A QM/MM Study on the Initiation Reaction of Firefly Bioluminescence—Enzymatic Oxidation of Luciferin
Abstract
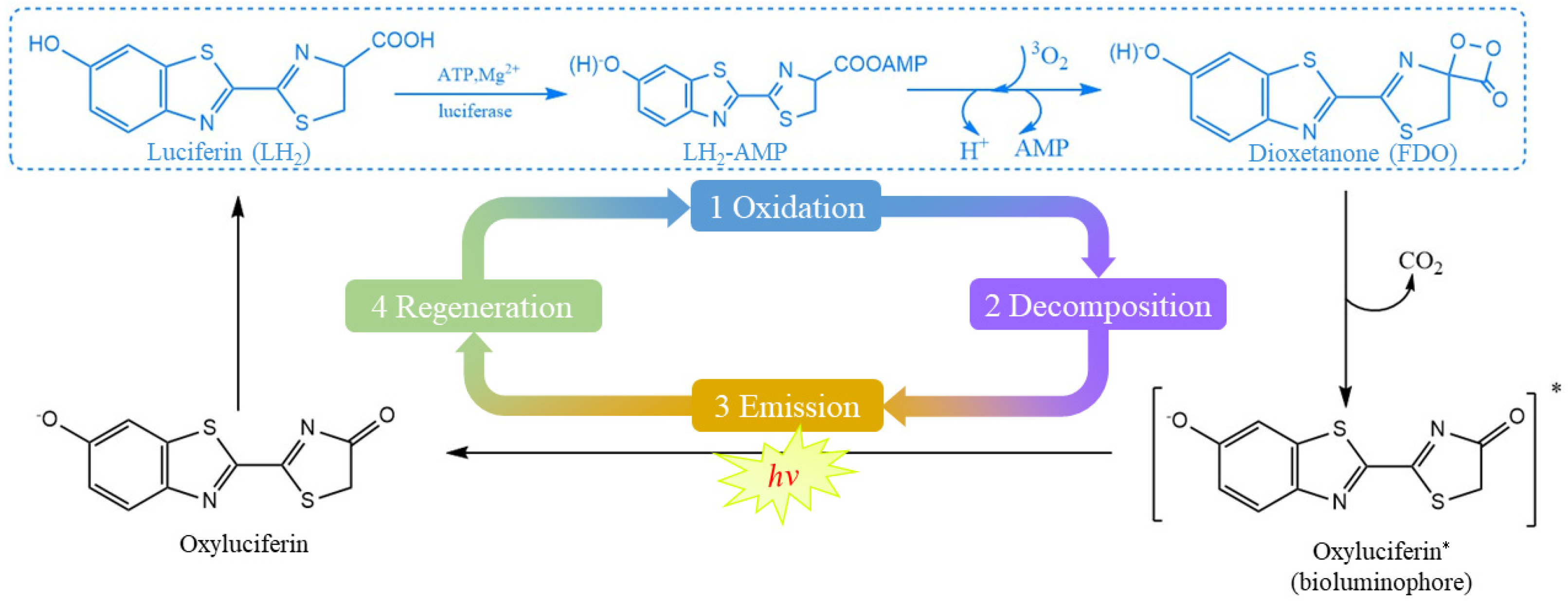
:1. Introduction
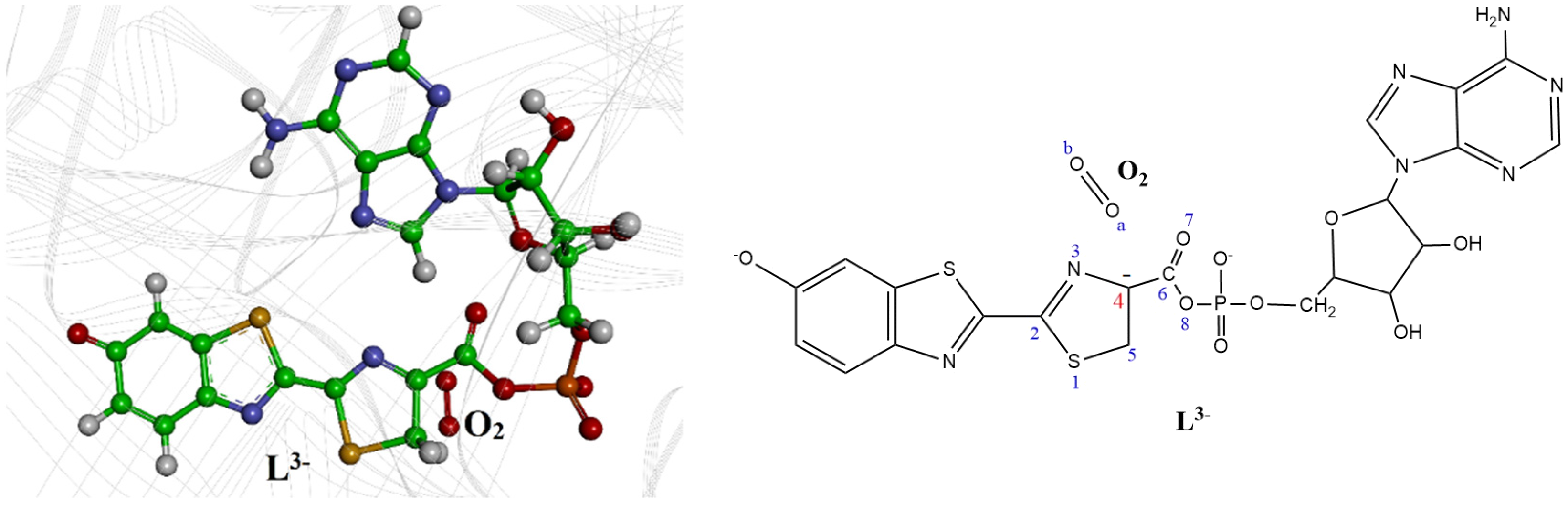
2. Computational Details
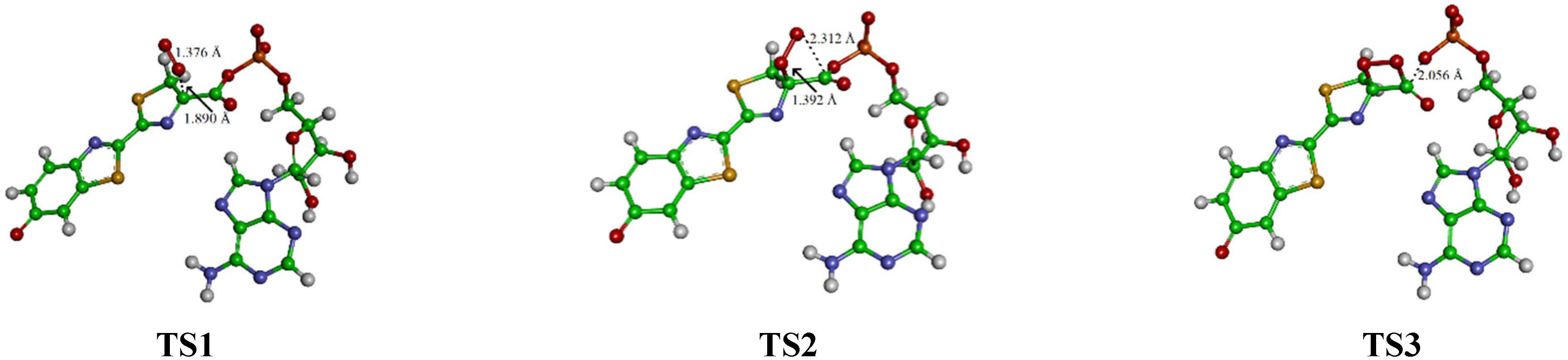
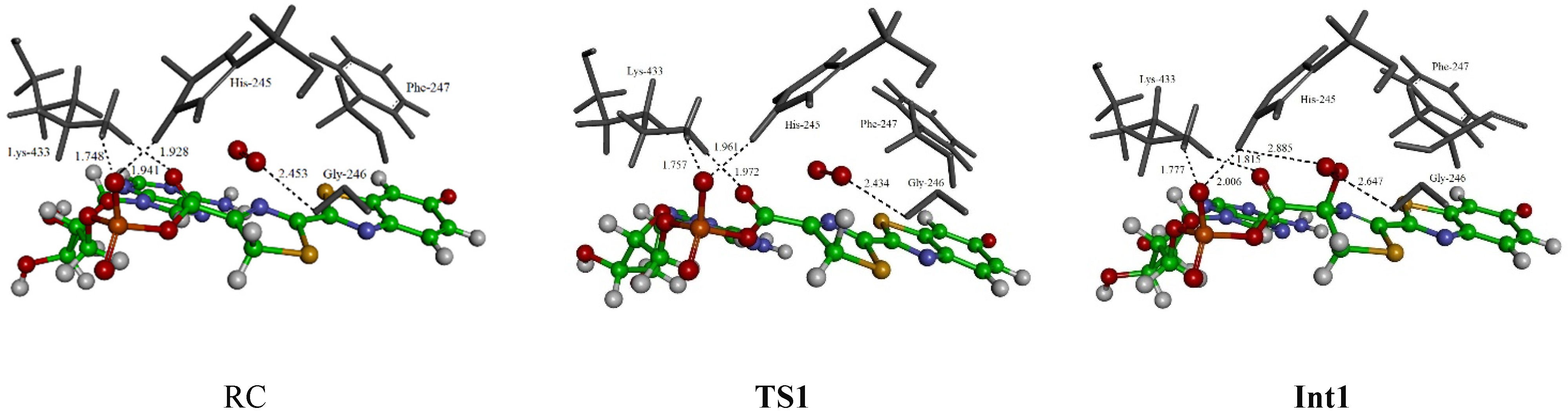
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ando, Y.; Niwa, K.; Yamada, N.; Enomot, T.; Irie, T.; Kubota, H.; Ohmiya, Y.; Akiyama, H. Firefly bioluminescence quantum yield and colour change by pH-sensitive green emission. Nat. Photonics. 2008, 2, 44–47. [Google Scholar] [CrossRef]
- Kuchimaru, T.; Iwano, S.; Kiyama, M.; Mitsumata, S.; Kadonosono, T.; Niwa, H.; Maki, S.; Kizaka-Kondoh, S. A luciferin analogue generating near-infrared bioluminescence achieves highly sensitive deep-tissue imaging. Nat. Commun. 2016, 7, 11856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negrin, R.S.; Contag, C.H. Innovation—In vivo imaging using bioluminescence: A tool for probing graft-versus-host disease. Nat. Rev. Immunol. 2006, 6, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Vacher, M.; Galvan, I.F.; Ding, B.-W.; Schramm, S.; Berraud-Pache, R.; Naumov, P.; Ferre, N.; Liu, Y.-J.; Navizet, I.; Roca-Sanjuan, D.; et al. Chemi- and Bioluminescence of Cyclic Peroxides. Chem. Rev. 2018, 118, 6927–6974. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.V. The chemical mechanism and evolutionary development of beetle bioluminescence. Photochem. Photobiol. 1995, 62, 662–673. [Google Scholar] [CrossRef]
- Schmidt, S.P.; Schuster, G.B. Dioxethanone Chemiluminescence by Chemically—Initiated Electron Exchange Pathway—Efficient Generation of Excited Singlet-States. J. Am. Chem. Soc. 1978, 100, 1966–1968. [Google Scholar] [CrossRef]
- Navizet, I.; Liu, Y.-J.; Ferre, N.; Roca-Sanjuan, D.; Lindh, R. The Chemistry of Bioluminescence: An Analysis of Chemical Functionalities. Chemphyschem 2011, 12, 3064–3076. [Google Scholar] [CrossRef]
- Navizet, I.; Liu, Y.-J.; Ferre, N.; Xiao, H.-Y.; Fang, W.-H.; Lindh, R. Color-Tuning Mechanism of Firefly Investigated by Multi-Configurational Perturbation Method. J. Am. Chem. Soc. 2010, 132, 706–712. [Google Scholar] [CrossRef]
- Chen, S.-F.; Liu, Y.-J.; Navizet, I.; Ferre, N.; Fang, W.-H.; Lindh, R. Systematic Theoretical Investigation on the Light Emitter of Firefly. J. Chem. Theory. Comput. 2011, 7, 798–803. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Esteves da Silva, J.C.G. Quantum/molecular mechanics study of firefly bioluminescence on luciferase oxidative conformation. Chem. Phys. Lett. 2014, 608, 45–49. [Google Scholar] [CrossRef]
- Orlova, G.; Goddard, J.D.; Brovko, L.Y. Theoretical study of the amazing firefly bioluminescence: The formation and structures of the light emitters. J. Am. Chem. Soc. 2003, 125, 6962–6971. [Google Scholar] [CrossRef]
- Yue, L.; Liu, Y.-J.; Fang, W.-H. Mechanistic Insight into the Chemiluminescent Decomposition of Firefly Dioxetanone. J. Am. Chem. Soc. 2012, 134, 11632–11639. [Google Scholar] [CrossRef]
- Yue, L.; Lan, Z.; Liu, Y.-J. The Theoretical Estimation of the Bioluminescent Efficiency of the Firefly via a Nonadiabatic Molecular Dynamics Simulation. J. Phys. Chem. Lett. 2015, 6, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-Y.; Liu, Y.-J. Luciferin Regeneration in Firefly Bioluminescence via Proton-Transfer-Facilitated Hydrolysis, Condensation and Chiral Inversion. Chem. Phys. Chem. 2019, 20, 1719–1727. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Esteves da Silva, J.C.G. Firefly Chemiluminescence and Bioluminescence: Efficient Generation of Excited States. Chem. Phys. Chem. 2012, 13, 2257–2262. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.P.; Esteves da Silva, J.C.G. Study on the Effects of Intermolecular Interactions on Firefly Multicolor Bioluminescence. Chem. Phys. Chem. 2011, 12, 3002–3008. [Google Scholar] [CrossRef] [PubMed]
- Dubinnyi, M.A.; Tsarkova, A.S.; Petushkov, V.N.; Kaskova, Z.M.; Rodionova, N.S.; Kovalchuk, S.I.; Ziganshin, R.H.; Baranov, M.S.; Mineev, K.S.; Yampolsky, I.V. Novel Peptide Chemistry in Terrestrial Animals: Natural Luciferin Analogues from the Bioluminescent Earthworm Fridericia heliota. Chem. Eur. J. 2015, 21, 3942–3947. [Google Scholar] [CrossRef]
- Winkler, U.K.; Sicher, J. Bioluminescence of animals, plants and bacteria. Naturwissenschaften 1996, 83, 312–320. [Google Scholar] [CrossRef]
- Shimomura, O.; Johnson, F.H. Structure of Latia luciferin. Biochemistry 1968, 7, 1734. [Google Scholar] [CrossRef]
- Ding, B.-W.; Naumov, P.; Liu, Y.-J. Mechanistic Insight into Marine Bioluminescence: Photochemistry of the Chemiexcited Cypridina (Sea Firefly) Lumophore. J. Chem. Theory. Comput. 2015, 11, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Branchini, B.R.; Behney, C.E.; Southworth, T.L.; Fontaine, D.M.; Gulick, A.M.; Vinyard, D.J.; Brudvig, G.W. Experimental Support for a Single Electron-Transfer Oxidation Mechanism in Firefly Bioluminescence. J. Am. Chem. Soc. 2015, 137, 7592–7595. [Google Scholar] [CrossRef] [PubMed]
- Berraud-Pache, R.; Lindh, R.; Navizet, I. QM/MM Study of the Formation of the Dioxetanone Ring in Fireflies Through a Superoxide Ion. J. Phys. Chem. B 2018, 122, 5173–5182. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Cheng, Y.; Liu, Y. Mechanistic Study of Oxygenation Reaction in Firefly Bioluminescence. Acta Chim. Sin. 2020, 78, 989–993. [Google Scholar] [CrossRef]
- Sundlov, J.A.; Fontaine, D.M.; Southworth, T.L.; Branchini, B.R.; Gulick, A.M. Crystal Structure of Firefly Luciferase in a Second Catalytic Conformation Supports a Domain Alternation Mechanism. Biochemistry 2012, 51, 6493–6495. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision B.01; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2007, 120, 215–241. [Google Scholar]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian-Basis Sets For Molecular Calculations 1. 2ND Row Atoms, Z=11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular-Orbital Methods 20. Basis Set for Correlated Wave-Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Case, D.A.; Betz, R.M.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Homeyer, N.; et al. AMBER 2016; University of California: San Francisco, CA, USA, 2016. [Google Scholar]

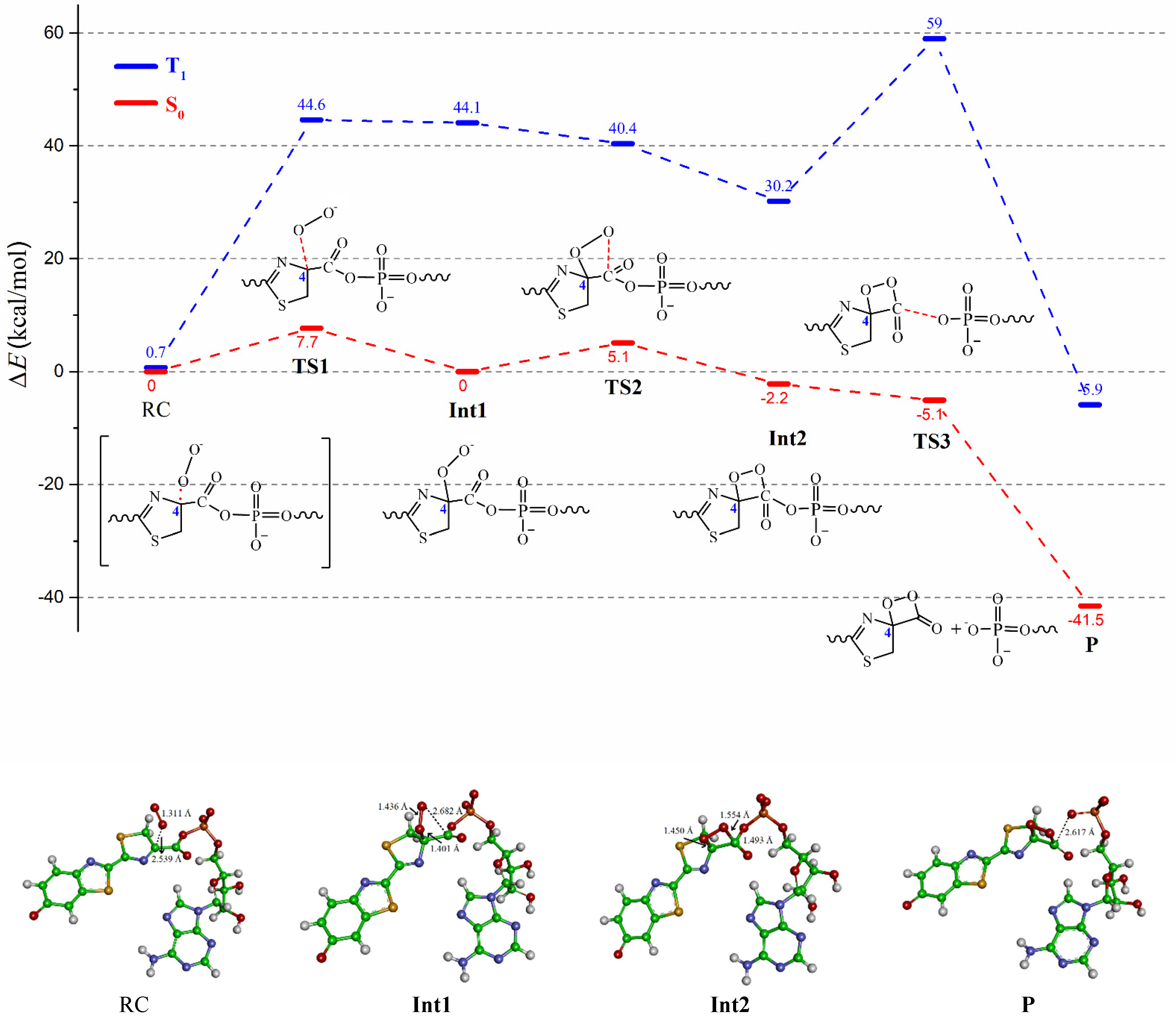
| Oa-Ob | C4-C6 | C6-O7 | C6-O8 | C4-Oa | C6-Ob | C6-C4-Oa-Ob | ρ(O2•−) | ρ(L•2−) | |
|---|---|---|---|---|---|---|---|---|---|
| 1RC | 1.311 | 1.462 | 1.206 | 1.412 | 2.539 | 3.358 | 119.8 | −0.88 | −2.12 |
| TS1 | 1.376 | 1.493 | 1.201 | 1.390 | 1.890 | 3.139 | 118.2 | −0.98 | −2.02 |
| Int1 | 1.436 | 1.568 | 1.200 | 1.370 | 1.401 | 2.682 | 63.0 | −1.01 | −1.99 |
| TS2 | 1.440 | 1.576 | 1.198 | 1.386 | 1.392 | 2.321 | 49.8 | −0.92 | −2.07 |
| Int2 | 1.446 | 1.598 | 1.232 | 1.493 | 1.450 | 1.554 | 17.9 | −0.69 | −2.31 |
| TS3 | 1.447 | 1.552 | 1.185 | 2.056 | 1.476 | 1.416 | 1.3 | −0.56 | −2.44 |
| P | 1.445 | 1.530 | 1.178 | 2.617 | 1.494 | 1.375 | −5.1 | −0.52 | −2.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Liu, Y. A QM/MM Study on the Initiation Reaction of Firefly Bioluminescence—Enzymatic Oxidation of Luciferin. Molecules 2021, 26, 4222. https://doi.org/10.3390/molecules26144222
Yu M, Liu Y. A QM/MM Study on the Initiation Reaction of Firefly Bioluminescence—Enzymatic Oxidation of Luciferin. Molecules. 2021; 26(14):4222. https://doi.org/10.3390/molecules26144222
Chicago/Turabian StyleYu, Mohan, and Yajun Liu. 2021. "A QM/MM Study on the Initiation Reaction of Firefly Bioluminescence—Enzymatic Oxidation of Luciferin" Molecules 26, no. 14: 4222. https://doi.org/10.3390/molecules26144222
APA StyleYu, M., & Liu, Y. (2021). A QM/MM Study on the Initiation Reaction of Firefly Bioluminescence—Enzymatic Oxidation of Luciferin. Molecules, 26(14), 4222. https://doi.org/10.3390/molecules26144222






