2.1. Development of a Table Grape Model for Evaluating Crop Protection against Smoke
Initial attempts by us [
20] and others [
16] at devising crop protection strategies to block the uptake of smoke-derived VPs into grape tissues were limited by both the number of vines that can reasonably be put into a smoke exposure apparatus (influencing the number of variables that may be tested) and the natural seasonal constraints imposed by the
Vitis vinifera growing season (limiting the window of time available for field work). Szeto et al. [
6] and Modesti et al. [
13] have successfully demonstrated that wine grapes exposed to smoke immediately after harvesting replicate much of the phenotype accounting for smoke taint, i.e., VP uptake and biochemical storage as glycosides and possibly other conjugates. Accordingly, it was hypothesized that off-vine model systems would enable the rapid testing of greater numbers of protective strategies, each with more replicates, while also uncoupling these proof-of-concept tests from the need to perform experiments on active, for-profit vineyards, while simultaneously permitting year-round smoke-taint research. Commercial, imported table grapes are available in most parts of North America year round, and it is known that these varietals have the capacity to alter their biosynthesis of phenylpropanoids (including glycosylated analogues) in response to temperature changes and CO
2 exposure [
21]. While both varietal-specific and harvest-related processes undoubtedly affect the cuticle and cell wall polysaccharides of table grapes, they nevertheless afford a vineyard-like model by which the physical-chemical interactions between smoke-derived VPs, grape cuticles/cell walls, and potential prophylactic sprays may be rapidly and systematically compared. Previously, Antolini et al. [
13] used table grapes to evaluate the impact of ozone treatments on smoke-taint intensity, although no attempts were made to evaluate VPs beyond guaiacol or 4-methylguaiacol, nor were VP glycosides assessed. Thus, we sought to test here whether table grapes could be used to reproduce the previously observed uptake of VPs into grapes in which they are subsequently stored as largely acid-labile conjugates, presumed to be glycosides. A parallel objective of these experiments was to reassess positive results from in-vineyard trials [
20] evaluating the ability of several approved agro-sprays to block VP uptake. Accordingly, individual clusters of commercial red table grapes were treated with three previously evaluated prophylactic sprays and exposed to simulated forest fire smoke, after which nine VPs were quantitated by GC–MS/MS (
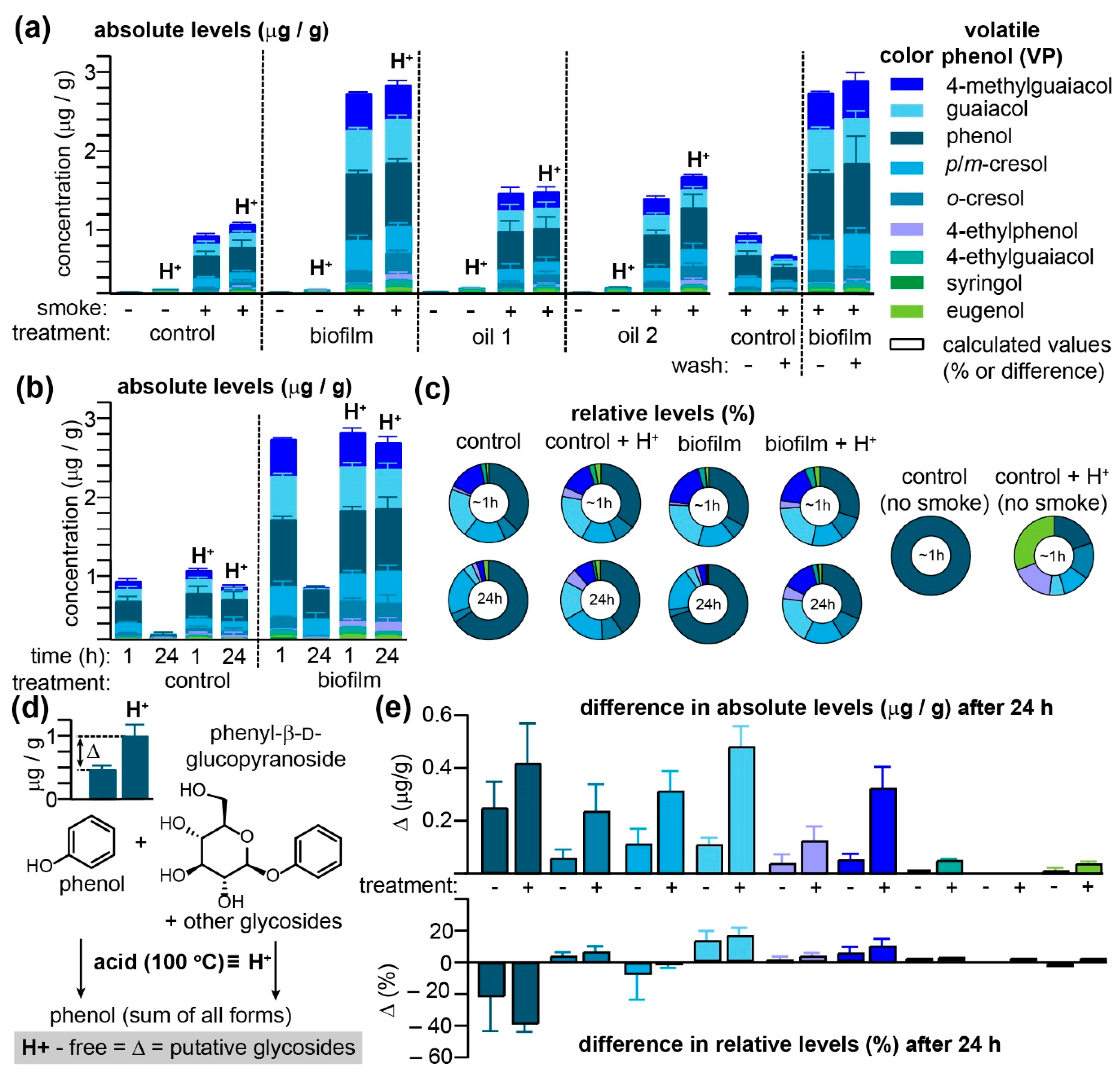
Figure 1 and
Supplementary Tables S1–S3), as described previously [
7,
10,
20]. A portion of each sample was subjected to acid hydrolysis prior to VP extraction, a treatment that hydrolyzes VP glycosides, thereby permitting all VPs, whether free or bound, to be quantitated [
1,
6,
7,
10], with the difference between the two VP measurements representing the VPs transformed by grapes into glycosides and/or alternative acid-labile conjugates.
In the absence of smoke, the free VP content in red table grapes, regardless of whether they had been treated with one of three agro-sprays, was, with a sole exception, restricted to barely detectible traces of phenol (
Figure 1a and
Supplementary Table S1). Subjecting samples to acid hydrolysis (H
+), conditions which have been shown to quantitatively release simple VP glycoconjugates [
10], reveals traces of
o- and
p- and/or m-cresol (
p- and
m-cresol co-eluted with the GC–MS/MS method employed), guaiacol, 4-ethylphenol, and eugenol. Interestingly, pre-treatment of grapes with oil2 (please refer to the Methods for a list of all three treatments) one day prior to smoke exposure significantly increased concentrations of these acid-labile VPs in non-smoked grapes (
Supplementary Table S1). Exposure to smoke for 1 h significantly elevated VPs in all samples, with concentrations in the biofilm-treated grapes exceeding those of the control samples by over two-fold, a result that is in direct contrast with previous field studies, demonstrating that this product exhibited significant reductions in VPs after smoke exposure [
20]. Oil1 and oil2 also exhibited significantly higher VP levels than control grapes, albeit not to the same level as the biofilm samples; we note that our initial evaluation of oil2 [
20] also revealed that it led to increased concentrations of VPs in treated grapes. Washing the grapes prior to processing did not significantly influence the VP concentrations detectible in biofilm-treated grapes, although modest decreases were observed in untreated samples for all VPs detected (
Figure 1a and
Supplementary Table S2), suggesting that the biofilm in this instance was either making the grapes more porous (permitting rapid VP absorption into grape tissues and hence protection from washing) or making the grape surface more hydrophobic and hence increasing their ability to adsorb hydrophobic VPs. The inability to wash VPs off smoke-exposed grapes mimicked previous observations made by us [
7] and others [
6] when using on-vine wine grapes. No significant differences between free and acid-labile VPs were discernable among the four treatment groups when grapes were processed and analyzed immediately (ca. 1 h) post-smoke exposure. However, for both control and biofilm-treated grapes, significant decreases in free VPs were observed when grapes were extracted 24 h after smoke exposure (
Figure 1b and
Supplementary Table S3). Either slow evaporation of VP adsorbed to the grape surface or VP metabolism within grape tissues would account for these losses. The equivalence in the concentrations of acid-labile VPs in grapes processed 1 or 24 h post-smoke exposure indicates that both VP absorption into grape tissues and biotransformation into molecules with chemical properties consistent with those of VP glycosides had occurred in smoke-exposed grocery store-sourced table grapes. This transformation was somewhat slower than previously observed for on-vine berries wherein the conversion of VPs into acid-labile conjugates occurred in >1 h [
7].
Since both control and biofilm-treated grapes were exposed to the same smoke, VP concentrations were normalized in order to deduce whether some were preferentially adsorbed to the surface of biofilm-treated grapes (
Figure 1c and
Supplementary Table S3). For the grapes processed and analyzed within 1 h of smoke exposure, only slight differences were observed in VP ratios between both treatment groups, independent of acid hydrolysis; this suggests that the biofilm treatment nonspecifically increased VP adsorption or absorption into table grapes. However, while VP ratios for the acid-hydrolyzed samples analyzed after 24 h closely mirrored those obtained immediately after smoke exposure, the pool of VPs remaining in their unconjugated forms after 24 h was found to be substantially enriched in phenol (rising from an average of both control and biofilm samples of 35.0% to 67.7%), at the expense of guaiacol (dropping from 20.8% to 3.8% of the total VPs over 24 h) and
o-cresol (6.1% to 3.5%). The differences between VPs quantitated after acid hydrolysis and without hydrolysis (Δ in
Figure 1d) were calculated for the control and biofilm-treated samples after 24 h (
Figure 1e) as this Δ corresponds to the concentration of putative VP glycosides accrued over this period. With the sole exception of syringol, all VPs exhibited a substantial Δ at 24 h, with the biofilm-treated samples consistently exceeding untreated grapes. This transformation of free VPs into acid-labile compounds, however, appeared to be concentration independent as the Δ with respect to relative VP concentrations was essentially equivalent between both groups of grapes (
Figure 1e). Note that for phenol, the large negative Δ value—which indicates that phenol was selectively enriched in the free VP pools after 24 h—was offset by relative increases in all other VPs with the exception of
p/
m-cresol, suggesting that some VPs (most notable for guaiacol) are selectively trapped as acid-labile conjugates within smoke-exposed table grapes. While these off-vine experiments failed to reproduce the protective effect of the biofilm with respect to VP accumulation in grapes (which might be due to numerous differences between this experiment and our prior pilot study [
20]), they demonstrate that table grapes do mimic the capacity of on-vine wine grapes to sequester VPs in grape tissues primarily as acid-labile conjugates.
2.2. Re-Evaluation of Biofilm and Establishment of the Duration of Crop-Protection Effects
As noted, prior pilot attempts by us [
20] and others [
18] to evaluate smoke-taint protection strategies are limited in the number of variables that may be evaluated by the physical constraints to the number of vines that may be enclosed in a smoking apparatus in a vineyard; thus, in our prior study, the most promising treatment (biofilm) was applied at only one time point (one week) prior to smoke exposure, and to only one row of grapes in a single vineyard. To permit a more robust evaluation of this form of crop protection, and to verify the contradictory results from the off-vine study (see
Section 2.1), we modified our previously described smoke-enclosure [
7,
20] such that four identical enclosures up to 33 m apart could be filled with smoke from a single source. Within each tent was one vine from the following four treatment groups: three biofilm spray treatments where the spray was applied either one, seven or 14 days prior to smoke exposure, and one treatment where the grapes were sprayed with water only. An equivalent set of grapevines was prepared as controls, except these were not tented nor were they exposed to the smoke. Thus, at each of three different vineyards grapes were collected from
n = 4 replicate vines × 4 treatment groups × smoke vs. no smoke = 32 separate vines, spread over an area of ca. 33 m × 33 m. Smoke exposure for a duration of 1 h occurred at two weeks post-
veraison, exactly as previously described, with grapes being exposed to smoke for 1.5 h. Grape samples (
n = 4/condition/vineyard) were collected for the quantitation of both free and acid-labile VPs immediately post-smoke exposure (T
1) and again at commercial maturity (T
2). The time-dependent results for vineyard 1 are summarized in
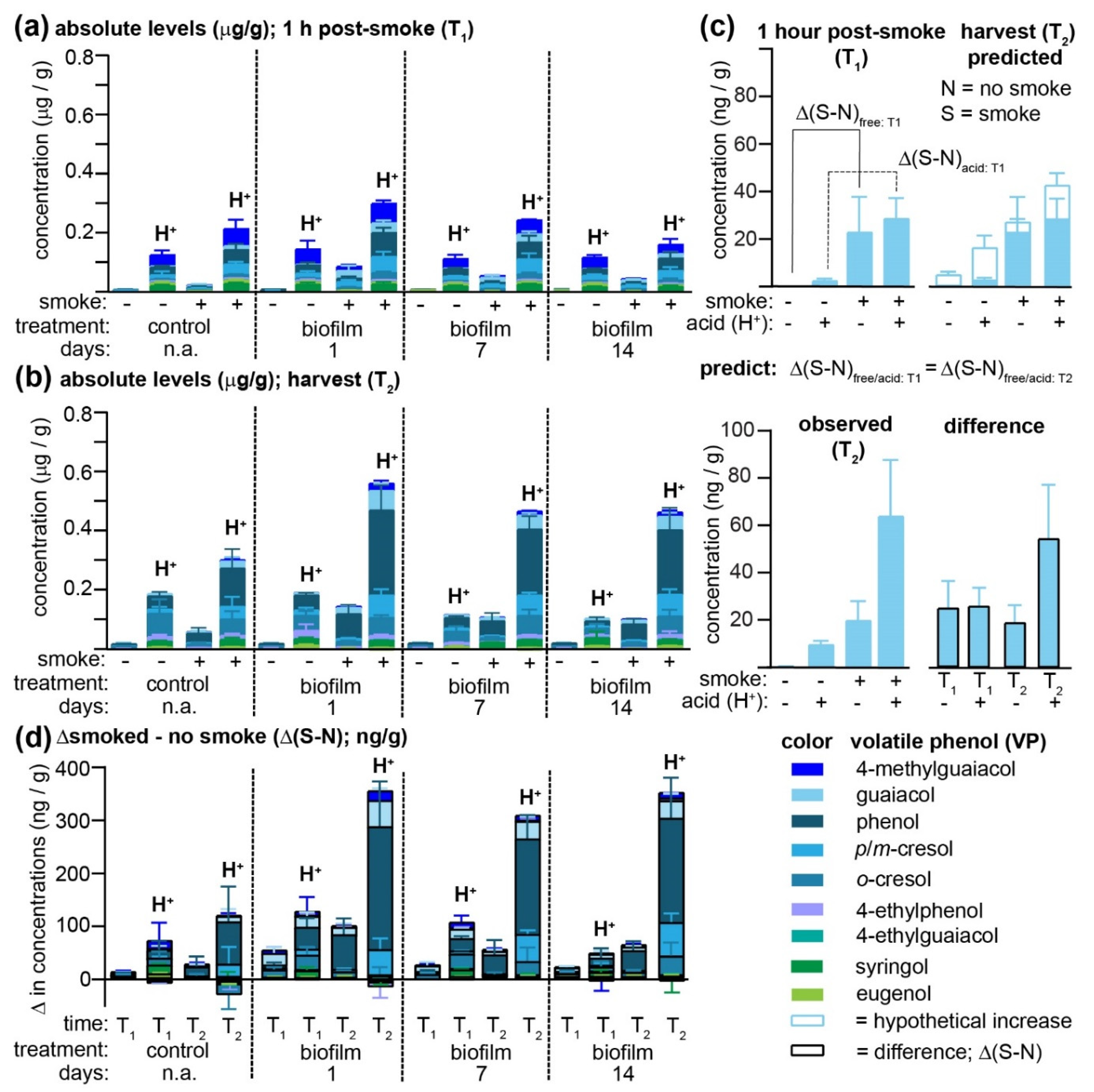
Figure 2a and
Supplementary Tables S4 and S5; results for vineyards 2 and 3 are found in
Figure 3 and
Supplementary Tables S6–S9.
At vineyard 1, smoke exposure led to a significant increase in the concentrations of all free VPs (with the exception of eugenol) in non-biofilm-treated Pinot Noir grapes (
Figure 2a), and concentrations of all VPs remained significantly elevated over controls at harvest (
Figure 2b). In almost all cases, acid hydrolysis permitted greater concentrations of VPs to be determined for both smoke-exposed and non-exposed grapes, consistent with earlier observations that VPs are rapidly conjugated to other molecules in-grape [
1,
4,
6,
7]. An unusual exception to this trend was the widely accepted smoke-taint marker 4-methylguaiacol [
22], which was found at high and essentially equivalent concentrations in all grape samples collected from vineyard 1 at T
1 that had been subjected to acid hydrolysis (H
+); thus, it is unlikely that this 4-methylguaiacol originated from the smoke. In agreement with the off-vine table grape experiments described above, but in contrast with the previous pilot study [
20], all of the biofilm treatments led to increased concentrations of free VPs present in smoke-exposed grapes, with a significant time dependence observed for the cresols, guaiacol, and 4-ethylphenol at T
1, and
p/m-cresol, guaiacol, 4-methylguaiacol, 4-ethylphenol, and 4-ethylguaiacol at T
2. More specifically, grapes to which the biofilm had been applied one day before smoke exposure had significantly higher concentrations of these VPs than those treated 14 days earlier. Likewise, the concentrations of VPs detected post-acid hydrolysis (H
+) were higher in the biofilm-treated grapes, again exhibiting a time dependence (i.e., treatments applied earlier contained lower VPs, like the non-sprayed controls) for phenol,
p/m-cresol, guaiacol, and 4-ethylphenol at T
1 and phenol, guaiacol, 4-methylguaiacol, and 4-ethylguaiacol at harvest (T
2;
Supplementary Table S5). A comparison of VP concentrations (both free and total) at both time points indicates that with only a few exceptions (of low magnitude), VP levels continued to rise for both control and smoke-exposed grapes over the duration of the ripening process (compare
Figure 2a,b). It was hypothesized that the difference in VP concentrations observed between smoke-exposed and control grapes (i.e., Δ(S-N), where S and N = smoked and non-smoked samples, respectively) would be equivalent in grapes analyzed immediately post-exposure (T
1) and at harvest (T
2;
Figure 2c). This hypothesis would assume that any ripening-associated changes, hypothetically indicated for guaiacol present in one-day biofilm-treated grapes with the non-filled histograms in
Figure 2c (top, right panel), would be identical for both smoke-exposed and control berries and thus that smoke-induced increases in VP concentrations would remain constant over time. While the differences in the free guaiacol concentration between smoke-exposed and control grapes did remain constant at both T
1 and T
2, this was not the case for the acid-labile forms (
Figure 2c; bottom panel; shaded histograms with black outline), in which the concentration difference almost doubled. This increase in Δ(S-N) might indicate that smoke exposure affected in-grape biosynthetic pathways yielding acid-labile guaiacyl conjugates (which is an explanation that is inconsistent with the accepted mechanisms of feedback inhibition); alternatively, it may be due to VP sequestration in grapes as initially non-acid-labile conjugates (which are therefore unable to be detected at T
1) which are then more slowly converted into glycoconjugates and/or other acid-labile forms over ripening (T
2). We note that Szeto and co-workers [
6] have recently provided evidence for the interconversion of in-grape VP storage forms, while Noestheden and co-workers [
7] have previously documented that VPs may be trapped within Pinot Noir grapes as base-labile conjugates under conditions in which VP glycosides remain stable. These ripening-associated changes in acid-labile VPs were observed for other VPs in addition to guaiacol (
Figure 2d), most notably phenol, followed by guaiacol,
m/
p-cresol, and 4-methylguaiacol. It is also possible that VP glycosides produced in other parts of smoke-exposed vines (e.g., leaves) may also be transported into the grapes during ripening [
19,
23].
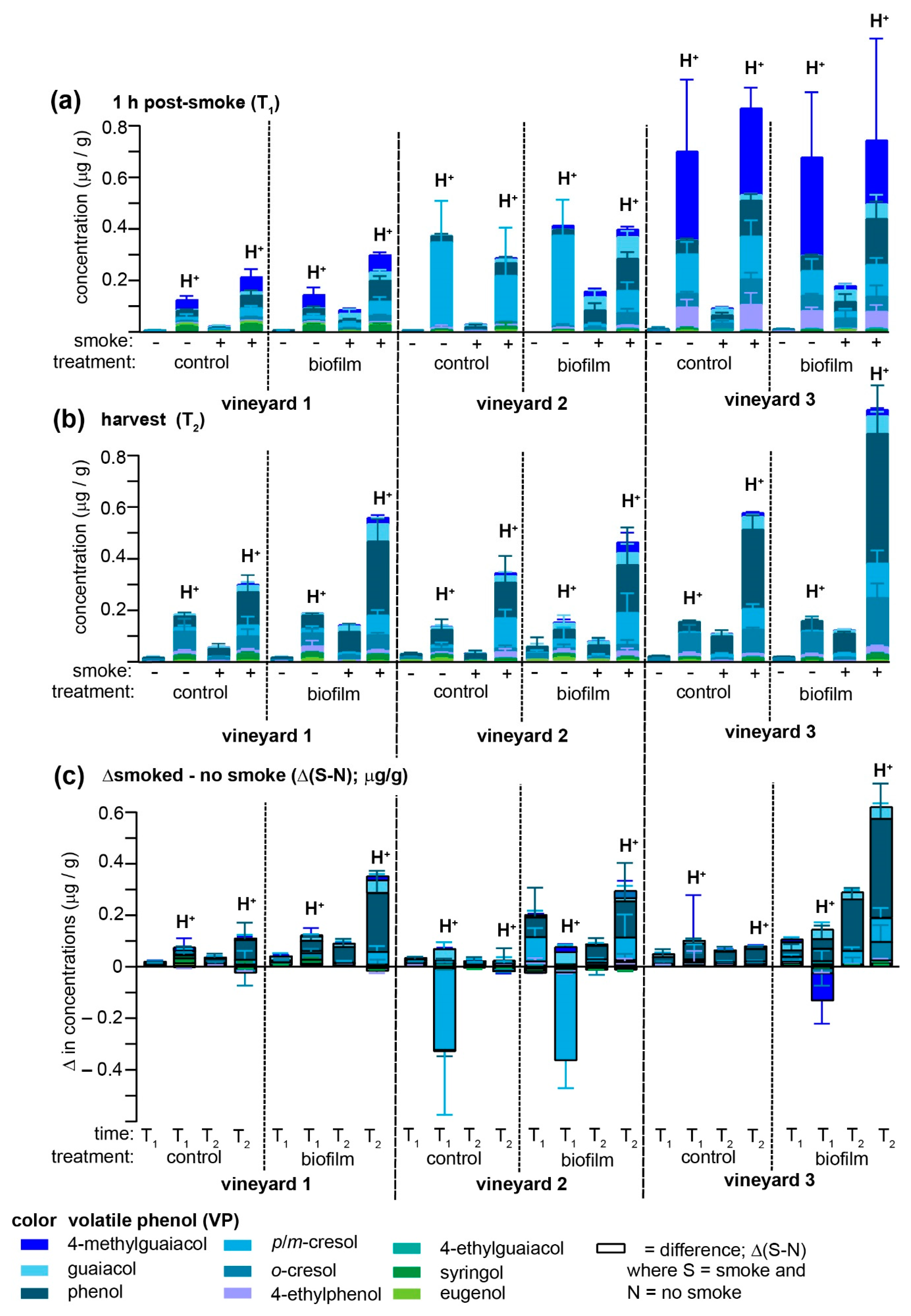
Biofilm and smoke applications were evaluated at three different vineyards to provide a more robust evaluation of its potential prophylactic applications (
Figure 3); for simplicity, only samples treated with biofilm one day prior to smoke exposure are depicted (but all data are summarized in
Supplementary Tables S4–S9). In no case did the biofilm treatment afford protection against smoke-derived VP accumulation, and in many instances both free and acid-labile VPs in the biofilm-treated samples significantly exceeded unsprayed vines. The consistent failure to replicate crop protection with the biofilm was surprising, since one of the vineyards (vineyard 2) was the site of our initial pilot study, albeit different Pinot Noir vines were used for each experiment and obviously the fuel/smoke differed. One reason for the opposite effects of the biofilm treatment may be that the two years over which treatments were applied differed considerably with respect to total precipitation (
Supplementary Table S10), with 2018 being much drier than 2019. Indeed, in all instances of biofilm treatment application in this study, rain was reported on or shortly after the date of biofilm application. Although the biofilm was initially developed to reduce instances of cuticular fractures in berries or soft fruit after rain showers [
24]—which, taken in isolation might reduce uptake of VPs—the increased VP adhesiveness of biofilm-treated red table grapes (
Figure 1a) and the general time-dependent decrease in this phenomenon in on-vine Pinot Noir grapes (
Figure 3a,b) are consistent with the hypothesis that rain water washes away biofilm components that most strongly interact with VPs. Further experiments, including off-vine experiments, are required to reconcile these inconsistencies.
There are several other notable observations to be made in comparing the effects of the biofilm treatments across three different vineyards. First, non-smoked grapes contained traces of free VPs but large amounts of acid-labile analogues at T
1 (which occurred approximately two weeks post-
veraison). However, the concentrations of the acid-labile VPs varied substantially across vineyards, with grapes at vineyard 3 containing very high amounts of 4-methylguaiacol (similar to vineyard 1) and moderate amounts of
p/
m-cresol and 4-ethylphenol, while non-smoked grapes at vineyard 2 possessed VP profiles dominated by
p/
m-cresol, exceeding 300 ng/g in all instances (
Figure 3a). These high levels of (presumably) endogenous acid-labile VPs appeared to be negatively impacted by smoke exposure as a negative Δ(S-N) value was observed at T
1 (
Figure 3c) for
p/
m-cresol for both control and biofilm-treated vines at vineyard 2, and 4-methylguaiacol for the biofilm-treated group at vineyard 3. Interestingly, over the ripening period (T
2), all three vineyards tended to converge toward relatively similar VP profiles (which was most noticeable among the acid-labile VPs present in smoke-exposed samples) dominated by phenol and
p/
m-cresol or
o-cresol and only traces of the previously (T
1) high 4-methylguaiacol (
Figure 3b). The biofilm-treated samples from vineyards 1 and 2 closely mirrored each other in that the Δ(S-N) from both biofilm-treated and non-treated samples exhibited a clear ripening-associated increase (
Figure 3c), most notable for phenol, followed by the cresols and guaiacol.
Some practical conclusions may be drawn from this set of field studies. First, environmental conditions appear to play a significant effect on the effectiveness of potential smoke-taint prophylactics. Second, substantial amounts of endogenous VPs, and conjugates thereof, exist in Vitis vinifera berries, indicating that it is imperative that grape growers define a “normal” range in the absence of smoke exposure as all comparisons in the presence of smoke must be made against this significant and variable background. Third, all three vineyards exhibited substantial changes in VP concentrations over the ripening process, independent of both smoke exposure or biofilm treatment, and two vineyards clearly demonstrated ripening-associated increases in the magnitude of the difference in VP concentrations present in smoke-exposed and non-exposed grapes (most notable for the acid-labile forms). Thus, it would be most prudent for wineries to test berries for smoke taint closer to harvest rather than immediately post-smoke exposure. Finally, commercial red table grapes mimic the effects of smoke on on-vine Pinot Noir grapes, in both the adverse effects of the biofilm and in terms of their capacity to transform VPs into acid-labile conjugates, and therefore represent an adequate model to rapidly screen future prophylactic treatments.
2.3. Fermentation-Associated Changes in VPs and Their Acid-Labile Conjugates
Although the biofilm failed to protect grapes from accumulating smoke-derived VPs, at the conclusion of this field study, we were in possession of a set of samples that, due to the differing biofilm treatments, all varied with respect to the impact of their smoke exposure. When grapes from equivalent treatments across all three vineyards were pooled and fermented (representing eight pooled samples), we reasoned that this set of paired grape/must and wine samples would permit (i) an analysis of the impact of fermentation on both endogenous and smoke-derived VPs and their acid-labile conjugates and (ii) enable us to determine which measurement (free VP vs. the total) most closely predicted the actual levels of free (thus perceptible by smell) VPs in the finished wines (
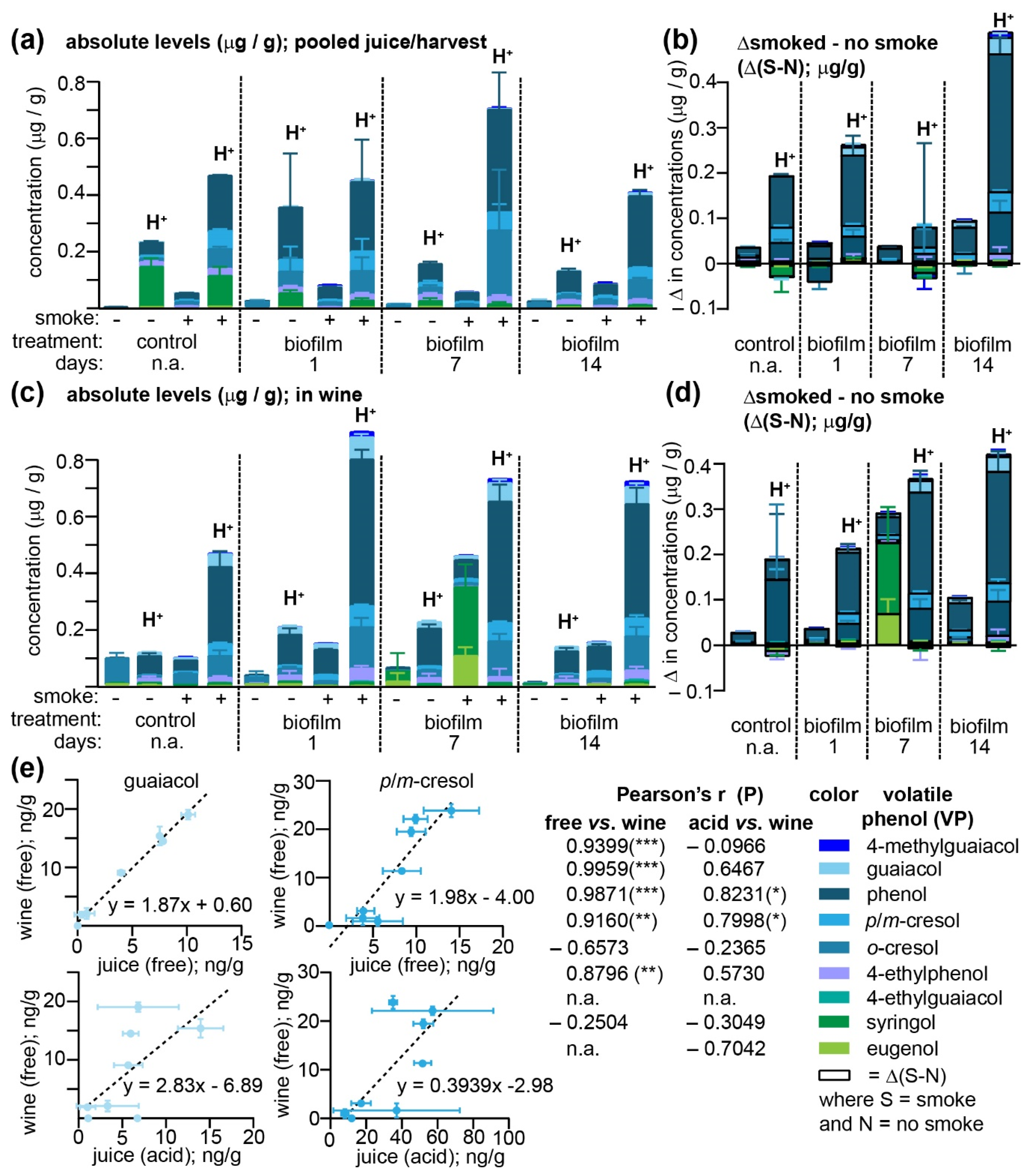
Figure 4 and
Supplementary Tables S12 and S13). Since differing amounts of grapes remained from each treatment group and vineyard after initial analyses (
Figure 2 and
Figure 3), the same trends with respect to biofilm treatment increasing VP uptake more when the duration between treatment and smoking was reduced were not apparent within pooled samples (
Figure 4a). Nevertheless, consistent with the VP levels detected from each vineyard at harvest (
Figure 3c), phenol, followed by
p/
m-cresol and
o-cresol were the most abundant of both free and acid-labile VPs detected in the eight wines; one exception to these trends was the syringol present in the control wines, which was present in high amounts in its free form. The cause of this notably apparent increase may be due to the fact that VPs from individual grape samples were quantitated in the supernatant obtained from a sample homogenate, whereas the pooled juice samples were collected immediately before inoculation and after a slow thawing process (grapes were frozen for one month prior to thawing and fermentation), followed by pressing and a 24 h cold soak to which SO
2 had been added, i.e., the increased syringol concentration may be due to both increased duration of extraction (from grape skins) and possibly post-extraction protection from oxidation by SO
2. The difference between smoke-exposed and non-exposed juices (Δ(S-N)) was larger for the acid-labile VPs—presumably VP glycoconjugates—than the free analogues (
Figure 4b) and it was hypothesized that the magnitude of Δ(S-N) would increase for the free forms after fermentation as yeast glycosyl-hydrolases cleave these glycoconjugates [
2,
4]. While the concentrations of the free VPs did indeed consistently increase in all wines—in agreement with previous studies [
2,
6,
7,
20] (
Figure 4c and
Supplementary Tables S12 and S13)—the concentrations of the corresponding acid-labile conjugates in some instances remained essentially constant (e.g., phenol and
o-cresol) or actually increased during fermentation; for example, total concentrations of all guaiacol conjugates in wines produced from the four smoke-exposed samples increased from a mean of 7.4 ± 1.18 ng/g in must to 52.2 ± 5.19 ng/g in the wines, while 4-methylguaiacol conjugates increased from 2.8 ± 0.70 to 14.44 ± 3.47 ng/g. Meanwhile, the Δ(S-N) for both the free and acid-labile VPs present in wines was largely unchanged with respect to the must (
Figure 4d). One notable exception to this trend was the free syringol and eugenol concentrations detected in wines produced from the samples treated with the biofilm seven days prior to smoke exposure; the high concentrations of these free VPs were detected despite both the free and acid-labile quantities of these VPs in the must being below their respective method detection limits. These anomalous data for syringol and eugenol, when paired with the increase in free VPs without an accompanying decrease (and in some instances, an increase) in the total acid-labile forms suggest that, as was observed over ripening (
Figure 3a,d), a fraction of VPs are sequestered as non-acid-labile conjugates and that these remaining non-acid-labile conjugates may, to some extent, be metabolized by yeast during fermentation. It is possible that these VP conjugates are present in the grape skins, which are currently not assessed by published VP quantitation methods [
6,
7,
10,
25], but are accessible to yeast during the fermentation process.
Regardless of their exact chemical form, it is clear that (i) free, odor-active VPs increased in concentration during the fermentation process and (ii) that a larger fraction of VPs found in grapes after smoke exposure were in their acid-labile rather than their free forms. Pearson’s correlation coefficients were therefore calculated between either the free or acid-labile VPs quantitated in each of the eight different must/juice samples, and the free VPs present in the resulting wines in an attempt to determine which in-must/juice measurements yielded the greatest predictive value (
Figure 4e). Even though the free VPs in must/juice samples were only a minor fraction of the total, they nevertheless correlated better with the free VPs present in wines than the total acid-labile levels did; significant positive correlations were exhibited for 4-methylguaiacol, guaiacol, phenol,
p/
m-cresol, and 4-ethyphenol when comparing free VP levels, while only two weakly positive (but significant) correlations were observed (phenol and
p/
m-cresol) when total acid-labile VPs in juice were correlated against their free analogues in wines (
Figure 4e). As discussed above, this lack of a clear correlation between total VPs—from which any increase in free VPs present in wine should have theoretically been attributable—would imply that either some of the free analogues in wines were originating from a conjugated VP pool that was not acid labile (and therefore unlikely to be a simple glycoside), or that the currently utilized hydrolysis methods fail to adequately detect some acid-labile VPs such as those present in grape cell walls that are omitted from the hydrolysis procedure but still included in fermentation. Further experiments are currently ongoing to investigate both of these possibilities. It is clear, however, that for the majority of VPs quantitated within this set of samples, high levels of free VPs in juice/berry samples directly correlated with high levels in wine and, based on these data, we would propose that this sole measurement of free VPs may be used by vineyards for smoke-taint risk assessment purposes, provided that the grapes are collected near commercial maturity (
vide supra;
Figure 3). Further research will be required in order to determine whether similar predictive correlations are apparent with other grape varietals, grapes grown in different regions (where fuel sources may influence VP profiles and viticulture or environmental factors may influence VP uptake or metabolism), or wines made with differing yeast strains or fermentation techniques.
In summary, our experiments to re-evaluate a biofilm that had previously exhibited the ability to prevent the in-grape accumulation of smoke-derived VPs demonstrated that this product increased VP concentrations in both red table grapes and Pinot Noir grapes grown on three different vineyards. While the source of these divergent field studies is still under investigation, with precipitation being one likely variable accounting for these discrepancies, the replication of some aspects of smoke-taint chemistry with table grapes suggests that the latter may afford a convenient way to rapidly assess how VPs interact with the grape cuticle, are biochemically transformed within grapes, and how both processes might be influenced by prophylactic treatments. Our data demonstrated that the differences in total VP levels observed between smoke-exposed and control vines on the same vineyards actually increased over the ripening process, indicating that immediately after smoke exposure, VPs might not be sequestered as acid-labile conjugates and that VP storage forms may be interconverted. We also demonstrated that endogenously occurring VPs vary considerably between vineyards (even within the same grape varietal), albeit they tend to converge at harvest, leading to the conclusion that the most accurate VP-based risk assessments (of smoke taint in wines) should utilize grape samples that are at, or close to, commercial maturity. The substantial increases in the concentrations of total (sum of both free and acid-labile) guaiacol and 4-methylguaiacol during the fermentation of smoke-exposed grapes leads us to conclude that at least some of the cryptic, non-acid-labile VPs sequestered within grapes may be metabolically accessed by yeast during the fermentation process. Nevertheless, the concentrations of free VPs in grapes, although only a minor fraction of the total, closely correlated with the final levels of the free, aroma-active VPs quantitated in the resulting wines.