Reduction of Breakdown Pressure by Filter Cake Removal Using Thermochemical Fluids and Solvents: Experimental and Numerical Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drilling Fluid Formulation
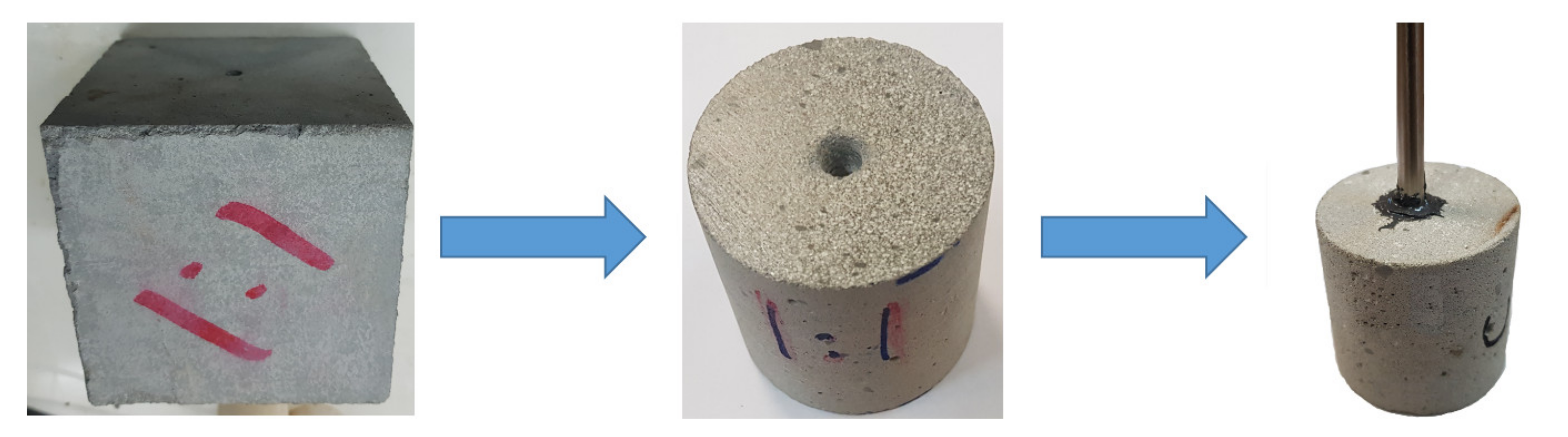
2.2. Core Preparation
2.3. Thermochemical Fluids
2.4. Chelating Agent
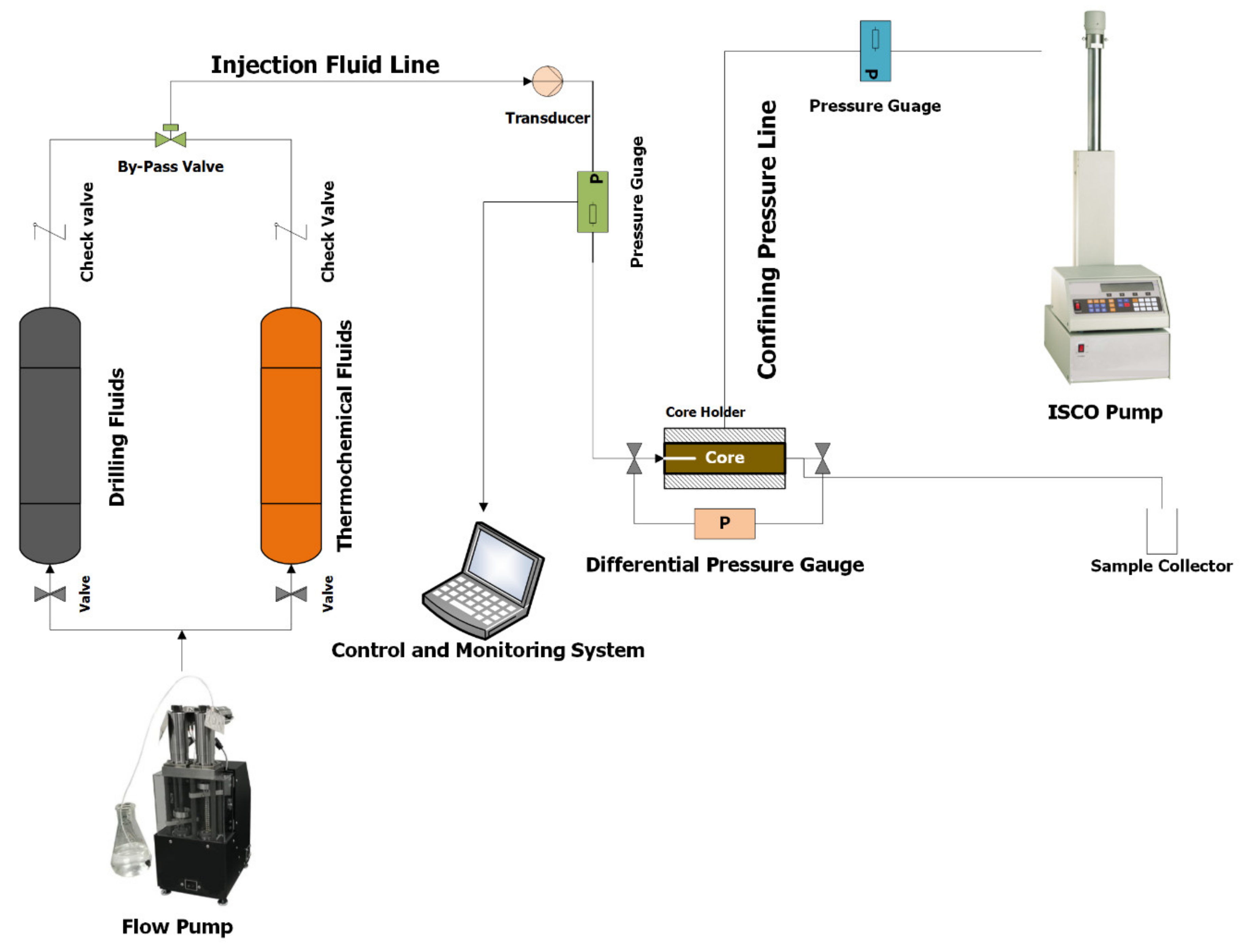
2.5. Experimental Setup
3. Results and Discussions
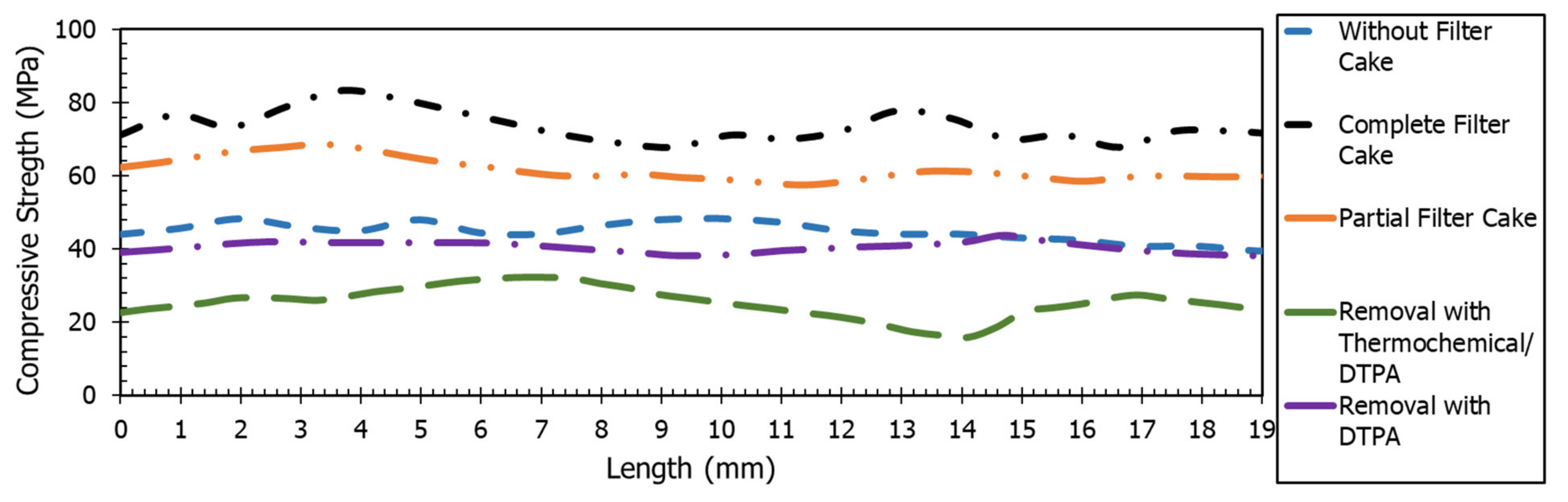
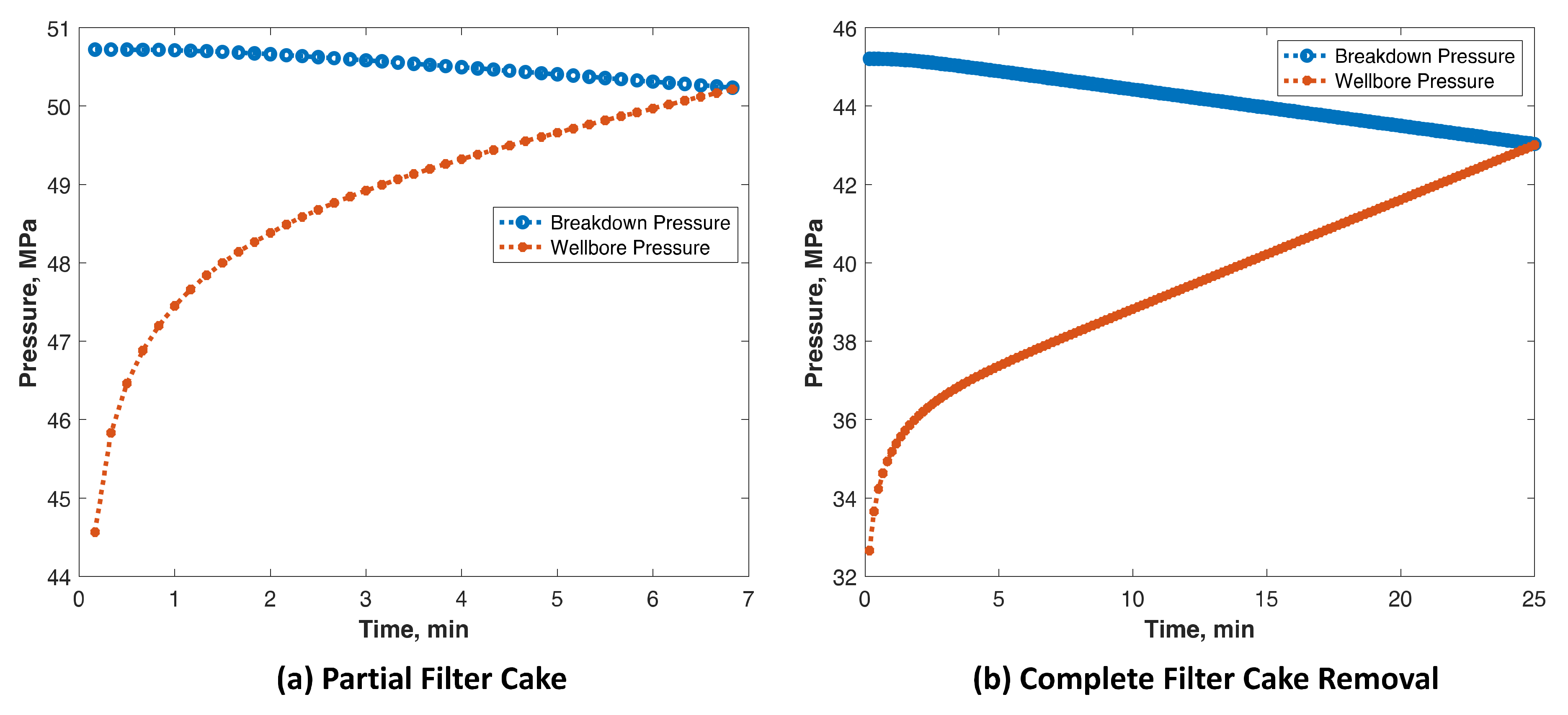
3.1. Breakdown Pressure Results
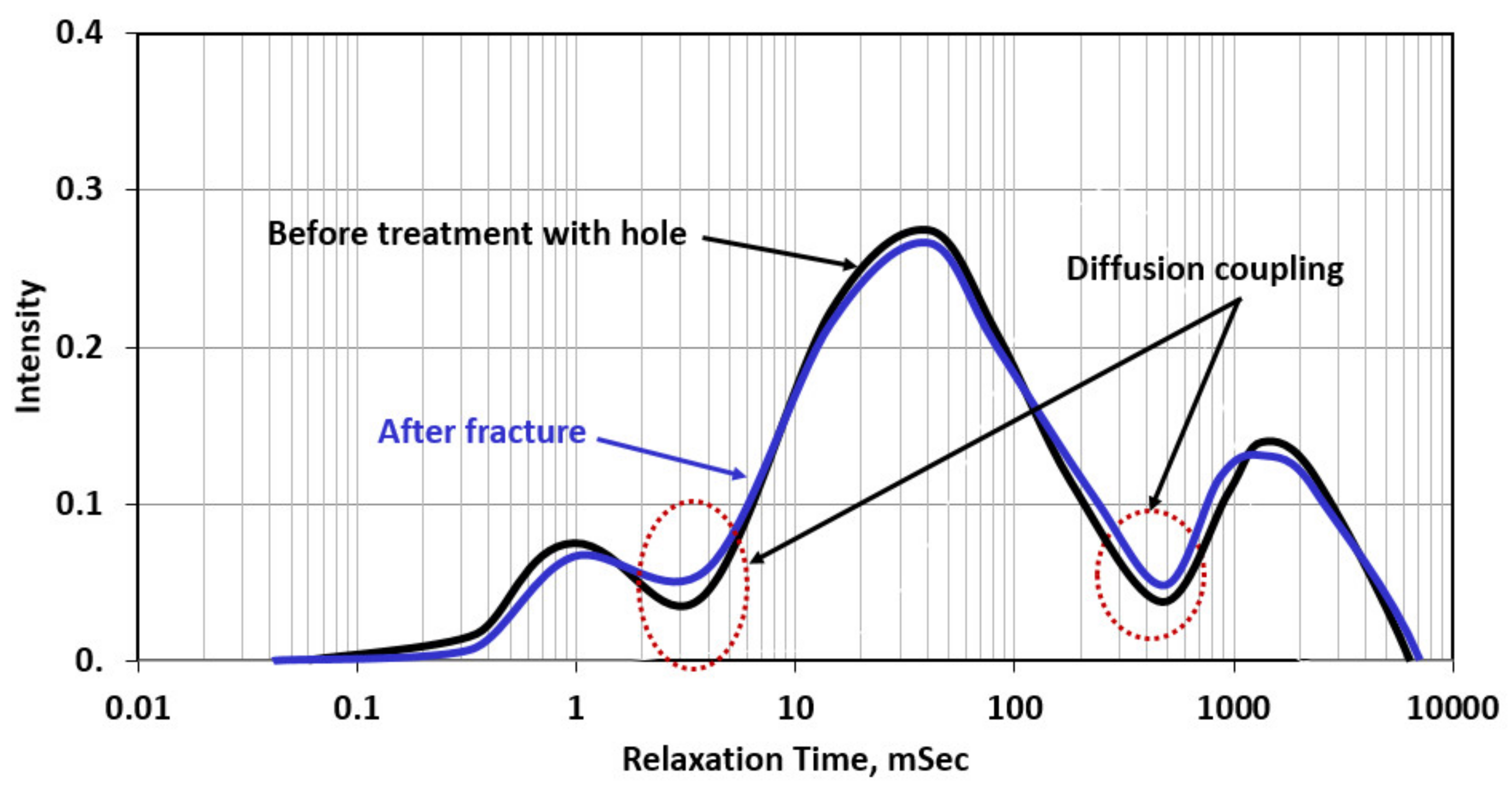
3.2. NMR Results
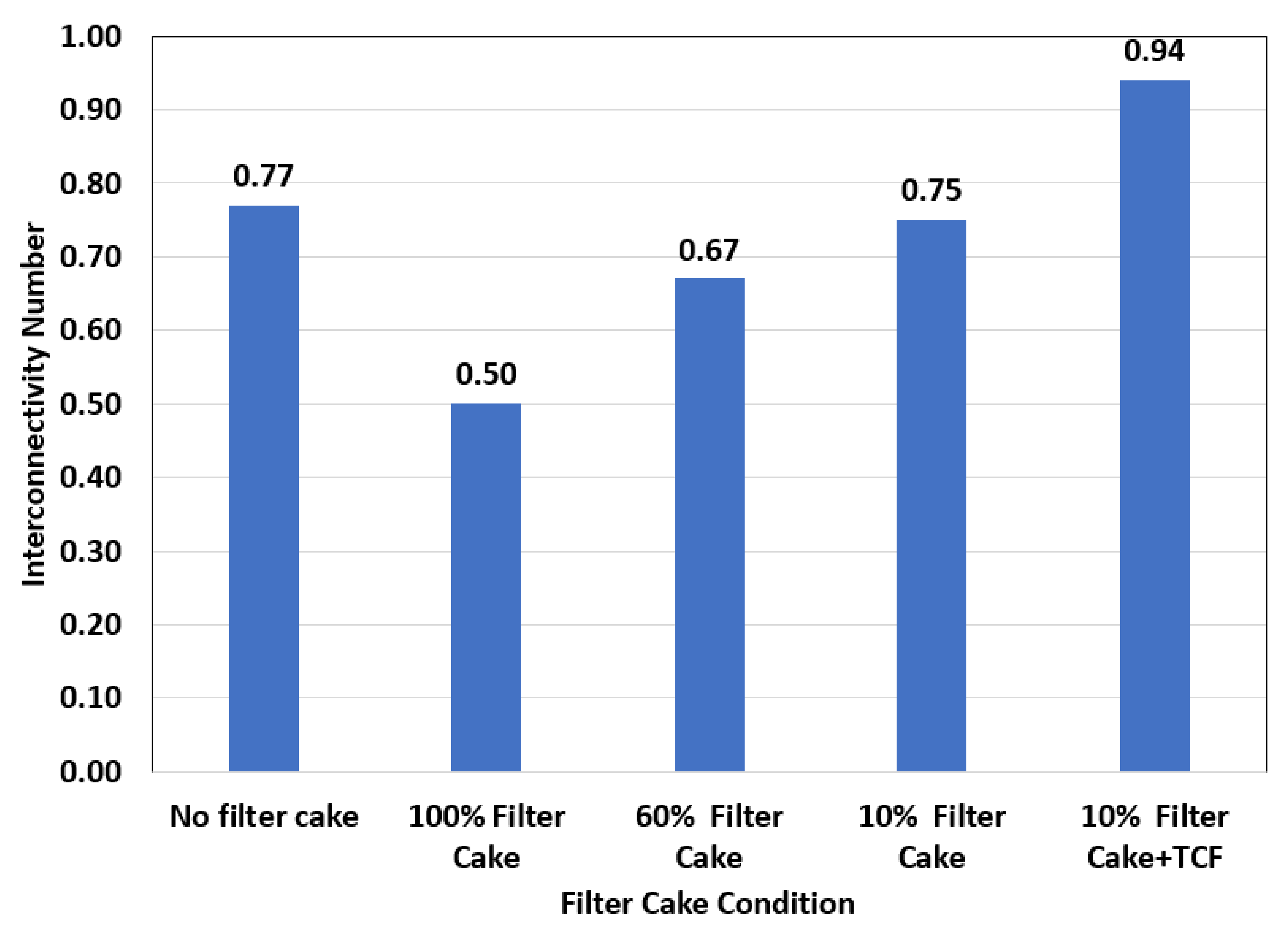
- Before treatment, in which the filter cake completely plugged the connection between the hole and the pore systems in the rock, ICN = 0/0.025 = zero;
- In the case of partial removal of the filter cake, ICN = 0.02/0.03 = 0.67;
- After creating the fracture (with water) through the partially removed filter cake, ICN = 0.045/0.65 = 0.70.
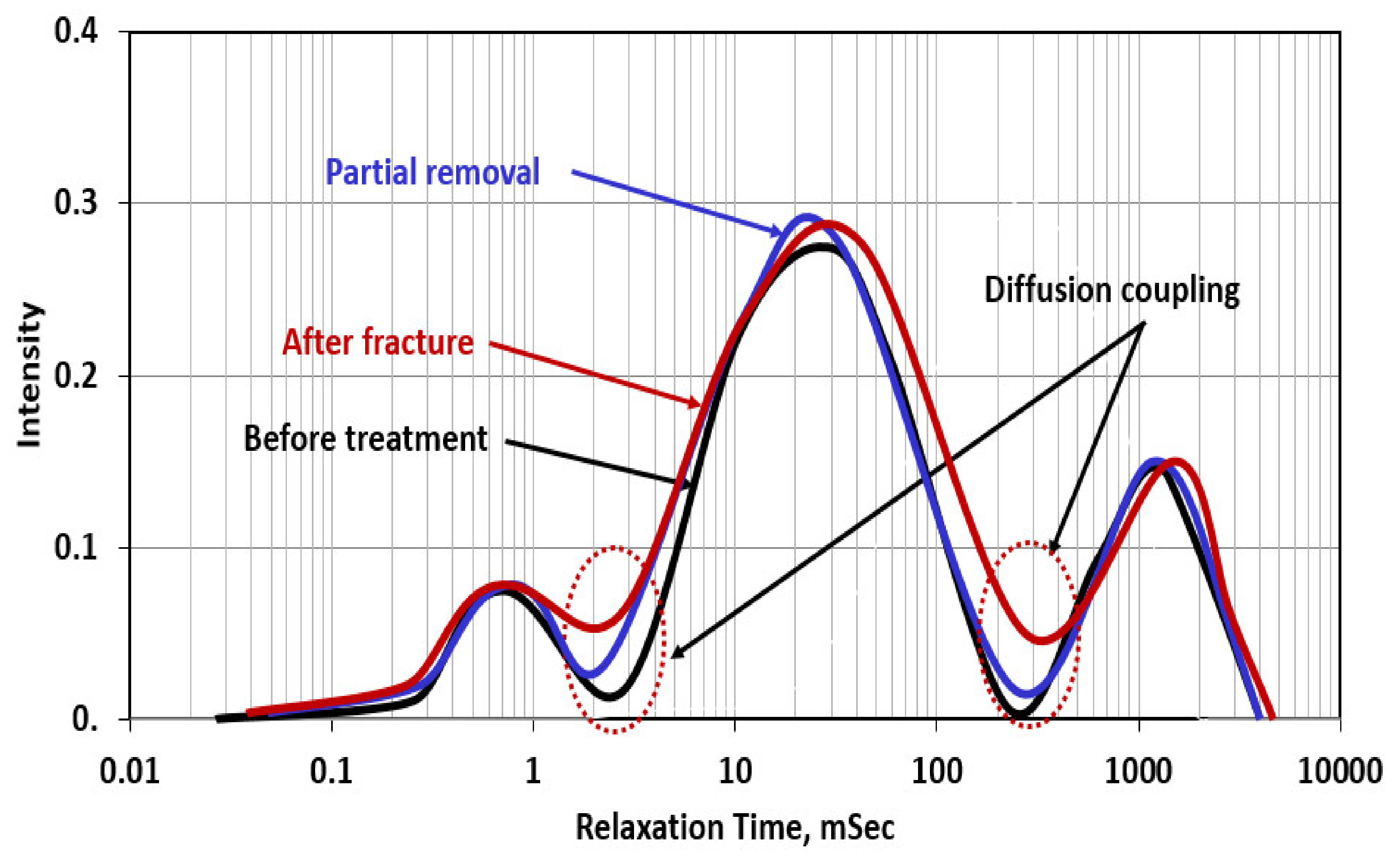
- Before treatment in which the filter cake completely plugged the connection and covered the wall of the hole, ICN = 0 /0.25 = zero;
- Complete removal of the filter cake: in this case, the remover fluid removed both the filter cake and the polymer; the used formulation consists of GLDA chelating agent that can break the polymer coat and DTPA + K2CO3 to remove the barite. In this case, the ICN = 0.3/0.4 = 0.75;
- After fracture (with water), the ICN = 0.45/0.60 = 0.75.

- Before treatment, in which the filter cake completely covered the hole, ICN = 0 / 0.02 = zero;
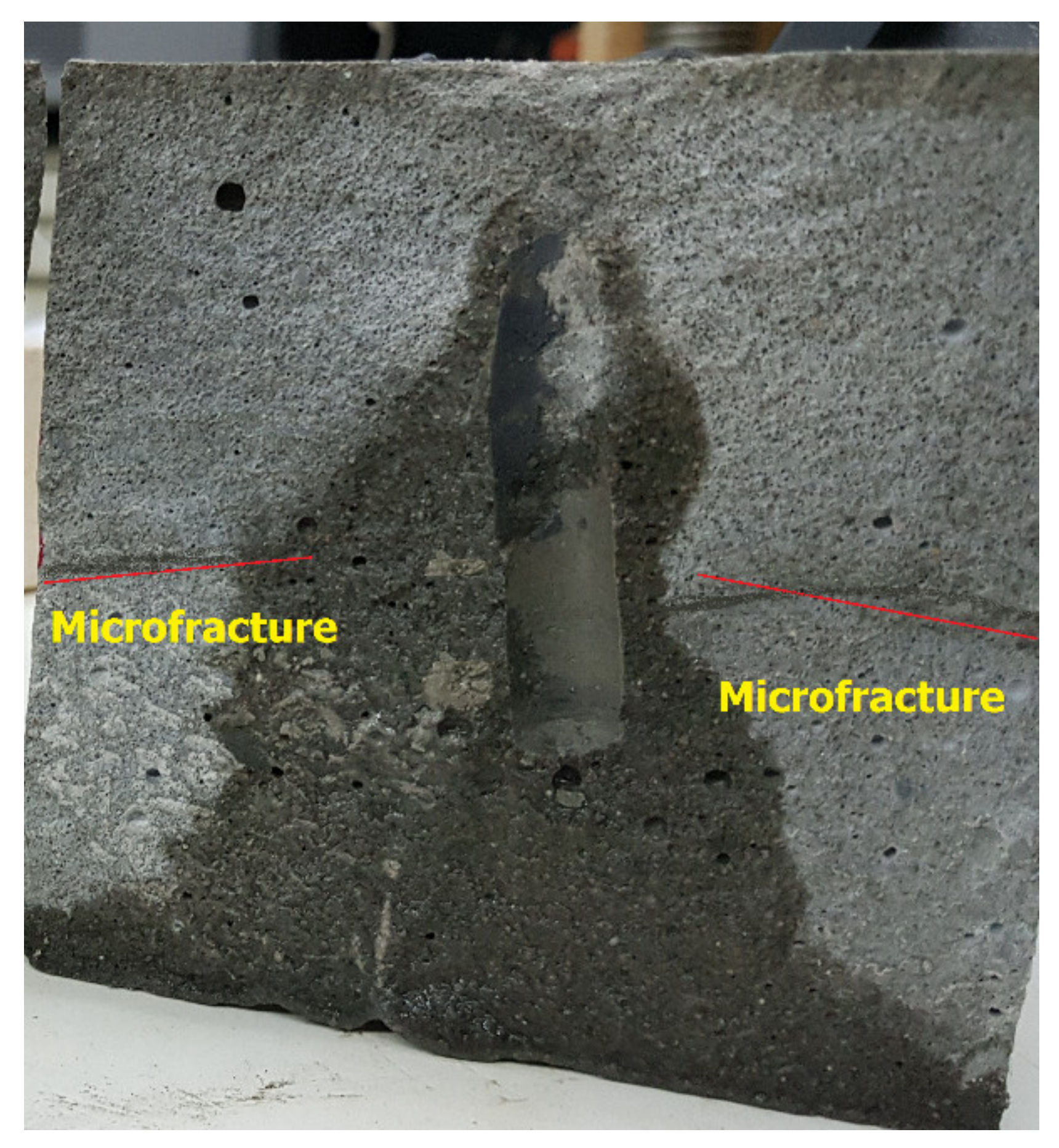
- Complete removal + fracture; in this case, the filter cake was completely removed, and TCF created a fracture and microfractures around the hole, ICN = 0.075 / 0.08 = 0.94. The interconnectivity number, in this case, was the highest among all cases presented in this study due to the creation of microfractures (as shown in Figure 8), in addition to the major fracture. In addition, the complete removal of the filter cake obtained from the thermochemical treatment enhanced the communication between the different pore systems in the rock.

4. Breakdown Pressure Modeling
Modeling Results
5. Conclusions
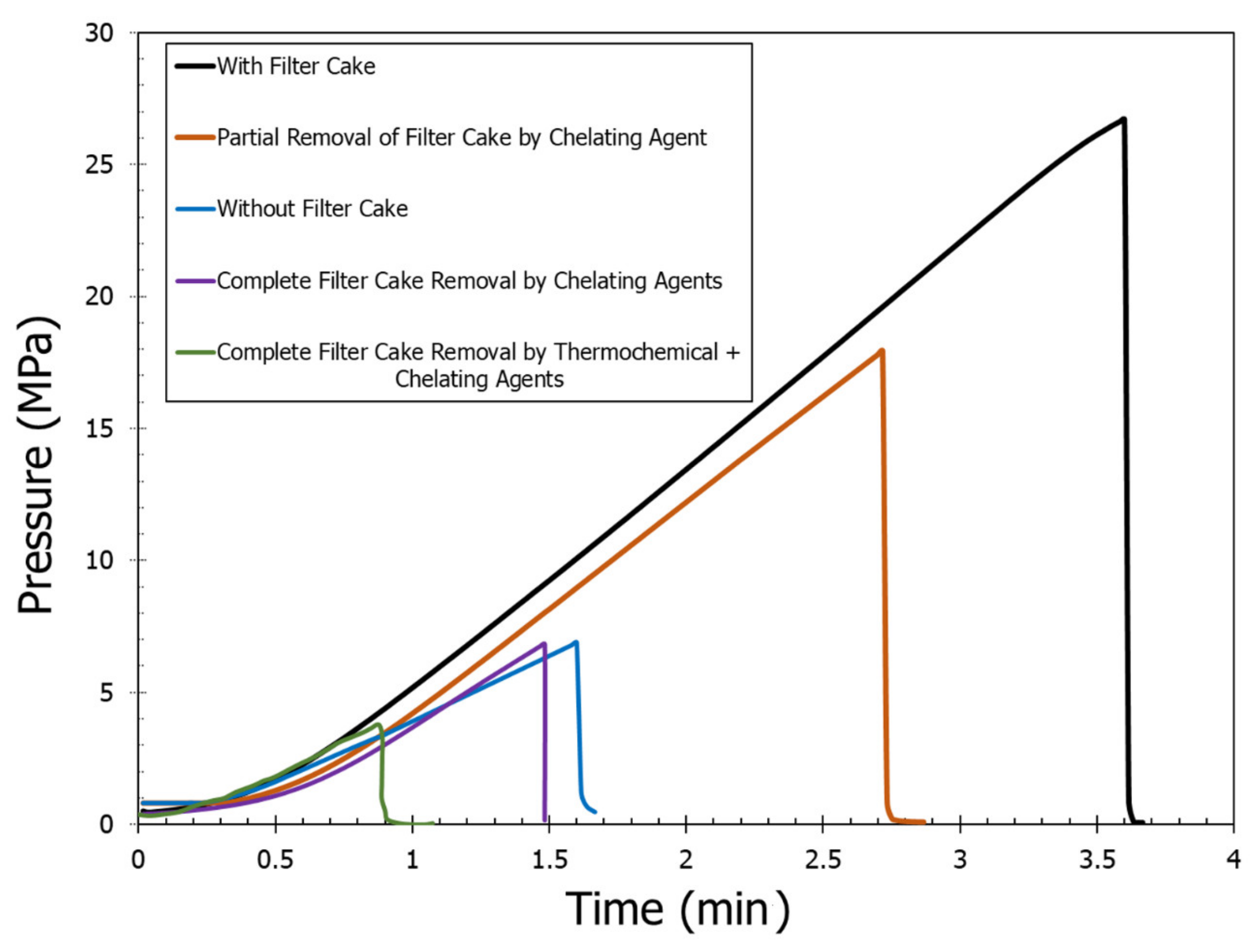
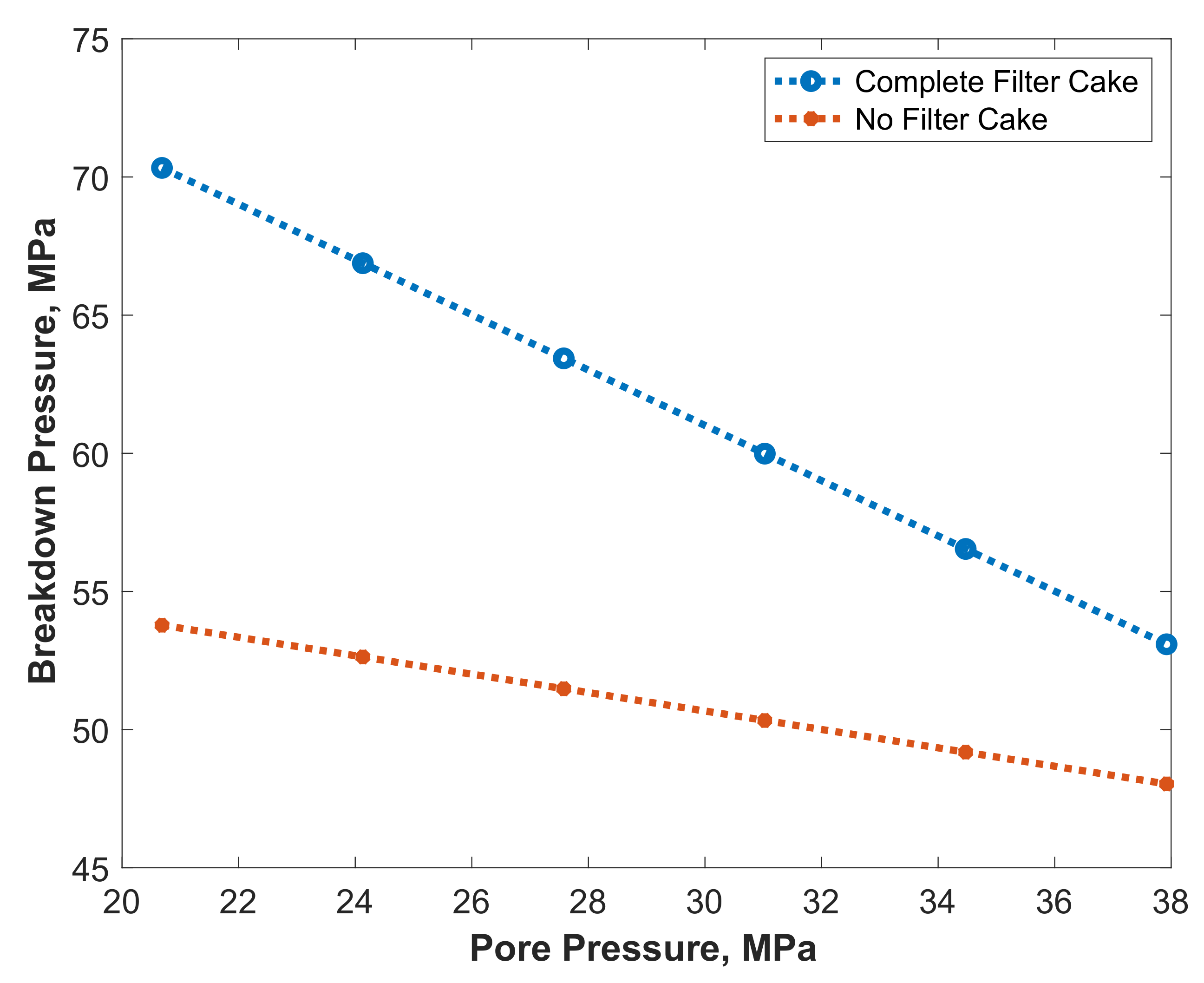
- Filter cake increased the breakdown pressure more than four times due to the wellbore strengthening effect. Breakdown pressure was increased from 6.9 MPa (case without filter cake) to 26.7 MPa (case with complete filter cake).
- Partial removal of the filter cake with 20 wt% DTPA resulted in 40% removal of the filter cake. This resulted in a 33% reduction in breakdown pressure from 26.7 MPa (case with complete filter cake) to 17.9 MPa (case with partial filter cake).
- The chelating agents such as 20 wt% DTPA, 10 wt% GLDA, and 9 wt% K2CO3 resulted in complete dissolution of the filter cake, and the required breakdown pressure of the rock was similar to the one without filter cake.
- Using thermochemical treatment combined with DTPA to remove the filter cake resulted in a further decrease of the breakdown pressure due to the complete removal of the filter cake in addition to the creation of microfractures.
- The compressive strength of the samples after fracturing experiments was aligned with the breakdown pressure.
- The diffusion coupling through NMR scans confirmed the higher interconnectivity between different pore systems after the combined thermochemical and chelating agent treatment.
- A strong relationship between the interconnectivity number (ICN) and fracture pressure was observed; lower breakdown pressure resulted in a very high interconnectivity number, and higher breakdown pressure yielded a very low interconnectivity number.
- A model was created to predict the breakdown pressure at a field scale. It shows that filter cake removal in terms of TCF treatment can reduce the breakdown pressure significantly.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yang, M.; Li, M.C.; Wu, Q.; Growcock, F.B.; Chen, Y. Experimental study of the impact of filter cakes on the evaluation of LCMs for improved lost circulation preventive treatments. J. Pet. Sci. Eng. 2020, 191, 107152. [Google Scholar] [CrossRef]
- Min, F.; Du, J.; Zhang, N.; Chen, X.; Lv, H.; Liu, L.; Yu, C. Experimental study on property change of slurry and filter cake of slurry shield under seawater intrusion. Tunn. Undergr. Space Technol. 2019, 88, 290–299. [Google Scholar] [CrossRef]
- Siddig, O.; Mahmoud, A.A.; Elkatatny, S. A review of different approaches for water-based drilling fluid filter cake removal. J. Pet. Sci. Eng. 2020, 192, 107346. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Li, X.; Lin, C. Experimental study on the supercritical CO2 fracturing of shale considering anisotropic effects. J. Pet. Sci. Eng. 2019, 173, 932–940. [Google Scholar] [CrossRef]
- Detournay, E.; Carbonell, R. Fracture-Mechanics Analysis of the Breakdown Process in Minifracture or Leakoff Test. SPE Prod. Facil. 1997, 12, 195–199. [Google Scholar] [CrossRef]
- Warpinski, N.R.; Wolhart, S.L.; Wright, C.A. Analysis and prediction of microseismicity induced by hydraulic fracturing. SPE J. 2004, 9, 24–33. [Google Scholar] [CrossRef]
- Patel, S.M.; Sondergeld, C.H.; Rai, C.S. Laboratory studies of hydraulic fracturing by cyclic injection. Int. J. Rock Mech. Min. Sci. 2017, 95, 8–15. [Google Scholar] [CrossRef]
- Song, X.; Guo, Y.; Zhang, J.; Sun, N.; Shen, G.; Chang, X.; Yu, W.; Tang, Z.; Chen, W.; Wei, W.; et al. Fracturing with Carbon Dioxide: From Microscopic Mechanism to Reservoir Application. Joule 2019, 3, 1913–1926. [Google Scholar] [CrossRef]
- Al-muntasheri, G.A.; Advanced, E.; Aramco, S. Nanoparticle-Enhanced Hydraulic-Fracturing Fluids: A Review. SPE Prod. Oper. 2016, 32, 186–195. [Google Scholar] [CrossRef]
- Warpinski, N.R.; Mayerhofer, M.J.; Vincent, M.C.; Cipolla, C.L.; Lolon, E.R. Stimulating unconventional reservoirs: Maximizing network growth while optimizing fracture conductivity. J. Can. Pet. Technol. 2009, 48, 39–51. [Google Scholar] [CrossRef]
- Rogala, A.; Ksiezniak, K.; Krzysiek, J.; Hupka, J. Carbon dioxide sequestration during shale gas recovery. Physicochem. Probl. Miner. Process. 2014. [Google Scholar] [CrossRef]
- Chu, Z.; Dreiss, C.A.; Feng, Y. Smart wormlike micelles. Chem. Soc. Rev. 2013, 42, 7174–7203. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, T.H. Integrative Understanding of Shale Gas Reservoirs; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-29295-3. [Google Scholar]
- Gomaa, A.M.; Qu, Q.; Nelson, S.; Maharidge, R. New insights into shale fracturing treatment design. In Proceedings of the Society of Petroleum Engineers—European Unconventional Resources Conference and Exhibition 2014: Unlocking European Potential, Vienna, Austria, 25–27 February 2014; Society of Petroleum Engineers: Richardson, TX, USA, 2014; Volume 2, pp. 658–672. [Google Scholar]
- Wu, X.; Xia, J.; Guan, B.; Yan, X.; Zou, L.; Liu, P.; Yang, L.; Hong, S.; Hu, S. Water availability assessment of shale gas production in the Weiyuan play, China. Sustainability 2019, 11, 940. [Google Scholar] [CrossRef] [Green Version]
- Nasr-El-Din, H.A.; Al-Mutairi, S.H.; Al-Hajji, H.H.; Lynn, J.D. Evaluation of a New Barite Dissolver: Lab Studies. In Proceedings of the SPE International Formation Damage Control Symposium Proceedings, Lafayette, LA, USA, 18–20 February 2004; Society of Petroleum Engineers: Richardson, TX, USA, 2004. [Google Scholar]
- Nasr-El-Din, H.A.; Al-Otaibi, M.B.; Al-Qahtani, A.A.; Al-Fuwaires, O.A. Filter-cake cleanup in MRC wells using enzyme/surfactant solutions. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 15–17 February 2006; pp. 667–680. [Google Scholar]
- Xiao, J.; Nasr-Ei-din, H.A.; Al-Bagoury, M. Evaluation of micronized llmenite as a weighting material in oil-based drilling fluids for HPHT applications. In Proceedings of the SPE—European Formation Damage Conference Proceedings, EFDC, Society of Petroleum Engineers, Richardson, TX, USA, 5–7 June 2013; Volume 2, pp. 1017–1030. [Google Scholar]
- Jiao, D.; Sharma, M.M. Formation Damage Due to Static and Dynamic Filtration of Water-Based Muds. In Proceedings of the SPE Formation Damage Control Symposium, Lafayette, LA, USA, 26–27 February 1992; Society of Petroleum Engineers: Richardson, TX, USA, 1992. [Google Scholar]
- Hajiabadi, S.H.; Aghaei, H.; Kalateh-Aghamohammadi, M.; Sanati, A.; Kazemi-Beydokhti, A.; Esmaeilzadeh, F. A comprehensive empirical, analytical and tomographic investigation on rheology and formation damage behavior of a novel nano-modified invert emulsion drilling fluid. J. Pet. Sci. Eng. 2019, 181, 106257. [Google Scholar] [CrossRef]
- Ba Geri, B.S.; Al-Mutairi, S.H.; Mahmoud, M.A. Different techniques for characterizing the filter cake. In Proceedings of the the SPE Unconventional Gas Conference and Exhibition, Muscat, Oman, 28–30 January 2013; pp. 104–116. [Google Scholar]
- Bageri, B.S.; Mahmoud, M.; Abdulraheem, A.; Al-Mutairi, S.H.; Elkatatny, S.M.; Shawabkeh, R.A. Single stage filter cake removal of barite weighted water based drilling fluid. J. Pet. Sci. Eng. 2017, 149, 476–484. [Google Scholar] [CrossRef]
- Mahmoud, M. Well Clean-Up Using a Combined Thermochemical/Chelating Agent Fluids. J. Energy Resour. Technol. 2019, 141. [Google Scholar] [CrossRef]
- Mahmoud, M.A.N.E.D.; Elkatatny, S. Removal of barite-scale and barite-weighted water- Or oil-based-drilling-fluid residue in a single stage. SPE Drill. Complet. 2019, 34, 16–26. [Google Scholar] [CrossRef]
- Mahadi, K.A.; Nizam, M.M.; Jadid, M.B.; Ogbonna, C.; Hashim, S.; Chen, Y.C. Use of acid precursor as alternative to acid treatment to drill-in fluid filter cake removal: FN case study. In Proceedings of the Society of Petroleum Engineers—International Petroleum Technology Conference 2014, IPTC 2014—Innovation and Collaboration: Keys to Affordable Energy, Kuala Lumpur, Malaysia, 10–12 December 2014; Volume 1, pp. 98–111. [Google Scholar]
- Howard, S.K. Formate brines for drilling and completion: State of the art. In Proceedings of the the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 22–25 October 1995; pp. 31–39. [Google Scholar]
- Al-Anzi, N.A.; Haider, B.Y.A.; Gohain, A.K.; Hussain, I.; Davidson, E.; Macmillan, B.H. Carefully designed water-based drill-in fluid and filter cake removal system improves reservoir producibility and reduce well completion costs. In Proceedings of the 8th European Formation Damage Conference, Scheveningen, The Netherlands, 27–29 May 2009. [Google Scholar]
- Zubail, M.A.; Al-Kuait, A.M.; Al-Yateem, K.S.; El Bialy, M.; Maghrabi, S.; Olivares, T.; Ezell, R.G. Improved Producibility after Delayed Filter Cake Breaker Treatment in the Safaniya Offshore Field in Saudi Arabia. In Proceedings of the SPE Kuwait International Petroleum Conference and Exhibition, Kuwait City, Kuwait, 10–12 December 2012. [Google Scholar]
- Al-Otaibi, M.A.; BinMoqbil, K.H.; Al-Rabba, A.S.; Abitrabi, A.N. Single-stage chemical treatment for oil-based mudcake cleanup: Field case and laboratory studies. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 10–12 February 2010. [Google Scholar]
- Quintero, L.; Jones, T.A.; Pietrangeli, G. Phase boundaries of microemulsion systems help to increase productivity. In Proceedings of the Society of Petroleum Engineers—9th European Formation Damage Conference, Noordwijk, The Netherlands, 7–10 June 2011; Society of Petroleum Engineers: Richardson, TX, USA, 2011; Volume 2, pp. 1275–1284. [Google Scholar]
- Brege, J.; El Sherbeny, W.; Quintero, L.; Jones, T. Using microemulsion technology to remove oil-based mud in wellbore displacement and remediation applications. In Proceedings of the North Africa Technical Conference and Exhibition, Cairo, Egypt, 20–22 February 2012; SPE: Richardson, TX, USA, 2012. [Google Scholar]
- Addagalla, A.K.; Kosandar, B.A.; Lawal, I.G.; Jadhav, P.B.; Imran, A.; Al Saqer, Q.R.; El Sherbeny, W.; Ansari, A.; Pino, R.; Gad-Alla, A.E.; et al. Overcoming OBM filter cake damage using micro-emulsion remediation technology across a high-temperature formation. In Proceedings of the SPE International Conference and Exhibition on Formation Damage Control, Lafayette, Louisiana, USA, 24–26 February 2016; SPE: Richardson, TX, USA, 2016. [Google Scholar]
- Tan, Y.; Chen, H.; Wang, Z.; Xue, C.; He, R. Performances of cement mortar incorporating superabsorbent polymer (SAP) using different dosing methods. Materials 2019, 12, 1619. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Gu, Q.; Ning, J.; Liu, X.; Jia, Z.; Huang, D. Uniaxial compression behavior of cement mortar and its damage-constitutive model based on energy theory. Materials 2019, 12, 1309. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.D.; Lee, J.W.; Jang, Y.I.; Jang, S.J.; Choi, W. Microstructure and mechanical properties of cement mortar containing phase change materials. Appl. Sci. 2019, 9, 943. [Google Scholar] [CrossRef] [Green Version]
- Dong, B.; Qiu, Q.; Xiang, J.; Huang, C.; Xing, F.; Han, N. Study on the carbonation behavior of cement mortar by electrochemical impedance spectroscopy. Materials 2014, 7, 218–231. [Google Scholar] [CrossRef] [Green Version]
- Yim, H.J.; Kim, J.H. Physical characterization of cementitious materials on casting and placing process. Materials 2014, 7, 3049–3064. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Liu, X.; Guo, L.; Duan, P. Evaluation of properties and microstructure of cement paste blended with metakaolin subjected to high temperatures. Materials 2019, 16, 941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.A.; Iwaniw, M.A.; Fogler, H.S. Kinetics and mechanism of the reaction between ammonium and nitrite ions: Experimental and theoretical studies. Chem. Eng. Sci. 2003, 58, 4351–4362. [Google Scholar] [CrossRef]
- Al-Nakhli, A.; Tariq, Z.; Mahmoud, M.; Abdulraheem, A.; Al-Shehri, D. A novel thermochemical fracturing approach to reduce fracturing pressure of high strength rocks. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 11–14 November 2019; Society of Petroleum Engineers: Richardson, TX, USA, 2019. [Google Scholar]
- Al-Nakhli, A.R.; Tariq, Z.; Mahmoud, M.; Abdulraheem, A. A state-of-the-art technology to reduce fracturing pressure in tight gas formations using thermochemical pulse. In Proceedings of the Unconventional Resources Technology Conference, Austin, TX, USA, 20–22 July 2020. [Google Scholar]
- Rocha, N.O.; Khalil, C.N.; Leite, L.F.; Goja, A.M. Thermochemical Process To Remove Sludge From Storage Tanks. SPE Proj. Facil. Constr. 2009, 4, 97–102. [Google Scholar] [CrossRef]
- Alade, O.S.; Mahmoud, M.; Hassan, A.; Al-Shehri, D.; Al-Nakhli, A.; Bataweel, M. Evaluation of Kinetics and Energetics of Thermochemical Fluids for Enhanced Recovery of Heavy Oil and Liquid Condensate. Energy Fuels 2019, 33, 5538–5543. [Google Scholar] [CrossRef]
- Tariq, Z.; Mahmoud, M.A.; Abdulraheem, A.; Al-Nakhli, A.; Bataweel, M. An experimental study to reduce the fracture pressure of high strength rocks using a novel thermochemical fracturing approach. Geofluids 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Tariq, Z.; Mahmoud, M.; Abdulraheem, A.; Al-Nakhli, A.; BaTaweel, M. An experimental study to reduce the breakdown pressure of the unconventional carbonate rock by cyclic injection of thermochemical fluids. J. Pet. Sci. Eng. 2020, 187, 106859. [Google Scholar] [CrossRef]
- Tariq, Z.; Mahmoud, M.; Alade, O.; Abdulraheem, A.; Mustafa, A.; Mokheimer, E.M.A.; Al-Jawad, M.; Al-Nakhli, A. Productivity Enhancement in Multilayered Unconventional Rocks Using Thermochemicals. J. Energy Resour. Technol. 2021, 143, 033001. [Google Scholar] [CrossRef]
- Mustafa, A.; Mahmoud, M.; Abdulraheem, A.; Tariq, Z.; Al-Nakhli, A. Improvement of petrophysical properties of tight sandstone and limestone reservoirs using thermochemical fluids. Petrophysics 2020, 61, 363–382. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Alzobaidi, S.; Li, Z. Application and Mechanisms of Self-Generated Heat Foam for Enhanced Oil Recovery. Energy Fuels 2018, 32, 9093–9105. [Google Scholar] [CrossRef]
- Ba Alawi, M.; Hassan, A.; Aljawad, M.S.; Kamal, M.S.; Mahmoud, M.; Al-Nakhli, A. A Novel Approach to Improve Acid Diversion in Carbonate Rocks Using Thermochemical Fluids: Experimental and Numerical Study. Molecules 2020, 25, 2976. [Google Scholar] [CrossRef] [PubMed]
- Aljawad, M.S.; Mahmoud, M.; Abu-Khamsin, S.A. Mass and Heat Transfer of Thermochemical Fluids in a Fractured Porous Medium. Molecules 2020, 25, 4179. [Google Scholar] [CrossRef] [PubMed]
- Barri, A.; Mahmoud, M.; Elkatatny, S. Evaluation of Rock Mechanical Properties Alteration During Matrix Stimulation With Chelating Agents. J. Energy Resour. Technol. 2016, 138, 032907. [Google Scholar] [CrossRef]
- Tariq, Z.; Aljawad, M.S.; Hassan, A.; Mahmoud, M.; Al-Ramadhan, A. Chelating Agents as Acid-Fracturing Fluids: Experimental and Modeling Studies. Energy Fuels 2021, 35, 2602–2618. [Google Scholar] [CrossRef]
- Mahmoud, M. Determination of the optimum wormholing conditions in carbonate acidizing using NMR. J. Pet. Sci. Eng. 2017, 159, 952–969. [Google Scholar] [CrossRef]
- Hubbert, M.K.; Willis, D.G. Mechanics Of Hydraulic Fracturing. Trans. AIME 1957, 210, 153–168. [Google Scholar] [CrossRef]
- Haimson, B.; Fairhurst, C. Hydraulic fracturing in porous-permeable materials. J. Pet. Technol. 1969, 21, 811–817. [Google Scholar] [CrossRef]
- Smith, M.B.; Montgomery, C.T. Hydraulic Fracturing; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781466566927. [Google Scholar]
- Detournay, E.; Cheng, A. Influence of pressurization rate on the magnitude of the breakdown pressure. In Proceedings of the 33rd U.S. Symposium on Rock Mechanics (USRMS), Santa Fe, NM, USA, 3–5 June 1992; Volume 30, pp. 325–333. [Google Scholar]


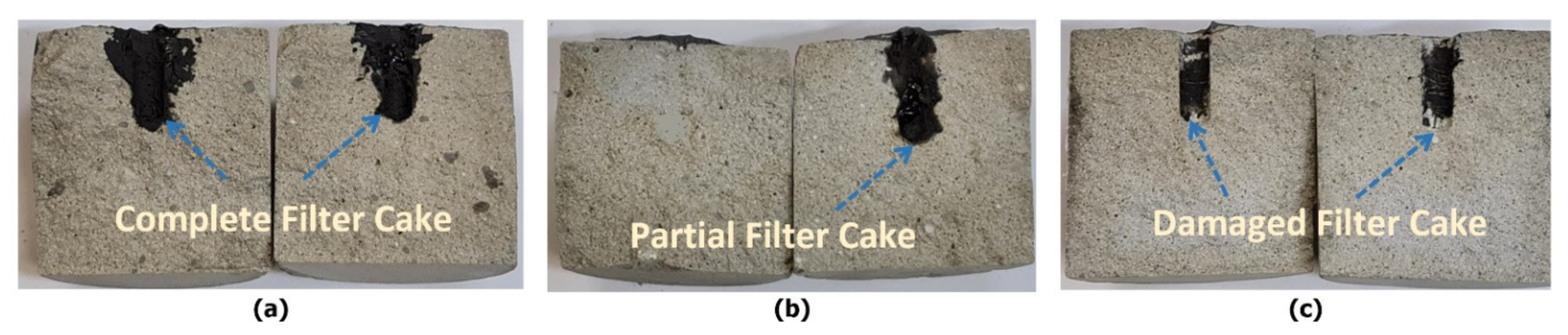



| Filter Cake Type | Filter Cake Remover | Comments | References |
|---|---|---|---|
| Barite water-based mud | DTPA chelating agent + K2CO3 + Enzyme | Removal efficiency reached 90% at 270 °F | [21] |
| EDTA chelating agent + K2CO3 + Enzyme | Removal efficiency reached 85% at 270 °F | [22] | |
| EDTA chelating + thermochemical | Removal efficiency reached more than 80% at different temperatures | [23] | |
| Barite Oil-based mud | Multi-stage (Solvent + GLDA + K2CO3 + HCl) | Removal efficiency reached 83% at different temperatures | [24] |
| EDTA chelating agent + K2CO3 + EGMBE | Removal efficiency reached 80% at different temperatures | [24] | |
| Calcium carbonate water-based, drill in fluid | Acid precursor of organic acids | Filter cake removed efficiently | [25] |
| Barite filter cake | Formate brines | Barite solubility reached 3500 mg/L at 100 °C | [26] |
| Invert emulsion filter cake | Oil wetting agents, a precursor of organic acid | Uniform removal of filter cake | [27,28] |
| Invert emulsion filter cake | Microemulsion | Filter cake removal efficiency reached 97% after 24 h | [29] |
| Invert emulsion filter cake | Microemulsion | Efficient removal of the oil-based filter cake | [30,31] |
| Synthetic and oil-based drilling filter cake | Microemulsion + surfactants/co-surfactants | Efficient removal of the oil-based filter cake | [32] |
| Rock Parameters | Values | Units |
|---|---|---|
| Permeability | 0.5 | mD |
| Porosity | 17 | % |
| Unconfined Compressive Strength | 41.3 | MPa |
| Tensile Strength | 8.9 | MPa |
| Compressional Wave Velocity | 3050 | m/s |
| Shear Wave Velocity | 1900 | m/s |
| Dynamic Poisson’s Ratio | 0.27 | - |
| Dynamic Young’s Modulus | 34 | GPa |
| Bulk Density | 2.7 | g/cc |
| Reaction Parameters | Values |
|---|---|
| 85–110 | |
| 0.1–0.6 | |
| 369 |
| Experiments | Fracturing Fluid | Filter Cake Formation | NMR Scan | Scratch Test | Comment |
|---|---|---|---|---|---|
| 1 | Water | No | Yes | Yes | Base case experiment without filter cake. |
| 2 | Water | Yes | Yes | Yes | Breakdown pressure experiment with water on filter cake |
| 3 | 20 wt% DTPA | Yes | Yes | Yes | Breakdown pressure experiment with DTPA chelating agent only on filter cake |
| 4 | 20 wt% DTPA + 10 wt% GLDA + 9 wt% K2CO3 | Yes | Yes | Yes | Breakdown pressure experiment with mixed chelating agent only on filter cake |
| 5 | Thermochemical + DTPA | Yes | Yes | Yes | Breakdown pressure experiment with chelating agent and thermochemical fluids only on filter cake |
| Input | SI Unit | Field Unit |
|---|---|---|
| 9.869 × 10−15 m2 | 10 mD | |
| 0.2 | ||
| 0.053 m3/s | 2 bpm | |
| 29.85 MPa | 4330 psi | |
| 1 mPa.s | 1 cp | |
| 1.45 × 10−4 1/MPa | 1 × 10−6 1/psi | |
| 41.4 MPa | 6000 psi | |
| 41.4 MPa | 6000 psi | |
| 8.3 MPa | 1200 psi | |
| 0.25 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tariq, Z.; Aljawad, M.S.; Mahmoud, M.; Alade, O.; Kamal, M.S.; Al-Nakhli, A. Reduction of Breakdown Pressure by Filter Cake Removal Using Thermochemical Fluids and Solvents: Experimental and Numerical Studies. Molecules 2021, 26, 4407. https://doi.org/10.3390/molecules26154407
Tariq Z, Aljawad MS, Mahmoud M, Alade O, Kamal MS, Al-Nakhli A. Reduction of Breakdown Pressure by Filter Cake Removal Using Thermochemical Fluids and Solvents: Experimental and Numerical Studies. Molecules. 2021; 26(15):4407. https://doi.org/10.3390/molecules26154407
Chicago/Turabian StyleTariq, Zeeshan, Murtada Saleh Aljawad, Mohamed Mahmoud, Olalekan Alade, Muhammad Shahzad Kamal, and Ayman Al-Nakhli. 2021. "Reduction of Breakdown Pressure by Filter Cake Removal Using Thermochemical Fluids and Solvents: Experimental and Numerical Studies" Molecules 26, no. 15: 4407. https://doi.org/10.3390/molecules26154407
APA StyleTariq, Z., Aljawad, M. S., Mahmoud, M., Alade, O., Kamal, M. S., & Al-Nakhli, A. (2021). Reduction of Breakdown Pressure by Filter Cake Removal Using Thermochemical Fluids and Solvents: Experimental and Numerical Studies. Molecules, 26(15), 4407. https://doi.org/10.3390/molecules26154407









