Free-Standing, Flexible Nanofeatured Polymeric Films Prepared by Spin-Coating and Anodic Polymerization as Electrodes for Supercapacitors
Abstract
1. Introduction
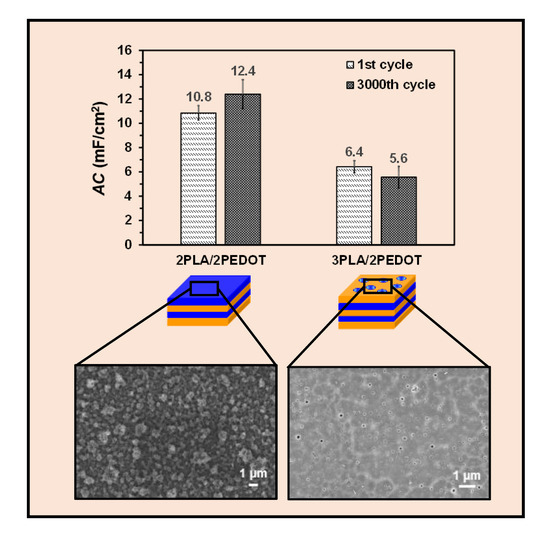
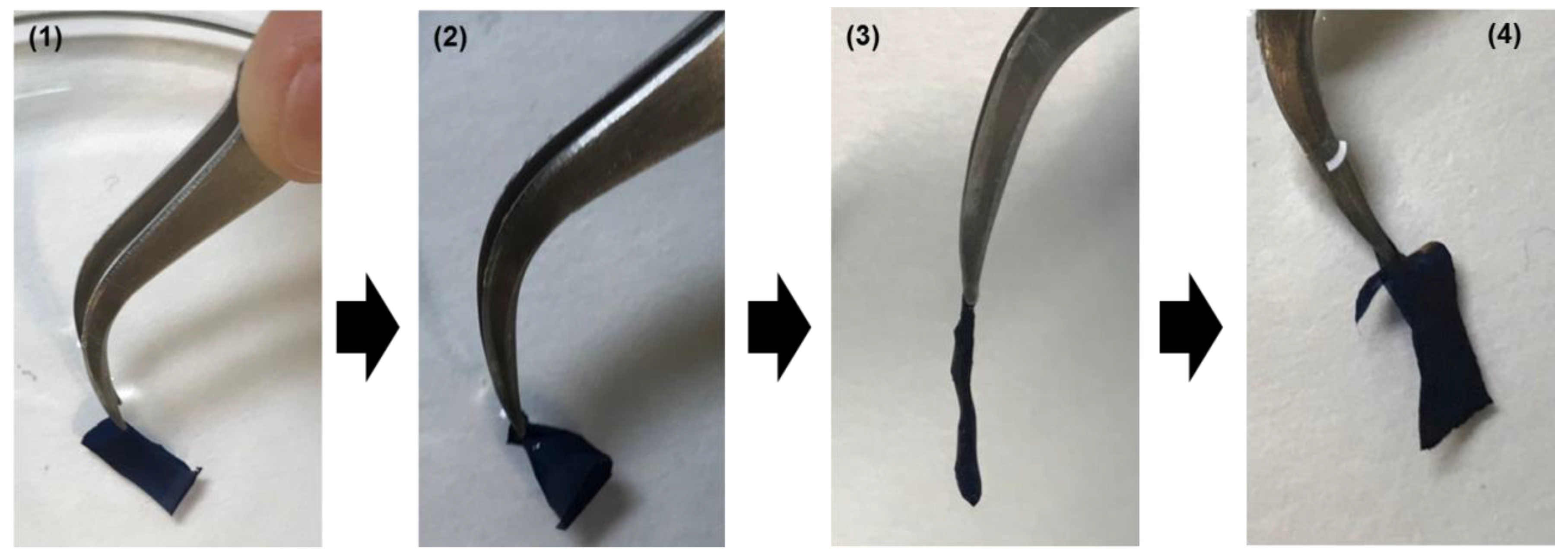
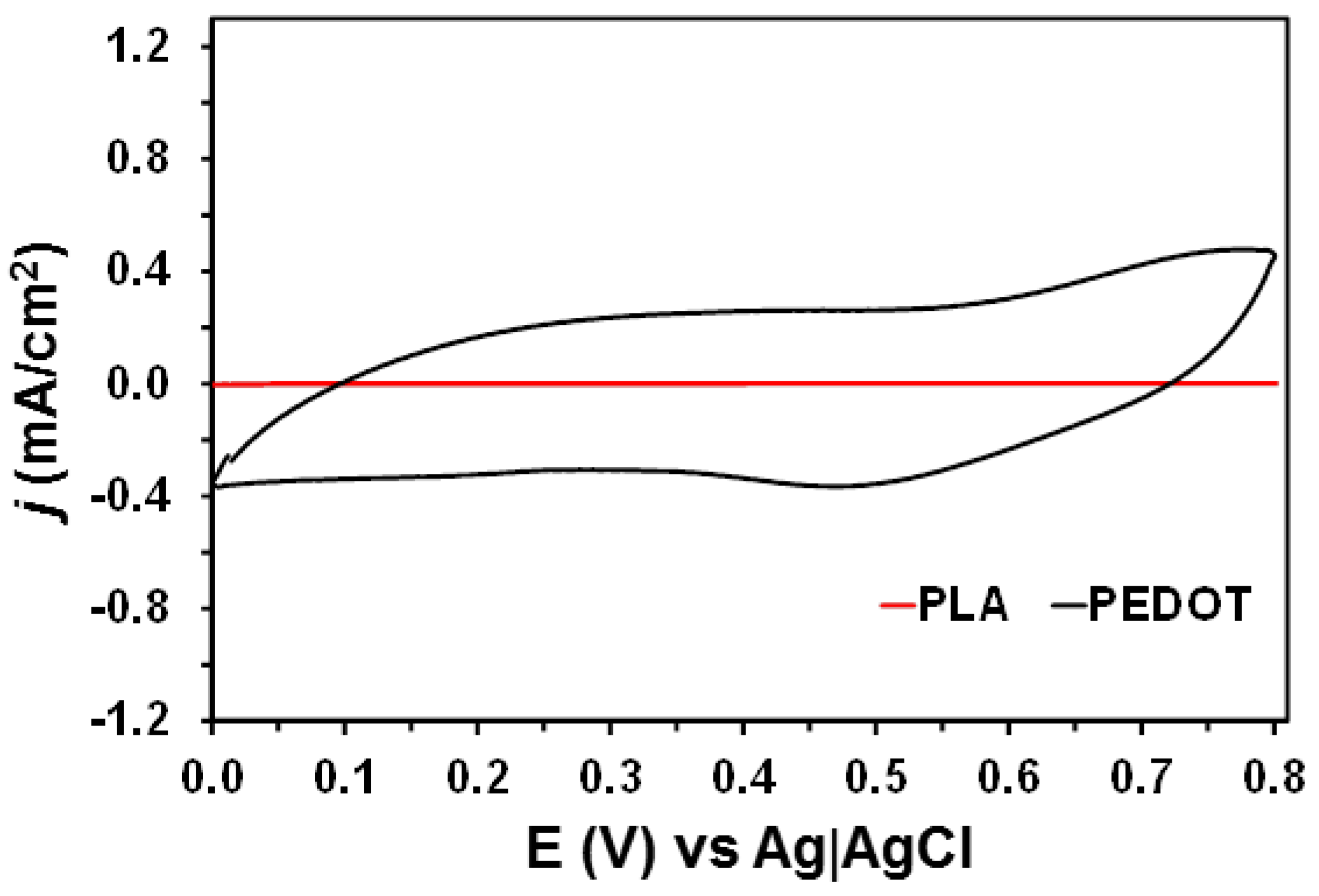
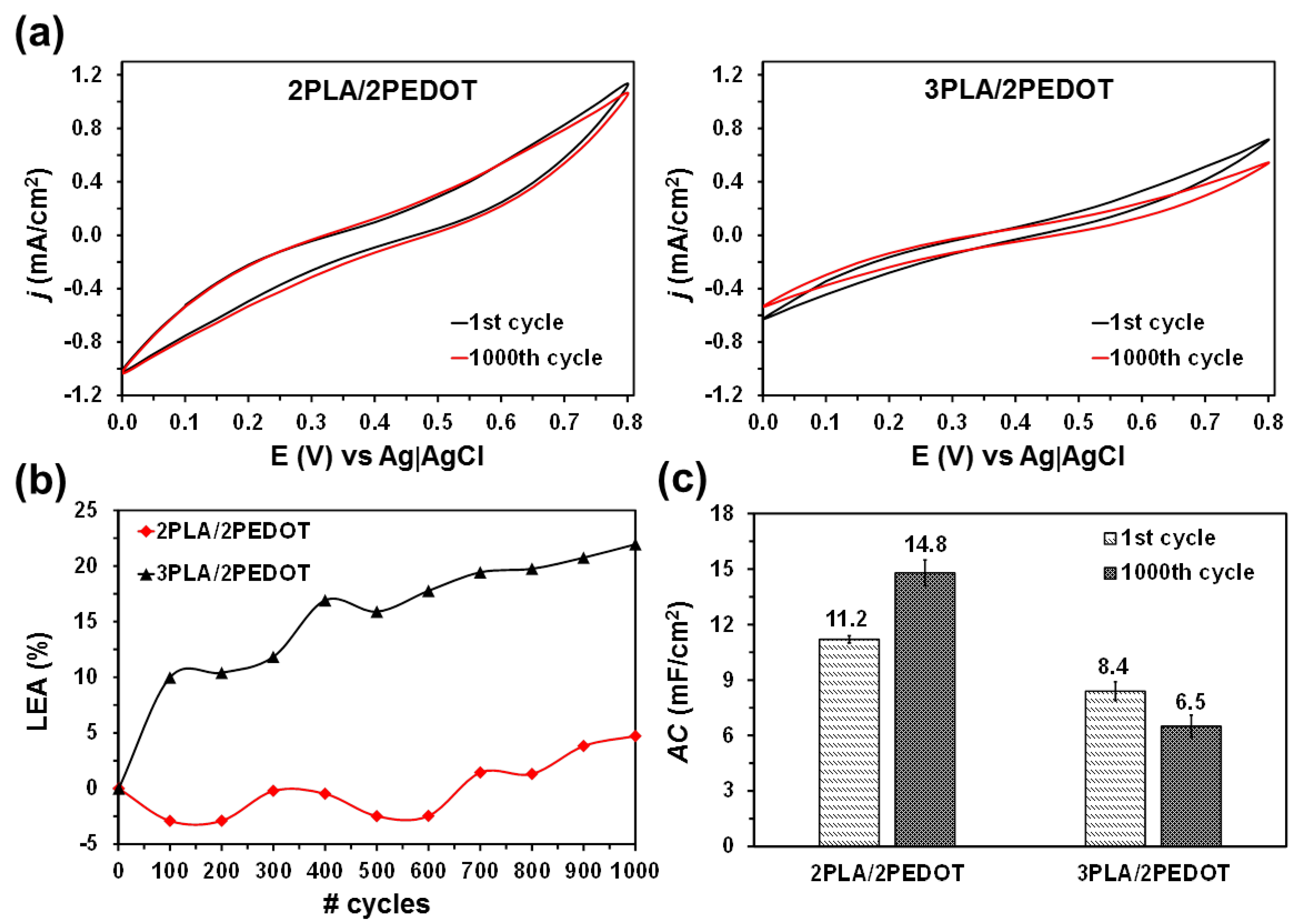
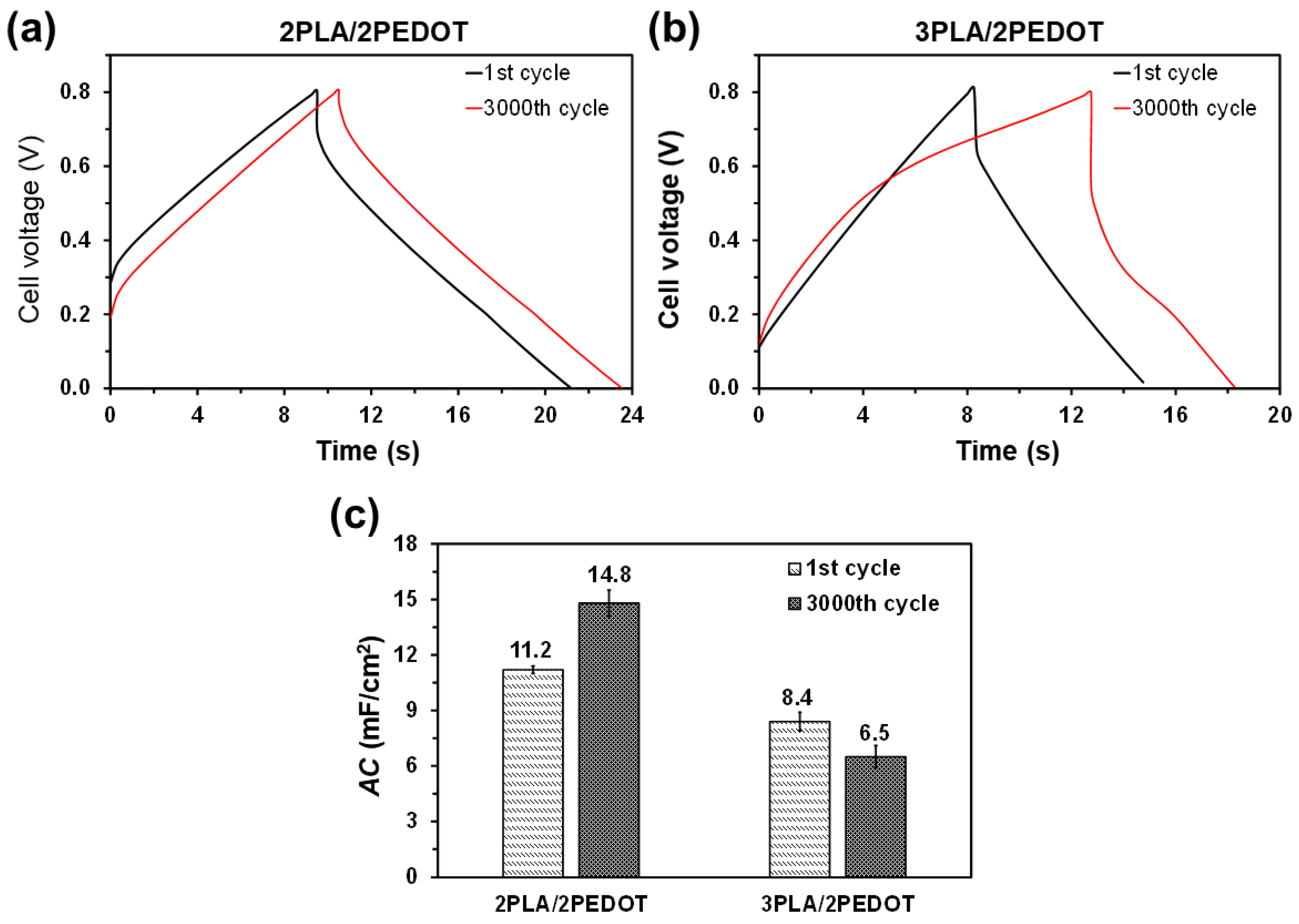
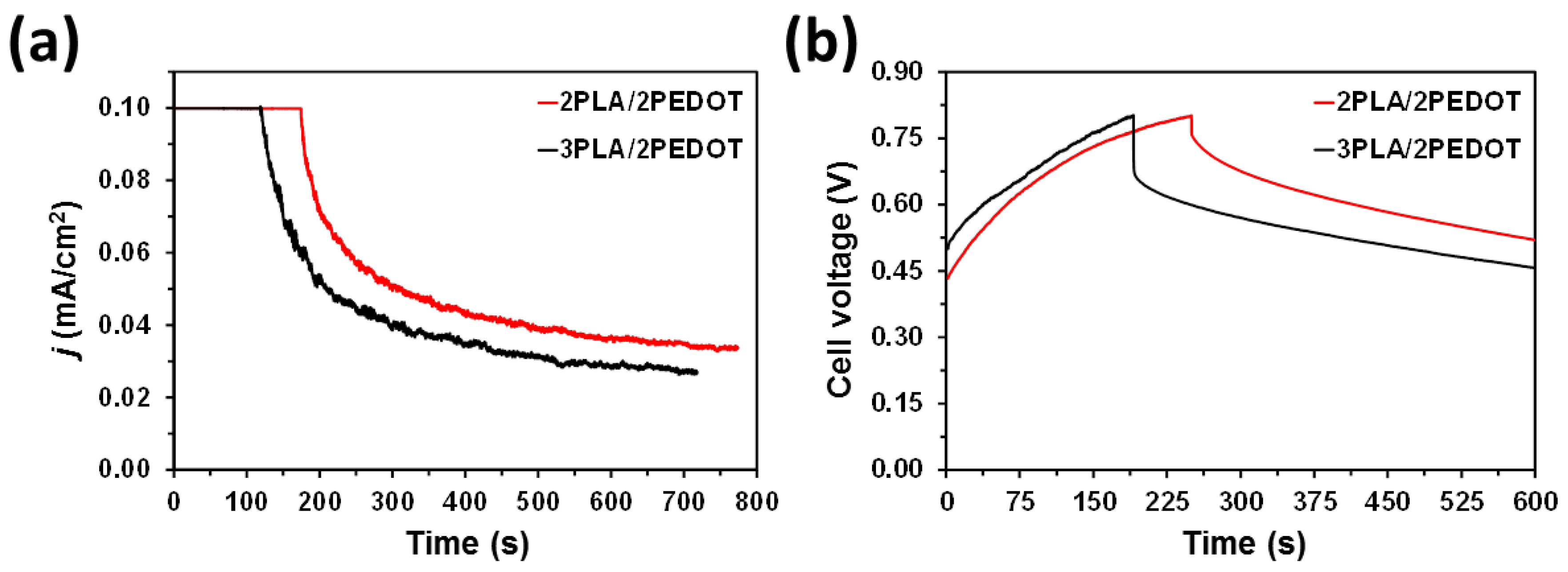
2. Results
3. Methods and Materials
3.1. Materials
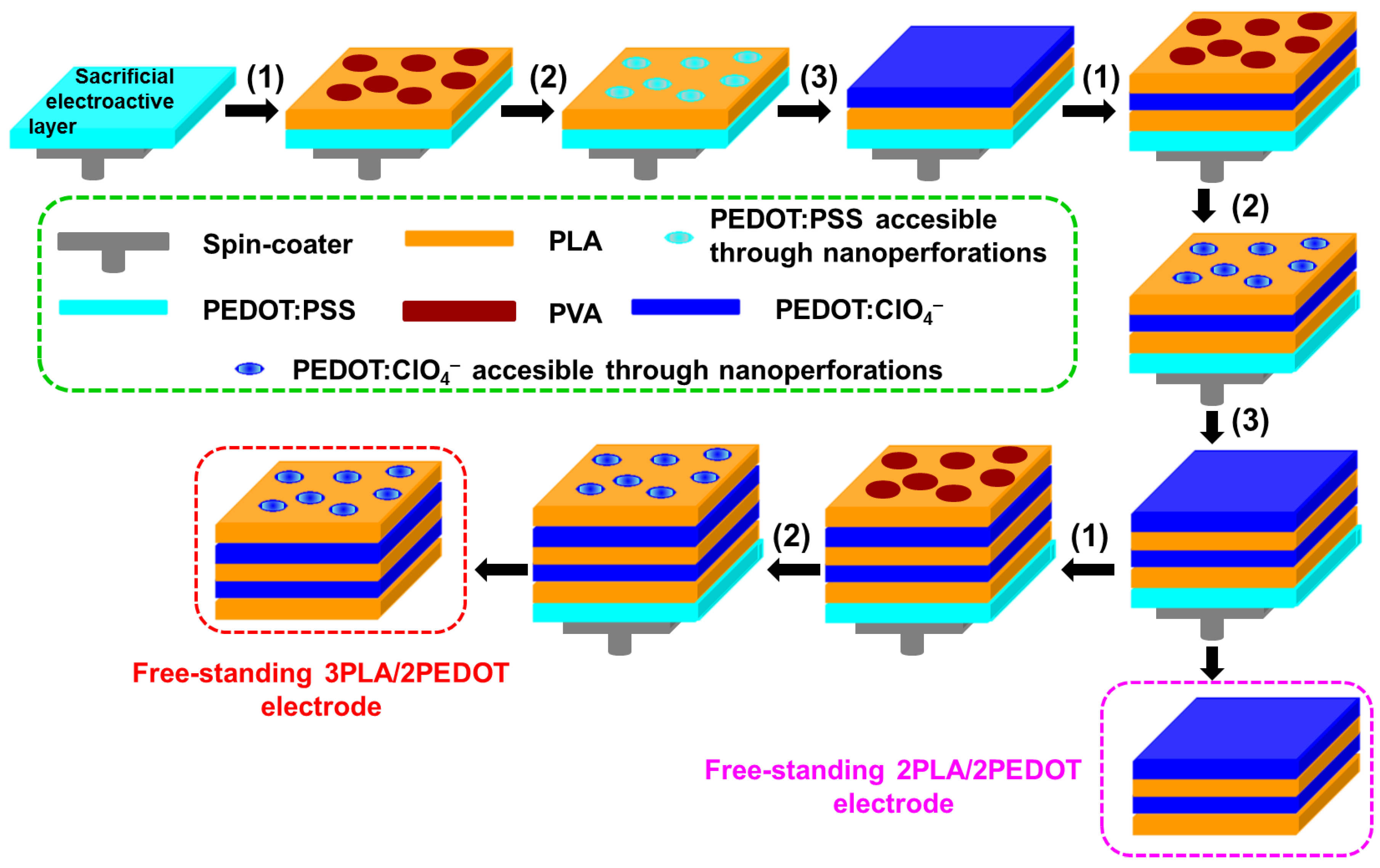
3.2. Preparation of the Films
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Miao, L.; Song, Z.; Zhu, D.; Li, L.; Gan, L.; Liu, M. Recent advances in carbon-based supercapacitors. Mater. Adv. 2020, 1, 945–966. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Chu, Y.; Zhou, Y.; Zhang, C.; Qu, S. Recent advances in stretchable supercapacitors enabled by low-dimensional nanomaterials. Small 2018, 14, e1803976. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Chen, X. Latest advances in flexible symmetric supercapacitors: From material engineering to wearable applications. Acc. Chem. Res. 2020, 18, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Armelin, E.; Pérez-Madrigal, M.M.; Alemán, C.; Díaz, D.D. Current status and challenges of biohydrogels for applications as supercapacitors and secondary batteries. J. Mater. Chem. A 2016, 4, 8952–8968. [Google Scholar] [CrossRef]
- Jost, K.; Dion, G.; Gogotsi, Y. Textile energy storage in perspective. J. Mater. Chem. A 2014, 2, 10776–10787. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Ji, J.; Liu, J.; Zhao, X.; Zhen, Y.; Lin, J.; Zhu, Y.; Ji, H.; Zhang, L.L.; Ruoff, R. In situ activation of nitrogen-doped graphene anchored on graphite foam for a high-capacity anode. ACS Nano 2015, 9, 8609–8616. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Yang, J.D.; Zhou, X.F.; Jiang, S.Q.; Chen, W.; Liu, Z.P. Highly crumpled graphene-like material as compression-resistant electrode material for high energy -power density supercapacitor. Chem. Eng. J. 2020, 397, 125525. [Google Scholar] [CrossRef]
- Guo, F.; Xiao, P.; Yan, B.Y.; Hahn, M.; Kong, Y.Y.; Zhang, W.; Piao, Y.Z.; Diao, G.W. One-pot synthesis of hydrazide-pillar[5]arene functionalized reduced graphene oxide for supercapacitor electrode. Chem. Eng. J. 2020, 391, 123511. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.X.; Acauan, L.; Kalfon-Cohen, E.; Ni, X.C.; Stein, Y.; Gleason, K.K.; Wardle, B.L. Ultrahigh-areal-capacitance flexible supercapacitor electrodes enabled by conformal P3MT on horizontally aligned carbon-nanotube arrays. Adv. Mater. 2019, 31, 1901916. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, C.; Zhao, J.; Juang, J.D.; Xu, H.; Gou, G.J. Creating a new electrode material of supercapacitors from the waste multi-walled carbon nanotubes. Electrochim. Acta 2020, 330, 135237. [Google Scholar]
- Yeo, T.; Lee, J.; Shin, D.; Park, S.; Hwang, H.; Choi, W. One-step fabrication of silver nanosphere-wetted carbon nanotube electrodes via electric-field-driven combustion waves for high-performance flexible supercapacitors. J. Mater. Chem. A 2019, 7, 9004–9018. [Google Scholar] [CrossRef]
- Zhu, J.H.; Zhang, Q.; Zhang, H.P.; Chen, H.P.; Zhang, R.Y.; Liu, L.F.; Yu, J.Y. Setaria viridis-inspired electrode with polyaniline decorated on porous heteroatom-doped carbon nanofibers for flexible supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 43634–43645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, L.; Zhao, J.; Guo, S.W. Effects of sodium alginate on the composition, morphology, and electrochemical properties of electrospun carbon nanofibers as electrodes for supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 632–640. [Google Scholar] [CrossRef]
- Gopalakrishan, A.; Sahatiya, P.; Badhulika, S. Template-assisted electrospinning of bubbled carbon nanofibers as binder-free electrodes for high-performance supercapacitors. Chem. Electro.Chem. 2018, 5, 531–539. [Google Scholar]
- Qu, K.Q.; You, Y.; Qi, H.J.; Shi, C.; Sun, Z.; Huang, Z.H.; Yuan, B.N.; Guo, Z.H. Fungus bran-derived porous n-doped carbon-zinc manganese oxide nanocomposite positive electrodes toward high-performance asymmetric supercapacitors. J. Phys. Chem. C 2020, 124, 15713–15722. [Google Scholar] [CrossRef]
- Biswas, S.; Sharma, V.; Mandal, D.; Chowdhurry, A.; Chalravarty, M.; Priva, S.; De, P.; Singh, I.; Chandra, A.; Gowda, C.C. Hollow nanostructures of metal oxides as emerging electrode materials for high performance supercapacitors. CrystEngComm. 2020, 22, 1633–1644. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, C.H.; Li, N.W.; Yuan, S.; Yu, L. Formation of Co-Mn mixed oxide double-shelled hollow spheres as advanced electrodes for hybrid supercapacitors. J. Mater. Chem. A 2019, 7, 25247–25253. [Google Scholar] [CrossRef]
- Aradilla, D.; Estrany, F.; Aleman, C. Ultraporous poly (3, 4-ethylenedioxythiophene) for nanometric electrochemical supercapacitor. Thin Solid Films 2012, 520, 4402–4409. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Estrany, F.; Armelin, E.; Díaz, D.D.; Alemán, C. Towards sustainable solid-state supercapacitors: Electroactive conducting polymers combined with biohydrogels. J. Mater. Chem. A 2016, 4, 1792–1805. [Google Scholar] [CrossRef]
- Li, K.; Wang, X.H.; Li, S.; Urbankowski, P.; Li, J.M.; Xu, Y.X.; Gogotsi, Y. An ultrafast conducting polymer@MXene positive electrode with high volumetric capacitance for advanced asymmetric supercapacitors. Small 2020, 16, 1906851. [Google Scholar] [CrossRef]
- Xia, C.; Chen, W.; Wang, X.B.; Hedhili, M.N.; Wei, N.N.; Alshareef, H.N. Highly stable supercapacitors with conducting polymer core-shell electrodes for energy storage applications. Adv. Energy Mater. 2015, 5, 1401805. [Google Scholar] [CrossRef]
- Kang, L.; Zhang, M.Y.; Zhang, J.; Liu, S.D.; Zhang, N.; Yao, W.J.; Ye, Y.; Luo, C.; Gong, Z.W.; Wang, C.L.; et al. Dual-defect surface engineering of bimetallic sulfide nanotubes towards flexible asymmetric solid-state supercapacitors. J. Mater. Chem. A 2020, 8, 24053–24064. [Google Scholar] [CrossRef]
- Zhao, W.W.; Jiang, M.Y.; Wang, W.K.; Liu, S.J.; Huang, W.; Zhao, Q. Flexible transparent supercapacitors: Materials and devices. Adv. Funct. Mater. 2020, 31, 2009136. [Google Scholar] [CrossRef]
- Liu, Q.F.; Qiu, J.H.; Yang, C.; Zang, L.M.; Zhang, G.H.; Sakai, E. High-performance PVA/PEDOT:PSS hydrogel electrode for all-gel-state flexible supercapacitors. Adv. Mater. Technol. 2021, 6, 2000919. [Google Scholar] [CrossRef]
- Chen, W.Y.; Wei, T.T.; Mo, L.E.; Wu, S.G.; Li, Z.Q.; Chen, S.H.; Zhang, X.X.; Hu, L.H. CoS2 nanosheets on carbon cloth for flexible all-solid-state supercapacitors. Chem. Eng. J. 2020, 400, 125856. [Google Scholar] [CrossRef]
- Yu, H.M.; Rouelle, N.; Qiu, A.D.; Oh, J.A.; Kempajah, D.M.; Whittle, J.D.; Aakyiir, M.; Xing, W.J.; Ma, J. Hydrogen bonding-reinforced hydrogel electrolyte for flexible, robust, and all-in-one supercapacitor with excellent low-temperature tolerance. ACS Appl. Mater. Interfaces 2020, 12, 37977–37985. [Google Scholar] [CrossRef]
- Ruano, G.; Tononi, J.; Curcó, D.; Puiggalí, J.; Torras, J.; Alemán, C. Doped photo-crosslinked polyesteramide hydrogels as solid electrolytes for supercapacitors. Soft Matter 2020, 16, 8033–8046. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.Y.; Zhou, F.L.; Zhang, Z.Z.; Yang, J. A self-healable and mechanical toughness flexible supercapacitor based on polyacrylic acid hydrogel electrolyte. Chem. Eng. J. 2019, 357, 428–434. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Edo, M.; Saborío, M.G.; Estrany, F.; Aleman, C. Pastes and hydrogels from carboxymethyl cellulose sodium salt as supporting electrolyte of solid electrochemical supercapacitors. Carbohydr. Polym. 2018, 200, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sardana, S.; Dalal, J.; Lather, S.; Maan, A.S.; Tripathi, R.; Punia, R.; Singh, K.; Ohlan, A. Nanostructured polyaniline/graphene/Fe2O3 composites hydrogel as a high-performance flexible supercapacitor electrode material. ACS Appl. Energy Mater. 2020, 3, 6434–6446. [Google Scholar] [CrossRef]
- Saborío, M.G.; Lanzalaco, S.; Fabregat, G.; Puiggalí, J.; Estrany, F.; Alemán, C. Flexible electrodes for supercapacitors based on the supramolecular assembly of biohydrogel and conducting polymer. J. Phys. Chem. C 2018, 122, 1078–1090. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.M.; Zhao, B.X. Electrically conductive hydrogels for flexible energy storage systems. Prog. Polym. Sci. 2019, 88, 220–240. [Google Scholar] [CrossRef]
- Ying, Z.R.; Zhang, Y.Z.; Lin, X.M.; Hui, S.J.; Wang, Y.X.; Yang, Y.B.; Li, Y.C. A biomass-derived super-flexible hierarchically porous carbon film electrode prepared via environment-friendly ice-microcrystal pore-forming for supercapacitors. Chem. Commun. 2020, 56, 10730–10733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhu, Q.Z.; Soomro, R.A.; He, S.Y.; Sun, N.; Qiao, N.; Xu, B. In situ ice template approach to fabricate 3D flexible MXene film-based electrode for high performance supercapacitors. Adv. Funct. Mater. 2020, 30, 2000922. [Google Scholar] [CrossRef]
- Wang, Q.F.; Ma, Y.; Liang, X.; Zhang, D.H.; Miao, M.H. Flexible supercapacitors based on carbon nanotube-MnO2 nanocomposite film electrode. Chem. Eng. J. 2019, 371, 145–153. [Google Scholar] [CrossRef]
- Kwak, C.S.; Ko, T.H.; Lee, J.H.; Kim, H.Y.; Kim, B.S. Flexible transparent symmetric solid-state supercapacitors based on NiO-decorated nanofiber-based composite electrodes with excellent mechanical flexibility and cyclability. ACS Appl. Energy Mater. 2020, 3, 2394–2403. [Google Scholar] [CrossRef]
- Samuel, E.; Joshi, B.; Kim, M.W.; Kim, Y.I.; Swithart, M.T.; Yoon, S.S. Hierarchical zeolitic imidazolate framework-derived manganese-doped zinc oxide decorated carbon nanofiber electrodes for high performance flexible supercapacitors. Chem. Eng. J. 2019, 371, 657–665. [Google Scholar] [CrossRef]
- Park, J.H.; Rana, H.H.; Lee, J.Y.; Park, H.S. Renewable flexible supercapacitors based on all-lignin-based hydrogel electrolytes and nanofiber electrodes. J. Mater. Chem. A 2019, 7, 16962–16968. [Google Scholar] [CrossRef]
- Levchenko, I.; Xu, S.; Baranov, O.; Bazaka, O.; Ivanova, E.P.; Bazaka, K. Plasma and Polymers: Recent Progress and Trends. Molecules 2021, 26, 4091. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar]
- Molina, B.G.; Cuesta, S.; Besharatloo, H.; Roa, J.J.; Armelin, E.; Alemán, C. Free-standing faradaic motors based on biocompatible nanoperforated poly(lactic acid) layers and electropolymerized poly(3,4-ethylenedioxythiophene). ACS Appl. Mater. Interfaces 2019, 11, 29427–29435. [Google Scholar] [CrossRef]
- Molina, B.; Cuesta, S.; Puiggalí-Jou, A.; del Valle, L.J.; Armelin, E.; Alemán, C. Perforated polyester nanomebranes as templates of electroactive and robust free-standing films. Eur. Polym. J. 2019, 114, 213–222. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Torres, J.; Crean, C. Multilayered flexible fibers with high performance for wearable supercapacitor applications. Adv. Sustain. Syst. 2018, 2, 1700143–1700152. [Google Scholar] [CrossRef]
- Aradilla, D.; Estrany, F.; Casellas, F.; Iribarren, J.I.; Aleman, C. All-polythiophene rechargeable batteries. Org. Electron. 2014, 15, 40–46. [Google Scholar] [CrossRef]
- Ujvári, M.; Takács, M.; Vesztergom, S.; Bazsó, F.; Ujhelyi, F.; Láng, G.G. Monitoring of the electrochemical degradation of PEDOT films on gold using the bending beam method. J. Solid State Electrochem. 2011, 15, 2341–2349. [Google Scholar] [CrossRef]
- Saborío, M.C.G.; Estrany, F.; Alemán, C. Properties of in situ polymerized poly(3,4-ethylenedioxythiophene)/alumina composites for energy storage applications. J. Polym. Sci. Part B: Polym. Phys. 2017, 55, 1131–1141. [Google Scholar] [CrossRef][Green Version]
- Andreas, H.A. Self-discharge in electrochemical capacitors: A perspective. J. Electrochem. Soc. 2015, 162, A5047. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruano, G.; Molina, B.G.; Torras, J.; Alemán, C. Free-Standing, Flexible Nanofeatured Polymeric Films Prepared by Spin-Coating and Anodic Polymerization as Electrodes for Supercapacitors. Molecules 2021, 26, 4345. https://doi.org/10.3390/molecules26144345
Ruano G, Molina BG, Torras J, Alemán C. Free-Standing, Flexible Nanofeatured Polymeric Films Prepared by Spin-Coating and Anodic Polymerization as Electrodes for Supercapacitors. Molecules. 2021; 26(14):4345. https://doi.org/10.3390/molecules26144345
Chicago/Turabian StyleRuano, Guillem, Brenda G. Molina, Juan Torras, and Carlos Alemán. 2021. "Free-Standing, Flexible Nanofeatured Polymeric Films Prepared by Spin-Coating and Anodic Polymerization as Electrodes for Supercapacitors" Molecules 26, no. 14: 4345. https://doi.org/10.3390/molecules26144345
APA StyleRuano, G., Molina, B. G., Torras, J., & Alemán, C. (2021). Free-Standing, Flexible Nanofeatured Polymeric Films Prepared by Spin-Coating and Anodic Polymerization as Electrodes for Supercapacitors. Molecules, 26(14), 4345. https://doi.org/10.3390/molecules26144345