Photosynthesis-Inhibiting Activity of N-(Disubstituted-phenyl)-3-hydroxynaphthalene-2-carboxamides †
Abstract
:1. Introduction
2. Results and Discussion
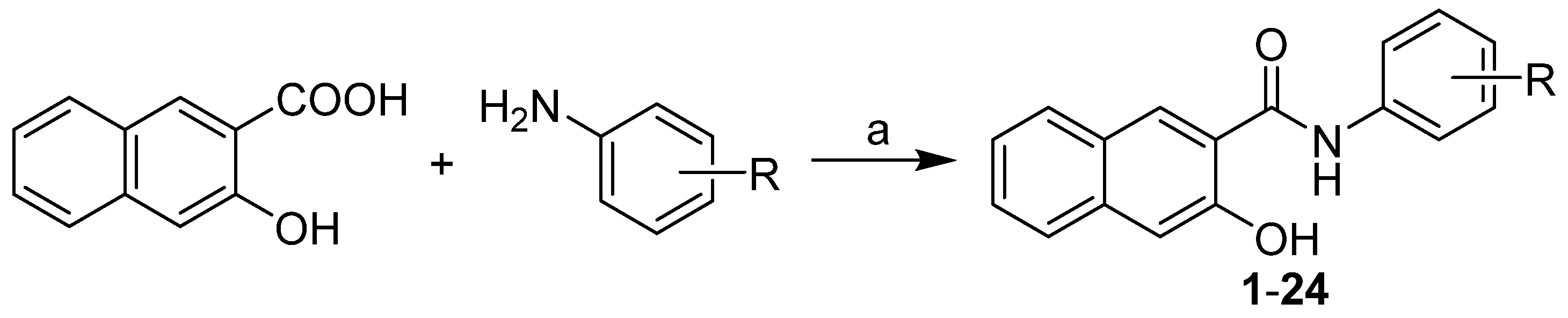
2.1. Chemistry
2.2. Inhibition of Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
3. Materials and Methods
3.1. General Information
3.2. Synthesis
General Procedure for the Synthesis of N-(Disubstituted phenyl)-3-hydroxynaphthalene-2-carboxamides 1–24
3.3. Study of Inhibition of Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Importance & Benefits of Pesticides. CropLife International: Brussels, Belgium, 2020. Available online: https://pesticidefacts.org/topics/necessity-of-pesticides/ (accessed on 19 May 2021).
- Gianessi, L.P. The increasing importance of herbicides in worldwide crop production. Pest. Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Gianessi, L.; Williams, A. The importance of herbicides for natural resource conservation in the USA. In Convergence of Food Security, Energy Security and Sustainable Agriculture; Songstad, D., Hatfield, J., Tomes, D., Eds.; Springer: Heidelberg, Germany, 2014; pp. 333–350. [Google Scholar]
- Classification of Herbicides. Weed Management in Horticulture Crops. Available online: http://ecoursesonline.iasri.res.in/mod/page/view.php?id=12032 (accessed on 19 May 2021).
- Forouzesh, A.; Zand, E.; Soufizadeh, S.; Foroushani, S.S. Classification of herbicides according to chemical family for weed resistance management strategies—An update. Weed Res. 2015, 55, 334–358. [Google Scholar] [CrossRef]
- Herbicide Resistance Action Committee. HRAC Mode of Action Classification 2020 Map. Available online: https://hracglobal.com/tools/hrac-mode-of-action-classification-2020-map (accessed on 19 May 2021).
- Draber, W.; Tietjen, K.; Kluth, J.F.; Trebst, A. Herbicides in photosynthesis research. Angew. Chem. 1991, 3, 1621–1633. [Google Scholar] [CrossRef]
- Tischer, W.; Strotmann, H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron-transport. Biochim. Biophys. Acta 1977, 460, 113–125. [Google Scholar] [CrossRef]
- Trebst, A.; Draber, W. Structure activity correlations of recent herbicides in photosynthetic reactions. In Advances in Pesticide Science; Greissbuehler, H., Ed.; Pergamon Press: Oxford, UK, 1979; pp. 223–234. [Google Scholar]
- Bowyer, J.R.; Camilleri, P.; Vermaas, W.F.J. Herbicides, Topics in Photosynthesis; Baker, N.R., Percival, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 27–85. [Google Scholar]
- Izawa, S. Acceptors and donors for chloroplast electron transport. In Methods in Enzymology; Part, C., Colowick, P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA; London, UK, 1980; pp. 413–434. [Google Scholar]
- Whitmarsh, J. Electron transport and energy transduction. In Photosynthesis: A Comprehensive Treatise; Raghavendra, A.S., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 87–110. [Google Scholar]
- Jablonkai, I. Molecular mechanism of action of herbicides. In Herbicides—Mechanisms and Mode of Action; Abd El-Ghany Hasaneen, M.N., Ed.; IntechOpen: Rijeka, Croatia, 2011; Chapter 1; Available online: https://www.intechopen.com/books/herbicides-physiology-of-action-and-safety/modes-of-action-of-different-classes-of-herbicides (accessed on 19 May 2021).
- Sherwani, S.I.; Arif, I.A.; Khan, H.A. Modes of action of different classes of herbicides. In Herbicides—Physiology of Action, and Safety; Price, A., Kelton, J., Sarunaite, L., Eds.; IntechOpen: Rijeka, Croatia, 2015; Chapter 8; Available online: https://www.intechopen.com/books/herbicides-physiology-of-action-and-safety/modes-of-action-of-different-classes-of-herbicides (accessed on 19 May 2021).
- Huppatz, J.L.; McFadden, H.G. Understanding the topography of the photosystem II herbicide binding niche: Does QSAR help? Z. Naturforsch. 1993, 48, 140–145. [Google Scholar] [CrossRef]
- Lambreva, M.D.; Russo, D.; Polticelli, F.; Scognamiglio, V.; Antonacci, A.; Zobnina, V.; Campi, G.; Rea, G. Structure/function/dynamics of photosystem II plastoquinone binding sites. Curr. Protein Pept. Sci. 2014, 15, 285–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trebst, A. Inhibitors in the functional dissection of the photosynthetic electron transport system. Photosynth. Res. 2007, 92, 217–224. [Google Scholar] [CrossRef]
- Teixeira, R.R.; de Andrade Barros, M.V.; Bressan, G.C.; Siqueira, R.P.; Dos Santos, F.S.; Bertazzini, M.; Kiralj, R.; Ferreira, M.M.C.; Forlani, G. Synthesis, theoretical studies, and effect on the photosynthetic electron transport of trifluoromethyl arylamides. Pest. Manag. Sci. 2017, 73, 2360–2371. [Google Scholar] [CrossRef]
- Dolezal, M.; Zitko, J.; Osicka, Z.; Kunes, J.; Vejsova, M.; Buchta, V.; Dohnal, J.; Jampilek, J.; Kralova, K. Synthesis, antimycobacterial, antifungal and photosynthesis-inhibiting activity of chlorinated N-phenylpyrazine-2-carboxamides. Molecules 2010, 15, 8567–8581. [Google Scholar] [CrossRef] [PubMed]
- Imramovsky, A.; Pesko, M.; Kralova, K.; Vejsova, M.; Stolarikova, J.; Vinsova, J.; Jampilek, J. Investigating spectrum of biological activity of 4- and 5-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides. Molecules 2011, 16, 2414–2430. [Google Scholar] [CrossRef] [Green Version]
- Imramovsky, A.; Pesko, M.; Monreal-Ferriz, J.; Kralova, K.; Vinsova, J.; Jampilek, J. Photosynthesis-inhibiting efficiency of 4-chloro-2-(chlorophenylcarbamoyl)phenyl alkyl-carbamates. Bioorg. Med. Chem. Lett. 2011, 21, 4564–4567. [Google Scholar] [CrossRef]
- Fajkusova, D.; Pesko, M.; Keltosova, S.; Guo, J.; Oktabec, Z.; Vejsova, M.; Kollar, P.; Coffey, A.; Csollei, J.; Kralova, K.; et al. Anti-infective and herbicidal activity of N-substituted 2-aminobenzothiazoles. Bioorg. Med. Chem. 2012, 20, 7059–7068. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Bobal, P.; Kollar, P.; Cizek, A.; Kralova, K.; et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013, 21, 6531–6541. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Pesko, M.; Dohanosova, J.; Oravec, M.; Liptaj, T.; Kralova, K.; Jampilek, J. Halogenated 1-Hydroxynaphthalene-2-Carboxanilides Affecting Photosynthetic Electron Transport in Photosystem II. Molecules 2017, 22, 1709. [Google Scholar] [CrossRef] [Green Version]
- Bak, A.; Pizova, H.; Kozik, V.; Vorcakova, K.; Kos, J.; Treml, J.; Odehnalova, K.; Oravec, M.; Imramovsky, A.; Bobal, P.; et al. SAR-mediated Similarity Assessment of the Property Profile for New, Silicon-Based AChE/BChE Inhibitors. Int. J. Mol. Sci. 2019, 20, 5385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattabiraman, V.R.; Bode, J.W. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Zadrazilova, I.; Nevin, E.; Kauerova, T.; Pesko, M.; Kos, J.; Oravec, M.; Kollar, P.; Coffey, A.; O’Mahony, J.; et al. Synthesis and biological evaluation of N-alkoxyphenyl-3-hydroxynaphthalene-2-carboxanilides. Molecules 2015, 20, 9767–9787. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.R.; Pereira, J.L.; Pereira, W.L. Photosynthetic inhibitors. In Applied Photosynthesis; Najafpour, M., Ed.; InTech: Rijeka, Croatia, 2012; pp. 3–22. [Google Scholar]
- Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Gonec, T.; Bobal, P.; Kauerova, T.; Oravec, M.; Kollar, P.; et al. Antibacterial and herbicidal activity of ring-substituted 3-hydroxynaphthalene-2-carboxanilides. Molecules 2013, 18, 7977–7997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Govender, R.; Keltosova, S.; Chambel, B.; Pereira, D.; Kollar, P.; Imramovsky, A.; et al. Antibacterial and herbicidal activity of ring-substituted 2-hydroxynaphthalene-1-carboxanilides. Molecules 2013, 18, 9397–9419. [Google Scholar] [CrossRef] [Green Version]
- Gonec, T.; Kralova, K.; Pesko, M.; Jampilek, J. Antimycobacterial N-alkoxyphenylhydroxynaphthalene-carboxamides affecting photosystem II. Bioorg. Med. Chem. Lett. 2017, 27, 1881–1885. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K.; Pesko, M.; Kos, J. Ring-substituted 8-hydroxyquinoline-2-carboxanilides as photosystem II inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 3862–3865. [Google Scholar] [CrossRef] [PubMed]
- Bak, A.; Kos, J.; Michnova, H.; Gonec, T.; Pospisilova, S.; Kozik, V.; Cizek, A.; Smolinski, A.; Jampilek, J. Similarity-driven pharmacophore mapping for series of N-(disubstituted-phenyl)-3-hydroxynaphthalene-2-carboxamides. Int. J. Mol. Sci. 2020, 21, 6583. [Google Scholar] [CrossRef] [PubMed]
- Kerns, E.H.; Di, L. Drug-Like Properties: Concepts. Structure Design and Methods: From ADME to Toxicity Optimization; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Jampilek, J. Potential of agricultural fungicides for antifungal drug discovery. Expert Opin. Drug Dis. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masarovicova, E.; Kralova, K. Approaches to measuring plant photosynthesis activity. In Handbook of Photosynthesis, 2nd ed.; Pessarakli, M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 617–656. [Google Scholar]


 | ||||
|---|---|---|---|---|
| Comp. | R | Clog P 2 | σ(Ar) 3 | PET Inhibition IC50 [µM] |
| 11 | 2,5-OCH3 | 3.9563 | 0.08 | 183 |
| 21 | 3,5-OCH3 | 4.5463 | 0.93 | 24.5 |
| 31 | 2,5-CH3 | 4.7942 | 0.59 | 11.6 |
| 41 | 2,6-CH3 | 4.1442 | 0.58 | 28.5 |
| 51 | 3,5-CH3 | 5.4442 | 0.59 | 9.9 |
| 61 | 2,5-F | 4.4799 | 1.24 | 11.2 |
| 71 | 2,6-F | 3.8799 | 1.44 | 78.7 |
| 81 | 3,5-F | 5.0799 | 1.12 | 9.8 |
| 91 | 2,5-Cl | 5.3699 | 1.22 | 321 |
| 101 | 2,6-Cl | 4.5199 | 1.33 | 156 |
| 111 | 3,4-Cl | 6.0999 | 1.19 | 47.5 |
| 121 | 3,5-Cl | 6.2199 | 1.11 | 39.2 |
| 13 | 2,4-Br | 5.6399 | 1.11 | 296 |
| 14 | 2,5-Br | 5.6399 | 1.23 | 161 |
| 151 | 3,5-CF3 | 6.8207 | 1.05 | 15.9 |
| 16 | 2-OCH3-5-F | 4.2725 | 0.14 | 79.1 |
| 17 | 2-F-6-OCH3 | 3.6725 | 0.16 | 507 |
| 18 | 3-F-5-OCH3 | 4.8625 | 0.99 | 31.6 |
| 19 | 2-Cl-5-OCH3 | 4.5825 | 1.13 | 171 |
| 20 | 2-F-4-Cl | 5.0499 | 1.17 | 1405 |
| 211 | 3-F-4-Br | 5.7999 | 1.16 | 527 |
| 221 | 3-F-5-CF3 | 6.0131 | 1.04 | 31.0 |
| 231 | 2-Cl-5-CF3 | 5.7331 | 1.19 | 13.2 |
| 241 | 2-Br-4-CF3 | 5.8531 | 1.32 | 621 |
| DCMU | – | – | – | 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kos, J.; Gonec, T.; Oravec, M.; Jendrzejewska, I.; Jampilek, J. Photosynthesis-Inhibiting Activity of N-(Disubstituted-phenyl)-3-hydroxynaphthalene-2-carboxamides . Molecules 2021, 26, 4336. https://doi.org/10.3390/molecules26144336
Kos J, Gonec T, Oravec M, Jendrzejewska I, Jampilek J. Photosynthesis-Inhibiting Activity of N-(Disubstituted-phenyl)-3-hydroxynaphthalene-2-carboxamides . Molecules. 2021; 26(14):4336. https://doi.org/10.3390/molecules26144336
Chicago/Turabian StyleKos, Jiri, Tomas Gonec, Michal Oravec, Izabela Jendrzejewska, and Josef Jampilek. 2021. "Photosynthesis-Inhibiting Activity of N-(Disubstituted-phenyl)-3-hydroxynaphthalene-2-carboxamides " Molecules 26, no. 14: 4336. https://doi.org/10.3390/molecules26144336
APA StyleKos, J., Gonec, T., Oravec, M., Jendrzejewska, I., & Jampilek, J. (2021). Photosynthesis-Inhibiting Activity of N-(Disubstituted-phenyl)-3-hydroxynaphthalene-2-carboxamides . Molecules, 26(14), 4336. https://doi.org/10.3390/molecules26144336








