Quantification of Major Bioactive Constituents, Antioxidant Activity, and Enzyme Inhibitory Effects of Whole Coffee Cherries (Coffea arabica) and Their Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification and Quantification of Major Bioactive Compounds in WCC, WCCE1, and WCCE2
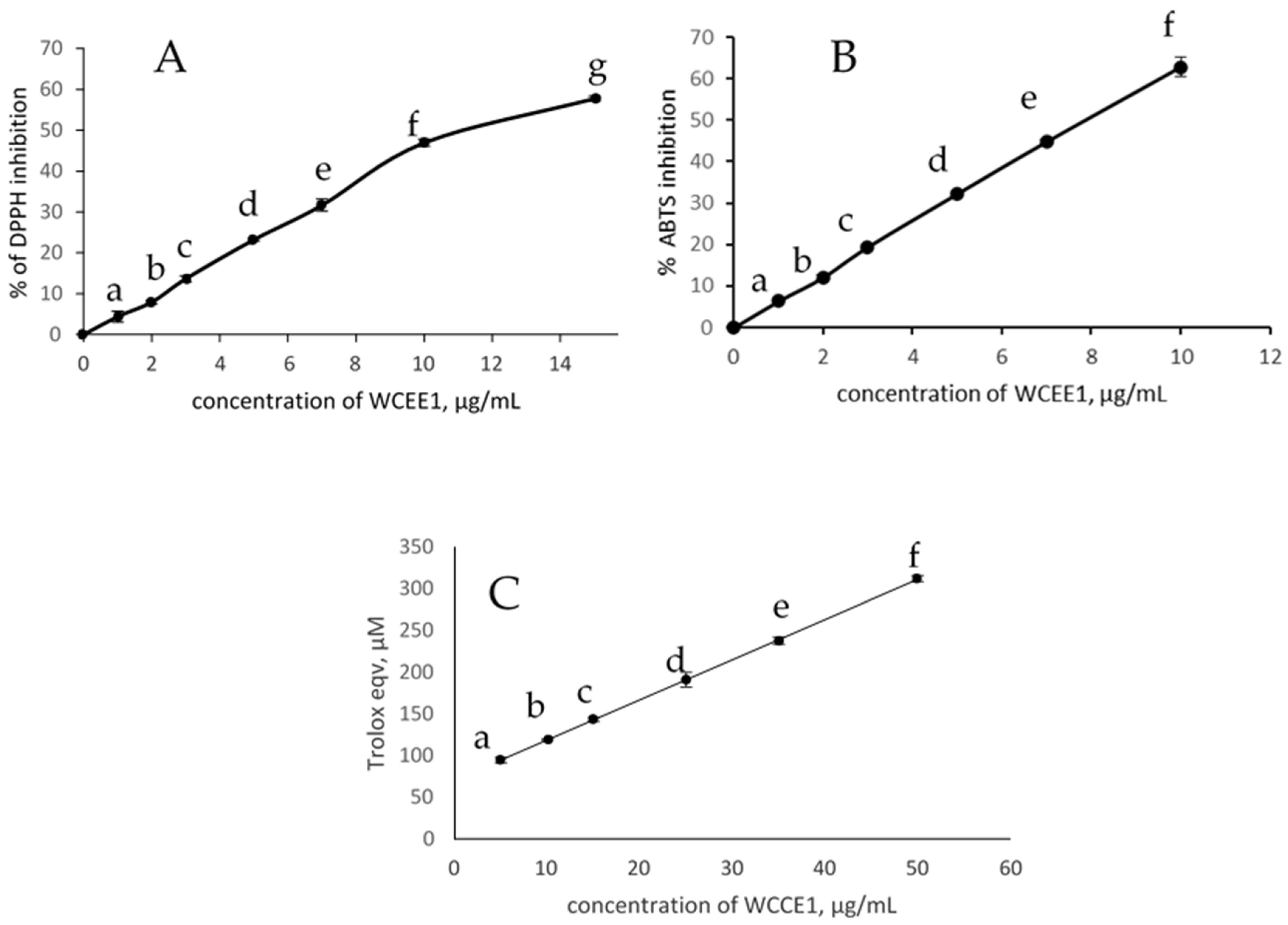
2.2. Antioxidant Activities
2.2.1. DPPH Radical Scavenging Activity
2.2.2. ABTS Radical Scavenging Activity
2.2.3. Ferric Reducing Power Activity (FRAP)
2.2.4. ORAC 5.0 Assay Antioxidant Capacity
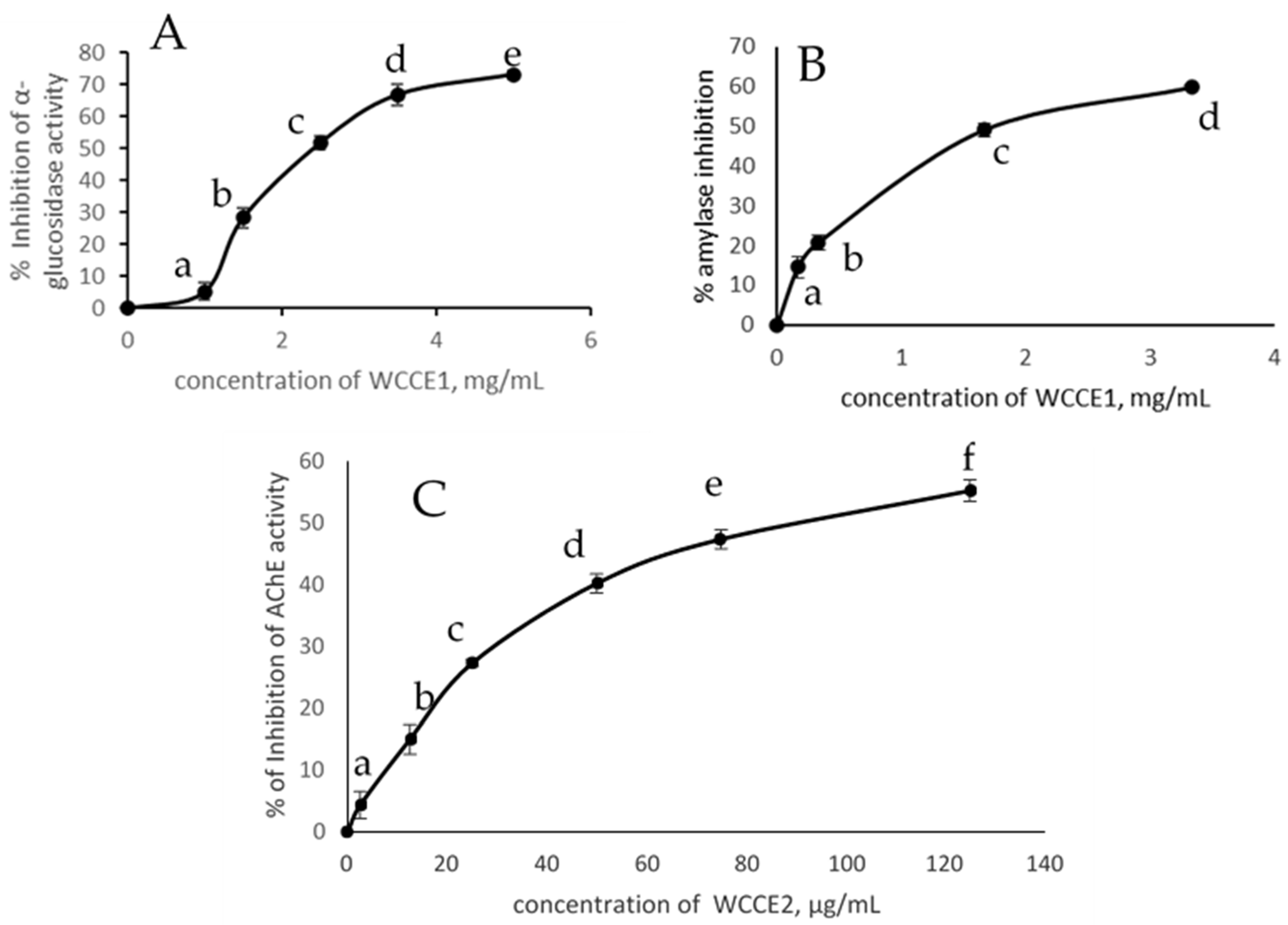
2.3. Inhibition of α-Glucosidase Activity, α-Amylase, and AChE Activities
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Identification of Compounds by LC-MS/MS Analysis
3.3. Quantification of Chlorogenic Acids by High-Performance Liquid Chromatography (HPLC)
3.4. Quantification of Caffeine and Trigonellin by HPLC
3.5. Measurements of Antioxidant Activity
3.5.1. ABTS Radical Scavenging Activity Assay
3.5.2. DPPH Radical Scavenging Activity Assay:
3.5.3. Ferric Reducing Antioxidant Power (FRAP)
3.5.4. ORAC Antioxidant Assay:
3.6. Measurement of Enzyme Inhibition Activity
3.6.1. Inhibition of α-Amylase Assay
3.6.2. Inhibition of α-Glucosidase Assay
3.6.3. Inhibition of Acetylcholinesterase (AChE) Assay
3.7. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Coffee-Around-the-World, National Coffee Association of U.S.A., Inc. Available online: https://www.ncausa.org/About-Coffee/Coffee-Around-the-World (accessed on 7 July 2021).
- Little, J.A.; Shanoff, H.F.; Csima, A.; Torornto, M.A.; Yano, R. Coffee and serum-lipids in coronary heart-disease. Lancet 1966, 287, 732–734. [Google Scholar] [CrossRef]
- Anderson, L.F.; Jacobs, D.R.J.; Carlsen, M.H.; Blomhoff, R. Consumption of coffee associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in Iowa Women’s Health study. Am. J. Nutr. 2006, 83, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- La Vecchia, C.; Tavani, A. Coffee and cancer risk: An update. Eur. J. Cancer. Prev. 2007, 16, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model system. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- De Melo, B.; Gomes, S.; da Silveria, G.A.; Carvalho, I. α- and β- glucosidase inhibitors: Chemicals structure and biological activity. Tetrahedron 2006, 62, 10277–10302. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural products as α-amylase α-glucosidase inhibitors and their hypoglycemic potential in the treatment of diabetes: An update. Min. Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Bolen, S.; Feldman, L.; Vassy, J.; Wilson, L.; Yeh, H.C.; Marinopolous, S.; Wiley, C.; Selvin, E.; Wilson, R.; Bass, E.; et al. Systematic review: Comparative effectiveness and safety oral medicines for type2 diabetes mellitus. Ann. Intern. Med. 2007, 147, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. α-glucosidase inhibitors. Endocrin. Metab. Clin. N. Am. 1997, 26, 539–551. [Google Scholar] [CrossRef]
- Rabassa-Lhoret, R.; Chiasson, L. Potential of alpha-glucosidase inhibitors in elderly patients with diabetes mellitus and impaired glucose tolerance. Drugs Aging 1998, 13, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.Y.; Qian, K.; Morris-Natschke, S.L.; Hsu, C.S.; Lee, K.H. Recent discovery of plant based anti-diabetic natural products. Nat. Prod. Rep. 2012, 29, 580–606. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.; Abshiru, N.; Al-Taher, F. Identification of phytochemical compounds in coffea arabica whole coffee cherries and their extracts by LC-MS/MS. J. Agric. Food Chem. 2021, 69, 3430–3438. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Serra, F.; Reniero, F.; Héberger, K. Botanical and geographical characterization of green coffee (Coffea arabica and Coffea canephora): Chemometric evaluation of phenolic and methylxanthine contents. J. Agric. Food Chem. 2009, 57, 4224–4235. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Nemzer, B.; Ou, B.; Stalmach, A.; Hunter, J.; Clifford, M.N.; Combet, E. The antioxidant and chlorogenic acid profiles of whole coffee fruits are influenced by the extraction procedures. J. Agric. Food. Chem. 2011, 59, 3754–3762. [Google Scholar] [CrossRef]
- Mullen, W.; Nemzer, B.; Stalmach, A.; Ali, S.; Combet, E. Polyphenolic and hydroxycinnamate contents of whole coffee fruits from China, India, and Mexico. J. Agric. Food. Chem. 2013, 61, 5298–5309. [Google Scholar] [CrossRef] [Green Version]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food. Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Jokura, H.; Watanabe, I.; Umeda, M.; Hase, T.; Shimotoyodome, A. Coffee polyphenol consumption improves hyperglycemia associated with impaired vascular endothelial function in healthy male adults. Nutr. Res. 2015, 35, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Dellalibera, O.; Lemaire, B.; Lafay, S. Svetol®, green coffee extract, induces weight loss and increases the lean to fat mass ratio in volunteers with overweight problem. Phytotherapie 2006, 4, 194–197. [Google Scholar] [CrossRef]
- Robinson, J.L.; Yanes, J.A.; Reid, M.A.; Murphy, J.E.; Busler, J.N.; Mumford, P.W.; Young, K.C.; Pietrzkowski, Z.J.; Nemzer, B.V.; Hunter, J.M.; et al. Neurophysiological effects of whole coffee cherry extract in older adults with subjective cognitive impairment: A randomized, double-blind, placebo-controlled, cross-over pilot study. Antioxidants 2021, 10, 144. [Google Scholar] [CrossRef]
- Reyes-Izquierdo, T.; Nemzer, B.; Shu, C.; Huynh, L.; Argumedo, R.; Keller, R.; Pietrzkowski, Z. Modulatory effect of coffee fruit extract on plasma levels of brain-derived neurotrophic factor in healthy subjects. Br. J. Nutr. 2013, 110, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Linag, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [Green Version]
- Zang, L.Y.; Cosma, G.; Gardner, H.; Castranova, V.; Vallyathan, V. Effect of chlorogenic acid on hydroxyl radical. Mol. Cell. Biochem. 2003, 247, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acidisomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Lee, H.K.; Kim, J.-A.; Hong, S.I.; Kim, H.C.; Jo, T.H.; Park, Y.I.; Lee, C.K.; Kim, Y.-B.; Lee, S.Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharm. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type2 diabetes (in vitro): A comparative study. J. Basic. Clin. Phyiol. Pharmacol. 2015, 26, 165–170. [Google Scholar]
- Xu, D.; Wang, Q.; Zhang, W.; Hu, B.; Zhou, L.; Zeng, X.; Sun, Y. Inhibitory activities of caffeoylquinic acid derivatives from Ilex kudingcha C.J. Tseng on glucosidase from Saccharomyces cerevisiae. J. Agric. Food Chem. 2015, 63, 3694–3703. [Google Scholar] [CrossRef]
- Chio, S.Y.; Sung, M.; Hunag, P.W.; Lin, S.D. Antioxidant, antidiabetic, and antihypertensive properties of Echinacea purpurea flower extract and caffeic acid derivatives using in vitro models. J. Med. Food 2017, 20, 171–179. [Google Scholar] [CrossRef]
- Alongi, M.; Celayeta, J.M.F.; Vriz, R.; Kinsella, G.K.; Rulikowska, A.; Anese, M. In vitro digestion nullified the differences triggered by roasting in phenolic composition and α-glucosidase inhibitory capacity of coffee. Food Chem. 2020, 22, 132. [Google Scholar] [CrossRef]
- Alongi, M.; Anese, M. Effect of coffee roasting on in invitro α-glucosidase activity: Inhibition and mechanism of action. Food Res. Int. 2018, 111, 480–487. [Google Scholar] [CrossRef]
- Duangjai, A.; Pontip, P.; Sumhem, S.; Kaweekul, W.; Utsingtong, M.; Ontawong, A.; Trisat, K.; SaoKaew, S. Phenolic acids from Coffee arabica L. suppress intestinal uptake of glucose and cholesterol. Biomed. Res. 2020, 31, 59–66. [Google Scholar]
- Ma, C.M.; Hattori, M.; Daneshtalab, M.; Wang, L. Chlorogenic acid derivatives with alkyl chains at different lengths and orientation: Potent α-glucosidase inhibitors. J. Med. Chem. 2008, 51, 6188–6194. [Google Scholar] [CrossRef] [PubMed]
- Herawati, D.; Giriwono, P.E.; Dewi, F.N.A.; Kashwagi, T.; Andarwulan, N. Antioxidant, anti-α-glucosidase and anti-glycation activities of coffee brew from Robusta coffee beans roasted at different levels. Int. Food Res. J. 2019, 26, 1305–1313. [Google Scholar]
- Herawati, D.; Giriwono, P.E.; Dewi, F.N.A.; Kashiwagi, T.; Andarwulan, N. Three major compounds showing significant antioxidative, glucosidase inhibition, and antiglycation activities in Robusta coffee brew. Int. J. Food Prop. 2019, 22, 994–1010. [Google Scholar] [CrossRef] [Green Version]
- Narita, Y.; Inouye, K. Inhibitory effects of chlorogenic acids form green coffee beans and cinnamate derivatives on the activity of porcine pancreas amylase isozyme I. Food Chem. 2011, 127, 1532–1539. [Google Scholar] [CrossRef] [Green Version]
- Narita, Y.; Inouye, K. Kinetic analysis and mechanism on the inhibition of chlorogenic acid and its components against porcine pancreas α-amylase isozymes I and II. J. Agric. Food Chem. 2009, 57, 9218–9225. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic amylase activity by chlorogenic acid. J. Funct. Food 2020, 64, 103587. [Google Scholar] [CrossRef]
- Karim, Z.; Holmes, M.; Orfila, C. Inhibitory effect of chlorogenic acid on digestion of potato starch. Food Chem. 2017, 217, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Nyambe-Silavwe, H.; Williamson, G. Chlorogenic and phenolic acids are only very weak inhibitor of human salivary amylase and rat intestinal maltase activities. Food Res. Int. 2018, 113, 452–455. [Google Scholar] [CrossRef]
- Kalita, D.; Holm, D.; LaBarbera, D.; Petrash, J.M.; Jayanty, S.S. Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PLoS ONE 2018, 13, e0191025. [Google Scholar] [CrossRef]
- Pohanka, M.; Dobes, P. Caffeine inhibits acetylcholinesterase, but not butylcholinesterase. Int. J. Mol. Sci. 2013, 14, 9873–9882. [Google Scholar] [CrossRef] [PubMed]
- Karadsheh, N.; Kussie, P.; Linthicum, D.C. Inhibition of acetylcholinesterase by caffeine, anabasine, methyl pyrrolidine and their derivatives. Toxic. Lett. 1991, 55, 335–342. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic acid and chlorogenic acids on the key enzymes linked to alzheimer’s disease and some pro-oxidnat induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipohililic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodhill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay sing fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, D.K.; Andreas, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
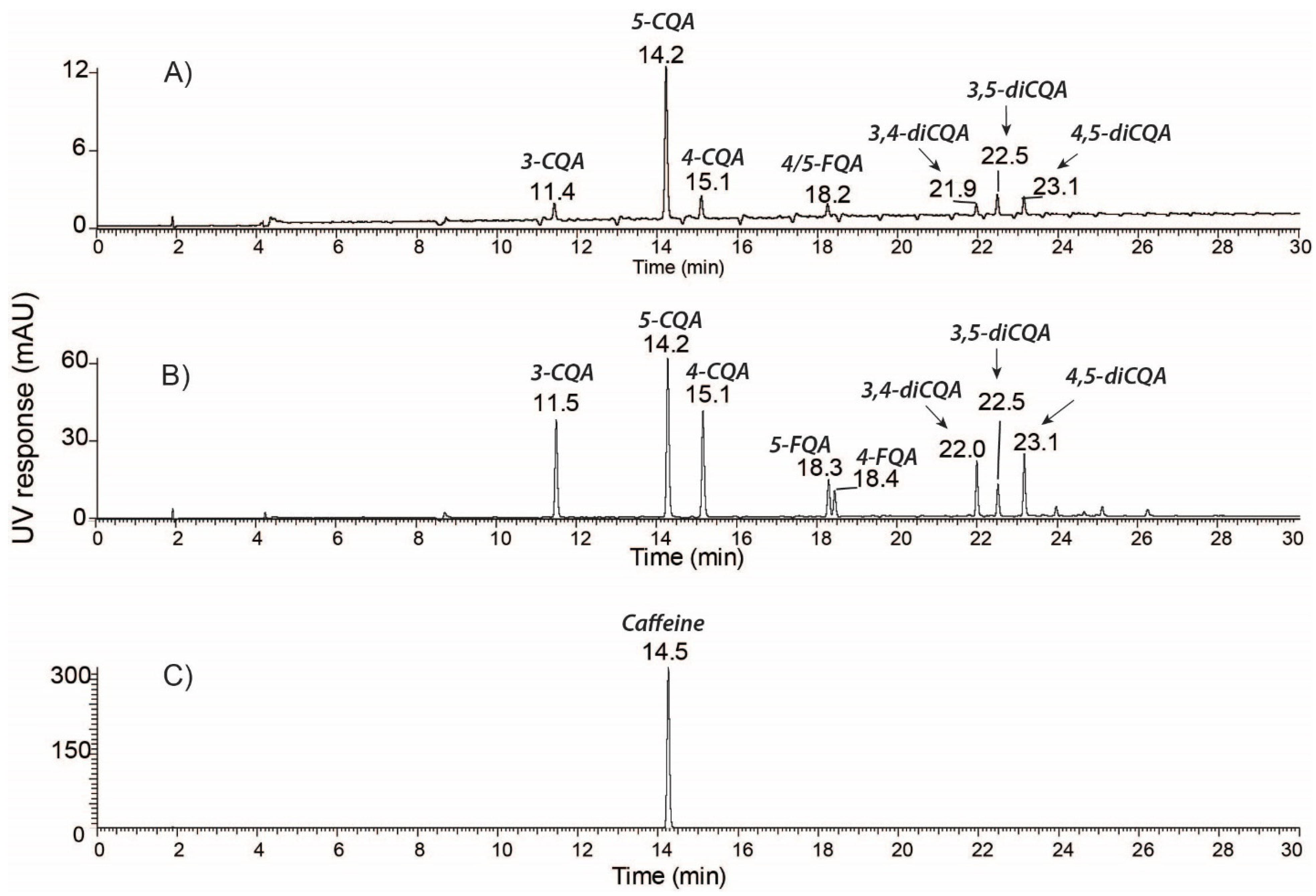
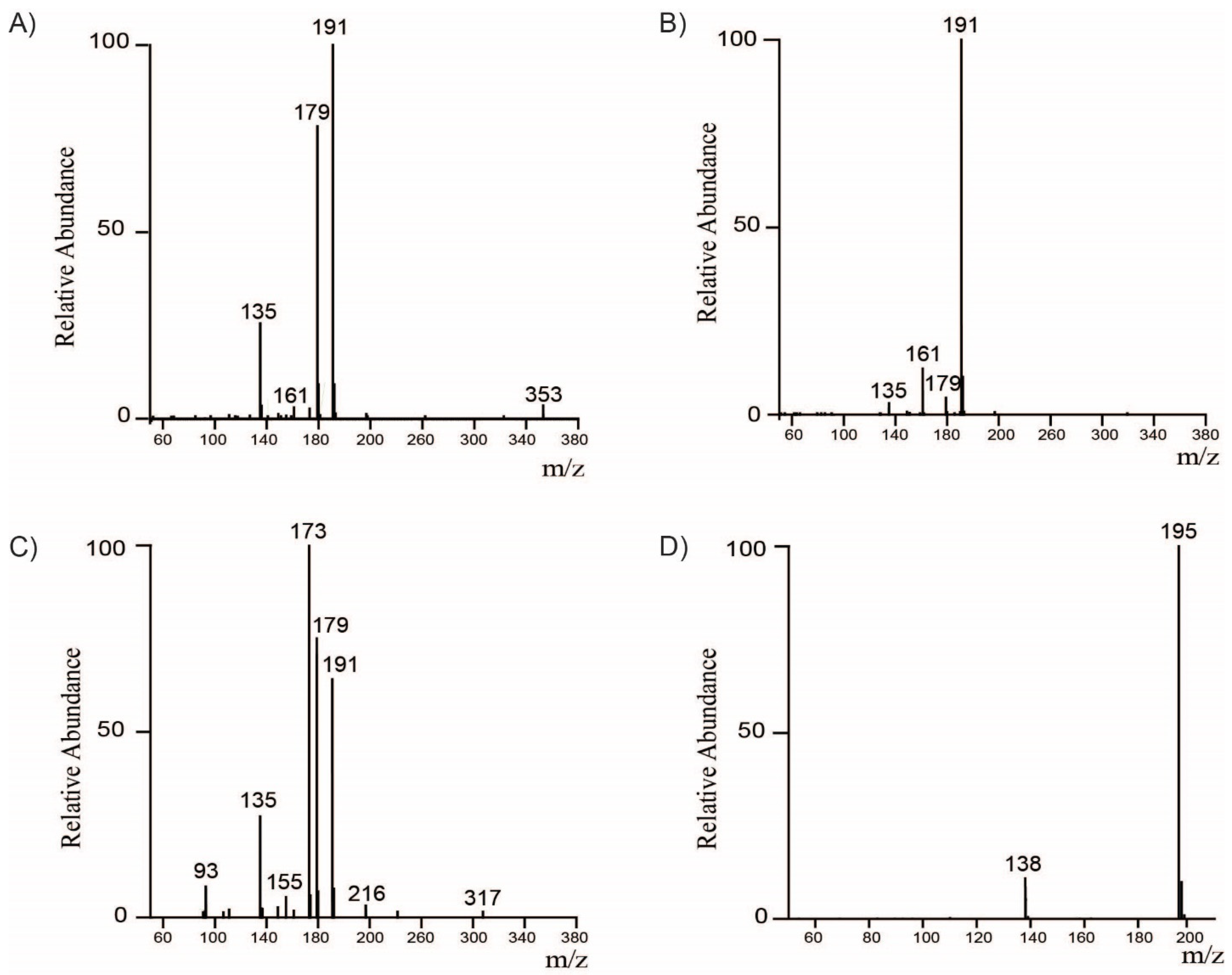
| [M−H]-m/z | [M+H]-m/z | Compound | MS/MS | Concentration (% w/w) | ||
|---|---|---|---|---|---|---|
| WCC | WCCE1 | WCCE2 | ||||
| 353.09 | - | 3-CQA | 135, 179, 191 | 1.02 ± 0.26 | 8.19 ± 0.10 | 1.22 ± 0.00 |
| 353.09 | - | 5-CQA | 191 | 2.95 ± 0.32 | 14.38 ± 0.07 | 2.30 ± 0.01 |
| 353.09 | - | 4-CQA | 135, 173, 179, 191 | 1.17 ± 0.16 | 9.41 ± 0.71 | 1.31 ± 0.00 |
| 367.10 | - | 5-FQA | 173, 191 | 0.41 ± 0.12 | 3.38 ± 0.01 | 0.28 ± 0.00 |
| 367.10 | - | 4-FQA | 173, 193, 191 | 0.18 ± 0.07 | 2.23 ± 0.02 | 0.16 ± 0.00 |
| 515.12 | - | 3,4-diCQA | 173, 179, 191, 353 | 0.39 ± 0.17 | 3.43 ± 0.01 | 0.33 ± 0.00 |
| 515.12 | - | 3,5-diCQA | 135, 179, 191, 353 | 0.23 ± 0.08 | 1.8 ± 0.00 | 0.17 ± 0.00 |
| 515.12 | - | 4,5-diCQA | 173, 179, 191, 353 | 0.41 ± 0.16 | 3.64 ± 0.01 | 0.33 ± 0.00 |
| Total chlorogenic acid | 6.76 ± 1.34 | 46.46 ± 0.93 | 6.1 ± 0.01 | |||
| - | 138.05 | Trigonelline | 94 | 0.4 ± 0.00 | 2.40 + 0.09 | <0.01 |
| - | 195.09 | Caffeine | 138 | 1.12 ± 0.17 | 0.90 ± 0.00 | 73.60 ± 0.65 |
| Sample | DPPH (IC50) (µg/mL) | ABTS (IC50) (µg/mL) |
|---|---|---|
| WCC | 300.00 | 140.00 |
| WCCE1 | 11.12 | 7.85 |
| WCCE2 | ND | ND |
| Antioxidant Activity (µmol TE/g) | ||||||
|---|---|---|---|---|---|---|
| Sample | ORAC | HORAC | NORAC | SORAC | SOAC | Total ORAC |
| WCC | 772 | 764 ± 45.70 | 128.33 ± 11.15 | 126.33 ± 10.60 | 237.66 ± 1 6.56 | 2028.32 |
| WCCE1 | 9029 | 11,089.67 ± 942.59 | 1547 ± 72.80 | 76.33 ± 9.45 | 8623 ± 314.72 | 30365 |
| WCCE2 | ND | 230.33 ± 30.03 | 350 ± 12.12 | ND | ND | 580.33 |
| Sample | IC50 α-Amylase (mg/mL) | IC50 α-Glucosidase (mg/mL) | IC50 AChE (mg/mL) |
|---|---|---|---|
| WCC | 15.00 | 33.00 | ND |
| WCCE1 | 1.74 | 2.42 | ND |
| WCCE2 | ND | ND | 0.09 |
| Caffeine | ND | ND | 0.065 |
| Chlorogenic acid | 2.8 | 2.1 | ND |
| Acarbose | 0.008 | 1.25 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemzer, B.; Kalita, D.; Abshiru, N. Quantification of Major Bioactive Constituents, Antioxidant Activity, and Enzyme Inhibitory Effects of Whole Coffee Cherries (Coffea arabica) and Their Extracts. Molecules 2021, 26, 4306. https://doi.org/10.3390/molecules26144306
Nemzer B, Kalita D, Abshiru N. Quantification of Major Bioactive Constituents, Antioxidant Activity, and Enzyme Inhibitory Effects of Whole Coffee Cherries (Coffea arabica) and Their Extracts. Molecules. 2021; 26(14):4306. https://doi.org/10.3390/molecules26144306
Chicago/Turabian StyleNemzer, Boris, Diganta Kalita, and Nebiyu Abshiru. 2021. "Quantification of Major Bioactive Constituents, Antioxidant Activity, and Enzyme Inhibitory Effects of Whole Coffee Cherries (Coffea arabica) and Their Extracts" Molecules 26, no. 14: 4306. https://doi.org/10.3390/molecules26144306
APA StyleNemzer, B., Kalita, D., & Abshiru, N. (2021). Quantification of Major Bioactive Constituents, Antioxidant Activity, and Enzyme Inhibitory Effects of Whole Coffee Cherries (Coffea arabica) and Their Extracts. Molecules, 26(14), 4306. https://doi.org/10.3390/molecules26144306





