New Composite Sorbent for Removal of Sulfate Ions from Simulated and Real Groundwater in the Batch and Continuous Tests
Abstract
:1. Introduction
2. Study Area
3. Models for Simulation of Experimental Measurements
3.1. Equilibrium Isotherm Models
- Freundlich model; Equation (1) can apply for multilayer sorption onto non-homogenous surfaces as follows:where KF is the constant of Freundlich and 1/n (<1) is the intensity of sorption.
- Langmuir model; Equation (2) applies for homogenous surfaces and monolayer sorption:where qmax is the maximum capacity of adsorption (mg/g) and b is the chemical intensity on the solid matrix.
3.2. Kinetic Models
- Pseudo first order:where qt and qe (mg/g) are the quantities of contaminant loaded on the solid particles at t and equilibrium times, respectively, and k1 is the rate constant (1/min).
3.3. Transport of Solute
4. Results and Discussion
4.1. Evaluation of Groundwater Quality
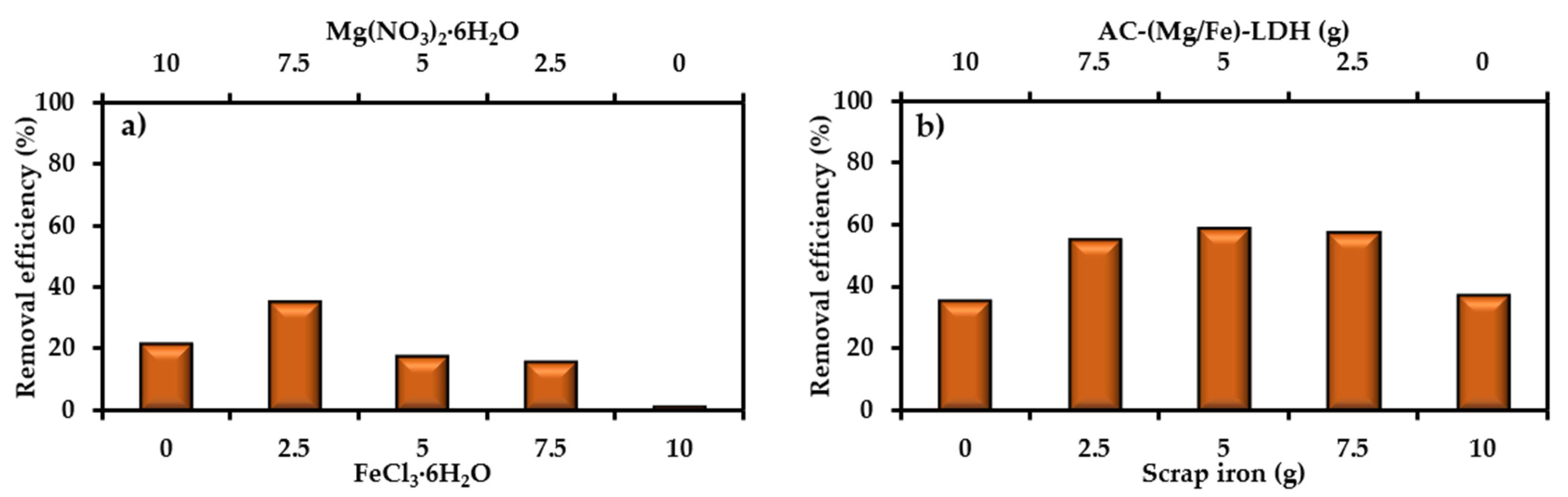
4.2. Preparation of Novel Sorbent
4.3. Effect of Operation Variables in Batch Experiments
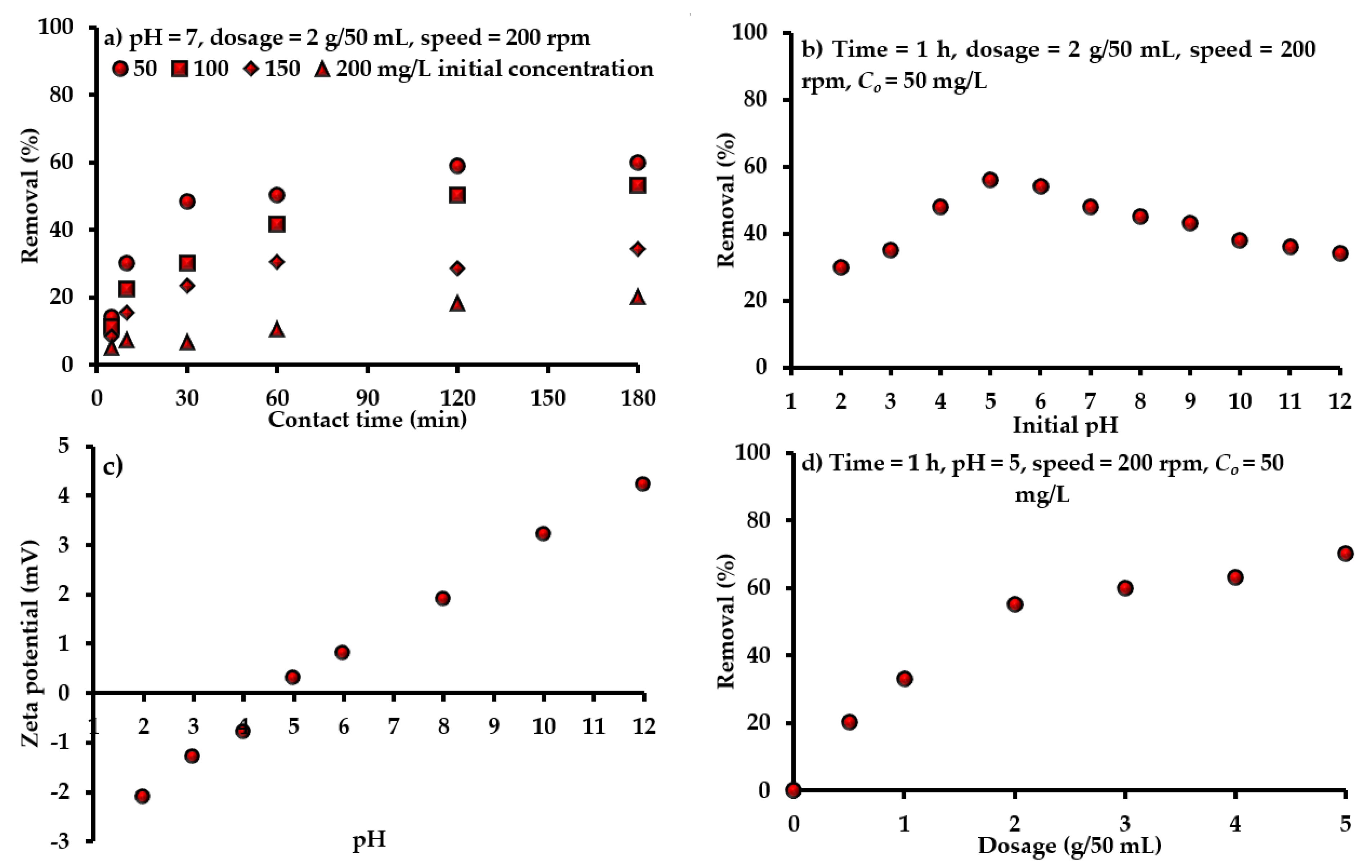
4.3.1. Time and Initial Concentration
4.3.2. Initial pH
4.3.3. Sorbent Mass
4.4. Sorption Isotherm and Kinetics Models
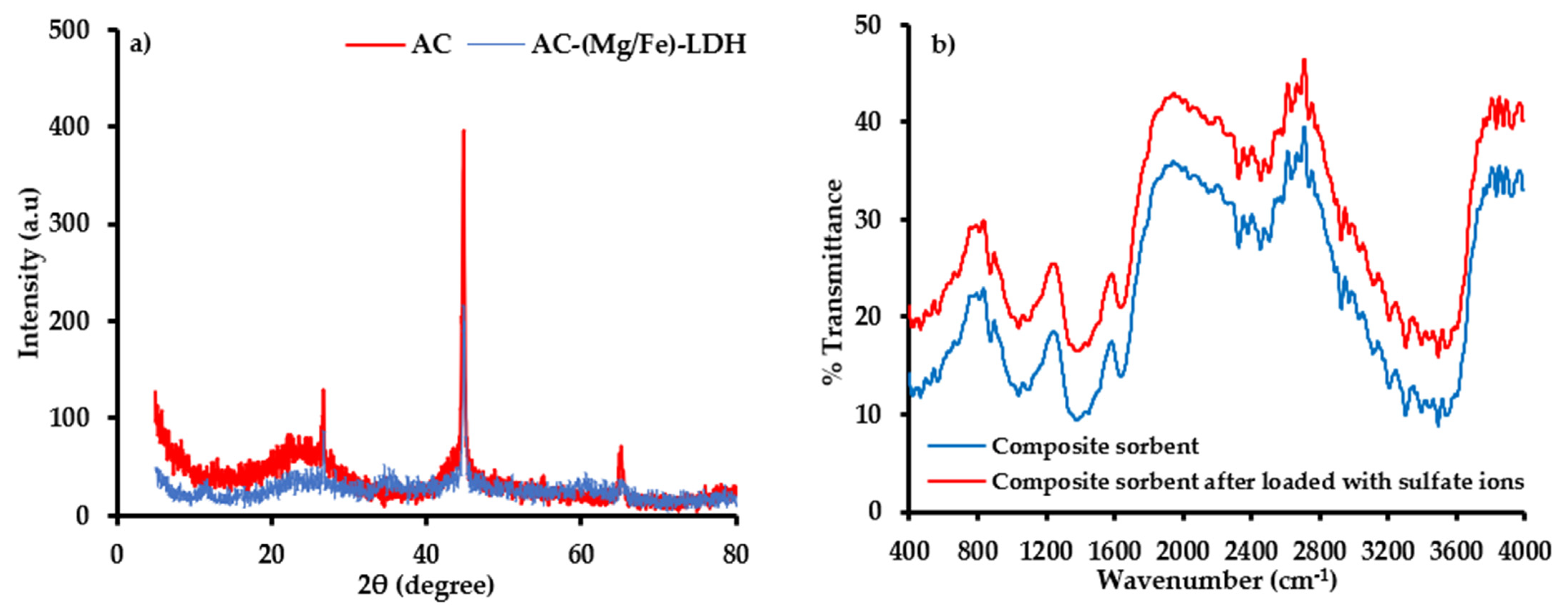
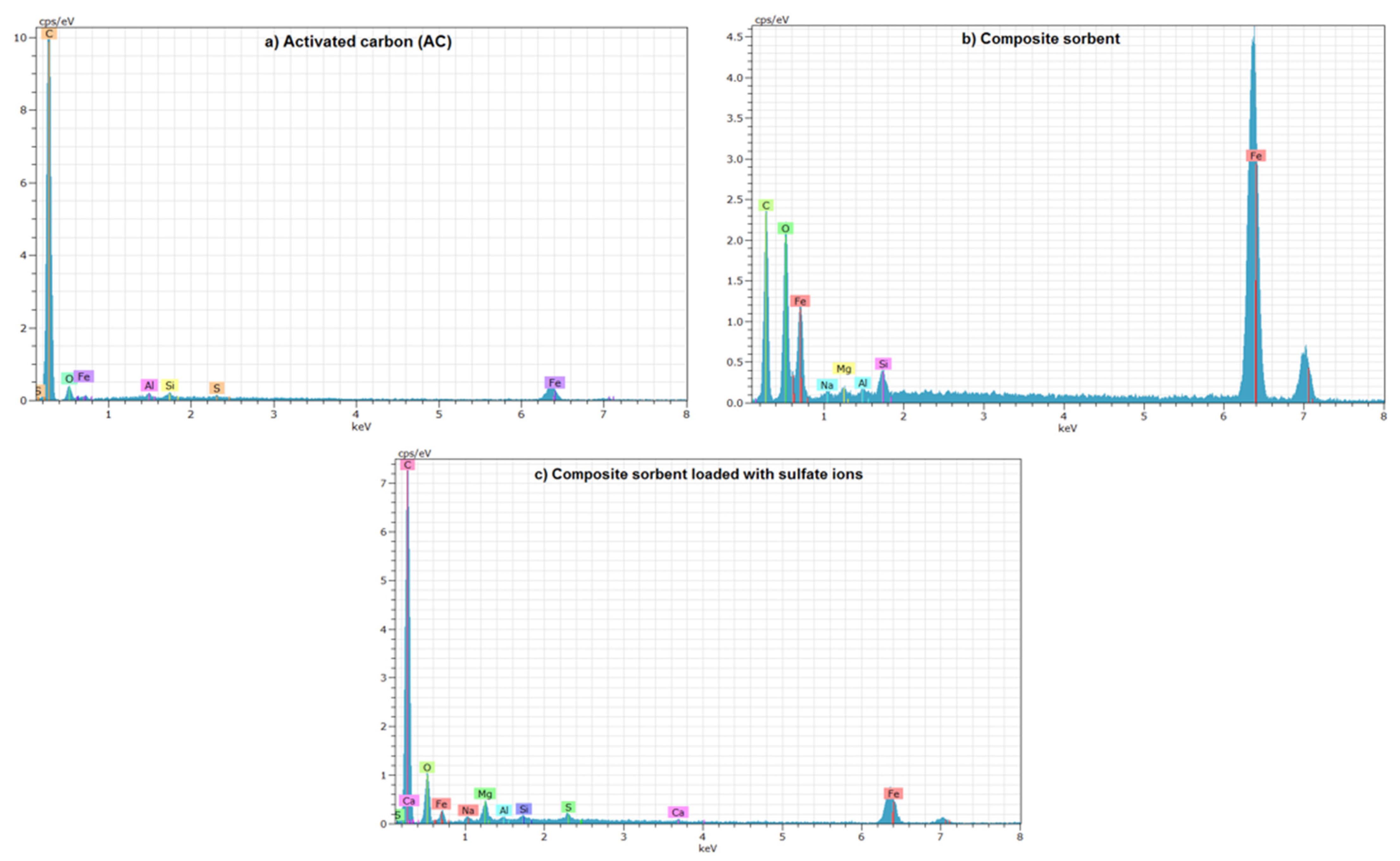
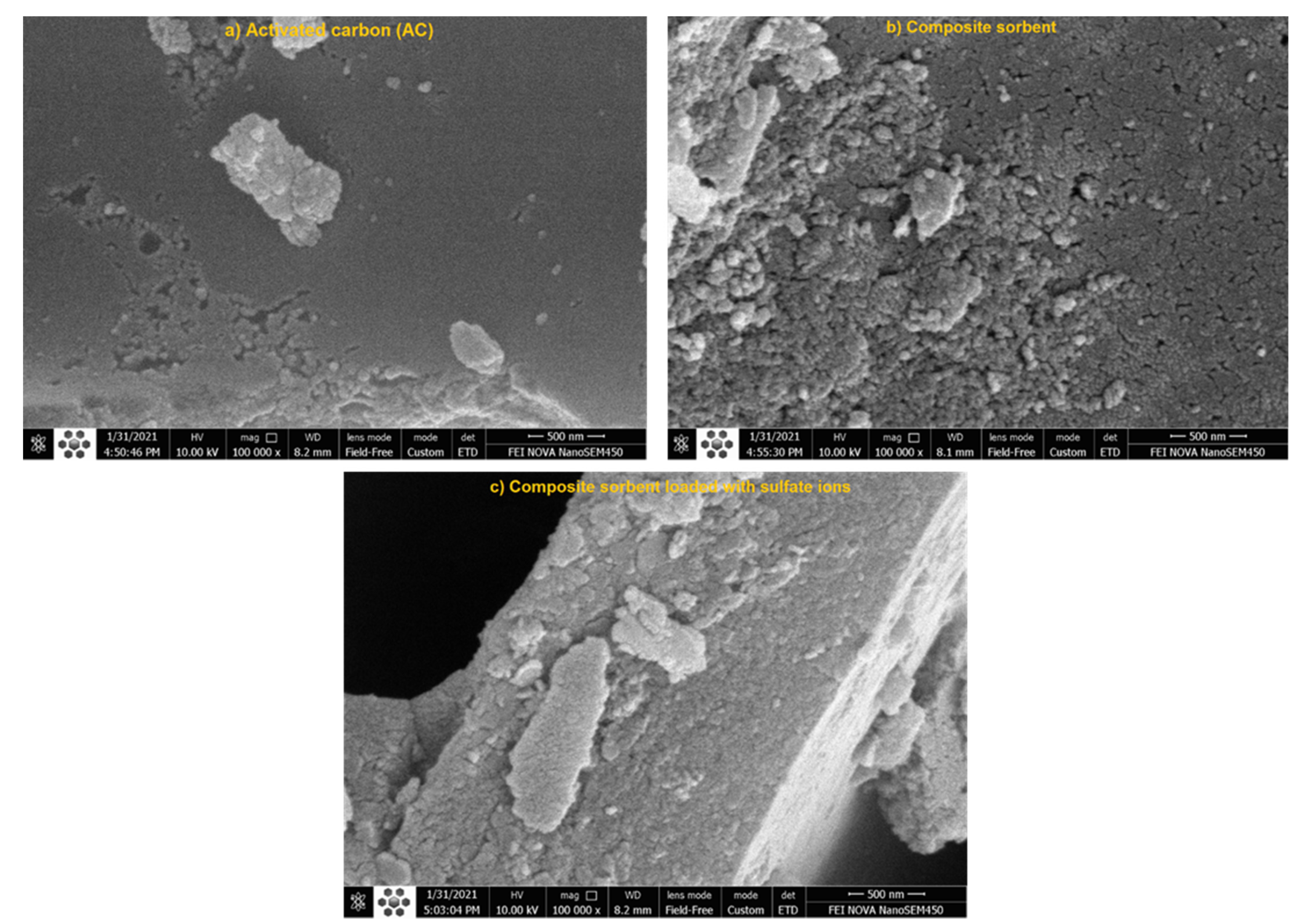
4.5. Characterization of Composite Sorbent
4.6. Dispersion Coefficient
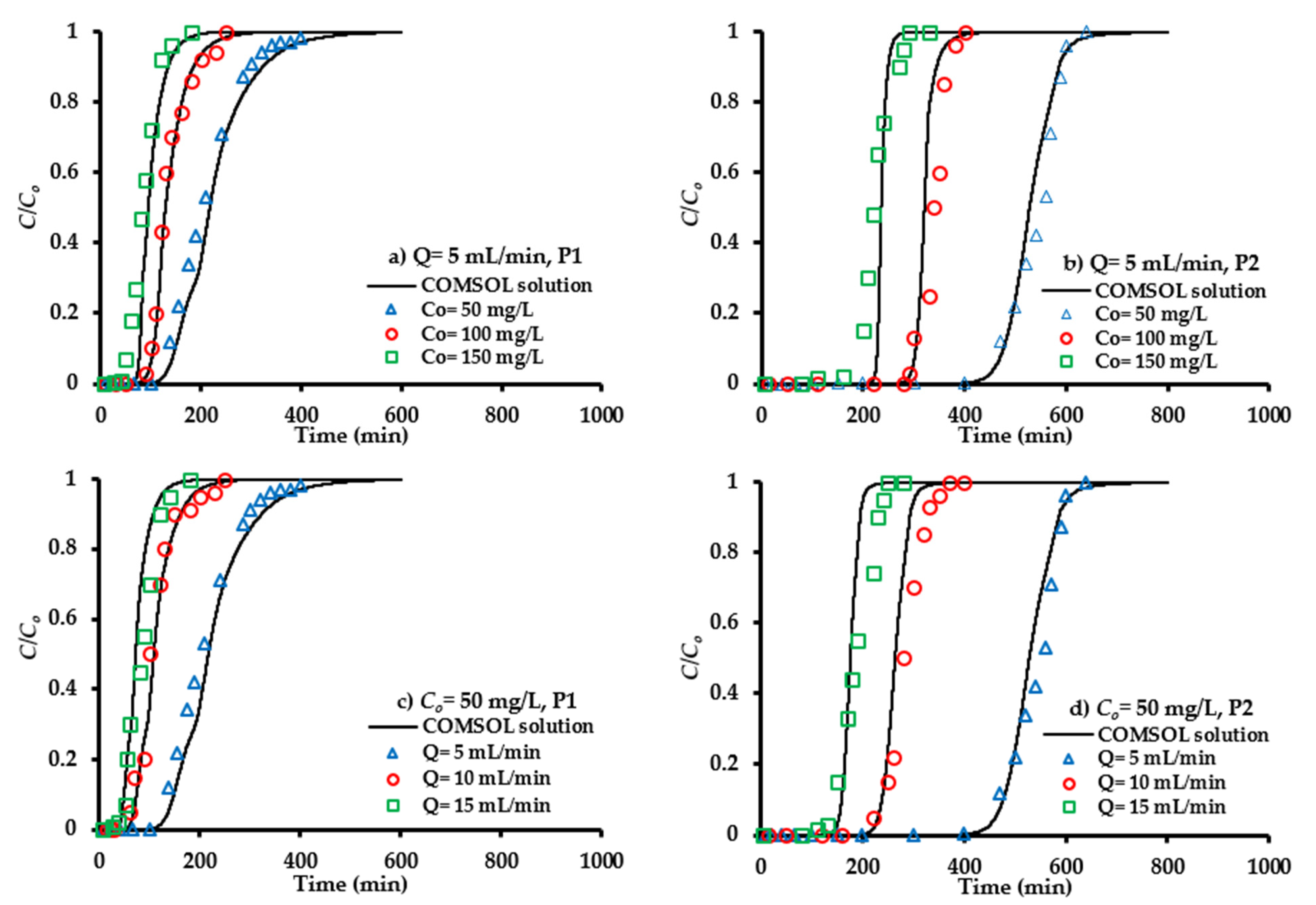
4.7. Breakthrough Curves in the Column Tests
4.7.1. Inlet Concentration
4.7.2. Flow Rate of Contaminated Water
4.7.3. Sorbent Quantity
4.7.4. Treatment of Real Groundwater Sample
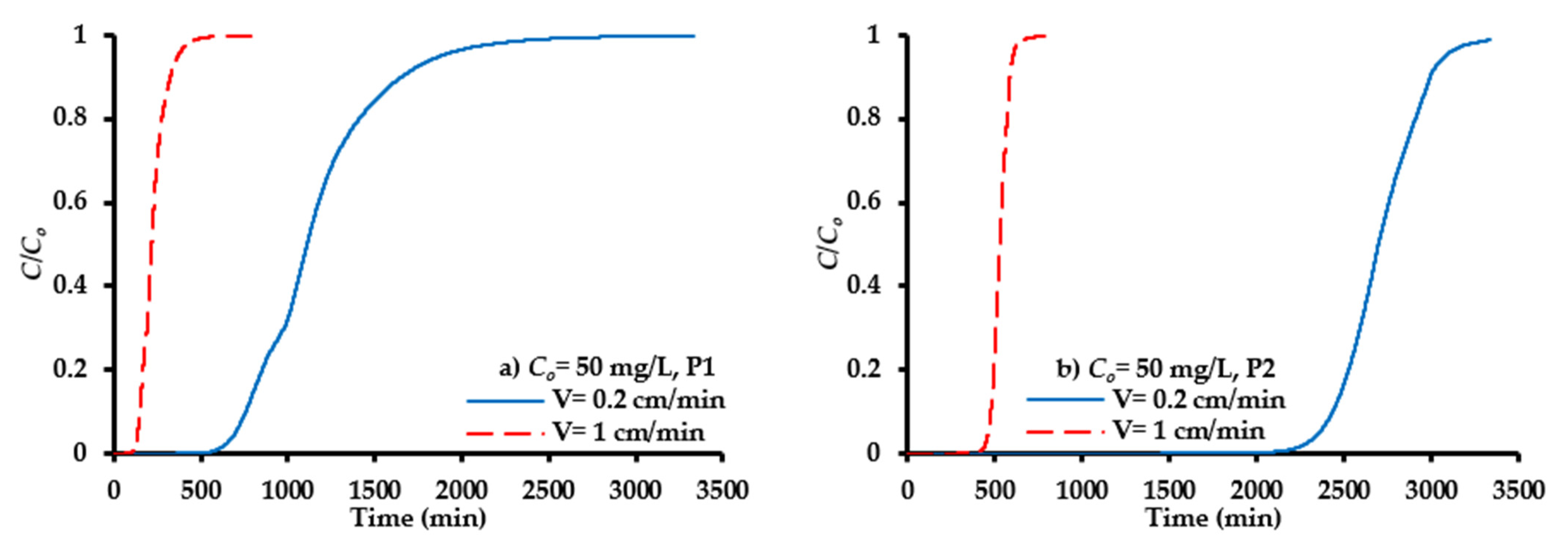
4.7.5. Hydraulic Conductivity
4.7.6. Numerical Modelling
5. Materials and Methods
5.1. Groundwater Quality
5.2. Materials
5.3. Synthesis of Sorbent
5.4. Characterization Analyses
5.5. Batch Mode Operation
5.6. Continuous Mode Operation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, X.H. Remediation techniques for soil and groundwater. Point Sources Pollut. Local Eff. Its Control 2009, II, 514. [Google Scholar]
- Połoński, M.; Pawluk, K.; Rybka, I. Optimization Model for the Design of Multi-layered Permeable Reactive Barriers. IOP Conf. Ser. Mater. Sci. Eng. 2017, 245. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Naushad, M. Adsorptive removal of noxious cadmium ions from aqueous medium using activated carbon/zirconium oxide composite: Isotherm and kinetic modelling. J. Mol. Liq. 2020, 310, 113025. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Al-Wakel, S.F.A.; Assi, H.A.; Naji, L.A.; Naushad, M. Waterworks sludge-filter sand permeable reactive barrier for removal of toxic lead ions from contaminated groundwater. J. Water Process Eng. 2020, 33. [Google Scholar] [CrossRef]
- Saad, N.; Abd Ali, Z.T.; Naji, L.A.; Faisal, A.A.A.H.; Al-Ansari, N. Development of Bi-Langmuir model on the sorption of cadmium onto waste foundry sand: Effects of initial pH and temperature. Environ. Eng. Res. 2020, 25, 677–684. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Abdul-Kareem, M.B.; Mohammed, A.K.; Naushad, M.; Ghfar, A.A.; Ahamad, T. Humic acid coated sand as a novel sorbent in permeable reactive barrier for environmental remediation of groundwater polluted with copper and cadmium ions. J. Water Process Eng. 2020, 36, 101373. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Al-Ridah, Z.A.; Naji, L.A.; Naushad, M.; El-Serehy, H.A. Waste Foundry Sand as Permeable and Low Permeable Barrier for Restriction of the Propagation of Lead and Nickel Ions in Groundwater. J. Chem. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Ahmed, D.N.; Faisal, A.A.H.; Jassam, S.H.; Naji, L.A.; Naushad, M. Kinetic Model for pH Variation Resulted from Interaction of Aqueous Solution Contaminated with Nickel Ions and Cement Kiln Dust. J. Chem. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Abdul-Kareem, M.B.; Mohammed, A.K.; Ghfar, A.A. Novel sorbent of sand coated with humic acid-iron oxide nanoparticles for elimination of copper and cadmium ions from contaminated water. J. Polym. Environ. 2021. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water, 3rd ed.; United States Geological Survey: Alexandria, Egypt, 1985; Volume 2254, pp. 1–264. [Google Scholar]
- Christensen, T.H.; Bjerg, P.L.; Banwart, S.A.; Jakobsen, R.; Heron, G.; Albrechtsen, H.J. Characterization of redox conditions in groundwater contaminant plumes. J. Contam. Hydrol. 2000, 45, 165–241. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, G.; Mao, Z.; Fang, Z.; Yang, C. Precipitation of valuable metals from bioleaching solution by biogenic sulfides. Miner. Eng. 2009, 22, 289–295. [Google Scholar] [CrossRef]
- Silva, R.; Cadorin, L.; Rubio, J. Sulphate ions removal from an aqueous solution: I. Co-precipitation with hydrolysed aluminum-bearing salts. Miner. Eng. 2010, 23, 1220–1226. [Google Scholar] [CrossRef]
- Bowell, R. Sulphate and salt minerals: The problem of treating mine waste. Min. Environ. Manag. 2000, 11–14. [Google Scholar]
- Alagha, O.; Manzar, M.S.; Zubair, M.; Anil, I.; Mu’azu, N.D.; Qureshi, A. Magnetic Mg-Fe/LDH intercalated activated carbon composites for nitrate and phosphate removal from wastewater: Insight into behavior and mechanisms. Nanomaterials 2020, 10, 1361. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.N.; Naji, L.A.; Faisal, A.A.H.; Al-Ansari, N.; Naushad, M. Waste foundry sand/MgFe-layered double hydroxides composite material for efficient removal of Congo red dye from aqueous solution. Sci. Rep. 2020, 10, 2042. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Shihab, A.H.; Naushad, M.; Ahamad, T.; Sharma, G.; Al-Sheetan, K.M. Green synthesis for novel sorbent of sand coated with (Ca/Al)-layered double hydroxide for the removal of toxic dye from aqueous environment. J. Environ. Chem. Eng. 2021, 9, 105342. [Google Scholar] [CrossRef]
- Lefèvre, G.; Lion, J.; Makolana, A. Extraction of tungsten as polyoxometalate anion using a layered double hydroxide: Selectivity and regeneration. Sep. Sci. Technol. 2018, 54, 549–558. [Google Scholar] [CrossRef]
- Hu, H.; Liu, J.; Xu, Z.; Zhang, L.; Cheng, B.; Ho, W. Applied Surface Science Hierarchical porous Ni/Co-LDH hollow dodecahedron with excellent adsorption property for Congo red and Cr(VI)ions. Appl. Surf. Sci. 2019, 478, 981–990. [Google Scholar] [CrossRef]
- Tran, H.N.; Lin, C.C.; Woo, S.H.; Chao, H.P. Efficient removal of copper and lead by Mg/Al layered double hydroxides intercalated with organic acid anions: Adsorption kinetics, isotherms, and thermodynamics. Appl. Clay Sci. 2018, 154, 17–27. [Google Scholar] [CrossRef]
- Ferreira, O.P.; de Moraes, S.G.; Durán, N.; Cornejo, L.; Alves, O.L. Evaluation of boron removal from water by hydrotalcite-like compounds. Chemosphere 2006, 62, 80–88. [Google Scholar] [CrossRef]
- Zhao, D.; Sheng, G.; Hu, J.; Chen, C.; Wang, X. The adsorption of Pb(II) on Mg2Al layered double hydroxide. Chem. Eng. J. 2011, 171, 167–174. [Google Scholar] [CrossRef]
- Dore, E.; Frau, F. Antimonate uptake by calcined and uncalcined layered double hydroxides: Effect of cationic composition and M2+/M3+ molar ratio. Environ. Sci. Pollut. Res. 2018, 25, 916–929. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.G.L.; Lopes, P.A.; Resende, J.A.; Pinto, F.G.; Tronto, J.; Guerreiro, M.C.; de Oliveira, L.C.A.; de Castro Nunes, W.; Neto, J.L. Performance of magnetite/layered double hydroxide composite for dye removal via adsorption, Fenton and photo-Fenton processes. Appl. Clay Sci. 2019, 179, 105152. [Google Scholar] [CrossRef]
- Gupta, N.K.; Saifuddin, M.; Kim, S.; Kim, K.S. Microscopic, spectroscopic, and experimental approach towards understanding the phosphate adsorption onto Zn–Fe layered double hydroxide. J. Mol. Liq. 2020, 297, 111935. [Google Scholar] [CrossRef]
- Zhang, Q.; Ji, F.; Zhao, T.; Shen, Q.; Fang, D.; Kuang, L.; Jiang, L.; Ding, S. Systematic screening of layered double hydroxides for phosphate removal and mechanism insight. Appl. Clay Sci. 2019, 174, 159–169. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Z.; Zhang, H.; Lu, H.; Zhang, W.; Qiu, Y.; Zhu, L.; Küppers, S. Calcined layered double hydroxides/reduced graphene oxide composites with improved photocatalytic degradation of paracetamol and efficient oxidation-adsorption of As(III). Appl. Catal. B Environ. 2018, 225, 550–562. [Google Scholar] [CrossRef]
- Hong, J.; Zhu, Z.; Lu, H.; Qiu, Y. Synthesis and arsenic adsorption performances of ferric-based layered double hydroxide with α-alanine intercalation. Chem. Eng. J. 2014, 252, 267–274. [Google Scholar] [CrossRef]
- Hudcová, B.; Erben, M.; Vítková, M.; Komárek, M. Antimonate adsorption onto Mg-Fe layered double hydroxides in aqueous solutions at different pH values: Coupling surface complexation modeling with solid-state analyses. Chemosphere 2019, 229, 236–246. [Google Scholar] [CrossRef]
- Ogata, F.; Nagai, N.; Kishida, M.; Nakamura, T.; Kawasaki, N. Interaction between phosphate ions and Fe-Mg type hydrotalcite for purification of wastewater. J. Environ. Chem. Eng. 2019, 7, 102897. [Google Scholar] [CrossRef]
- Mandel, K.; Drenkova-Tuhtan, A.; Hutter, F.; Gellermann, C.; Steinmetz, H.; Sextl, G. Layered double hydroxide ion exchangers on superparamagnetic microparticles for recovery of phosphate from waste water. J. Mater. Chem. A 2013, 1, 1840–1848. [Google Scholar] [CrossRef]
- Jiang, D.; Yu, Q.; Huang, C.; Chen, S.; Wu, Y.; Lin, J.; Chen, J.; Zhu, P. Preparation of mesoporous spherical magnesium hydroxide particles via the static self-assembled method. J. Mol. Struct. 2019, 1175, 858–864. [Google Scholar] [CrossRef]
- Vafaeifard, M.; Ibrahim, S.; Wong, K.T.; Pasbakhsh, P.; Pichiah, S.; Choi, J.; Yoon, Y.; Jang, M. Novel self-assembled 3D flower-like magnesium hydroxide coated granular polyurethane: Implication of its potential application for the removal of heavy metals. J. Clean. Prod. 2019, 216, 495–503. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, J.; Wang, Y.; Liu, Z.; Lian, J.; Liu, M. Highly efficient and selective removal of low-concentration antibiotics from aqueous solution by regenerable Mg(OH)2. J. Environ. Sci. 2020, 87, 228–237. [Google Scholar] [CrossRef]
- Qiu, H.; Ni, W.; Zhang, H.; Chen, K.; Yu, J. Fabrication and evaluation of a regenerable HFO-doped agricultural waste for enhanced adsorption affinity towards phosphate. Sci. Total Environ. 2019, 703, 135493. [Google Scholar] [CrossRef]
- Shemer, H.; Armush, A.; Semiat, R. Reusability of iron oxyhydroxide agglomerates adsorbent for repetitive phosphate removal. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 579, 123680. [Google Scholar] [CrossRef]
- Drenkova-Tuhtan, A.; Schneider, M.; Franzreb, M.; Meyer, C.; Gellermann, C.; Sextl, G.; Mandel, K.; Steinmetz, H. Pilot-scale removal and recovery of dissolved phosphate from secondary wastewater effluents with reusable ZnFeZr adsorbent @ Fe3O4/SiO2 particles with magnetic harvesting. Water Res. 2017, 109, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Drenkova-Tuhtan, A.; Sheeleigh, E.K.; Rott, E.; Meyer, C.; Sedlak, D.L. Sorption of recalcitrant phosphonates in reverse osmosis concentrates and wastewater effluents—Influence of metal ions. Water Sci. Technol. 2021, 83, 934–947. [Google Scholar] [CrossRef]
- Wu, J.; Lin, J.; Zhan, Y. Interception of phosphorus release from sediments using Mg/Fe-based layered double hydroxide (MF-LDH) and MF-LDH coated magnetite as geo-engineering tools. Sci. Total Environ. 2020, 739, 139749. [Google Scholar] [CrossRef] [PubMed]
- Faisal, A.A.H.; Sulaymon, A.H.; Khaliefa, Q.M. A review of permeable reactive barrier as passive sustainable technology for groundwater remediation. Int. J. Environ. Sci. Technol. 2018, 15, 1123–1138. [Google Scholar] [CrossRef]
- Tillman, G.M. Water Treatment: Troubleshooting and Problem Solving, 1st ed.; Lewis Publishers: Richmond, VA, USA, 1996; ISBN 978-1575040011. Available online: https://www.amazon.com/Water-Wastewater-Examination-Manual-Adams/dp/0873711998 (accessed on 27 October 1990).
- Naushad, M. Surfactant assisted nano-composite cation exchanger: Development, characterization and applications for the removal of toxic Pb2+ from aqueous medium. Chem. Eng. J. 2014, 235, 100–108. [Google Scholar] [CrossRef]
- Sharma, G.; Pathania, D.; Naushad, M.; Kothiyal, N.C. Fabrication, characterization and antimicrobial activity of polyaniline Th(IV) tungstomolybdophosphate nanocomposite material: Efficient removal of toxic metal ions from water. Chem. Eng. J. 2014, 251, 413–421. [Google Scholar] [CrossRef]
- Al-Ansari, N.; Saleh, S.; Abdullahand, T.; Ali Abed, S. Quality of Surface Water and Groundwater in Iraq. J. Earth Sci. Geotech. Eng. 2020, 11, 161–199. [Google Scholar] [CrossRef]
- Al-Jiburi, H.K.; Al-Basrawi, N.H. The Hydrogeology of Iraqi western and southern desert. West. Iraqi Desert J. 2007, 77–91. [Google Scholar]
- Zheng, H.; Liu, D.; Zheng, Y.; Liang, S.; Liu, Z. Sorption isotherm and kinetic modeling of aniline on Cr-bentonite. J. Hazard. Mater. 2009, 167, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Puranik, P.R.; Modak, J.M.; Paknikar, K.M. A comparative study of the mass transfer kinetics of metal biosorption by microbial biomass. Hydrometallurgy 1999, 52, 189–197. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Liu, J.; Yue, X.; Yu, Y.; Guo, Y. Adsorption of sulfate from natural water on calcined Mg–Fe layered double hydroxides. Desalin. Water Treat. 2015, 56, 274–283. [Google Scholar] [CrossRef]
- Adegoke, H.I.; Adekola, F.A.; Bdulraheem, M.N. Kinetic and thermodynamic studies on adsorption of sulphate from aqueous solution by magnetite, activated carbon and magnetite-activated carbon composites. Niger. J. Chem. Res. 2017, 22, 39–69. [Google Scholar]
- Rahmati, M.; Yeganeh, G.; Esmaeili, H. Sulfate ion removal from water using activated carbon powder prepared by Ziziphus Spina-Christi Lotus Leaf. Acta Chim. Slov. 2019, 66, 888–898. [Google Scholar] [CrossRef]
- Amarasinghe, B.M.W.P.K.; Williams, R.A. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem. Eng. J. 2007, 132, 299–309. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Alquzweeni, S.S.; Naji, L.A.; Naushad, M. Predominant mechanisms in the treatment of wastewater due to interaction of benzaldehyde and iron slag byproduct. Int. J. Environ. Res. Public Health 2020, 17, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naji, L.A.; Jassam, S.H.; Yaseen, M.J.; Faisal, A.A.H.; Al-Ansari, N. Modification of Langmuir model for simulating initial pH and temperature effects on sorption process. Sep. Sci. Technol. 2020, 55, 2729–2736. [Google Scholar] [CrossRef] [Green Version]
- Namasivayam, C.; Sangeetha, D. Application of coconut coir pith for the removal of sulfate and other anions from water. Desalination 2008, 219, 1–13. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, Z.; Qiu, Y.; Zhao, J. Adsorption of arsenate on Cu/Mg/Fe/La layered double hydroxide from aqueous solutions. J. Hazard. Mater. 2012, 239–240, 279–288. [Google Scholar] [CrossRef]
- Yang, T.; Lua, A.C. Characteristics of activated carbons prepared from pistachio-nut shells by physical activation. J. Colloid Interface Sci. 2003, 267, 408–417. [Google Scholar] [CrossRef]
- Gulmine, J.; Janissek, P.; Heise, H.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Hesenov, A.; Meryemoğlu, B.; Içten, O. Electrolysis of coal slurries to produce hydrogen gas: Effects of different factors on hydrogen yield. Int. J. Hydrogen Energy 2011, 36, 12249–12258. [Google Scholar] [CrossRef]
- Delleur, J. The Handbook of Groundwater Engineering; CRC Press LLC, Springer: Boca Raton, FL, USA, 1999; ISBN 3-540-64745-7. [Google Scholar]
- Liao, P.; Zhan, Z.; Dai, J.; Wu, X.; Zhang, W.; Wang, K.; Yuan, S. Adsorption of tetracycline and chloramphenicol in aqueous solutions by bamboo charcoal: A batch and fixed-bed column study. Chem. Eng. J. 2013, 228, 496–505. [Google Scholar] [CrossRef]
- Ko, D.C.K.; Porter, J.F.; McKay, G. Optimised correlations for the fixed-bed adsorption of metal ions on bone char. Chem. Eng. Sci. 2000, 55, 5819–5829. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. As(III) removal from aqueous medium in fixed bed using iron oxide-coated cement (IOCC): Experimental and modeling studies. Chem. Eng. J. 2007, 129, 123–131. [Google Scholar] [CrossRef]
- Marzbali, M.H.; Esmaieli, M. Fixed bed adsorption of tetracycline on a mesoporous activated carbon: Experimental study and neuro-fuzzy modeling. J. Appl. Res. Technol. 2017, 15, 454–463. [Google Scholar] [CrossRef]
- Todd, D.K.; Mays, L.W. Groundwater Hydrology, 3rd ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2005; ISBN 978-0-471-05937-0. [Google Scholar]
- US EPA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 1975; pp. 1–496.
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Irrig. Drain. Pap. 29, Rev. 1; FAO: Rome, Italy, 1994; pp. 1–130. [Google Scholar]
- Faisal, A.A.H.; Ali, I.M.; Naji, L.A.; Madhloom, H.M.; Al-ansari, N. Using different materials as a permeable reactive barrier for remediation of groundwater contaminated with landfill’s leachate. Desalin. Water Treat. 2020, 24890, 1–12. [Google Scholar] [CrossRef]
- Gupta, V.K. Equilibrium uptake, sorption dynamics, process development, and column operations for the removal of copper and nickel from aqueous solution and wastewater using activated slag, a low-cost adsorbent. Ind. Eng. Chem. Res. 1998, 37, 192–202. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Y.; He, M.; Chen, L.; Yu, X. Characterization of GMZ bentonite and its application in the adsorption of Pb(II) from aqueous solutions. Appl. Clay Sci. 2009, 43, 164–171. [Google Scholar] [CrossRef]
- Ujfaludi, L. Le comportement de dispersion longitudinale des sols à granulométrie non-uniforme. Hydrol. Sci. J. 1986, 31, 467–474. [Google Scholar] [CrossRef] [Green Version]
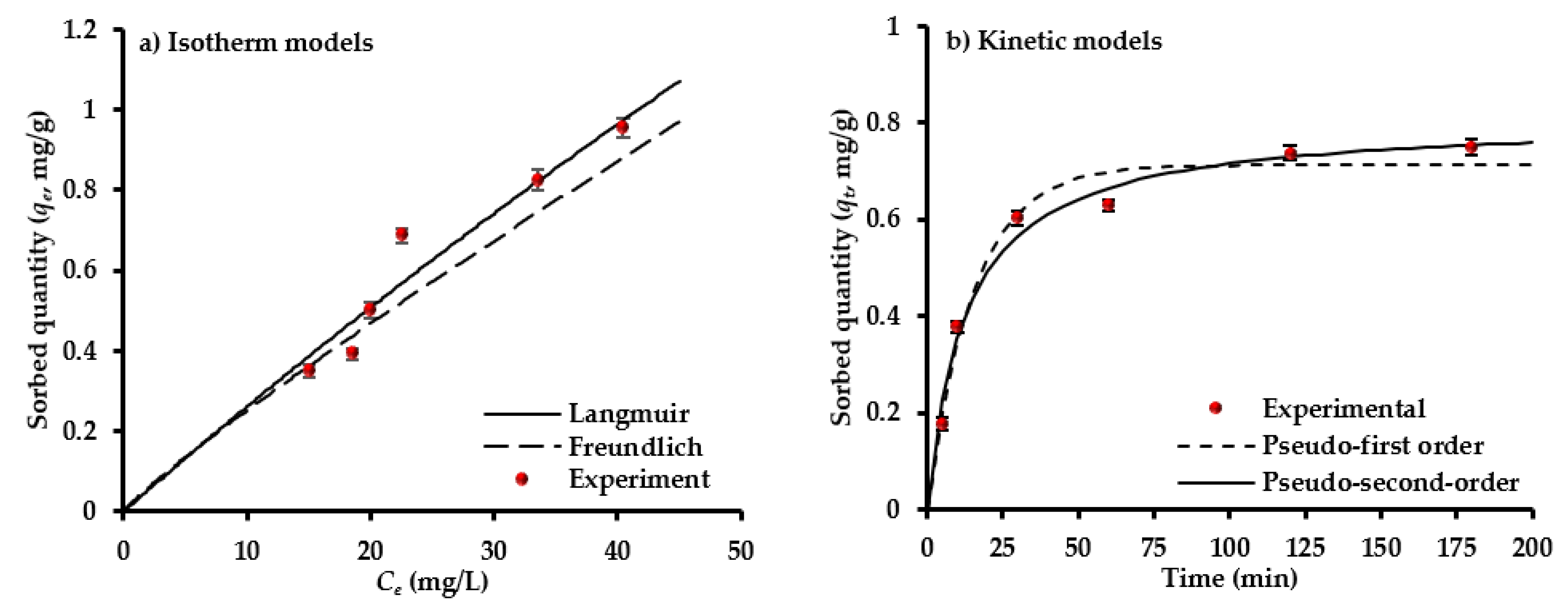

| Model | Parameter | Value |
|---|---|---|
| Freundlich | KF (mg/g)(L/mg)1/n | 0.0319 |
| 1/n | 0.8963 | |
| R2, SSE | 0.9253, 0.0430 | |
| Langmuir | qmax (mg/g) | 9.5 |
| b (L/mg) | 0.0028 | |
| R2, SSE | 0.9265, 0.0222 | |
| Pseudo first-order | qexp. (mg/g) | 0.7700 |
| k1 (min−1) | 0.0653 | |
| qe (mg/g) | 0.7224 | |
| R2, SSE | 0.9656, 0.0087 | |
| Pseudo second-order | k2 (g/mg min) | 0.0963 |
| qe (mg/g) | 0.8072 | |
| R2, SSE | 0.9782, 0.0058 |
| Element (%) | AC | Composite Sorbent | |
|---|---|---|---|
| Before Sorption | After Sorption | ||
| C | 90.72 | 56.14 | 79.25 |
| O | 8.5 | 32.75 | 18.69 |
| Mg | 0.0 | 1.03 | 0.56 |
| Al | 0.07 | 0.17 | 0.05 |
| Si | 0.06 | 0.42 | 0.05 |
| S | 0.03 | 0 | 1.34 |
| Fe | 0.62 | 9.49 | 1.05 |
| Parameter | Raw Groundwater | Treated Groundwater |
|---|---|---|
| pH | 7 | 8.3 |
| EC (µS/cm) | 5770 | 4561 |
| TDS (mg/L) | 1220 | 1170 |
| TSS (mg/L) | 21 | 16 |
| SO42− (mg/L) | 2801 | 709 |
| Ca2+ (mg/L) | 370 | 188 |
| Mg2+ (mg/L) | 34 | 29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, W.; Faisal, A.; Abed, E.; Al-Ansari, N.; Saleh, B. New Composite Sorbent for Removal of Sulfate Ions from Simulated and Real Groundwater in the Batch and Continuous Tests. Molecules 2021, 26, 4356. https://doi.org/10.3390/molecules26144356
Hassan W, Faisal A, Abed E, Al-Ansari N, Saleh B. New Composite Sorbent for Removal of Sulfate Ions from Simulated and Real Groundwater in the Batch and Continuous Tests. Molecules. 2021; 26(14):4356. https://doi.org/10.3390/molecules26144356
Chicago/Turabian StyleHassan, Waqed, Ayad Faisal, Enas Abed, Nadhir Al-Ansari, and Bahaa Saleh. 2021. "New Composite Sorbent for Removal of Sulfate Ions from Simulated and Real Groundwater in the Batch and Continuous Tests" Molecules 26, no. 14: 4356. https://doi.org/10.3390/molecules26144356
APA StyleHassan, W., Faisal, A., Abed, E., Al-Ansari, N., & Saleh, B. (2021). New Composite Sorbent for Removal of Sulfate Ions from Simulated and Real Groundwater in the Batch and Continuous Tests. Molecules, 26(14), 4356. https://doi.org/10.3390/molecules26144356









