Effect of Single and Dual Hydrothermal Treatments on the Resistant Starch Content and Physicochemical Properties of Lotus Rhizome Starches
Abstract
1. Introduction
2. Results and Discussion
2.1. Proximate Compositions
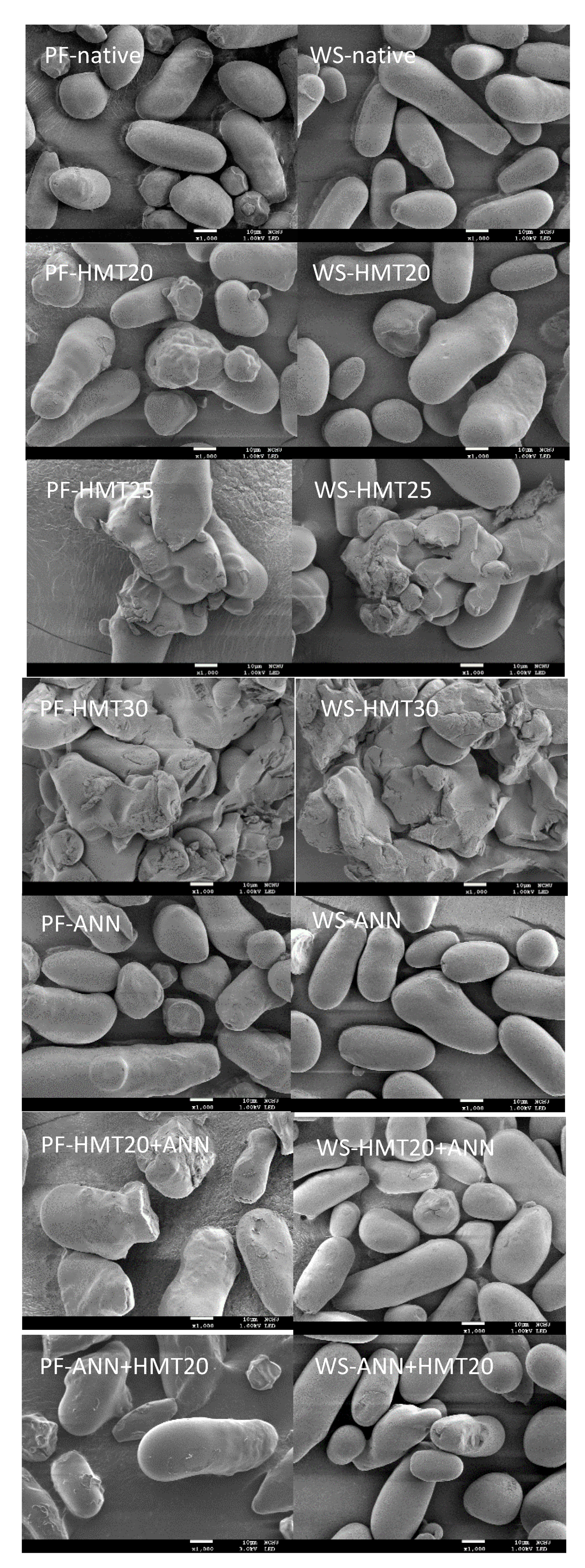
2.2. Granule Morphology
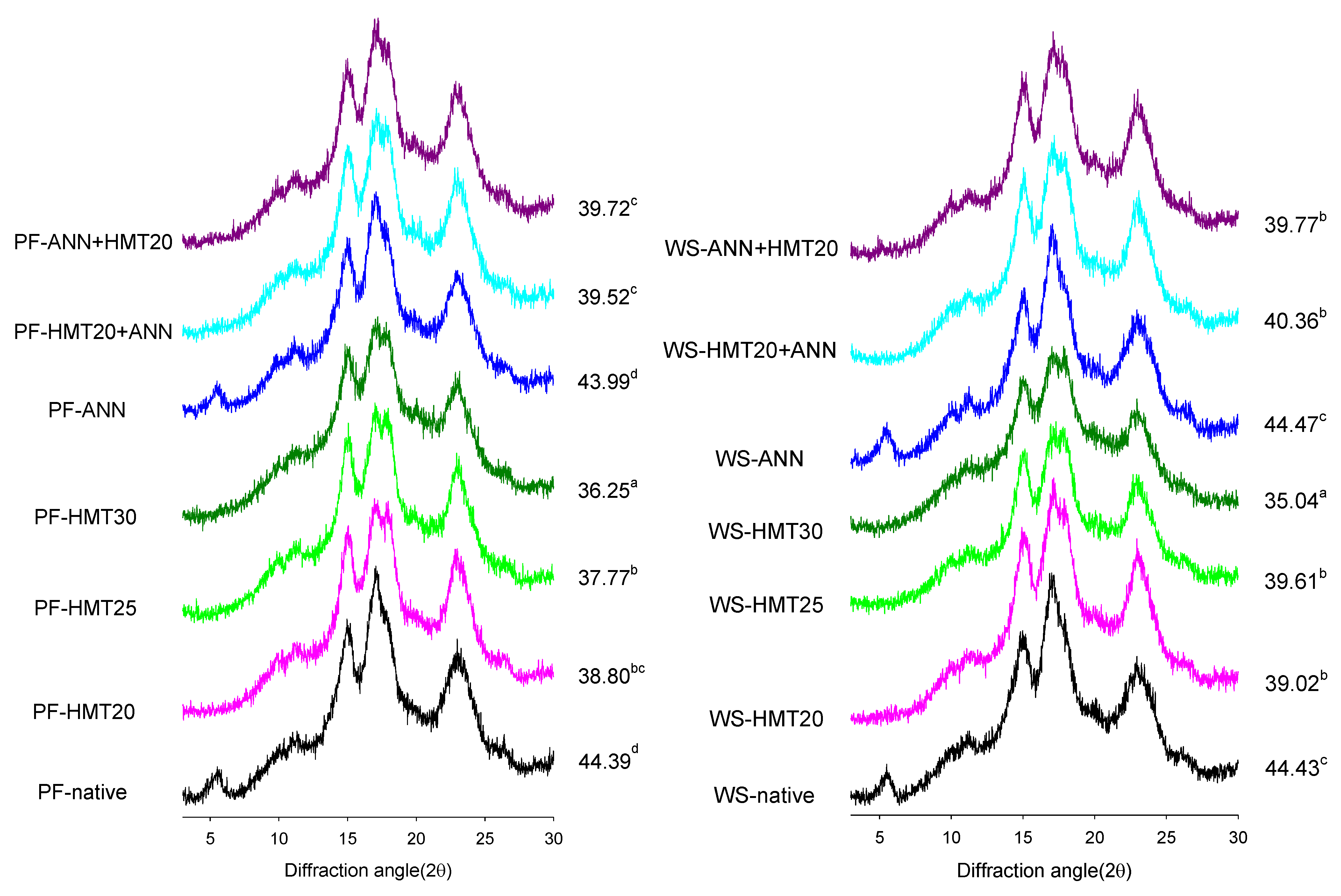
2.3. X-ray Diffraction (XRD) and Relative Crystallinity (RC)
2.4. Damage Starch and Amylose Content
2.5. Resistant Starch (RS) Content
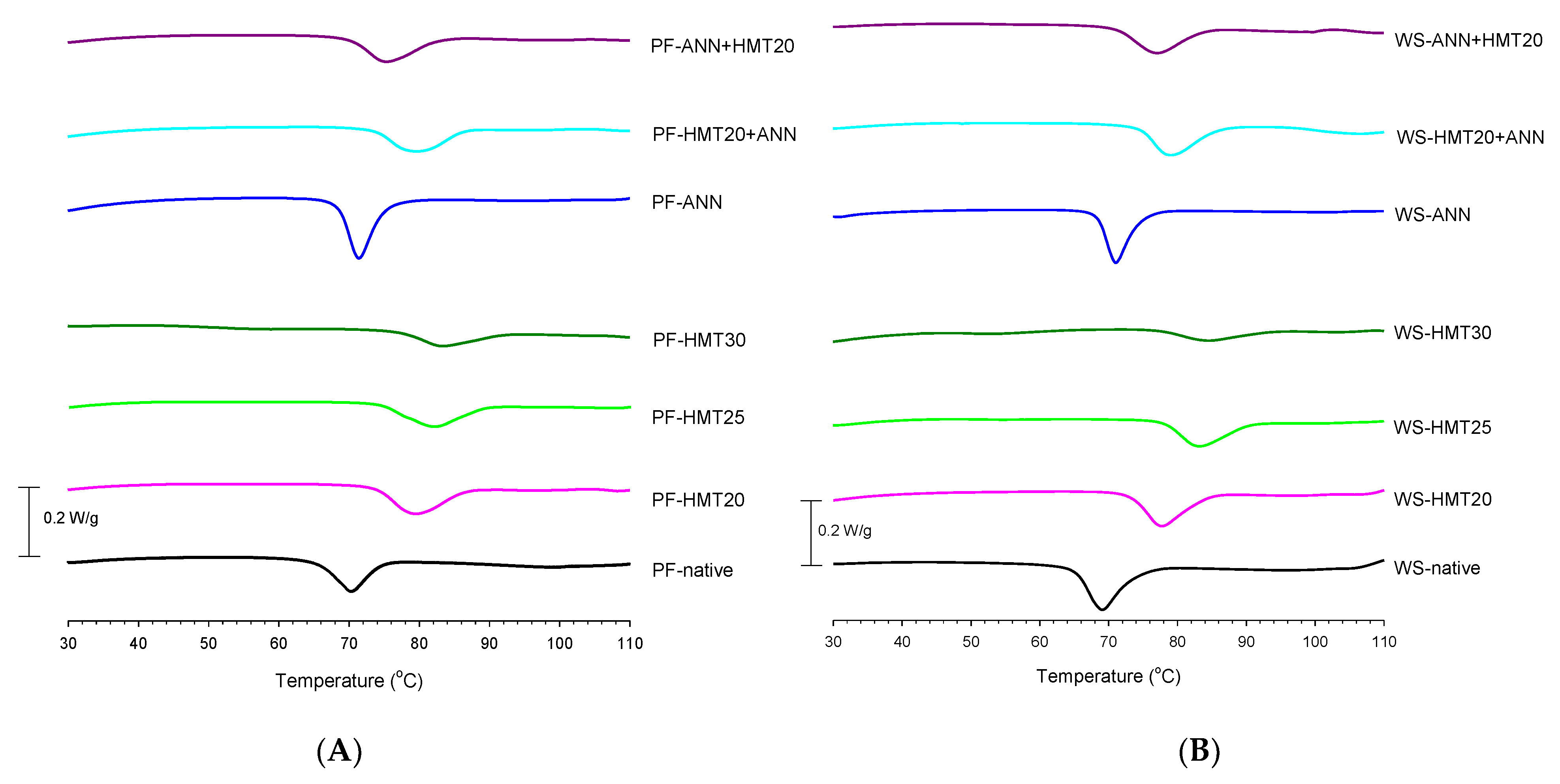
2.6. Thermal Properties
2.7. Pasting Properties
3. Materials and Methods
3.1. Materials
3.2. Starch Isolation
3.3. Hydrothermal Treatments
3.3.1. Annealing Treatment (ANN)
3.3.2. Heat-Moisture Treatment (HMT)
3.3.3. Dual Hydrothermal Modification
3.4. Proximate Compositions
3.5. Morphological Observation
3.5.1. Light Micrograph
3.5.2. Scanning Electron Micrograph
3.6. Crystallinity by X-ray Diffraction (XRD)
3.7. Damage Starch and Amylose Content
3.8. Resistant Starch (RS) Content
3.9. Thermal Properties by Differential Scanning Calorimetry (DSC)
3.10. Pasting Properties by Rapid Visco Analysis (RVA)
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayakody, L.; Hoover, R. Effect of annealing on the molecular structure and physicochemical properties of starches from different botanical origins—A review. Carbohydr. Polym. 2008, 74, 691–703. [Google Scholar] [CrossRef]
- Hoover, R. The impact of heat-moisture treatment on molecular structures and properties of starches isolated from different botanical sources. Crit. Rev. Food Sci. Nutr. 2010, 50, 835–847. [Google Scholar] [CrossRef]
- Varatharajan, V.; Hoover, R.; Liu, Q.; Seetharaman, K. The impact of heat-moisture treatment on the molecular structure and physicochemical properties of normal and waxy potato starches. Carbohydr. Polym. 2010, 81, 466–475. [Google Scholar] [CrossRef]
- Cai, C.; Cai, J.; Man, J.; Yang, Y.; Wang, Z.; Wei, C. Allomorph distribution and granule structure of lotus rhizome C-type starch during gelatinization. Food Chem. 2014, 142, 408–415. [Google Scholar] [CrossRef]
- Lee, H.K.; Choi, Y.M.; Noh, D.O.; Suh, H.J. Antioxidant effect of Korean traditional Lotus Liquor (Yunyupju). Int. J. Food Sci. Technol. 2005, 40, 709–715. [Google Scholar] [CrossRef]
- Zhong, G.; Chen, Z.D.; We, Y.M. Physicochemical properties of lotus (Nelumbo nucifera Gaertn.) and kudzu (Pueraria hirsute Matsum.) starches. Int. J. Food Sci. Technol. 2007, 42, 1449–1455. [Google Scholar]
- Suzuki, A.; Kaneyama, M.; Shibanuma, K.; Takeda, Y.; Abe, J.; Hizukuri, S. Characterization of lotus starch. Cereal Chem. 1992, 69, 309–315. [Google Scholar]
- Man, J.; Cai, J.; Cai, C.; Xu, B.; Huai, H.; Wei, C. Comparison of physicochemical properties of starches from seed and rhizome of lotus. Carbohydr. Polym. 2012, 88, 676–683. [Google Scholar] [CrossRef]
- Ali, N.A.; Dash, K.K.; Routray, W. Physicochemical characterization of modified lotus seed starch obtained through acid and heat moisture treatment. Food Chem. 2020, 319, 126513. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, T.; Bian, X.; Hua, Z.; Chen, G.; Wu, X. Structural characterization and physicochemical properties of starch from four aquatic vegetable varieties in China. Int. J. Biol. Macromol. 2021, 172, 542–549. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Ma, C. Preparation, physicochemical characterization and application of acetylated lotus rhizome starches. Carbohydr. Polym. 2016, 135, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Chang, S.; Yang, C.; Lii, C. Studies on the starches in Taiwan 3. Lotus tuber and seed starches. Food Sci. 1978, 5, 88–98. [Google Scholar]
- Lin, H.-M.; Chang, Y.-H.; Lin, J.-H.; Jane, J.-L.; Sheu, M.-J.; Lu, T.-J. Heterogeneity of lotus rhizome starch granules as revealed by α-amylase degradation. Carbohydr. Polym. 2006, 66, 528–536. [Google Scholar] [CrossRef]
- Akiko, K.; Naoaki, T.; Emako, M.; Shigeru, S.; Toshiaki, K.; Kenichi, K. Microscopic observation and X-ray diffractometry of heat/moisture-treated starch granules. Starch Stärke 1994, 46, 463–469. [Google Scholar]
- Li, S.; Ward, R.; Gao, Q. Effect of heat-moisture treatment on the formation and physicochemical properties of resistant starch from mung bean (Phaseolus radiatus) starch. Food Hydrocoll. 2011, 25, 1702–1709. [Google Scholar] [CrossRef]
- Chung, H.-J.; Liu, Q.; Hoover, R. Effect of single and dual hydrothermal treatments on the crystalline structure, thermal properties, and nutritional fractions of pea, lentil, and navy bean starches. Food Res. Int. 2010, 43, 501–508. [Google Scholar] [CrossRef]
- Kiseleva, V.I.; Krivandin, A.V.; Fornal, J.; Błaszczak, W.; Jeliński, T.; Yuryev, V.P. Annealing of normal and mutant wheat starches. LM, SEM, DSC, and SAXS studies. Carbohydr. Res. 2005, 340, 75–83. [Google Scholar] [CrossRef]
- Liu, H.; Lv, M.; Wang, L.; Li, Y.; Fan, H.; Wang, M. Comparative study: How annealing and heat-moisture treatment affect the digestibility, textural, and physicochemical properties of maize starch. Starch Stärke 2016, 68, 1158–1168. [Google Scholar] [CrossRef]
- Cahyana, Y.; Wijaya, E.; Halimah, T.S.; Marta, H.; Suryadi, E.; Kurniati, D. The effect of different thermal modifications on slowly digestible starch and physicochemical properties of green banana flour (Musa acuminata colla). Food Chem. 2019, 274, 274–280. [Google Scholar] [CrossRef]
- Costa, S.D.S.; Almeida, M.C.B.D.M.; Almeida, E.; Cavalcanti, M.T. Effects of low heat-moisture treatment in Prata Green Banana Starch (Musa AAB-Prata). Food Bioprocess. Technol. 2019, 12, 1938–1944. [Google Scholar] [CrossRef]
- Trung, P.T.B.; Ngoc, L.B.B.; Hoa, P.N.; Tien, N.N.T.; Van Hung, P. Impact of heat-moisture and annealing treatments on physicochemical properties and digestibility of starches from different colored sweet potato varieties. Int. J. Biol. Macromol. 2017, 105, 1071–1078. [Google Scholar] [CrossRef]
- Zavareze, E.D.R.; Storck, C.R.; de Castro, L.A.S.; Schirmer, M.A.; Dias, A.R.G. Effect of heat-moisture treatment on rice starch of varying amylose content. Food Chem. 2010, 121, 358–365. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Li, W.; Wang, X.; Lv, M.; Peng, Q.; Wang, M. Changes in physicochemical properties and in vitro digestibility of common buckwheat starch by heat-moisture treatment and annealing. Carbohydr. Polym. 2015, 132, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, A.; Hoover, R. Effect of heat-moisture treatment on the structure and physicochemical properties of tuber and root starches. Carbohydr. Polym. 2002, 49, 425–437. [Google Scholar] [CrossRef]
- Vermeylen, R.; Goderis, B.; Delcour, J.A. An X-ray study of hydrothermally treated potato starch. Carbohydr. Polym. 2006, 64, 364–375. [Google Scholar] [CrossRef]
- Piecyk, M.; Druzynska, B.; Ołtarzewska, A.; Wołosiak, R.; Worobiej, E.; Ostrowska-Ligęza, E. Effect of hydrothermal modifications on properties and digestibility of grass pea starch. Int. J. Biol. Macromol. 2018, 118, 2113–2120. [Google Scholar] [CrossRef]
- Molavi, H.; Razavi, S.M.A.; Farhoosh, R. Impact of hydrothermal modifications on the physicochemical, morphology, crystallinity, pasting and thermal properties of acorn starch. Food Chem. 2018, 245, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-J.; Hoover, R.; Liu, Q. The impact of single and dual hydrothermal modifications on the molecular structure and physicochemical properties of normal corn starch. Int. J. Biol. Macromol. 2009, 44, 203–210. [Google Scholar] [CrossRef]
- Bettge, A.D.; Morris, C.F.; Greenblatt, G.A. Assessing genotypic softness in single wheat kernels using starch granule-associated friabilin as a biochemical marker. Euphytica 1995, 86, 65–72. [Google Scholar] [CrossRef]
- Onyango, C.; Mewa, E.A.; Mutahi, A.W.; Okoth, M.W. Effect of heat-moisture-treated cassava starch and amaranth malt on the quality of sorghum-cassava-amaranth bread. Afr. J. Food Sci. 2013, 7, 80–86. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Jin, F.; Yu, J. Pea starch annealing: New insights. Food Bioprocess. Technol. 2013, 6, 3564–3575. [Google Scholar] [CrossRef]
- O’Brien, S.; Wang, Y.-J. Susceptibility of annealed starches to hydrolysis by α-amylase and glucoamylase. Carbohydr. Polym. 2008, 72, 597–607. [Google Scholar] [CrossRef]
- Jiranuntakul, W.; Puttanlek, C.; Rungsardthong, V.; Puncha-Arnon, S.; Uttapap, D. Amylopectin structure of heat-moisture treated starches. Starch Stärke 2012, 64, 470–480. [Google Scholar] [CrossRef]
- Kohyama, K.; Sasaki, T. Differential scanning calorimetry and a model calculation of starches annealed at 20 and 50°C. Carbohydr. Polym. 2006, 63, 82–88. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- Chung, H.-J.; Liu, Q.; Hoover, R. Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinized corn, pea and lentil starches. Carbohydr. Polym. 2009, 75, 436–447. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch–A review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Warren, F.J.; Butterworth, P.J.; Ellis, P.R.; Gidley, M.J. Mechanisms of starch digestion by α-amylase—Structural basis for kinetic properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 875–892. [Google Scholar] [CrossRef]
- Jane, J.-L.; Wong, K.-S.; McPherson, A.E. Branch-structure difference in starches of A- and B-type X-ray patterns revealed by their Naegeli dextrins. Carbohydr. Res. 1997, 300, 219–227. [Google Scholar] [CrossRef]
- Gérard, C.; Colonna, P.; Buléon, A.; Planchot, V. Amylolysis of maize mutant starches. J. Sci. Food Agric. 2001, 81, 1281–1287. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Flanagan, B.M.; Gilbert, E.; Gidley, M.J. A novel approach for calculating starch crystallinity and its correlation with double helix content: A combined XRD and NMR study. Biopolymers 2008, 89, 761–768. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Hoover, R.; Donner, E.; Liu, Q. Starch chain interactions within the amorphous and crystalline domains of pulse starches during heat-moisture treatment at different temperatures and their impact on physicochemical properties. Food Chem. 2014, 143, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cheng, L.; Yin, J.; Yan, S.; Liu, K.; Zhang, F.; Xu, B.; Li, L. Structure and physicochemical properties of starches in lotus (Nelumbo nucifera Gaertn.) rhizome. Food Sci. Nutr. 2013, 1, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, H.; Delcour, J. Hydrothermal modifications of granular starch, with retention of the granular structure: A review. J. Agric. Food Chem. 1998, 46, 2895–2905. [Google Scholar] [CrossRef]
- Tester, R.; Debon, S.; Sommerville, M. Annealing of maize starch. Carbohydr. Polym. 2000, 42, 287–299. [Google Scholar] [CrossRef]
- Stute, R. Hydrothermal modification of starches: The difference between annealing and heat/moisture—Treatment. Starch Stärke 1992, 44, 205–214. [Google Scholar] [CrossRef]
- Rahman, S.M.M.; Wheatley, C.; Rakshit, S.K. Selection of sweet potato variety for high starch extraction. Int. J. Food Prop. 2003, 6, 419–430. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- Wang, S.; Yu, J.; Zhu, Q.; Yu, J.; Jin, F. Granular structure and allomorph position in C-type Chinese yam starch granule revealed by SEM, 13C CP/MAS NMR and XRD. Food Hydrocoll. 2009, 23, 426–433. [Google Scholar] [CrossRef]
- AACC. Approved Methods of American Association of Cereal Chemists, 10th ed.; AACC: St Paul, MI, USA, 2000. [Google Scholar]



| Sample | Crude Lipid | Crude Protein | Ash | N.F.E 3 |
|---|---|---|---|---|
| (%, d.b.) | ||||
| PF starch 1 | 0.03 ± 0.00 | 0.91 ± 0.13 | 0.08 ± 0.01 | 98.99 ± 0.13 |
| WS starch | 0.11 ± 0.02 | 0.21 ± 0.03 | 0.04 ± 0.01 | 99.65 ± 0.01 |
| Sample Code 1 | Damage Starch | Amylose | Resistant Starch |
|---|---|---|---|
| (%, d.b.) | |||
| PF-native | 1.59 ± 0.02 a | 18.38 ± 0.03 ab | 27.74 ±0.15 f |
| PF-HMT20 | 2.53 ± 0.15 b | 18.79 ± 0.58 abc | 8.29 ± 0.14 d |
| PF-HMT25 | 3.40 ± 0.04 c | 19.25 ± 0.87 bcd | 5.40 ± 0.11 b |
| PF-HMT30 | 23.56 ± 0.61 g | 19.52 ± 0.28 cd | 2.69 ± 0.04 a |
| PF-ANN | 6.19 ± 0.00 d | 18.03 ± 1.01 a | 20.02 ± 0.86 e |
| PF-HMT20 + ANN | 8.65 ± 0.12 f | 20.02 ± 0.27 d | 7.11 ± 0.22 c |
| PF-ANN + HMT20 | 7.11 ± 0.21 e | 19.29 ± 0.31 bcd | 3.35 ± 0.09 a |
| WS-native | 0.53 ± 0.01 a | 16.43 ± 0.45 a | 35.39 ± 0.56 d |
| WS-HMT20 | 2.39 ± 0.06 b | 17.50 ± 0.85 ab | 5.57 ± 0.06 b |
| WS-HMT25 | 3.23 ± 0.05 c | 20.90 ± 0.27 d | 3.35 ± 0.05 a |
| WS-HMT30 | 21.15 ± 0.35 f | 19.97 ± 0.87 d | 2.68 ± 0.10 a |
| WS-ANN | 3.07 ± 0.11 c | 17.46 ± 0.81 ab | 16.52 ± 1.43 c |
| WS-HMT20 + ANN | 6.29 ± 0.03 e | 17.74 ± 0.52 bc | 5.73 ± 0.05 b |
| WS-ANN + HMT20 | 3.80 ± 0.11 d | 18.59 ± 0.27 c | 5.17 ± 0.07 b |
| Sample Code 1 | To (°C) 2 | Tp (°C) | Tc (°C) | Tc-To (°C) | ∆H (J/g) |
|---|---|---|---|---|---|
| PF-native | 65.84 ± 0.19 a | 70.04 ± 0.15 a | 74.98 ± 0.27 a | 9.15 ± 0.20 b | 3.96 ± 0.09 d |
| PF-HMT20 | 73.06 ± 0.59 d | 79.62 ± 0.37 d | 86.12 ± 0.15 c | 13.07 ± 0.58 c | 3.30 ± 0.16 c |
| PF-HMT25 | 74.74 ± 0.05 f | 81.49 ± 0.26 e | 88.24 ± 0.29 e | 13.50 ± 0.25 d | 2.91 ± 0.08 b |
| PF-HMT30 | 77.72 ± 0.79 g | 82.67 ± 0.50 f | 90.72 ± 0.75 f | 13.00 ± 0.04 cd | 1.44 ± 0.02 a |
| PF-ANN | 68.47 ± 0.16 b | 71.38 ± 0.26 b | 75.08 ± 0.46 a | 6.60 ± 0.30 a | 3.83 ± 0.25 d |
| PF-HMT20 + ANN | 73.86 ± 0.21 e | 79.35 ± 0.21 d | 86.77 ± 0.64 d | 12.91 ± 0.56 cd | 3.36 ± 0.38 c |
| PF-ANN + HMT20 | 70.23 ± 0.14 c | 75.11 ± 0.02 c | 82.98 ± 0.45 b | 12.75 ± 0.33 c | 3.35 ± 0.21 c |
| WS-native | 65.05 ± 0.18 a | 68.82 ± 0.21 a | 73.80 ± 0.29 a | 8.75 ± 0.13 b | 4.09 ± 0.20 d |
| WS-HMT20 | 73.32 ± 0.18 d | 77.69 ± 0.21 d | 83.90 ± 0.16 c | 10.58 ± 0.09 c | 3.34 ± 0.30 c |
| WS-HMT25 | 78.44 ± 0.27 f | 83.21 ± 0.21 f | 90.10 ± 0.53 e | 11.66 ± 0.64 d | 2.79 ± 0.26 b |
| WS-HMT30 | 78.41 ± 1.00 f | 84.81 ± 0.98 g | 93.78 ± 1.46 f | 15.37 ± 0.47 e | 1.60 ± 0.06 a |
| WS-ANN | 68.18 ± 0.01 b | 70.76 ± 0.01 b | 74.42 ± 0.10 a | 6.24 ± 0.10 a | 4.06 ± 0.13 d |
| WS-HMT20 + ANN | 74.39 ± 0.06 e | 78.80 ± 0.10 e | 85.18 ± 0.29 d | 10.92 ± 0.18 c | 3.48 ± 0.25 c |
| WS-ANN + HMT20 | 71.13 ± 0.33 c | 76.76 ± 0.04 c | 83.16 ± 0.02 b | 12.03 ± 0.35 d | 3.25 ± 0.17 c |
| Sample Code 1 | Peak Time (min) | Pasting Temperature (°C) | Peak Viscosity | Breakdown | Holding Strength | Setback | Final Viscosity |
|---|---|---|---|---|---|---|---|
| (cP) | |||||||
| PF-native | 4.10 ± 0.10 a | 75.53 ± 0.04 a | 2844.54 ± 23.34 f | 1025.46 ± 2.12 e | 1818.96 ± 25.46 f | 663.00 ± 16.97 e | 2481.96 ± 8.49 e |
| PF-HMT20 | 5.87 ± 0.19 bc | 82.33 ± 0.04 c | 259.00 ± 5.66 b | −5.00 ± 4.24 c | 264.00 ± 1.41 b | 167.00 ± 5.66 a | 431.00 ± 7.07 a |
| PF-HMT25 | 6.00 ± 0.00 c | 85.73 ± 0.53 d | 407.33 ± 2.08 c | −49.33 ± 1.53 a | 456.67 ± 2.52 d | 212.00 ± 5.00 b | 668.67 ± 7.51 c |
| PF-HMT30 | 6.00 ± 0.00 c | 86.58 ± 0.04 e | 710.00 ± 7.07 d | −24.5 ± 0.71 b | 734.50 ± 6.36 e | 302.00 ± 5.66 d | 1036.50 ± 12.02 d |
| PF-ANN | 5.70 ± 0.10 b | 75.80 ± 0.07 a | 2121.00 ± 29.70 e | 149.50 ± 12.02 d | 1971.50 ± 17.68 g | 771.50 ± 30.41 f | 2743.00 ± 48.08 f |
| PF-HMT20 + ANN | 5.97 ± 0.00 c | 81.85 ± 0.64 c | 184.00 ± 2.83 a | −42.50 ± 0.71 a | 226.50 ± 3.54 a | 169.00 ± 4.24 a | 395.50 ± 0.71 a |
| PF-ANN + HMT20 | 5.97 ± 0.00 c | 80.60 ± 0.00 b | 270.50 ± 7.78 b | −44.00 ± 9.90 a | 314.50 ± 2.12 c | 259.50 ± 3.54 c | 574.00 ± 1.41 b |
| WS-native | 4.37 ± 0.05 a | 74.35 ± 0.50 a | 2614.98 ± 29.36 e | 745.00 ± 19.11 e | 1869.98 ± 25.62 d | 698.99 ± 28.08 c | 2569.03 ± 16.06 e |
| WS-HMT20 | 6.00 ± 0.00 d | 83.50 ± 0.00 d | 147.00 ± 1.41 a | −25.50 ± 0.71 ab | 172.50 ± 2.12 ab | 90.50 ± 3.54 ab | 263.00 ± 1.41 ab |
| WS-HMT25 | 5.97 ± 0.00 cd | 87.03 ± 0.11 e | 200.50 ± 7.78 b | −2.50 ± 3.54 bc | 203.00 ± 4.24 b | 74.50 ± 3.54 a | 277.50 ± 7.78 b |
| WS-HMT30 | 5.92 ± 0.02 bc | 88.33 ± 0.04 f | 376.50 ± 0.71 c | 2.00 ± 2.00 c | 374.50 ± 3.54 c | 129.50 ± 13.44 b | 504.00 ± 16.97 d |
| WS-ANN | 5.85 ± 0.03 b | 75.43 ± 0.11 b | 2093.00 ± 38.18 d | 57.50 ± 7.78 d | 2035.50 ± 30.41 e | 861.00 ± 39.60 d | 2896.50 ± 9.19 f |
| WS-HMT20 + ANN | 5.97 ± 0.00 cd | 83.48 ± 0.11 d | 116.50 ± 3.54 a | −21.00 ± 1.41 abc | 137.50 ± 4.95 a | 101.50 ± 10.61 ab | 239.00 ± 15.56 a |
| WS-ANN + HMT20 | 5.99 ± 0.02 cd | 81.88 ± 0.04 c | 146.50 ± 10.61 a | −41.50 ± 0.71 a | 188.00 ± 11.31 b | 121.00 ± 2.83 ab | 309.00 ± 14.14 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, Y.; Lai, L.-S. Effect of Single and Dual Hydrothermal Treatments on the Resistant Starch Content and Physicochemical Properties of Lotus Rhizome Starches. Molecules 2021, 26, 4339. https://doi.org/10.3390/molecules26144339
Yeh Y, Lai L-S. Effect of Single and Dual Hydrothermal Treatments on the Resistant Starch Content and Physicochemical Properties of Lotus Rhizome Starches. Molecules. 2021; 26(14):4339. https://doi.org/10.3390/molecules26144339
Chicago/Turabian StyleYeh, Yun, and Lih-Shiuh Lai. 2021. "Effect of Single and Dual Hydrothermal Treatments on the Resistant Starch Content and Physicochemical Properties of Lotus Rhizome Starches" Molecules 26, no. 14: 4339. https://doi.org/10.3390/molecules26144339
APA StyleYeh, Y., & Lai, L.-S. (2021). Effect of Single and Dual Hydrothermal Treatments on the Resistant Starch Content and Physicochemical Properties of Lotus Rhizome Starches. Molecules, 26(14), 4339. https://doi.org/10.3390/molecules26144339






