Effective Extraction of Limonene and Hibaene from Hinoki (Chamaecyparis obtusa) Using Ionic Liquid and Deep Eutectic Solvent
Abstract
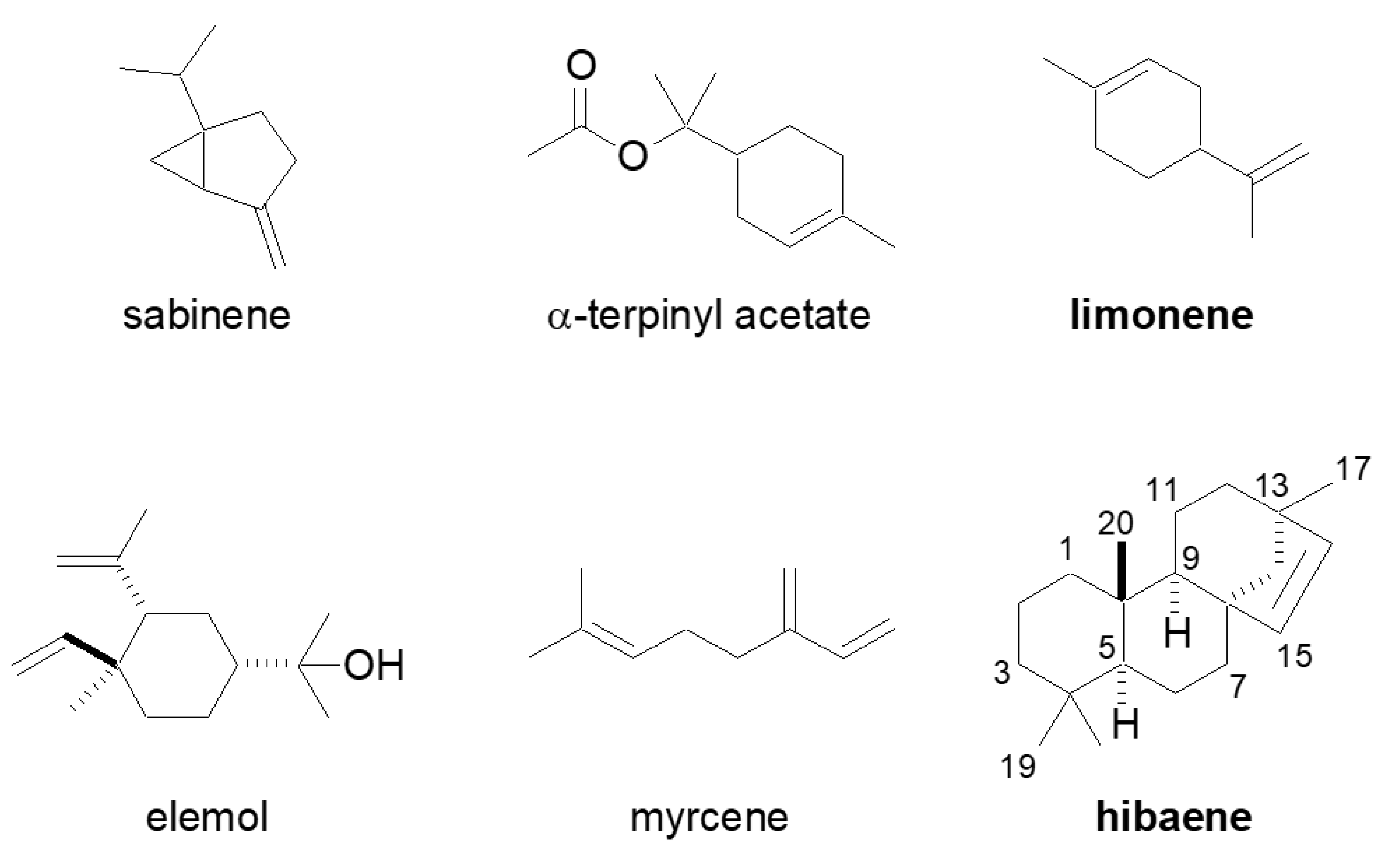
:1. Introduction
2. Results and Discussion
2.1. Extraction Yields Using IL
2.2. Extraction Yields Using DESs
3. Materials and Methods
3.1. General
3.2. Isolation of Hibaene
3.3. Organic Solvent Extraction
3.4. IL-Assisted Extraction
3.5. Preparation of Deep Eutectic Solvents (DESs)
3.6. Extraction Using DESs
3.7. GC-MS Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yang, J.K.; Choi, M.S.; Seo, W.T.; Rinker, D.L.; Han, S.W.; Cheong, G.W. Chemical composition and antimicrobial activity of Chamaecyparis obtusa leaf essential oil. Fitoterapia 2007, 78, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological effect of olfactory stimulation by Hinoki cypress (Chamaecyparis obtusa) leaf oil. J. Physiol. Anthropol. 2015, 34, 44. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.S.; Kang, S.Y.; Kim, Y.H.; Lee, Y.D.; Choi, N.Y.; You, Y.O.; Kim, K.J. Chamaecyparis obtusa Essential Oil Inhibits Methicillin-Resistant Staphylococcus aureus Biofilm Formation and Expression of Virulence Factors. J. Med. Food 2015, 18, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Chen, B.; Ko, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Muhammad, N.; Man, Z.; Mutalib, M.I.A.; Bustam, M.A.; Wilfred, C.D.; Khan, A.S.; Ullah, Z.; Gonfa, G.; Nasrullahet, A. Dissolution and Separation of Wood Biopolymers Using Ionic Liquids. ChemBioEng Rev. 2015, 2, 257–278. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P. Use of Ionic Liquids in Chitin Biorefinery: A Systematic Review. Front. Bioeng. Biotechnol. 2020, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Fukaya, Y.; Hayashi, K.; Wada, M.; Ohno, H. Cellulose dissolution with polar ionic liquids under mild conditions: Required factors for anions. Green Chem. 2008, 10, 44–46. [Google Scholar] [CrossRef]
- Munakata, K.; Yoshizawa-Fujita, M.; Rikukawa, M.; Usuki, T. Improved Extraction Yield of Citral from Lemon Myrtle Using a Cellulose-Dissolving Ionic Liquid. Aust. J. Chem. 2017, 70, 699–704. [Google Scholar] [CrossRef]
- Syahmina, A.; Usuki, T. Ionic Liquid-Assisted Extraction of Essential Oils from Thujopsis dolobrata (Hiba). ACS Omega 2020, 45, 29618–29622. [Google Scholar] [CrossRef]
- Hou, Y.; Gu, Y.; Zhang, S.; Yang, F.; Ding, H.; Shan, Y. Novel binary eutectic mixtures based on imidazole. J. Mol. Liq. 2008, 143, 154–159. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 2003, 70–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Guo, X.; Xu, T.; Fan, L.; Zhou, X.; Wu, S. Ionic deep eutectic solvents for the extraction and separation of natural products. J. Chromatogr. A 2019, 1598, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, M.; Razboršek, M.I.; Kolar, M. Innovative Extraction Techniques, Using Deep Eutectic Solvents and Analytical Methods for the Isolation and Characterization of Natural Bioactive Compounds from Plant Material. Plants 2020, 9, 1428. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, X.L.; Quan, H.F.; Yan, L.; Pei, X.Y.; Wang, R.; Peng, X.D. Effects of Betaine on LPS-Stimulated Activation of Microglial M1/M2 Phenotypes by Suppressing TLR4/NF-κB Pathways in N9 Cells. Molecules 2019, 24, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Yang, J.; Ning, Z.; Zhang, X. 1H NMR-Based Metabolic Profiling of Urine from Mice Fed Lentinula edodes-Derived Polysaccharides. Pol. J. Food Nutr. Sci. 2018, 68, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J. Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Hao, C.; Chen, L.; Dong, H.; Xing, W.; Xue, F.; Cheng, Y. Extraction of Flavonoids from Scutellariae Radix using Ultrasound-Assisted Deep Eutectic Solvents and Evaluation of Their Anti-Inflammatory Activities. ACS Omega 2020, 5, 23140–23147. [Google Scholar] [CrossRef]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.L.; Guo, L.; Li, P.; Liu, E.H. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Dai, Y.; Spronsen, J.V.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]

| Extraction Solvent | Average Yield (%, w/w) | |
|---|---|---|
| Limonene | Hibaene | |
| hexane | 0.02923 ± 0.01067 | 0.00423 ±0.00035 |
| EtOAc | 0.08763 ± 0.00884 | 0.00667 ± 0.00047 |
| acetone | 0.09758 ± 0.00475 | 0.00577 ± 0.00032 |
| IL IL/hexane | 0.06511 ±0.01695 0.09903 ± 0.02795 | 0.00732 ± 0.000205 0.00671 ± 0.00097 |
| IL/EtOAc | 0.12501 ± 0.03357 | 0.00954 ± 0.00156 |
| IL/acetone | 0.14782 ± 0.01978 | 0.01068 ± 0.00036 |
| Extraction Solvent | Average Yield (%, w/w) | |
|---|---|---|
| Limonene | Hibaene | |
| hexane | 0.02923 ± 0.01067 | 0.00423 ± 0.00035 |
| EtOAc | 0.08763 ± 0.00884 | 0.00667 ± 0.00047 |
| acetone | 0.09758 ± 0.00475 | 0.00577 ± 0.00032 |
| BetGly&H2O/hexane BetGly&H2O/EtOAc | 0.05112 ± 0.00032 0.07961 ± 0.00754 | 0.00481 ± 0.00026 0.00695 ± 0.00047 |
| BetGly&H2O/acetone | 0.07434 ± 0.00401 | 0.00568 ± 0.00030 |
| BetLac&H2O/hexane | 0.06574 ± 0.00474 | 0.00668 ± 0.00030 |
| BetLac&H2O/EtOAc | 0.11201 ± 0.00572 | 0.00903 ± 0.00125 |
| BetLac&H2O/acetone | 0.12451 ± 0.00702 | 0.00934 ± 0.00060 |
| BetSuc&H2O/hexane | 0.07493 ± 0.00138 | 0.00845 ± 0.00072 |
| BetSuc&H2O/EtOAc | 0.10342 ± 0.00260 | 0.00882 ± 0.00089 |
| BetSuc&H2O/acetone | 0.06147 ± 0.00537 | 0.00502 ± 0.00038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasutomi, R.; Anzawa, R.; Urakawa, M.; Usuki, T. Effective Extraction of Limonene and Hibaene from Hinoki (Chamaecyparis obtusa) Using Ionic Liquid and Deep Eutectic Solvent. Molecules 2021, 26, 4271. https://doi.org/10.3390/molecules26144271
Yasutomi R, Anzawa R, Urakawa M, Usuki T. Effective Extraction of Limonene and Hibaene from Hinoki (Chamaecyparis obtusa) Using Ionic Liquid and Deep Eutectic Solvent. Molecules. 2021; 26(14):4271. https://doi.org/10.3390/molecules26144271
Chicago/Turabian StyleYasutomi, Rina, Riki Anzawa, Masamitsu Urakawa, and Toyonobu Usuki. 2021. "Effective Extraction of Limonene and Hibaene from Hinoki (Chamaecyparis obtusa) Using Ionic Liquid and Deep Eutectic Solvent" Molecules 26, no. 14: 4271. https://doi.org/10.3390/molecules26144271
APA StyleYasutomi, R., Anzawa, R., Urakawa, M., & Usuki, T. (2021). Effective Extraction of Limonene and Hibaene from Hinoki (Chamaecyparis obtusa) Using Ionic Liquid and Deep Eutectic Solvent. Molecules, 26(14), 4271. https://doi.org/10.3390/molecules26144271







