H NMR study. Chem. Phys. Lett. 2011, 514, 341–346. [Google Scholar] [CrossRef]
- Pessine, F.B.T.; Calderini, A.; Alexandrino, G.L. Review: Cyclodextrin Inclusion Complexes Probed by NMR Techniques; Kim, D.-H., Ed.; InTech: London, UK, 2012; pp. 237–264. Available online: http://www.intechopen.com/books/magneticresonance-spectroscopy/review-study-of-inclusion-complexes-with-cyclodextrins-by-mrs (accessed on 10 June 2021).
- Ulatowski, F.; Dąbrowa, K.; Bałakier, T.; Jurczak, J. Recognizing the Limited Applicability of Job Plots in Studying Host−Guest Interactions in Supramolecular Chemistry. J. Org. Chem. 2016, 81, 1746–1756. [Google Scholar] [CrossRef]
- Hibbert, D.B.; Thordarson, P. The death of the Job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun. 2016, 52, 12792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renny, J.S.; Tomasevich, L.L.; Tallmadge, E.H.; David, B. Collum Method of Continuous Variations: Applications of Job Plots to the Study of Molecular Associations in Organometallic Chemistry. Angew. Chem. Int. Ed. 2013, 52, 11998–12013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chankvetadze, B. Capillary Electrophoresis in Chiral Analysis; John Wiley: Chichester, UK, 1997; pp. 71–133, 141–228. [Google Scholar]
- Petrović, I.; Milovanović, B.; Etinski, M.; Petković, M. Theoretical scrutinization of nine benzoic acid dimers: Stability and energy decomposition analysis. Int. J. Quantum Chem. 2019, 119, e25918. [Google Scholar] [CrossRef]
- Meshkov, A.N.; Gamov, G.A. KEV: A free software for calculating the equilibrium composition and determining the equilibrium constants using UV-Vis and potentiometric data. Talanta 2019, 198, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Bondarev, N.V. Exploratory Analysis in Thermodynamics of Equilibria. Classification and Prediction of Benzoic Acid Strength in Aqueous-Organic Solvents. Russ. J. Gen. Chem. 2016, 86, 1221–1228. [Google Scholar] [CrossRef]
- Terekhova, I.V. Comparative thermodynamic study on complex formation of native and hydroxypropylated cyclodextrins with benzoic acid. Thermochim. Acta 2011, 526, 118–121. [Google Scholar] [CrossRef]
- Terekhova, I.V.; Kumeev, R.S.; Alper, G.A. Inclusion complex formation of α- and β-cyclodextrins with aminobenzoic acids in aqueous solution studied by 1H NMR. J. Incl. Phenom. Macrocycl. Chem. 2007, 59, 301–306. [Google Scholar] [CrossRef]
- Terekhova, I.V. Volumetric and calorimetric study on complex formation of cyclodextrins with aminobenzoic acids. Mendeleev Commun. 2009, 19, 110–112. [Google Scholar] [CrossRef]
- Terekhova, I.V.; Obukhova, N.A. Study on inclusion complex formation of m-aminobenzoic acid with native and substituted β-cyclodextrins. J. Solut. Chem. 2007, 6, 1167–1176. [Google Scholar] [CrossRef]
- Zielenkiewicz, W.; Terekhova, I.V.; Wszelaka-Rylik, M.; Kumeev, R.S. Thermodynamics of inclusion complex formation of hydroxypropylated α- and β-cyclodextrins with aminobenzoic acids in water. J. Therm. Anal. Calorim. 2010, 101, 15–23. [Google Scholar] [CrossRef]
- Terekhova, I.V. Inclusion Complex Formation of Cyclodextrins with Aminobenzoic Acids in Aqueous Solution. Ch. 8 in Macrocyclic Chemistry: New Research Developments. Изд-вo; Nova Science Publishers Inc.: New York, NY, USA, 2010; pp. 263–286. [Google Scholar]
- Sankaranarayanan, R.K.; Siva, S.; Prabhu, A.A.M.; Rajendiran, N. A study on the inclusion complexation of 3,4,5-trihydroxybenzoic acid with β-cyclodextrin at different Ph. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 461–470. [Google Scholar] [CrossRef]
- Rajamohan, R.; Nayaki, S.K.; Swaminathan, M. Study on Host–Guest Complexation of 5-Amino-2-Mercaptobenzimidazole with β-Cyclodextrin. J. Solut. Chem. 2011, 40, 803–817. [Google Scholar] [CrossRef]
- Hendrickson, K.; Easton, C.J.; Lincoln, S.F. Cyclodextrin and termethylated cyclodextrin complexation of aromatic carboxylic acids and their conjugate bases in aqueous solution: The effect of size, hydrophobicity and charge. Aust. J. Chem. 1995, 48, 1125–1132. [Google Scholar] [CrossRef]
- Simova, S.; Schneider, H.-J. NMR analyses of cyclodextrin complexes with substituted benzoic acids and benzoate anions. J. Chem. Soc. Perkin Trans. 2000, 8, 1717–1722. [Google Scholar] [CrossRef]
- Rotich, M.K.; Brown, M.E.; Glass, B.D. Thermal studies on mixture of benzoic and salicylic acids with cyclodextrins. J. Therm. Anal. Calorim. 2003, 73, 671–686. [Google Scholar] [CrossRef]
- Roik, N.V.; Belyakova, L.A. Study of complex formation in the «β-cyclodextrin-p aminobenzoic acid» system. Surface 2009, 15, 69–79. [Google Scholar]
- Stalin, T.; Rajendiran, N. Intramolecular charge transfer effects on 3-aminobenzoic acid. Chem. Phys. 2006, 322, 311–322. [Google Scholar] [CrossRef]
- Stalin, T.; Shanthi, B.; Rani, P.V.; Rajendiran, N. Solvatochromism, prototropism and complexation of para-aminobenzoic acid. J. Incl. Phenom. Macrocycl. Chem. 2006, 55, 21–29. [Google Scholar] [CrossRef]
- Rajendiran, N.; Thulasidhasan, J.; Jenita, M.J. Guest-Host Inclusion Complex Formation of 2-, 3-, and 4-Aminobenzoic Acids with Native and Modified Cyclodextrins, International Letters of Chemistry. Phys. Astron. 2016, 69, 10–21. [Google Scholar]
- Lewis, E.A.; Hansen, L.D. Thermodynamics of binding of guest molecules to α- and β-cyclodextrins. J. Chem. Soc. Perkin Trans. 1973, 2, 2081–2085. [Google Scholar] [CrossRef]
- Mendez, S.G.; Espinar, F.J.O.; Alvarez, A.L.; Longhi, M.R.; Quevedo, M.A.; Zoppi, A. Ternary complexation of benzoic acid with β-cyclodextrin and aminoacids. Experimental and theoretical studies. J. Incl. Phenom. Macrocycl. Chem. 2016, 85, 33–48. [Google Scholar] [CrossRef]
- Huang, M.-J.; Watts, J.D.; Bodor, N. Theoretical studies of inclusion complexes of α- and β-cyclodextrin with benzoic acid and phenol. Int. J. Quantum Chem. 1997, 65, 1135–1152. [Google Scholar] [CrossRef]
- Liu, L.; Song, K.-S.; Li, X.-S.; Guo, Q.-X. Charge-transfer interaction: A driving force for cyclodextrin inclusion complexation. J. Incl. Phenom. Macrocycl. Chem. 2001, 40, 35–39. [Google Scholar] [CrossRef]
- Lu, C.-S.; Hu, C.-J.; Yu, Y.; Meng, Q.-J. The inclusion compounds of β-cyclodextrin with 4-substituted benzoic acid and benzaldehyde drugs studied by proton nuclear magnetic resonance spectroscopy. Chem. Pharm. Bull. 2000, 48, 56–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roik, N.V.; Belyakova, L.A.; Spectroscopy, I.R. X-Ray Diffraction and thermal analysis studies of solid β-cyclodextrin-para-aminobenzoic acid inclusion complex. Phys. Chem. Solid State 2011, 12, 168–173. [Google Scholar]
- Zhang, Y.; Yu, S.; Bao, F. Crystal structure of cyclomaltoheptaose (β-cyclodextrin) complexes with p-aminobenzoic acid and o-aminobenzoic acid. Carbohydr. Res. 2008, 343, 2504–2508. [Google Scholar] [CrossRef]
- Kacsó, I.; Borodi, G.; Farcas, S.I.; Hernanz, A.; Bratu, I. Host–guest system of Vitamin B10 in β-cyclodextrin: Characterization of the interaction in solution and in solid state. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 175–182. [Google Scholar] [CrossRef]
- Lehner, S.J.; Muller, B.W.; Seydel, J.K. Interaction between p-hydroxybenzoic acid esters and hydroxypropyl-β-cyclodextrin and their antimicrobial effect against Candida albicans. Int. J. Pharm. 1993, 93, 201–208. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Kabirov, D.N.; Alister, D.A.; Zavalishin, M.N.; Gamov, G.A.; Thi, L.P.; Xuan, M.V.; Thuan, Z.N. Thermodynamics of complexation of benzoic acid with β- and γ-cyclodextrins in a water-dimethyl sulfoxide solvent. Russ. Chem. Bull. 2020, 69, 1692–1696. (In Russian) [Google Scholar] [CrossRef]
- Borodin, V.A. Processing of the results of calorimetric measurements on a digital computer in the study of complex equilibria in solutions. J. Inorg. Chem. 1982, 27, 2169–2172. (In Russian) [Google Scholar]
- Usacheva, T.R.; Ledenkov, S.F.; Sharnin, V.A. Complex formation of Ag+ with polyether 18-crown-6. Calorimetric and potentiometric methods. J. Therm. Anal. Calorim. 2002, 70, 379–385. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Thi, L.P.; Sharnin, V.A. Calorimetric study of the molecular complex formation of glycyl–glycyl–glycine with 18-crown-6 in aqueous organic solvents. Russ. J. Gen. Chem. 2017, 87, 591–599. [Google Scholar] [CrossRef]
- Harata, K. Induced circular dichroism of cycloamylose complexes with meta- and para-distributed benzenes. Bioorg. Chem. 1981, 10, 255–265. [Google Scholar] [CrossRef]
- Usacheva, T.; Pham, L.T.; Nguyen, D.T.; Kabirov, D.; Alister, D.; Minh, X.; Le, T.; Sharnin, V.; Giancola, C. Host–guest inclusion complex of β-cyclodextrin and benzoic acid in water–ethanol solvents: Spectroscopic and thermodynamic characterization of complex formation. J. Therm. Anal. Calorim. 2020, 142, 2015–2024. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Thi, L.P.; Sharnin, V.A.; Baranskiy, A. Molecular complexation of some amino acids and triglycine with 18-crown-6 ester in H2O–EtOH solvents at 298.15 K. Russ. J. Inorg. Chem. 2013, 58, 1264–1268. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Thi, L.P.; Sharnin, V.A. Influence of the composition of the H2O–EtOH solvent on the formation reactions of molecular complexes of triglycine and glycine with the 18-crown-6 ether at T = 298.15 K. J. Phys. Chem. 2014, 88, 602–606. (In Russian) [Google Scholar]
- Matteoli, E.; Lepori, L.; Usacheva, T.R.; Sharnin, V.A. Thermodynamics of complex formation in mixed solvents K and ΔH for the formation reaction of [Gly18C6] at 298.15 K. J. Therm. Anal. Calorim. 2009, 97, 811–817. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Thi, L.P.; Sharnin, V.A. Thermodynamics of formation for the 18-crown-6-triglycine molecular complex in water-dimethylsulfoxide solvents. Russ. J. Phys. Chem. A 2014, 88, 908–912. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Kuzmina, I.A.; Sharnin, V.A.; Chernov, I.V.; Matteoli, E. The influence of the composition of an aqueous-acetone solvent on the thermodynamic characteristics of complex formation of 18-crown-6 ether with glycine. Russ. J. Phys. Chem. A 2011, 85, 948–951. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Chernov, I.V.; Sharnin, V.A.; Matteoli, E.; Terekhova, I.V.; Kumeev, R.S. The influence of water–ethanol mixture on the thermodynamics of complex formation between 18-crown-6 ether and L–phenylalanine. Chem. Phys. Lett. 2012, 543, 153–158. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Chernov, I.V.; Sharnin, V.A.; Voronina, S.I.; Matteoli, E. Molecular complex formation between L-phenylalanine and 18-crown-6 in H2O–DMSO solvents studied by titration calorimetry at T = 298.15 K. J. Therm. Anal. Calorim. 2013, 112, 399–405. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Sharnin, V.A.; Matteoli, E. Influence of solvation on the thermodynamics of the formation of molecular complexes of 18-crown-6 ester with glycine in water–dimethyl sulfoxide solutions. J. Phys. Chem. 2011, 85, 2038–2042. (In Russian) [Google Scholar]
- Usacheva, T.R. Molecular complexation of glycine by 18-crown-6 in aqueous-organic solvent: A solvation-thermodynamic study. In Glycine: Biosynthesis, Physiological Functions and Commercial Uses; Usacheva, T.R., Sharnin, V.A., Matteoli, E., Vojak, W., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2013; pp. 1–33. [Google Scholar]
- Krestov, G.A. Ionic Solvation; Ellis Horwood: London, UK, 1994; p. 264. [Google Scholar]
- Turyan, Y.I.; Shtipelman, R.Y. Polarographic study of thiocyanate complexes of lead in aqueous, water-methanol, methanol, water-ethanol solvents. J. Inorg. Chem. 1959, 4, 804–812. (In Russian) [Google Scholar]
- Turyan, Y.I.; Zhantalay, V.P. Polarographic study of cadmium chloride complexes in aqueous, water-methanol, methanol, water-ethanol solvents. J. Inorg. Chem. 1960, 5, 1748–1753. (In Russian) [Google Scholar]
- Shulman, V.M.; Larionov, S.V.; Kramareva, T.V.; Arykova, E.I.; Yudina, V.V. On the stability of complex compounds of zinc, cadmium, cobolt and nickel with thiourea in aqueous acetone solutions. J. Inorg. Chem. 1966, 11, 1076–1080. (In Russian) [Google Scholar]
- Nazarova, L.V.; Budu, G.I. Stability of nickel and cobalt pyridinates in water-alcohol and water-acetone solutions. J. Inorg. Chem. 1970, 14, 3072–3075. (In Russian) [Google Scholar]
- Mayer, U. Ionic equilibria in donor solvents. Pure Anal. Chem. 1979, 41, 1697–1712. [Google Scholar] [CrossRef]
- Beck, M.T.; Nagypal, I. Chemistry of Complex Equilibria; Akademiai Kiado: Budapest, Hungary, 1989; p. 402. [Google Scholar]
- Krestov, G.A.; Berezin, B.D. Basic Concepts of Modern Chemistry, 2nd ed.; Chemistry Publishing House: Leningrad, Russia, 1986; p. 104. (In Russian) [Google Scholar]
- Quist, A.S.; Marshall, W.L. Ionization equilibria in ammonia-water solutions to 700.deg. and to 4000 bars of pressure. J. Phys. Chem. 1968, 72, 3122–3128. [Google Scholar] [CrossRef]
- Belevantsev, V.I.; Fedorov, V.A. On the change in the equilibrium constants of complexation depending on the composition of the aqueous-organic solvent. Coord. Chem. 1977, 3, 638–642. (In Russian) [Google Scholar]
- Belevantsev, V.I.; Peschevitsky, B.I. Study of Complex Equilibria in Solutions; Science: Novosibirsk, Russia, 1978; p. 256. (In Russian) [Google Scholar]
- Hu, X.G.; Lin, R.S.; Zong, H.X. Enthalpies and entropies of dissolution and dissociation of benzoic acid in EtOH-H2O and i-PrOH-H2O mixtures. J. Acta Phys.-Chim. Sin. 1999, 15, 838–844. [Google Scholar]
- Chatjigakis, A.K.; Donze, C.; Coleman, A.W.; Cardot, P. Solubility Behavior of β-Cyciodextrin in Water-Cosolvent Mixtures. Anal. Chem. 1992, 64, 1632–1634. [Google Scholar] [CrossRef]
- Belica, S.; Sadowska, M.; Stepniak, A.; Graca, A.; Palecz, B. Enthalpy of solution of α- and β-cyclodextrin in water and in some organic solvents. J. Chem. Thermodyn. 2014, 69, 112–117. [Google Scholar] [CrossRef]
- Kuzmina, I.A.; Volkova, M.A.; Marov, A.S.; Thi, L.P.; Usacheva, T.R. Thermodynamics of the solvation of β-cyclodextrin in water–dimethylsulfoxide solvents. Russ. J. Phys. Chem. A 2020, 94, 2034–2037. [Google Scholar] [CrossRef]
- Vasiliev, V.P. Thermodynamic Properties of Electrolyte Solutions; Higher School: Moscow, Russia, 1982; p. 262. (In Russian) [Google Scholar]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A Robust and Accurate Tight-Binding Quantum Chemical Method for Structures, Vibrational Frequencies, and Noncovalent Interactions of Large Molecular Systems Parametrized for All spd-Block Elements (Z = 1–86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [Green Version]
- Pracht, P.; Caldeweyher, E.S.; Grimme, S. A Robust Non-Self-Consistent Tight-Binding Quantum Chemistry Method for large Molecules. ChemRxiv 2019. [Google Scholar] [CrossRef]
- Pracht, P.; Bohle, F.; Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef]
- Spicher, S.; Grimme, S. Robust Atomistic Modeling of Materials. Organomet. Biochem. Syst. 2020, 59, 15665–15673. [Google Scholar] [CrossRef]
- Spicher, S.; Bursch, M.; Grimme, S. Efficient Calculation of Small Molecule Binding in Metal-Organic Frameworks and Porous Organic Cages. J. Phys. Chem. C 2020, 124, 27529–27541. [Google Scholar] [CrossRef]
- Thi, L.P.; Usacheva, T.R.; Kuz’mina, I.A.; Nguyen, N.; Thai, H.; Volkova, M.; Le, H.; Nguyen, T.; Volynkin, V.; Tran, D. Effect of Cyclodextrin types and reagents solvation on the stability of complexes between β-Cyclodextrins and rutin in water-ethanol solvents. J. Mol. Liq. 2020, 318, 114308. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Kuz’mina, I.A.; Sharnin, V.A.; Sidorenko, N.S.; Voronina, S. The influence of solvation on the formation of Ag+ complexes with 18-crown-6 ether in water-dimethyl sulfoxide solvents. Russ. J. Phys. Chem. A 2011, 85, 952–954. [Google Scholar] [CrossRef]

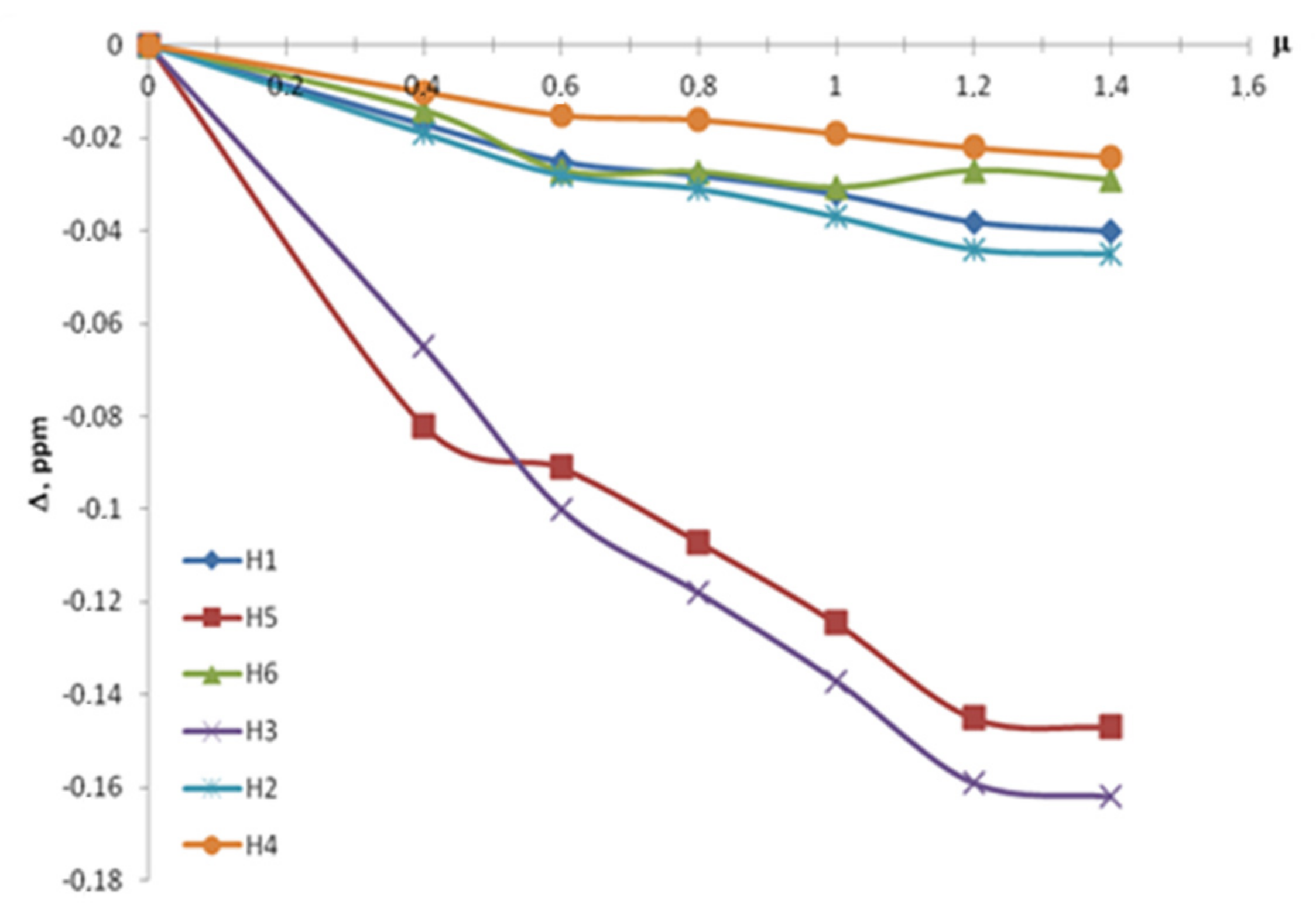
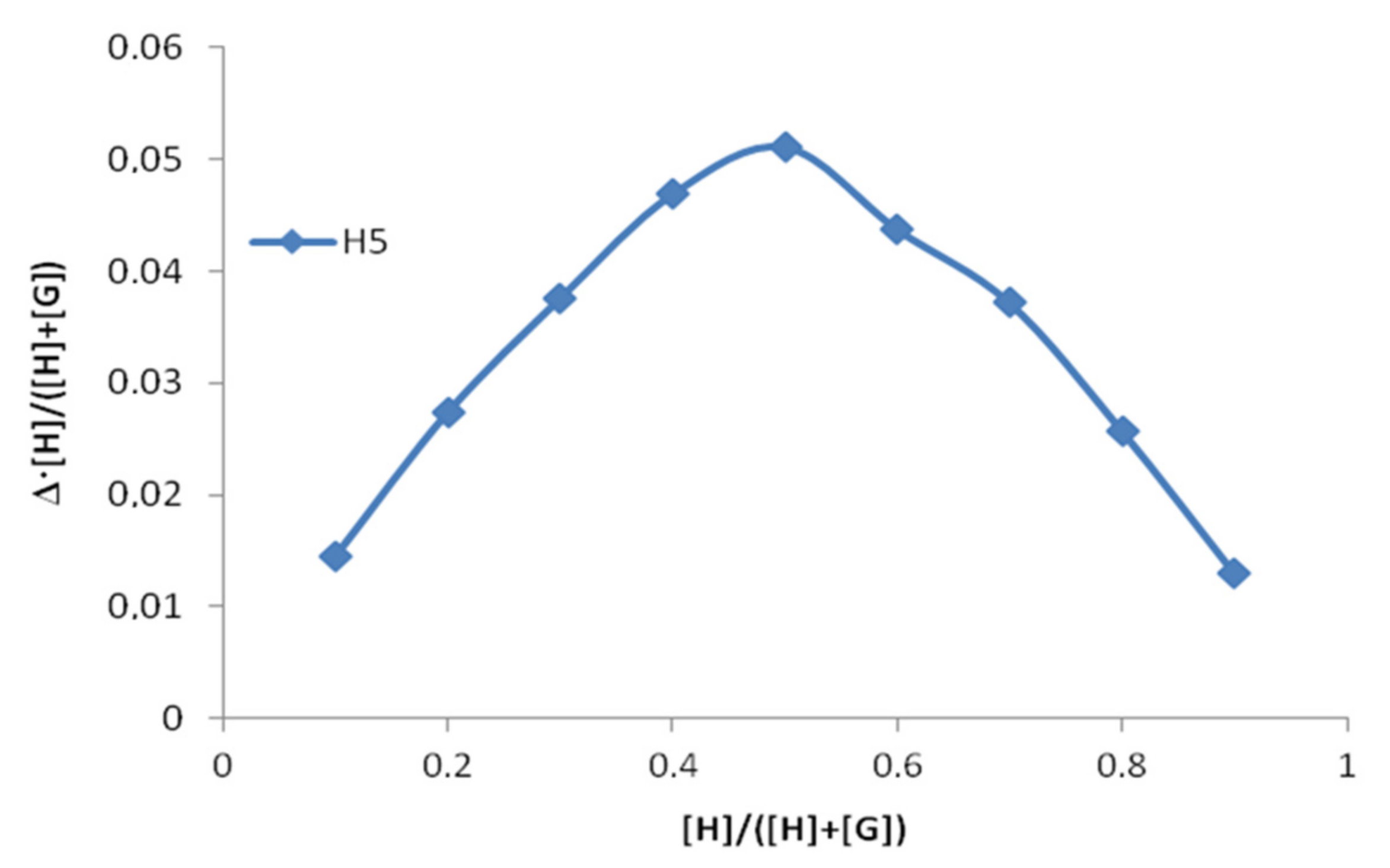
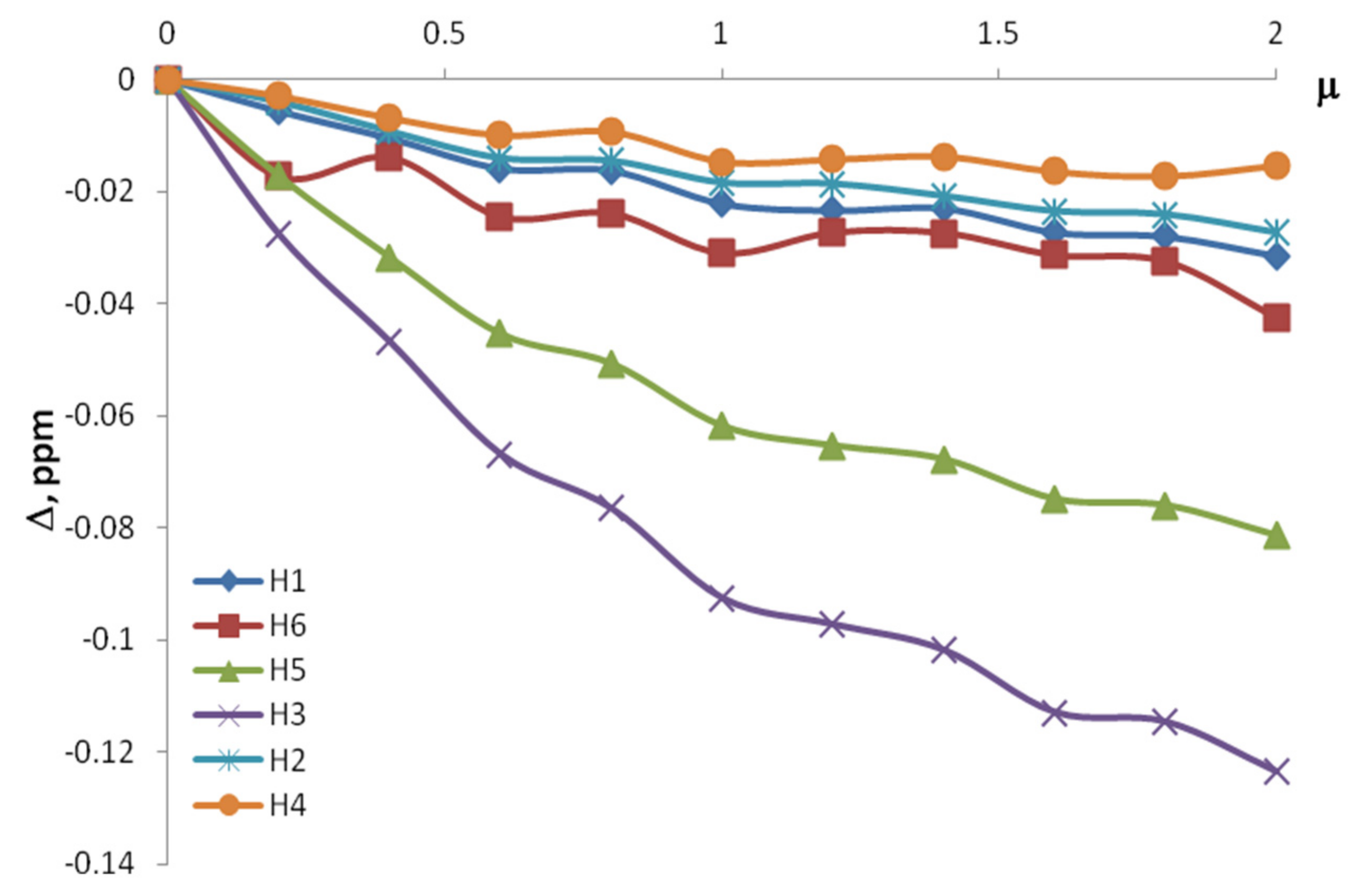
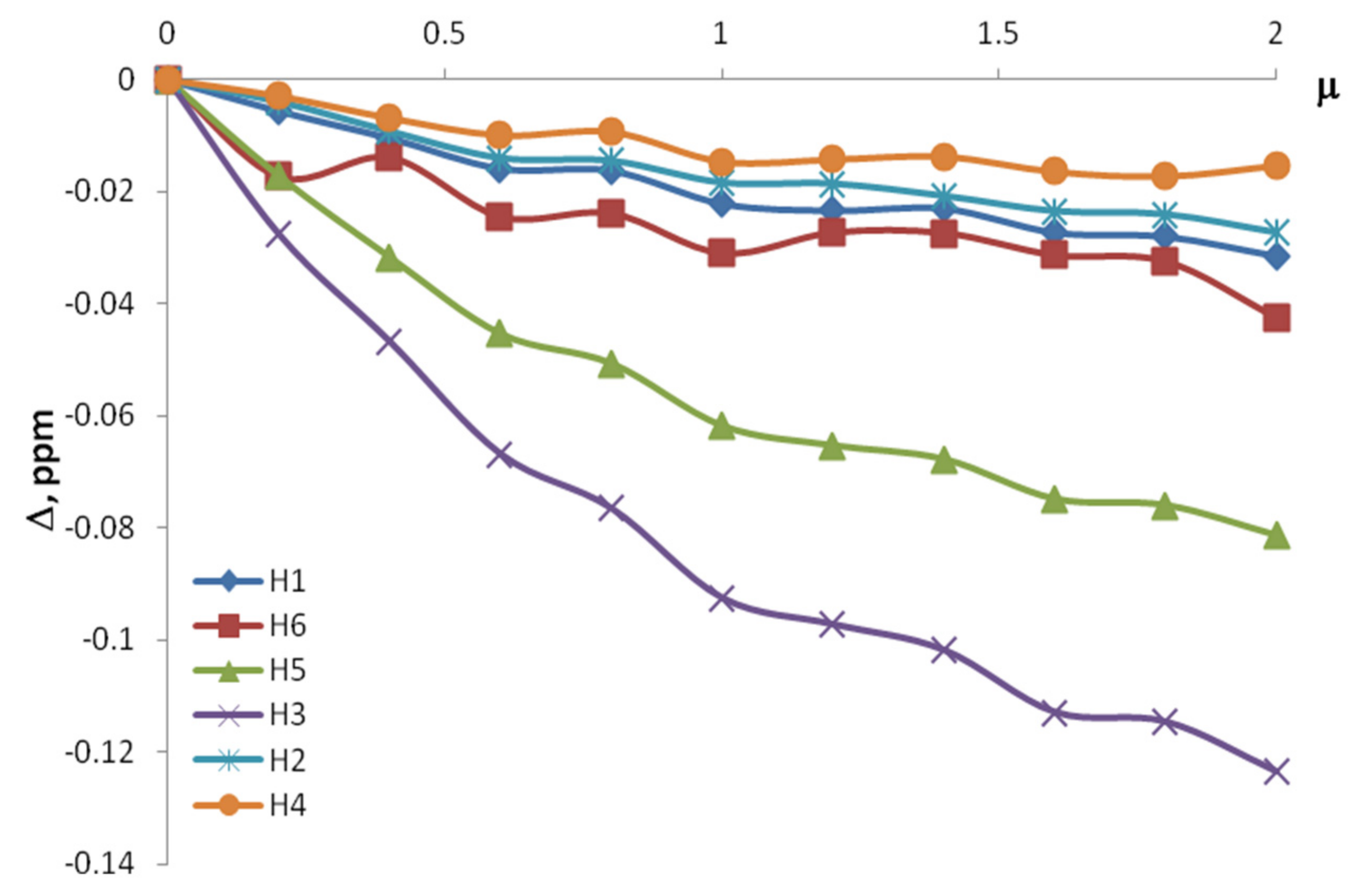






| CBA/Cβ-CD | Δ, ppm | |||||
|---|---|---|---|---|---|---|
| H1 | H5 | H6 | H3 | H2 | H4 | |
| 0.4 | −0.017 | −0.082 | −0.014 | −0.065 | −0.019 | −0.01 |
| 0.6 | −0.025 | −0.091 | −0.027 | −0.1 | −0.028 | −0.015 |
| 0.8 | −0.028 | −0.1071 | −0.0273 | −0.118 | −0.031 | −0.016 |
| 1 | −0.032 | −0.1245 | −0.0307 | −0.137 | −0.037 | −0.019 |
| 1.2 | −0.038 | −0.145 | −0.027 | −0.159 | −0.044 | −0.022 |
| 1.4 | −0.04 | −0.147 | −0.029 | −0.162 | −0.045 | −0.024 |
| X(DMSO-d6), mol. fr. | K, M−1 | Δ(H5), ppm | Δ(H3), ppm |
|---|---|---|---|
| 0 | 388.5 | −0.1986 | −0.2147 |
| 0.05 | 368.6 | −0.0900 | −0.1409 |
| 0.075 | 157.5 | −0.1013 | −0.1537 |
| 0.10 | 30.8 | −0.1068 | −0.1963 |
| X(DMSO-d6), mol. fr. | K | Δ(H5) | Δ(H3) |
| 0 | 388.5 | −0.1986 | −0.2147 |
| 0.05 | 368.6 | −0.0900 | −0.1409 |
| 0.075 | 157.5 | −0.1013 | −0.1537 |
| 0.10 | 30.8 | −0.1068 | −0.1963 |
| CBA/Cβ-CD | Δ(BA), ppm | Δ(β-CD), ppm | ||||||
|---|---|---|---|---|---|---|---|---|
| 2,6 | 4 | 3,5 | H1 | 5H | H6 | H3 | H2 | |
| 0.2 | 0.0058 | 0.0604 | 0.0399 | −0.0113 | −0.0256 | −0.0162 | −0.0172 | −0.0081 |
| 0.4 | 0.0118 | 0.0699 | 0.0396 | −0.0145 | −0.0421 | −0.0232 | −0.0269 | −0.0089 |
| 0.6 | 0.0132 | 0.0591 | 0.0339 | −0.0233 | −0.0567 | −0.0329 | −0.0390 | −0.0165 |
| 0.8 | 0.0122 | 0.0589 | 0.0354 | −0.0246 | −0.0664 | −0.0332 | −0.0479 | −0.0138 |
| 1 | 0.0116 | 0.0514 | 0.0311 | −0.0272 | −0.0722 | −0.0325 | −0.0529 | −0.0154 |
| 1.2 | 0.0056 | 0.0469 | 0.0318 | −0.0267 | −0.0779 | −0.0386 | −0.0552 | −0.0091 |
| 1.4 | 0.0149 | 0.0460 | 0.0316 | −0.0236 | −0.0809 | −0.0394 | −0.0549 | −0.0081 |
| 1.6 | 0.0101 | 0.0466 | 0.0293 | −0.0258 | −0.0837 | −0.0419 | −0.0562 | −0.0120 |
| 1.8 | 0.0167 | 0.0426 | 0.0319 | −0.0308 | −0.0839 | −0.0358 | −0.0579 | −0.0145 |
| 2 | 0.0096 | 0.0337 | 0.0219 | −0.0304 | −0.0883 | −0.0381 | −0.0629 | −0.0141 |
| X(EtOH), mol. fr. | K, M−1 | Δ(H5), ppm | Δ(H3), ppm |
|---|---|---|---|
| 0 | 388.5 | −0.1986 | −0.2147 |
| 0.05 | 292.3 | −0.1341 | −0.1851 |
| 0.075 | 249.4 | −0.0750 | −0.1179 |
| 0.10 | 193.1 | −0.1201 | −0.1499 |
| X(EtOH), mol. fr. | K | Δ(H5), ppm | Δ(H3), ppm |
| 0 | 388.5 | −0.1986 | −0.2147 |
| 0.05 | 292.3 | −0.1341 | −0.1851 |
| 0.075 | 249.4 | −0.0750 | −0.1179 |
| 0.10 | 193.1 | −0.1201 | −0.1499 |
| Configuration | HF | PR | TF |
|---|---|---|---|
| in vacuum | |||
| Eint,kJ mol−1 | −59.09 | −60.20 | −102.86 |
| in water | |||
| Eint,kJ mol−1 | −44.41 | −38.27 | −53.29 |
| in methanol | |||
| Eint,kJ mol−1 | −32.61 | −25.44 | −33.30 |
| in DMSO | |||
| Eint,kJ mol−1 | −2.54 | −8.32 | −19.09 |
| Parameter | BA | BA in TF (in Vacuum) | BA in TF (in Water) | BA in TF (in Methanol) | BA in TF (in DMSO) |
|---|---|---|---|---|---|
| d(H1-O2), Å | 0.969 | 0.988 | 0.977 | 0.974 | 0.974 |
| BO(H1-O2) | 0.868 | 0.803 | 0.798 | 0.824 | 0.818 |
| d(O2-C4), Å | 1.344 | 1.331 | 1.326 | 1.330 | 1.334 |
| <H1O2C4C5, degree | 180.00 | 175.96 | 178.70 | 178.89 | 176.97 |
| <O3C4C5C6, degree | 179.88 | 161.72 | 174.20 | 179.99 | 177.97 |
| q(BA), e | 0.000 | −0.021 | 0.007 | 0.008 | 0.004 |
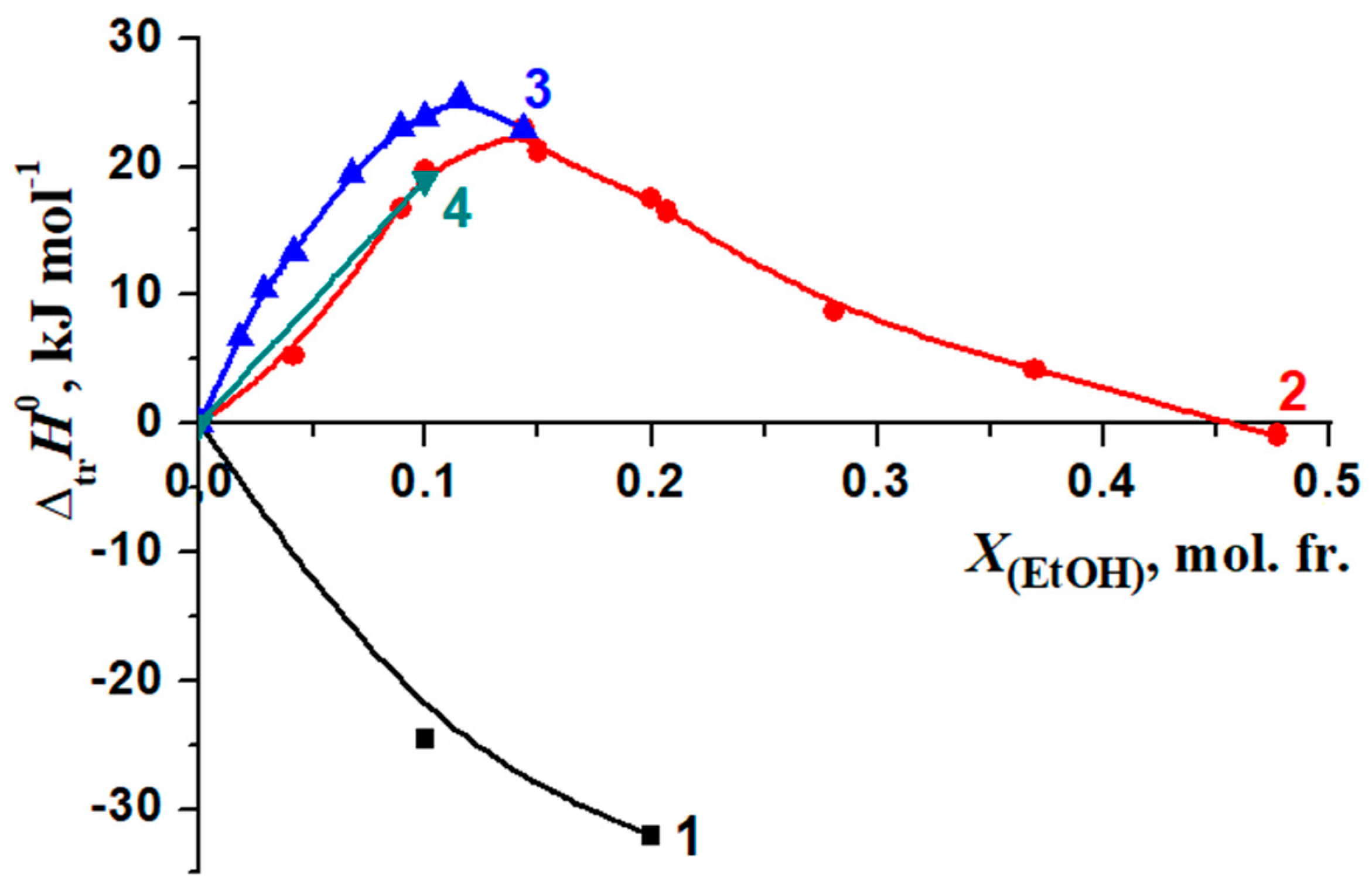
| XDMSO, mol. fr. | [BA]H2O-DMSO × 104, mol/L | [BA]Hex × 105, mol/L | K1 | K2 | ΔtrG0(BA), kJ/mol |
|---|---|---|---|---|---|
| 0.0 | 4.108 4.058 | 6.860 7.078 | 0.17 | - | 0 |
| 0.05 | 5.422 5.380 5.421 | 4.289 4.666 4.470 | - | 0.08 | −1.70 |
| 0.1 | 6.387 6.356 6.376 | 4.592 4.905 4.696 | - | 0.07 | −2.07 |
| 0.15 | 5.320 5.271 5.280 | 5.478 5.975 5.875 | 0.11 | −1.12 | |
| 0.2 | 5.371 5.415 5.426 | 4.731 4.287 4.176 | - | 0.08 | −1.91 |
| 0.3 | 5.410 5.316 5.529 | 5.452 6.393 4.262 | - | 0.10 | −1.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usacheva, T.R.; Volynkin, V.A.; Panyushkin, V.T.; Lindt, D.A.; Pham, T.L.; Nguyen, T.T.H.; Le, T.M.H.; Alister, D.A.; Kabirov, D.N.; Kuranova, N.N.; et al. Complexation of Cyclodextrins with Benzoic Acid in Water-Organic Solvents: A Solvation-Thermodynamic Approach. Molecules 2021, 26, 4408. https://doi.org/10.3390/molecules26154408
Usacheva TR, Volynkin VA, Panyushkin VT, Lindt DA, Pham TL, Nguyen TTH, Le TMH, Alister DA, Kabirov DN, Kuranova NN, et al. Complexation of Cyclodextrins with Benzoic Acid in Water-Organic Solvents: A Solvation-Thermodynamic Approach. Molecules. 2021; 26(15):4408. https://doi.org/10.3390/molecules26154408
Chicago/Turabian StyleUsacheva, Tatyana R., Vitaly A. Volynkin, Viktor T. Panyushkin, Dmitry A. Lindt, Thi Lan Pham, Thi Thu Ha Nguyen, Thi My Hanh Le, Diana A. Alister, Dzhovidon N. Kabirov, Natalya N. Kuranova, and et al. 2021. "Complexation of Cyclodextrins with Benzoic Acid in Water-Organic Solvents: A Solvation-Thermodynamic Approach" Molecules 26, no. 15: 4408. https://doi.org/10.3390/molecules26154408
APA StyleUsacheva, T. R., Volynkin, V. A., Panyushkin, V. T., Lindt, D. A., Pham, T. L., Nguyen, T. T. H., Le, T. M. H., Alister, D. A., Kabirov, D. N., Kuranova, N. N., Gamov, G. A., Kushnir, R. A., Biondi, M., Giancola, C., & Sharnin, V. A. (2021). Complexation of Cyclodextrins with Benzoic Acid in Water-Organic Solvents: A Solvation-Thermodynamic Approach. Molecules, 26(15), 4408. https://doi.org/10.3390/molecules26154408










