The Effects of Chemical Bonding at Subatomic Resolution: A Case Study on α-Boron
Abstract
:1. Introduction
2. Results and Discussion
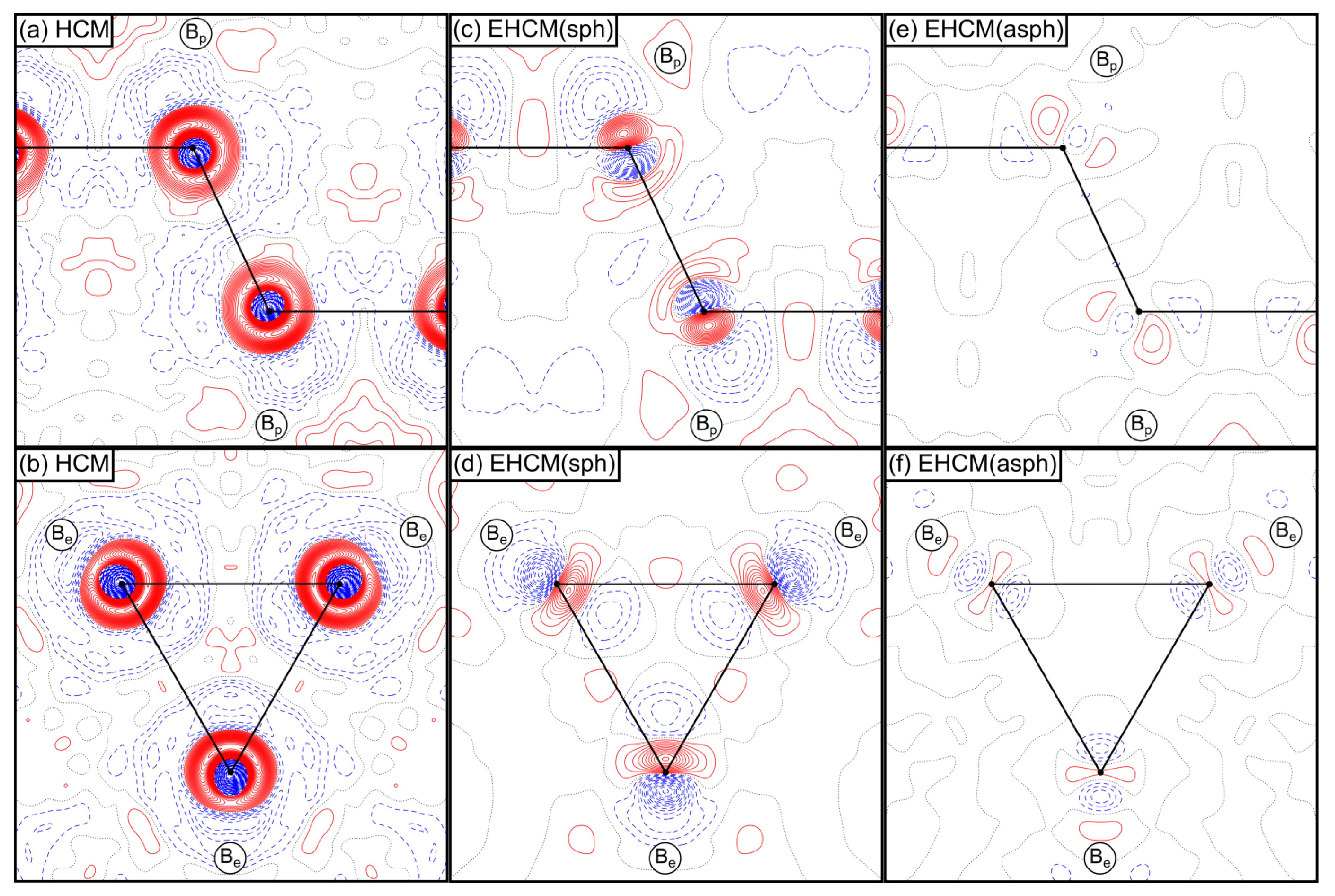
2.1. QTAIM-Analysis and HCM Refinements of Experimental Structure Factors
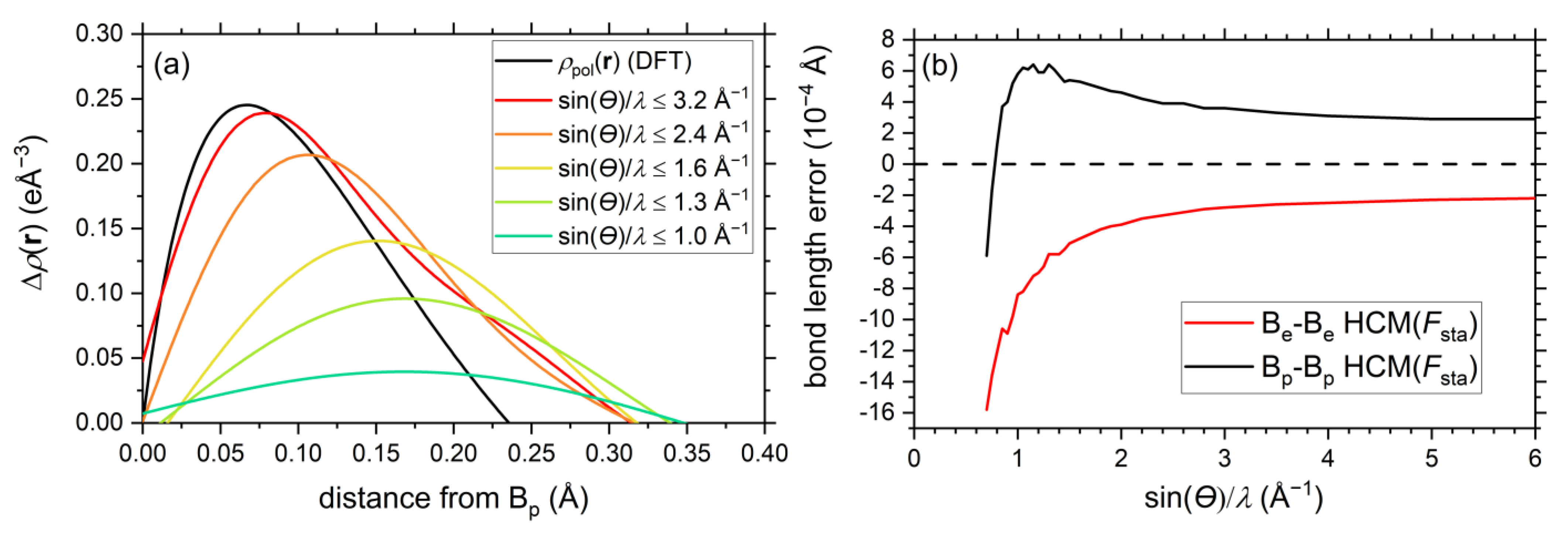
2.2. EHCM Refinements of Calculated Structure Factors and Resolution Dependence of Core Asphericity Shifts
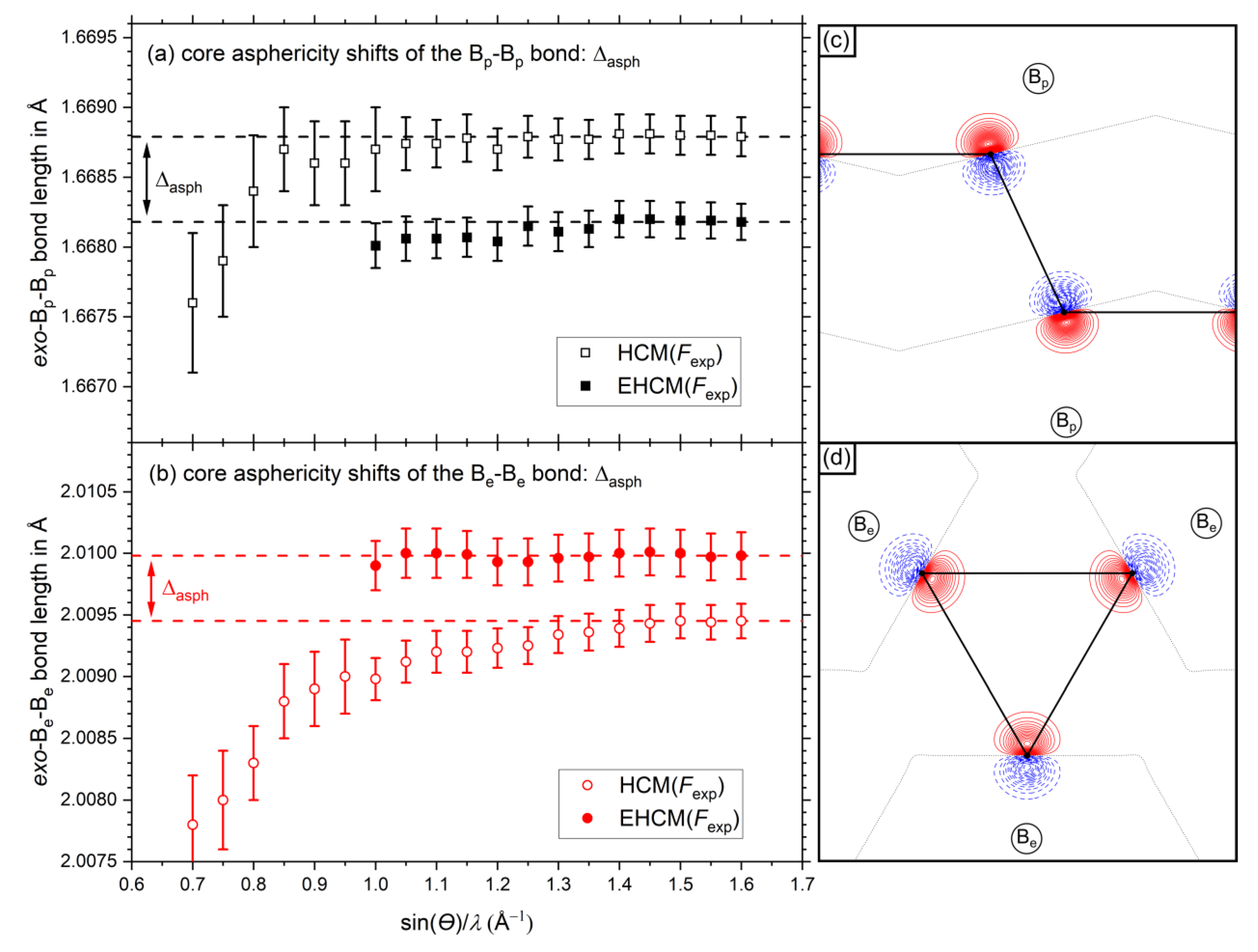
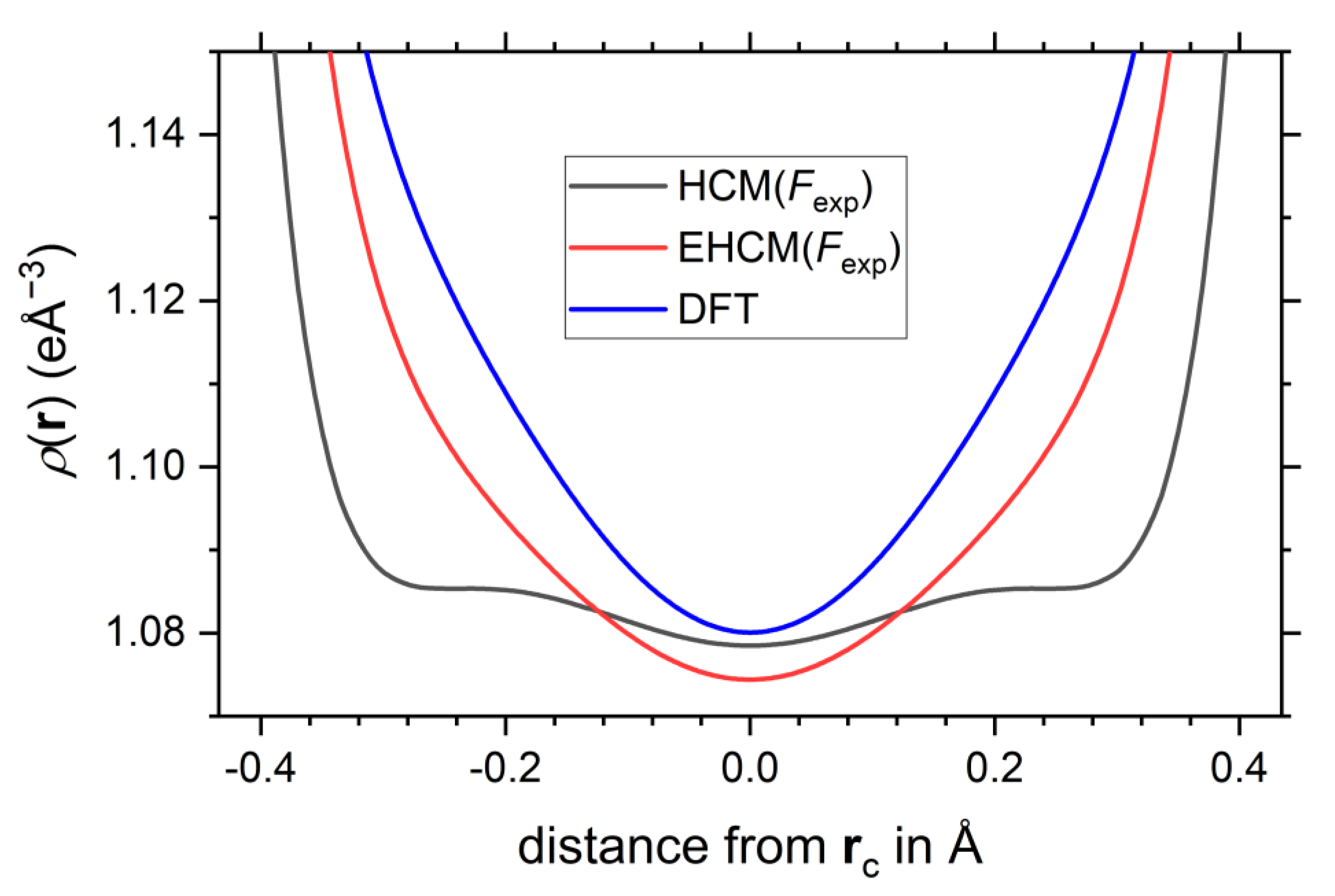
2.3. Experimental EHCM Refinements and Correction of Core Asphericity Shifts
3. Materials and Methods
3.1. Synthesis
3.2. Charge Density Study
3.3. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
Appendix B
References
- Cochran, W. A Comparison of Calculated and Measured Electron Distributions in the Benzene Ring. Acta Crystallogr. 1956, 9, 924–928. [Google Scholar] [CrossRef]
- Tomiie, Y. The Electron Distribution and the Location of the Bonded Hydrogen Atom in Crystals. J. Phys. Soc. Jpn. 1958, 13, 1030–1037. [Google Scholar] [CrossRef]
- Coppens, P. Combining X-ray and neutron diffraction: The study of charge density distributions in solids. In Neutron Diffraction; Dachs, H., Ed.; Topics in Current Physics; Springer: Berlin/Heidelberg Germany; New York, NY, USA, 1978; pp. 71–111. ISBN 978-3-540-08710-6. [Google Scholar]
- Coppens, P. X-ray Charge Densities and Chemical Bonding; International Union of Crystallography/Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Allen, F.H. A Systematic Pairwise Comparison of Geometric Parameters Obtained by X-ray and Neutron Diffraction. Acta Crystallogr. B 1986, 42, 515–522. [Google Scholar] [CrossRef]
- Hansen, N.K.; Coppens, P. Testing Aspherical Atom Refinements on Small-Molecule Data Sets. Acta Crystallogr. Sect. A 1978, 34, 909–921. [Google Scholar] [CrossRef]
- Coppens, P. Evidence for Systematic Errors in X-ray Temperature Parameters Resulting from Bonding Effects. Acta Crystallogr. B 1968, 24, 1272–1274. [Google Scholar] [CrossRef]
- Reisinger, A.; Trapp, N.; Krossing, I.; Altmannshofer, S.; Herz, V.; Presnitz, M.; Scherer, W. Homoleptic Silver(I) Acetylene Complexes. Angew. Chem. Int. Ed. 2007, 46, 8295–8298. [Google Scholar] [CrossRef] [PubMed]
- Himmel, D.; Trapp, N.; Krossing, I.; Altmannshofer, S.; Herz, V.; Eickerling, G.; Scherer, W. Reply. Angew. Chem. Int. Ed. 2008, 47, 7798–7801. [Google Scholar] [CrossRef]
- Batke, K.; Eickerling, G. Topology of the Electron Density of d0 Transition Metal Compounds at Subatomic Resolution. J. Phys. Chem. A 2013, 117, 11566–11579. [Google Scholar] [CrossRef]
- Bentley, J.; Stewart, R.F. Core Deformation Studies by Coherent X-ray Scattering. Acta Crystallogr. Sect. A 1974, 30, 60–67. [Google Scholar] [CrossRef]
- Bentley, J.; Stewart, R.F. Diatomic Generalized X-ray Scattering Factors: Results from Hartree-Fock Electron Density Functions. J. Chem. Phys. 1975, 63, 3794–3803. [Google Scholar] [CrossRef]
- Bentley, J.; Stewart, R.F. Pseudoatoms in Diatomic Molecules: Restricted Radial Functions. Acta Crystallogr. Sect. A 1976, 32, 910–914. [Google Scholar] [CrossRef]
- Chandler, G.S.; Spackman, M.A. Pseudoatom Expansions of the First-Row Diatomic Hydride Electron Densities. Acta Crystallogr. Sect. A 1982, 38, 225–239. [Google Scholar] [CrossRef]
- Hirshfeld, F.L.; Rzotkiewicz, S. Electrostatic Binding in the First-Row AH and A2 Diatomic Molecules. Mol. Phys. 1974, 27, 1319–1343. [Google Scholar] [CrossRef]
- Spackman, M.A.; Maslen, E.N. Electron Density and the Chemical Bond. A Reappraisal of Berlin’s Theorem. Acta Crystallogr. A 1985, 41, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Hirshfeld, F.L. Electron Density Distributions in Molecules. Crystallogr. Rev. 1991, 2, 169–200. [Google Scholar] [CrossRef]
- Autschbach, J.; Schwarz, W.H.E. Where Do the Forces in Molecules Come from? A Density Functional Study of N2 and HCl. J. Phys. Chem. A 2000, 104, 6039–6046. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Hellmann-Feynman Constraint on Charge Densities, an Experimental Test. Acta Crystallogr. B 1984, 40, 613–615. [Google Scholar] [CrossRef]
- Jauch, W.; Schultz, A.J.; Stewart, R.F. Anharmonicity in Thermal Motion and Electrostatic Forces on Nuclei: Pulsed Neutron Diffraction from MnF2. Phys. Rev. B 1999, 59, 373–380. [Google Scholar] [CrossRef]
- Svendsen, H.; Overgaard, J.; Busselez, R.; Arnaud, B.; Rabiller, P.; Kurita, A.; Nishibori, E.; Sakata, M.; Takata, M.; Iversen, B.B. Multipole Electron-Density Modelling of Synchrotron Powder Diffraction Data: The Case of Diamond. Acta Crystallogr. Sect. A 2010, 66, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Tiana, D.; Scherer, W.; Batke, K.; Eickerling, G.; Svendsen, H.; Bindzus, N.; Iversen, B.B. Experimental and Theoretical Charge Density Studies at Subatomic Resolution. J. Phys. Chem. A 2011, 115, 13061–13071. [Google Scholar] [CrossRef]
- Scherer, W.; Fischer, A.; Eickerling, G. The Experimental Density Perspective of Chemical Bonding. In The Chemical Bond; Frenking, G., Shaik, S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 309–344. ISBN 978-3-527-66469-6. [Google Scholar]
- Bindzus, N.; Straasø, T.; Wahlberg, N.; Becker, J.; Bjerg, L.; Lock, N.; Dippel, A.-C.; Iversen, B.B. Experimental Determination of Core Electron Deformation in Diamond. Acta Crystallogr. Sect. Found. Adv. 2014, 70, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Svane, B.; Tolborg, K.; Kato, K.; Iversen, B.B. Multipole Electron Densities and Structural Parameters from Synchrotron Powder X-ray Diffraction Data Obtained with a MYTHEN Detector System (OHGI). Acta Crystallogr. Sect. Found. Adv. 2021, 77. [Google Scholar] [CrossRef]
- Wahlberg, N.; Bindzus, N.; Bjerg, L.; Becker, J.; Christensen, S.; Dippel, A.-C.; Jørgensen, M.R.V.; Iversen, B.B. Powder X-ray Diffraction Electron Density of Cubic Boron Nitride. J. Phys. Chem. C 2015, 119, 6164–6173. [Google Scholar] [CrossRef]
- Wahlberg, N.; Bindzus, N.; Bjerg, L.; Becker, J.; Dippel, A.-C.; Iversen, B.B. Synchrotron Powder Diffraction of Silicon: High-Quality Structure Factors and Electron Density. Acta Crystallogr. Sect. A 2016, 72, 28–35. [Google Scholar] [CrossRef]
- Tolborg, K.; Jørgensen, M.R.V.; Christensen, S.; Kasai, H.; Becker, J.; Walter, P.; Dippel, A.-C.; Als-Nielsen, J.; Iversen, B.B. Accurate Charge Densities from Powder X-ray Diffraction—A New Version of the Aarhus Vacuum Imaging-Plate Diffractometer. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2017, 73, 521–530. [Google Scholar] [CrossRef]
- Svane, B.; Tolborg, K.; Jørgensen, L.R.; Roelsgaard, M.; Jørgensen, M.R.V.; Brummerstedt Iversen, B. Multipole Electron Densities and Atomic Displacement Parameters in Urea from Accurate Powder X-ray Diffraction. Acta Crystallogr. Sect. Found. Adv. 2019, 75, 600–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasai, H.; Tolborg, K.; Sist, M.; Zhang, J.; Hathwar, V.R.; Filsø, M.Ø.; Cenedese, S.; Sugimoto, K.; Overgaard, J.; Nishibori, E.; et al. X-ray Electron Density Investigation of Chemical Bonding in van Der Waals Materials. Nat. Mater. 2018, 17, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Koritsanszky, T.; Volkov, A. Atomic Density Radial Functions from Molecular Densities. Chem. Phys. Lett. 2004, 385, 431–434. [Google Scholar] [CrossRef]
- Koritsanszky, T.; Volkov, A.; Chodkiewicz, M. New Directions in Pseudoatom-Based X-ray Charge Density Analysis. In Electron Density and Chemical Bonding II: Theoretical Charge Density Studies; Stalke, D., Ed.; Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–25. ISBN 978-3-642-30808-6. [Google Scholar]
- Michael, J.R.; Koritsanszky, T. On the Error in the Nucleus-Centered Multipolar Expansion of Molecular Electron Density and Its Topology: A Direct-Space Computational Study. J. Chem. Phys. 2017, 146, 204105. [Google Scholar] [CrossRef] [Green Version]
- Capelli, S.C.; Bürgi, H.-B.; Dittrich, B.; Grabowsky, S.; Jayatilaka, D. Hirshfeld Atom Refinement. IUCrJ 2014, 1, 361–379. [Google Scholar] [CrossRef] [Green Version]
- Woińska, M.; Jayatilaka, D.; Spackman, M.A.; Edwards, A.J.; Dominiak, P.M.; Woźniak, K.; Nishibori, E.; Sugimoto, K.; Grabowsky, S. Hirshfeld Atom Refinement for Modelling Strong Hydrogen Bonds. Acta Crystallogr. Sect. Found. Adv. 2014, 70, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Fugel, M.; Jayatilaka, D.; Hupf, E.; Overgaard, J.; Hathwar, V.R.; Macchi, P.; Turner, M.J.; Howard, J.a.K.; Dolomanov, O.V.; Puschmann, H.; et al. Probing the Accuracy and Precision of Hirshfeld Atom Refinement with HARt Interfaced with Olex2. IUCrJ 2018, 5, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.F. Electron Population Analysis with Rigid Pseudoatoms. Acta Crystallogr. A 1976, 32, 565–574. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. XVII. Spatial Partitioning of Charge Density. Isr. J. Chem. 1977, 16, 198–201. [Google Scholar] [CrossRef]
- Kleemiss, F.; Dolomanov, O.V.; Bodensteiner, M.; Peyerimhoff, N.; Midgley, L.; Bourhis, L.J.; Genoni, A.; Malaspina, L.A.; Jayatilaka, D.; Spencer, J.L.; et al. Accurate Crystal Structures and Chemical Properties from NoSpherA2. Chem. Sci. 2021, 12, 1675–1692. [Google Scholar] [CrossRef]
- Albert, B.; Hillebrecht, H. Boron: Elementary Challenge for Experimenters and Theoreticians. Angew. Chem. Int. Ed. 2009, 48, 8640–8668. [Google Scholar] [CrossRef]
- Will, G.; Kiefer, B.; Morosin, B.; Slack, G.A. Electron Deformation Density Distribution in α-Boron. MRS Online Proc. Libr. 1987, 97, 151–156. [Google Scholar] [CrossRef]
- Will, G.; Kiefer, B. Electron Deformation Density in Rhombohedral α-Boron. Z. Anorg. Allg. Chem. 2001, 627, 2100–2104. [Google Scholar] [CrossRef]
- Morosin, B.; Mullendore, A.W.; Emin, D.; Slack, G.A. Rhombohedral Crystal Structure of Compounds Containing Boron-Rich Icosahedra. AIP Conf. Proc. 1986, 140, 70–86. [Google Scholar] [CrossRef]
- Horn, F.H. On the Crystallization of Simple Rhombohedral Boron from Platinum. J. Electrochem. Soc. 1959, 106, 905–906. [Google Scholar] [CrossRef]
- Mondal, S.; Van Smaalen, S.; Parakhonskiy, G.; Prathapa, S.J.; Noohinejad, L.; Bykova, E.; Dubrovinskaia, N.; Chernyshov, D.; Dubrovinsky, L. Experimental Evidence of Orbital Order in α-B12 and γ-B28 Polymorphs of Elemental Boron. Phys. Rev. B 2013, 88, 024118. [Google Scholar] [CrossRef] [Green Version]
- Fujimori, M.; Nakata, T.; Nakayama, T.; Nishibori, E.; Kimura, K.; Takata, M.; Sakata, M. Peculiar Covalent Bonds in α-Rhombohedral Boron. Phys. Rev. Lett. 1999, 82, 4452–4455. [Google Scholar] [CrossRef]
- Collins, D.M. Electron Density Images from Imperfect Data by Iterative Entropy Maximization. Nature 1982, 298, 49–51. [Google Scholar] [CrossRef]
- Hosoi, S.; Kim, H.; Nagata, T.; Kirihara, K.; Soga, K.; Kimura, K.; Kato, K.; Takata, M. Electron Density Distributions in Derivative Crystals of α-Rhombohedral Boron. J. Phys. Soc. Jpn. 2007, 76, 044602. [Google Scholar] [CrossRef]
- Nishibori, E.; Hyodo, H.; Kimura, K.; Takata, M. Revisit: High Resolution Charge Density Study of α-Rhombohedral Boron Using Third-Generation SR Data at SPring-8. Solid State Sci. 2015, 47, 27–31. [Google Scholar] [CrossRef]
- Lee, S.; Bylander, D.M.; Kleinman, L. Bands and Bonds of B12. Phys. Rev. B 1990, 42, 1316–1320. [Google Scholar] [CrossRef]
- He, J.; Wu, E.; Wang, H.; Liu, R.; Tian, Y. Ionicities of Boron-Boron Bonds in B12 Icosahedra. Phys. Rev. Lett. 2005, 94, 015504. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K.; Dekura, H.; Mori, Y.; Fujii, Y.; Hyodo, H.; Kimura, K. Structural Study of α-Rhombohedral Boron at High Pressures. J. Phys. Soc. Jpn. 2011, 80, 084601. [Google Scholar] [CrossRef]
- Sagawe, V. Chemische Bindungen in Boriden—Theoretische Und Experimentelle Untersuchung. Ph.D. Thesis, Albert-Ludwigs-Universität Freiburg im Breisgau, Freiburg, Germany, 2013. [Google Scholar]
- Decker, B.F.; Kasper, J.S. The Crystal Structure of a Simple Rhombohedral Form of Boron. Acta Crystallogr. 1959, 12, 503–506. [Google Scholar] [CrossRef]
- Pendás, M.A.; Costales, A.; Luaña, V. Ions in Crystals: The Topology of the Electron Density in Ionic Materials. I. Fundamentals. Phys. Rev. B 1997, 55, 4275–4284. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Legare, D.A. Properties of Atoms in Molecules: Structures and Reactivities of Boranes and Carboranes. Can. J. Chem. 1992, 70, 657–676. [Google Scholar] [CrossRef]
- Zhurov, V.V.; Pinkerton, A.A. Charge Density Analysis of an Organic Ferroelectric. Croconic Acid: An Experimental and Theoretical Study. Z. Anorg. Allg. Chem. 2013, 639, 1969–1978. [Google Scholar] [CrossRef]
- Volkov, A.; Coppens, P. Critical Examination of the Radial Functions in the Hansen-Coppens Multipole Model through Topological Analysis of Primary and Refined Theoretical Densities. Acta Crystallogr. Sect. A 2001, 57, 395–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoyanov, E.; Häussermann, U.; Leinenweber, K. Large-Volume Multianvil Cells Designed for Chemical Synthesis at High Pressures. High Press. Res. 2010, 30, 175–189. [Google Scholar] [CrossRef]
- Parakhonskiy, G.; Dubrovinskaia, N.; Dubrovinsky, L.; Mondal, S.; Van Smaalen, S. High Pressure Synthesis of Single Crystals of α-Boron. J. Cryst. Growth 2011, 321, 162–166. [Google Scholar] [CrossRef]
- Macchi, P.; Bürgi, H.-B.; Chimpri, A.S.; Hauser, J.; Gál, Z. Low-Energy Contamination of Mo Microsource X-ray Radiation: Analysis and Solution of the Problem. J. Appl. Crystallogr. 2011, 44, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Bruker. SADABS; Version 2014/2; Bruker AXS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Volkov, A.; Macchi, P.; Farrugia, L.J.; Gatti, C.; Mallinson, P.R.; Richter, R.; Koritsanszky, T. XD2006: A Computer Program for Multipole Refinement, Topological Analysis of Charge Densities, and Evaluation of Intermolecular Energies from Experimental or Theoretical Structure Factors; Version 5.42; 2007. Available online: https://www.chem.gla.ac.uk/~louis/xd-home/ (accessed on 13 July 2021).
- Becker, P.J.; Coppens, P. Extinction within the Limit of Validity of the Darwin Transfer Equations. I. General Formalism for Primary and Secondary Extinction and Their Applications to Spherical Crystals. Acta Crystallogr. Sect. A 1974, 30, 129–147. [Google Scholar] [CrossRef]
- Becker, P.J.; Coppens, P. Extinction within the Limit of Validity of the Darwin Transfer Equations. II. Refinement of Extinction in Spherical Crystals of SrF2 and LiF. Acta Crystallogr. Sect. A 1974, 30, 148–153. [Google Scholar] [CrossRef]
- Krause, L.; Niepötter, B.; Schürmann, C.J.; Stalke, D.; Herbst-Irmer, R. Validation of Experimental Charge-Density Refinement Strategies: When Do We Overfit? IUCrJ 2017, 4, 420–430. [Google Scholar] [CrossRef]
- The Elk Code. Available online: http://elk.sourceforge.net/ (accessed on 26 May 2021).
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otero-de-la-Roza, A.; Johnson, E.R.; Luaña, V. Critic2: A Program for Real-Space Analysis of Quantum Chemical Interactions in Solids. Comput. Phys. Commun. 2014, 185, 1007–1018. [Google Scholar] [CrossRef]
- Stewart, R.F.V. One-Electron Density Functions and Many-Centered Finite Multipole Expansions. Isr. J. Chem. 1977, 16, 124–131. [Google Scholar] [CrossRef]
- Clementi, E.; Raimondi, D.L. Atomic Screening Constants from SCF Functions. J. Chem. Phys. 1963, 38, 2686–2689. [Google Scholar] [CrossRef]

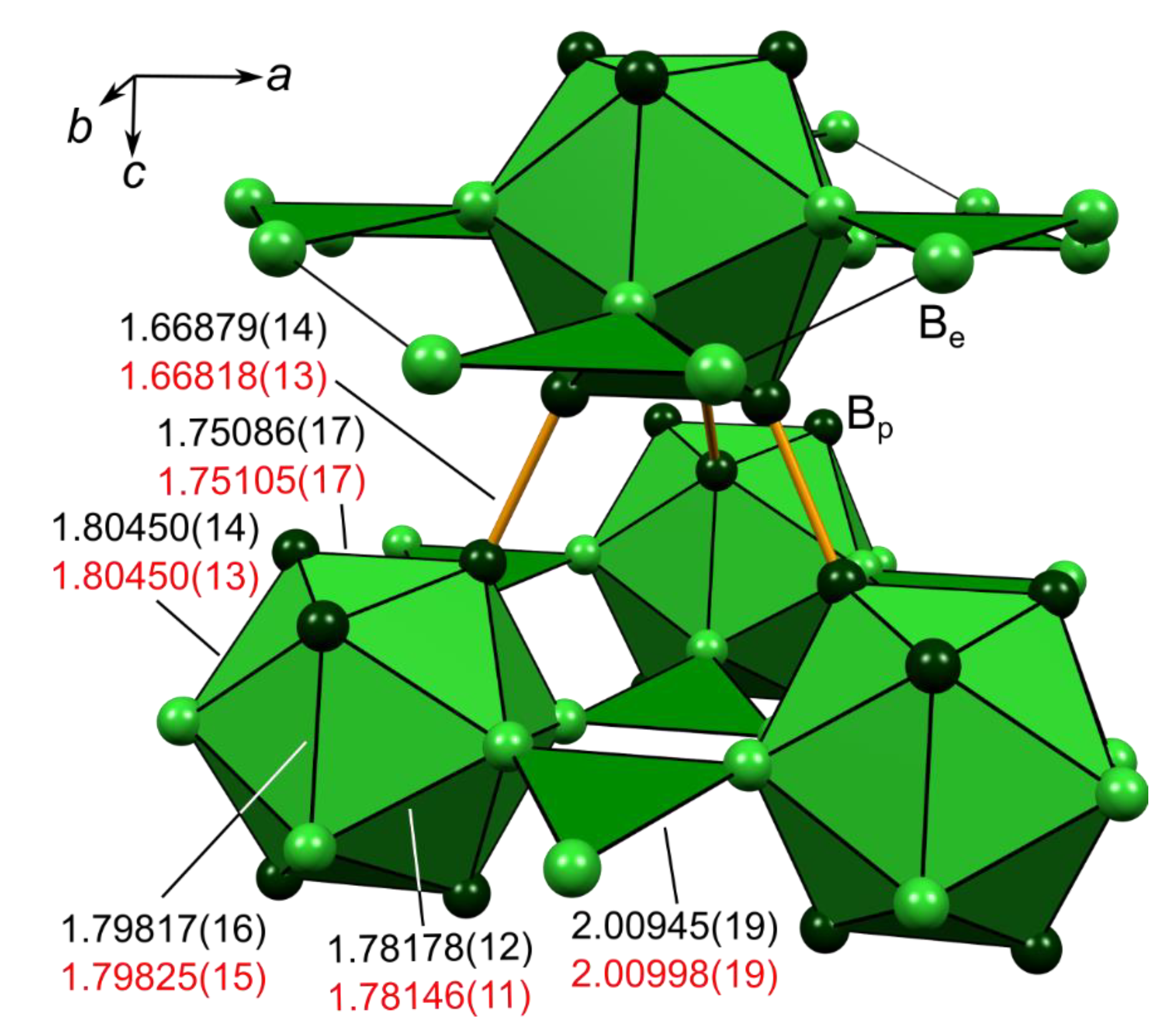
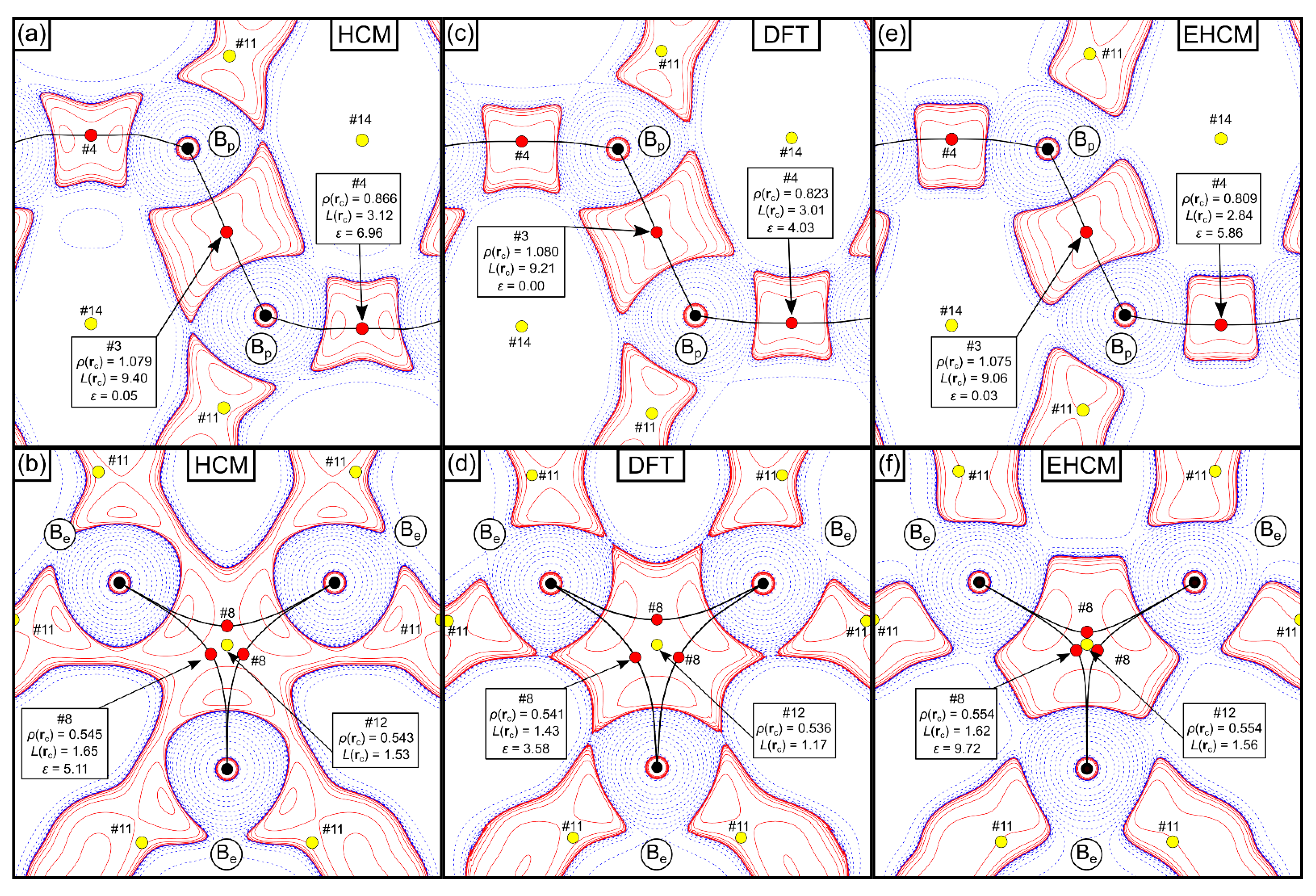
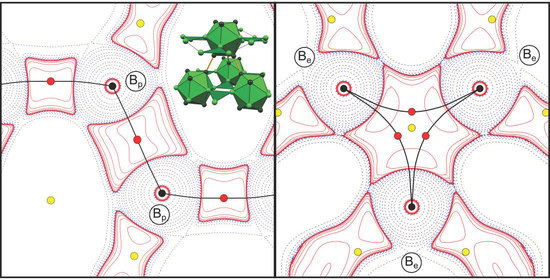
| Cp # | Study/Model | Rank | m | ρ(rc)(eÅ−3) | L(rc)(eÅ−5) | ε | λ3(eÅ−3) | Description |
|---|---|---|---|---|---|---|---|---|
| 3 | HCM | (3,−1) | 3 | 1.079 | 9.40 | 0.05 | 0.69 | Bpa-Bpb (exo) |
| EHCM(asph) | 1.075 | 9.06 | 0.03 | 1.18 | ||||
| Mondal et al. | {1.104} | {9.57} | {-} | {-} | ||||
| DFT | [1.080] | [9.21] | [0.00] | [1.69] | ||||
| 4 | HCM | (3,−1) | 6 | 0.866 | 3.12 | 6.96 | 1.09 | Bpa-Bpc (endo) |
| EHCM(asph) | 0.809 | 2.84 | 5.86 | 1.00 | ||||
| Mondal et al. | {0.820} | {2.26} | {-} | {-} | ||||
| DFT | [0.823] | [3.01] | [4.03] | [1.33] | ||||
| 5 | HCM | (3,−1) | 6 | 0.817 | 3.02 | 2.31 | 1.32 | Bea-Bed (endo) |
| EHCM(asph) | 0.803 | 3.06 | 4.52 | 0.98 | ||||
| Mondal et al. | {0.804} | {2.47} | {-} | {-} | ||||
| DFT | [0.796] | [2.87] | [2.70] | [1.57] | ||||
| 6 | HCM | (3,−1) | 6 | 0.756 | 2.58 | 4.41 | 1.01 | Bpa-Bee (endo) |
| EHCM(asph) | 0.774 | 2.81 | 7.16 | 0.85 | ||||
| Mondal et al. | {0.764} | {1.95} | {-} | {-} | ||||
| DFT | [0.768] | [2.60] | [3.45] | [1.45] | ||||
| 7 | HCM | (3,−1) | 12 | 0.756 | 1.93 | 3.93 | 1.44 | Bpa-Bea (endo) |
| EHCM(asph) | 0.774 | 2.60 | 8.78 | 0.77 | ||||
| Mondal et al. | {0.745} | {1.39} | {-} | {-} | ||||
| DFT | [0.764] | [2.39] | [3.93] | [1.50] | ||||
| 8 | HCM | (3,−1) | 6 | 0.545 | 1.65 | 5.11 | 1.07 | Bea-Bef (exo) |
| EHCM(asph) | 0.554 | 1.62 | 9.72 | 0.47 | ||||
| Mondal et al. | {0.561} | {1.24} | {-} | {-} | ||||
| DFT | [0.541] | [1.43] | [3.58] | [1.18] | ||||
| 9 | HCM | (3,+1) | 2 | 0.863 | 2.76 | - | - | Bpa-Bpc-Bph |
| EHCM(asph) | 0.800 | 2.20 | ||||||
| Mondal et al. | {0.795} | {1.16} | ||||||
| DFT | [0.807] | [2.15] | ||||||
| 12 | HCM | (3,+1) | 2 | 0.543 | 1.53 | - | - | Bea-Bef-Beg |
| EHCM(asph) | 0.554 | 1.56 | ||||||
| Mondal et al. | {0.557} | {1.06} | ||||||
| DFT | [0.536] | [1.17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, A.; Eickerling, G.; Scherer, W. The Effects of Chemical Bonding at Subatomic Resolution: A Case Study on α-Boron. Molecules 2021, 26, 4270. https://doi.org/10.3390/molecules26144270
Fischer A, Eickerling G, Scherer W. The Effects of Chemical Bonding at Subatomic Resolution: A Case Study on α-Boron. Molecules. 2021; 26(14):4270. https://doi.org/10.3390/molecules26144270
Chicago/Turabian StyleFischer, Andreas, Georg Eickerling, and Wolfgang Scherer. 2021. "The Effects of Chemical Bonding at Subatomic Resolution: A Case Study on α-Boron" Molecules 26, no. 14: 4270. https://doi.org/10.3390/molecules26144270
APA StyleFischer, A., Eickerling, G., & Scherer, W. (2021). The Effects of Chemical Bonding at Subatomic Resolution: A Case Study on α-Boron. Molecules, 26(14), 4270. https://doi.org/10.3390/molecules26144270