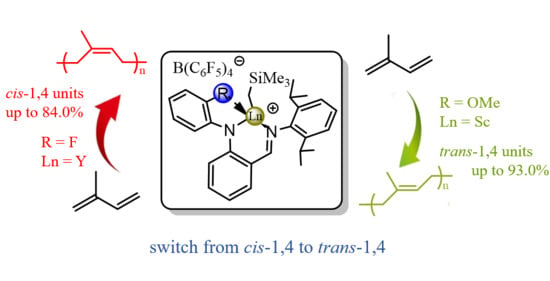
Side-Arm Assisted Anilido-Imine Based Rare-Earth Metal Complexes for Isoprene Stereoselective Polymerization
Abstract
:1. Introduction
2. Results and Discussion
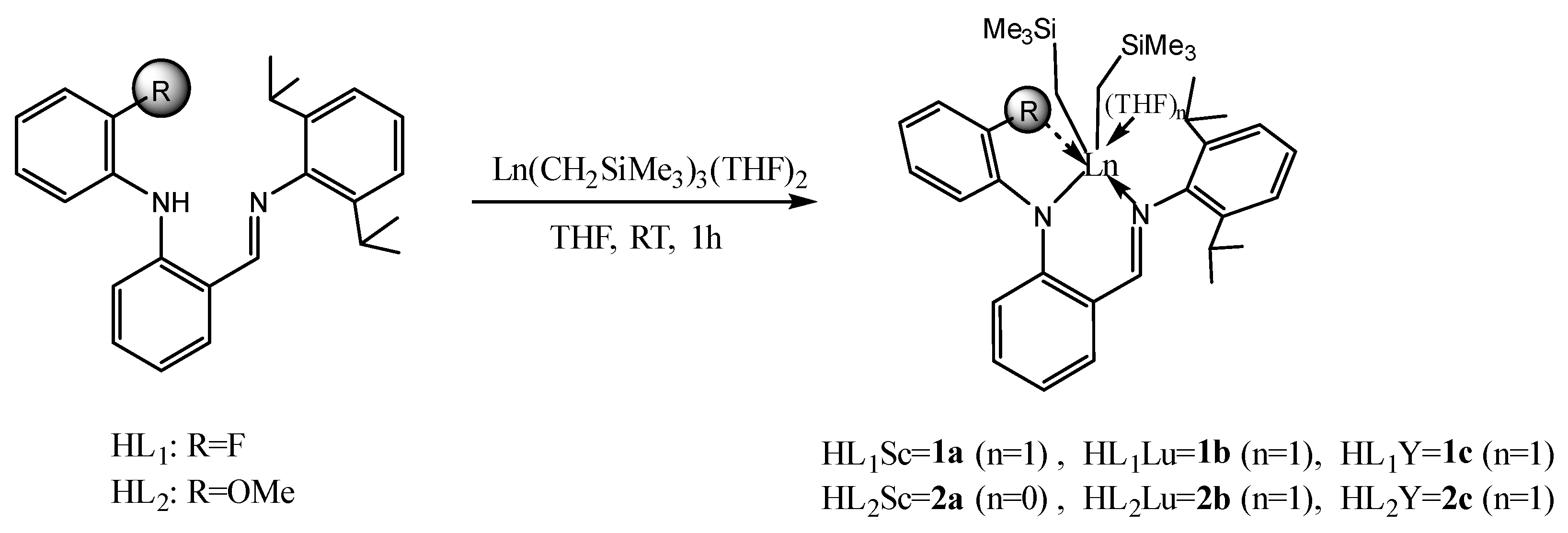
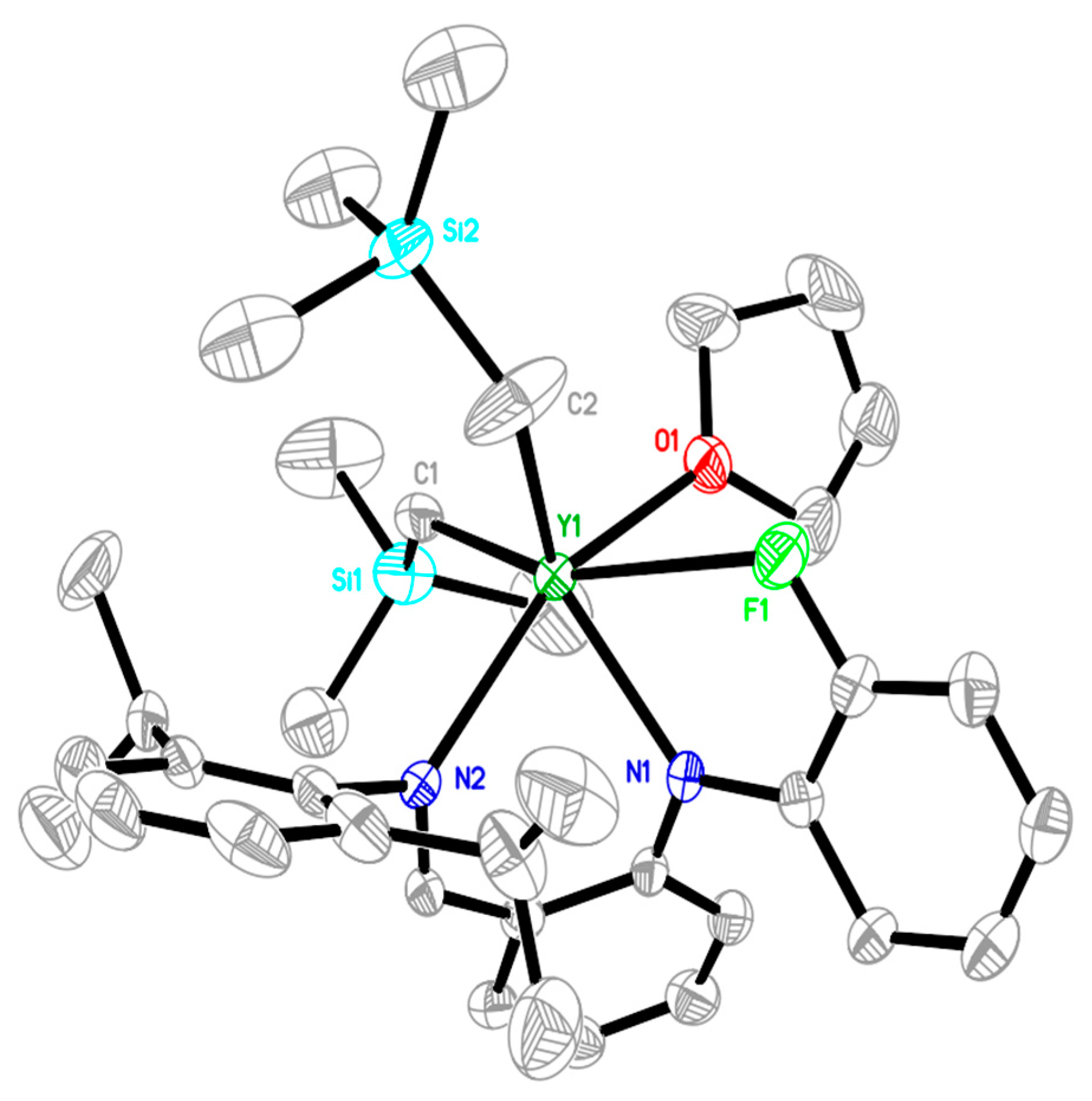
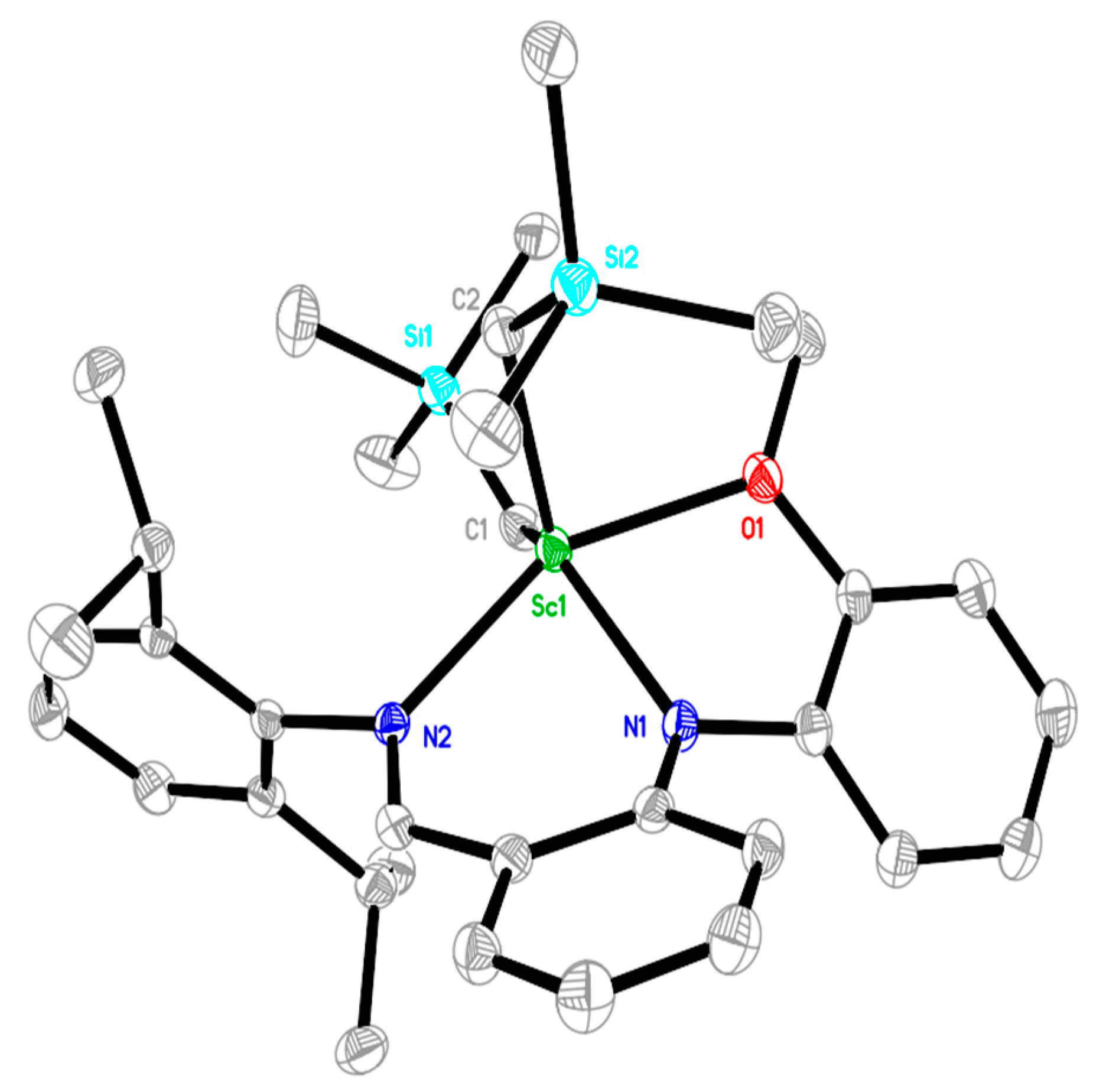
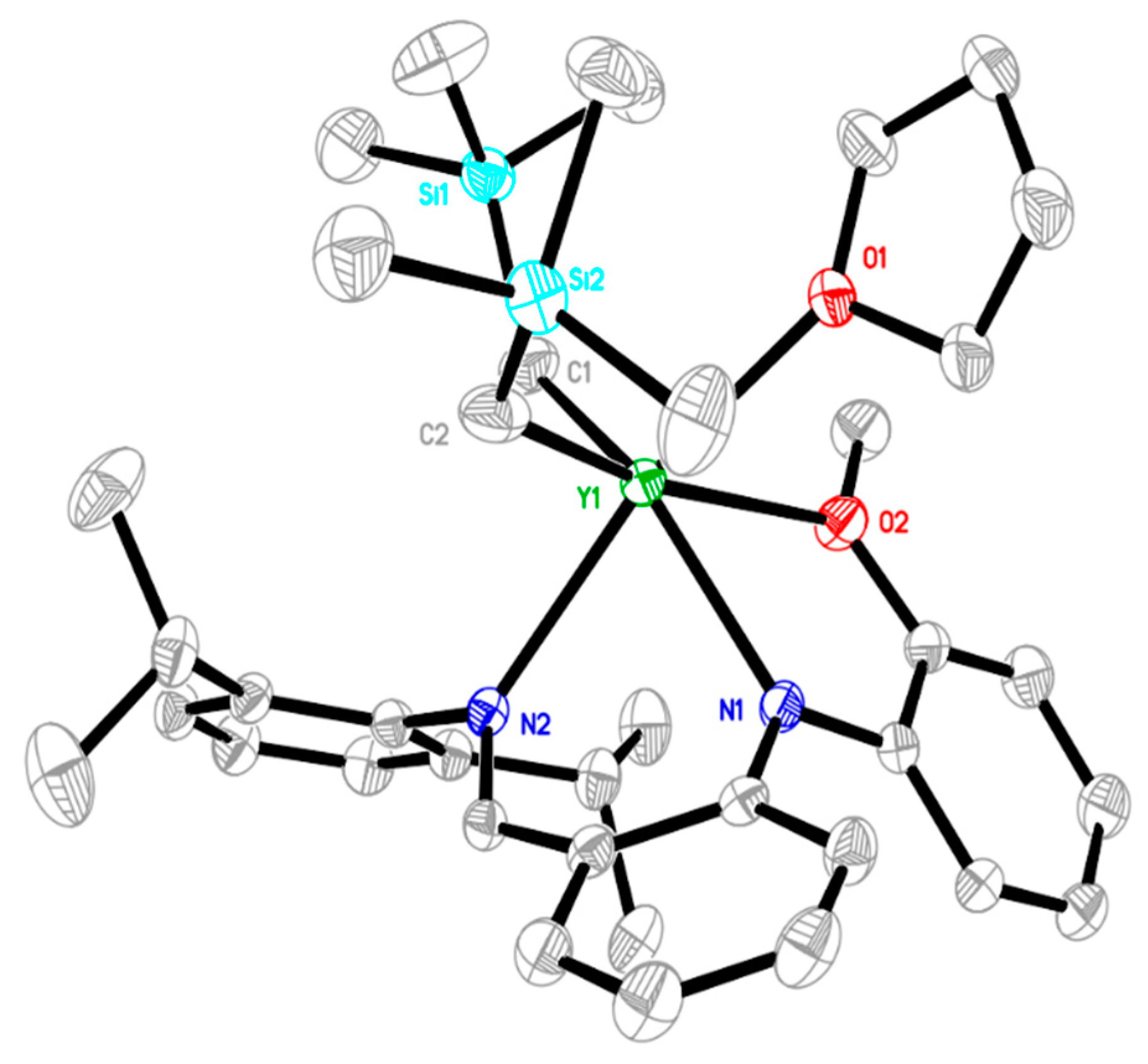
2.1. Synthesis and Characterization of Rare-Earth-Metal Complexes
2.2. Polymerization of Isoprene
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.2.1. Synthesis of o-C6H4NH(C6H4-F-o)(CH=NC6H3-i-Pr2-2,6) (L1)
3.2.2. Synthesis of o-C6H4NH(C6H4-OMe-o)(CH=NC6H3-i-Pr2-2,6) (L2)
3.2.3. Synthesis of L1Sc(CH2SiMe3)2(THF) (1a)
3.2.4. Synthesis of L1Lu(CH2SiMe3)2(THF) (1b)
3.2.5. Synthesis of L1Y(CH2SiMe3)2(THF) (1c)
3.2.6. Synthesis of L2Sc(CH2SiMe3)2 (2a)
3.2.7. Synthesis of L2Lu(CH2SiMe3)2(THF) (2b)
3.2.8. Synthesis of L2Y(CH2SiMe3)2(THF) (2c)
3.3. Polymerization of Isoprene
3.4. X-ray Crystallographic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rong, W.; Liu, D.; Zuo, H.; Pan, Y.; Jian, Z.; Li, S.; Cui, D. Rare-Earth-Metal Complexes Bearing Phosphazene Ancillary Ligands: Structures and Catalysis toward Highly Trans-1,4-Selective (Co)Polymerizations of Conjugated Dienes. Organometallics 2012, 32, 1166–1175. [Google Scholar] [CrossRef]
- Liu, B.; Sun, G.; Li, S.; Liu, D.; Cui, D. Isoprene Polymerization with Iminophosphonamide Rare-Earth-Metal Alkyl Complexes: Influence of Metal Size on the Regio- and Stereoselectivity. Organometallics 2015, 34, 4063–4068. [Google Scholar] [CrossRef]
- Evans, W.J.; Giarikos, D.G. Chloride Effects in Lanthanide Carboxylate Based Isoprene Polymerization. Macromolecules 2004, 37, 5130–5132. [Google Scholar] [CrossRef]
- Evans, W.J.; Giarikos, D.G.; Ziller, J.W. Lanthanide Carboxylate Precursors for Diene Polymerization Catalysis: Syntheses, Structures, and Reactivity with Et2AlCl. Organometallics 2001, 20, 5751–5758. [Google Scholar] [CrossRef]
- Fischbach, A.; Klimpel, M.G.; Widenmeyer, M.; Herdtweck, E.; Scherer, W.; Anwander, R. Stereospecific Polymerization of Isoprene with Molecular and MCM-48-Grafted Lanthanide(III) Tetraalkylaluminates. Angew. Chem. Int. Ed. 2004, 43, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Ajellal, N.; Furlan, L.; Thomas, C.M.; Jr, O.L.C.; Carpentier, J.-F. Mixed Aluminum-Magnesium-Rare Earth Allyl Catalysts for Controlled Isoprene Polymerization: Modulation of Stereocontrol. Macromol. Rapid Commun. 2006, 27, 338–343. [Google Scholar] [CrossRef]
- Zhang, L.; Suzuki, T.; Luo, Y.; Nishiura, M.; Hou, Z. Cationic Alkyl Rare-Earth Metal Complexes Bearing an Ancillary Bis(phosphinophenyl)amido Ligand: A Catalytic System for Livingcis-1,4-Polymerization and Copolymerization of Isoprene and Butadiene. Angew. Chem. Int. Ed. 2007, 46, 1909–1913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Nishiura, M.; Yuki, M.; Luo, Y.; Hou, Z. Isoprene Polymerization with Yttrium Amidinate Catalysts: Switching the Regio- and Stereoselectivity by Addition of AlMe3. Angew. Chem. Int. Ed. 2008, 47, 2642–2645. [Google Scholar] [CrossRef]
- Zhang, G.; Deng, B.; Wang, S.; Wei, Y.; Zhou, S.; Zhu, X.; Huang, Z.; Mu, X. Di and trinuclear rare-earth metal complexes supported by 3-amido appended indolyl ligands: Synthesis, characterization and catalytic activity towards isoprene 1,4-cis polymerization. Dalton Trans. 2016, 45, 15445–15456. [Google Scholar] [CrossRef]
- Pan, Y.; Li, W.; Wei, N.-N.; So, Y.-M.; Li, Y.; Jiang, K.; He, G. Anilido-oxazoline-ligated rare-earth metal complexes: Synthesis, characterization and highly cis-1,4-selective polymerization of isoprene. Dalton Trans. 2019, 48, 3583–3592. [Google Scholar] [CrossRef]
- Li, L.; Wu, C.; Liu, D.; Li, S.; Cui, D. Binuclear Rare-Earth-Metal Alkyl Complexes Ligated by Phenylene-Bridged β-Diketiminate Ligands: Synthesis, Characterization, and Catalysis toward Isoprene Polymerization. Organometallics 2013, 32, 3203–3209. [Google Scholar] [CrossRef]
- Jian, Z.; Tang, S.; Cui, N. Highly Regio- and Stereoselective Terpolymerization of Styrene, Isoprene and Butadiene with Lutetium-Based Coordination Catalyst. Macromolecules 2011, 44, 7675–7681. [Google Scholar] [CrossRef]
- Gao, W.; Cui, D. Highly cis-1,4 Selective Polymerization of Dienes with Homogeneous Ziegler–Natta Catalysts Based on NCN-Pincer Rare Earth Metal Dichloride Precursors. J. Am. Chem. Soc. 2008, 130, 4984–4991. [Google Scholar] [CrossRef]
- Lv, K.; Cui, D. CCC-Pincer Bis(carbene) Lanthanide Dibromides. Catalysis on Highly cis-1,4-Selective Polymerization of Isoprene and Active Species. Organometallics 2010, 29, 2987–2993. [Google Scholar] [CrossRef]
- Wang, L.; Cui, D.; Hou, Z.; Li, W.; Li, Y. Highly Cis-1,4-Selective Living Polymerization of 1,3-Conjugated Dienes and Copolymerization with ε-Caprolactone by Bis(phosphino)carbazolide Rare-Earth-Metal Complexes. Organometallics 2011, 30, 760–767. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Z.; Cui, D.; Liu, X. Precisely Controlled Polymerization of Styrene and Conjugated Dienes by Group 3 Single-Site Catalysts. ChemCatChem 2017, 10, 42–61. [Google Scholar] [CrossRef]
- Yao, C.; Liu, D.; Li, P.; Wu, C.; Li, S.; Liu, B.; Cui, D. Highly 3,4-Selective Living Polymerization of Isoprene and Copolymerization with ε-Caprolactone by an Amidino N-Heterocyclic Carbene Ligated Lutetium Bis(alkyl) Complex. Organometallics 2014, 33, 684–691. [Google Scholar] [CrossRef]
- Li, S.; Cui, D.; Li, D.; Hou, Z. Highly 3,4-Selective Polymerization of Isoprene with NPN Ligand Stabilized Rare-Earth Metal Bis(alkyl)s. Structures and Performances. Organometallics 2009, 28, 4814–4822. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.; Hou, Z. Unprecedented Isospecific 3,4-Polymerization of Isoprene by Cationic Rare Earth Metal Alkyl Species Resulting from a Binuclear Precursor. J. Am. Chem. Soc. 2005, 127, 14562–14563. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Lv, K.; Wang, L.; Wang, Y.; Cui, D. Isoprene polymerization with aminopyridinato ligand supported rare-earth metal complexes. Switching of the regio- and stereoselectivity. Chem. Commun. 2010, 46, 6150–6152. [Google Scholar] [CrossRef]
- Liu, D.; Cui, D. Highly trans-1,4 selective (co-)polymerization of butadiene and isoprene with quinolyl anilido rare earth metal bis(alkyl) precursors. Dalton Trans. 2011, 40, 7755–7761. [Google Scholar] [CrossRef]
- Bonnet, F.; Jones, C.E.; Semlali, S.; Bria, M.; Visseaux, M.; Roussel, P.; Arnold, P.L. Tuning the catalytic properties of rare earth borohydrides for the polymerisation of isoprene. Dalton Trans. 2013, 42, 790–801. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, F.; Visseaux, M.; Pereira, A.; Barbier-Baudry, D. Highlytrans-Stereospecific Isoprene Polymerization by Neodymium Borohydrido Catalystst. Macromolecules 2005, 38, 3162–3169. [Google Scholar] [CrossRef] [Green Version]
- Litlabø, R.; Enders, M.; Törnroos, K.W.; Anwander, R. Bis(tetramethylaluminate) Complexes of Yttrium and Lanthanum Supported by a Quinolyl-Substituted Cyclopentadienyl Ligand: Synthesis and Performance in Isoprene Polymerization. Organometallics 2010, 29, 2588–2595. [Google Scholar] [CrossRef]
- Diether, D.; Tyulyunov, K.; Maichle-Mössmer, C.; Anwander, R. Fluorenyl Half-Sandwich Bis(tetramethylaluminate) Complexes of the Rare-Earth Metals: Synthesis, Structure, and Isoprene Polymerization. Organometallics 2017, 36, 4649–4659. [Google Scholar] [CrossRef]
- Liu, H.; He, J.; Liu, Z.; Lin, Z.; Du, G.; Zhang, S.; Li, X. Quasi-Livingtrans-1,4-Polymerization of Isoprene by Cationic Rare Earth Metal Alkyl Species Bearing a Chiral (S,S)-Bis(oxazolinylphenyl)amido Ligand. Macromolecules 2013, 46, 3257–3265. [Google Scholar] [CrossRef]
- Kaita, S.; Yamanaka, M.; Horiuchi, A.C.; Wakatsuki, Y. Butadiene Polymerization Catalyzed by Lanthanide Metallocene–Alkylaluminum Complexes with Cocatalysts: Metal-Dependent Control of 1,4-Cis/Trans Stereoselectivity and Molecular Weight. Macromolecules 2006, 39, 1359–1363. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Z.; Sun, X.; Tang, Y. Synthesis, characterization, and catalytic behaviours of β-carbonylenamine-derived [O−NS]TiCl3 complexes in ethylene homo- and copolymerization. Dalton Trans. 2009, 41, 8945–8954. [Google Scholar] [CrossRef]
- Liao, S.; Sun, X.-L.; Tang, Y. Side Arm Strategy for Catalyst Design: Modifying Bisoxazolines for Remote Control of Enantioselection and Related. Accounts Chem. Res. 2014, 47, 2260–2272. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; An, H.; Gao, W.; Mu, Y. Chromium(III) Complexes with Chelating Anilido-Imine Ligands: Synthesis, Structures, and Catalytic Properties for Ethylene Polymerization. Eur. J. Inorg. Chem. 2010, 2010, 3360–3364. [Google Scholar] [CrossRef]
- Gao, H.; Guo, W.; Bao, F.; Gui, G.; Zhang, J.; Zhu, A.F.; Wu, Q. Synthesis, Molecular Structure, and Solution-Dependent Behavior of Nickel Complexes Chelating Anilido–Imine Donors and Their Catalytic Activity toward Olefin Polymerization. Organometallics 2004, 23, 6273–6280. [Google Scholar] [CrossRef]
- Li, S.; Miao, W.; Tang, T.; Dong, W.; Zhang, X.; Cui, D. New Rare Earth Metal Bis(alkyl)s Bearing an Iminophosphonamido Ligand. Synthesis and Catalysis toward Highly 3,4-Selective Polymerization of Isoprene. Organometallics 2008, 27, 718–725. [Google Scholar] [CrossRef]
- Kang, X.; Luo, Y.; Zhou, G.; Wang, X.; Yu, X.; Hou, Z.; Qu, J. Theoretical Mechanistic Studies on thetrans-1,4-Specific Polymerization of Isoprene Catalyzed by a Cationic La–Al Binuclear Complex. Macromolecules 2014, 47, 4596–4606. [Google Scholar] [CrossRef]
- Tobisch, S. Mechanistic insight into the selective trans-1,4-polymerization of butadiene by terpyridine–iron(II) complexes—A computational study. Can. J. Chem. 2009, 87, 1392–1405. [Google Scholar] [CrossRef]
- Porri, L.; Giarrusso, A.; Ricci, G. Recent views on the mechanism of diolefin polymerization with transition metal initiator systems. Prog. Polym. Sci. 1991, 16, 405–441. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.; Liu, X.; Gao, W.; Cui, D. Thiophene-NPN Ligand Supported Rare-Earth Metal Bis(alkyl) Complexes. Synthesis and Catalysis toward Highly trans-1,4 Selective Polymerization of Butadiene. Organometallics 2008, 27, 6531–6538. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, D.; Cui, D. Highly Selective Polymerization of 1,3-Conjugated Dienes by Rare Earth Organometallic Complexes. Acta Polym. Sin. 2015, 989–1009. [Google Scholar] [CrossRef]
- Jian, Z.; Cui, D.; Hou, Z.; Li, X. Living catalyzed-chain-growth polymerization and block copolymerization of isoprene by rare-earth metal allyl precursors bearing a constrained-geometry-conformation ligand. Chem. Commun. 2010, 46, 3022–3024. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, B.; Lin, F.; Wang, M.; Cui, D. cis -1,4-Selective Copolymerization of Ethylene and Butadiene: A Compromise between Two Mechanisms. Angew. Chem. Int. Ed. 2017, 56, 6975–6979. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Wu, C.; Cui, D. Sequence-controlled ethylene/styrene copolymerization catalyzed by scandium complexes. Polym. Chem. 2018, 10, 235–243. [Google Scholar] [CrossRef]
- Piers, W.E.; Chivers, T. Pentafluorophenylboranes: From obscurity to applications. Chem. Soc. Rev. 1997, 26, 345–354. [Google Scholar] [CrossRef]

| Entry | Cat | AliBu3/Cat | T (°C) | Time (h) | Conv. (%) | Microstructure (%) cis-1,4/trans-1,4/3,4 | Mnb(104) | PDI b | Tg/Tmc(°C) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1a | 10 | 25 | 0.5 | >99 | 57.3/25.6/17.1 | 4.03 | 1.62 | −55.9/-- |
| 2 | 1b | 10 | 25 | 0.5 | >99 | 64.2/21.7/14.1 | 5.83 | 1.64 | −60.3/-- |
| 3 | 1c | 10 | 25 | 0.5 | >99 | 84.0/6.7/9.3 | 6.24 | 1.12 | −59.9/-- |
| 4 | 2a | 10 | 25 | 0.5 | >99 | 1.7/93.0/5.3 | 2.05 | 1.54 | −69.7/36.7 |
| 5 | 2a | 10 | −30 | 4 | 98 | -/97.0/3.0 | 2.03 | 1.34 | −68.3/37.8 |
| 6 | 2b | 10 | 25 | 0.5 | >99 | 36.3/58.3/5.4 | 5.76 | 1.29 | −66.5/-- |
| 7 | 2c | 10 | 25 | 0.5 | >99 | 83.3/15.2/1.5 | 6.05 | 1.16 | −64.8/-- |
| 8 | 2a | 2 | 25 | 0.5 | >99 | 1.4/94.1/4.5 | 4.58 | 1.51 | −69.6/36.5 |
| 9 | 2a | 5 | 25 | 0.5 | >99 | 1.5/93.6/4.9 | 3.08 | 1.61 | −69.3/37.6 |
| 10 | 2a | 20 | 25 | 0.5 | >99 | 1.8/92.8/5.4 | 1.34 | 1.56 | −69/36.4 |
| 11 | 2a | 40 | 25 | 2 | >99 | 2.0/92.5/5.5 | 0.85 | 1.53 | −69.5/36.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Liu, X.; Cui, D. Side-Arm Assisted Anilido-Imine Based Rare-Earth Metal Complexes for Isoprene Stereoselective Polymerization. Molecules 2021, 26, 4154. https://doi.org/10.3390/molecules26144154
Wu Y, Liu X, Cui D. Side-Arm Assisted Anilido-Imine Based Rare-Earth Metal Complexes for Isoprene Stereoselective Polymerization. Molecules. 2021; 26(14):4154. https://doi.org/10.3390/molecules26144154
Chicago/Turabian StyleWu, Yi, Xinli Liu, and Dongmei Cui. 2021. "Side-Arm Assisted Anilido-Imine Based Rare-Earth Metal Complexes for Isoprene Stereoselective Polymerization" Molecules 26, no. 14: 4154. https://doi.org/10.3390/molecules26144154
APA StyleWu, Y., Liu, X., & Cui, D. (2021). Side-Arm Assisted Anilido-Imine Based Rare-Earth Metal Complexes for Isoprene Stereoselective Polymerization. Molecules, 26(14), 4154. https://doi.org/10.3390/molecules26144154