Unlocking the Real Potential of Black Soldier Fly (Hermetia illucens) Larvae Protein Derivatives in Pet Diets
Abstract
1. Introduction
2. Results
2.1. Glucosamine Content
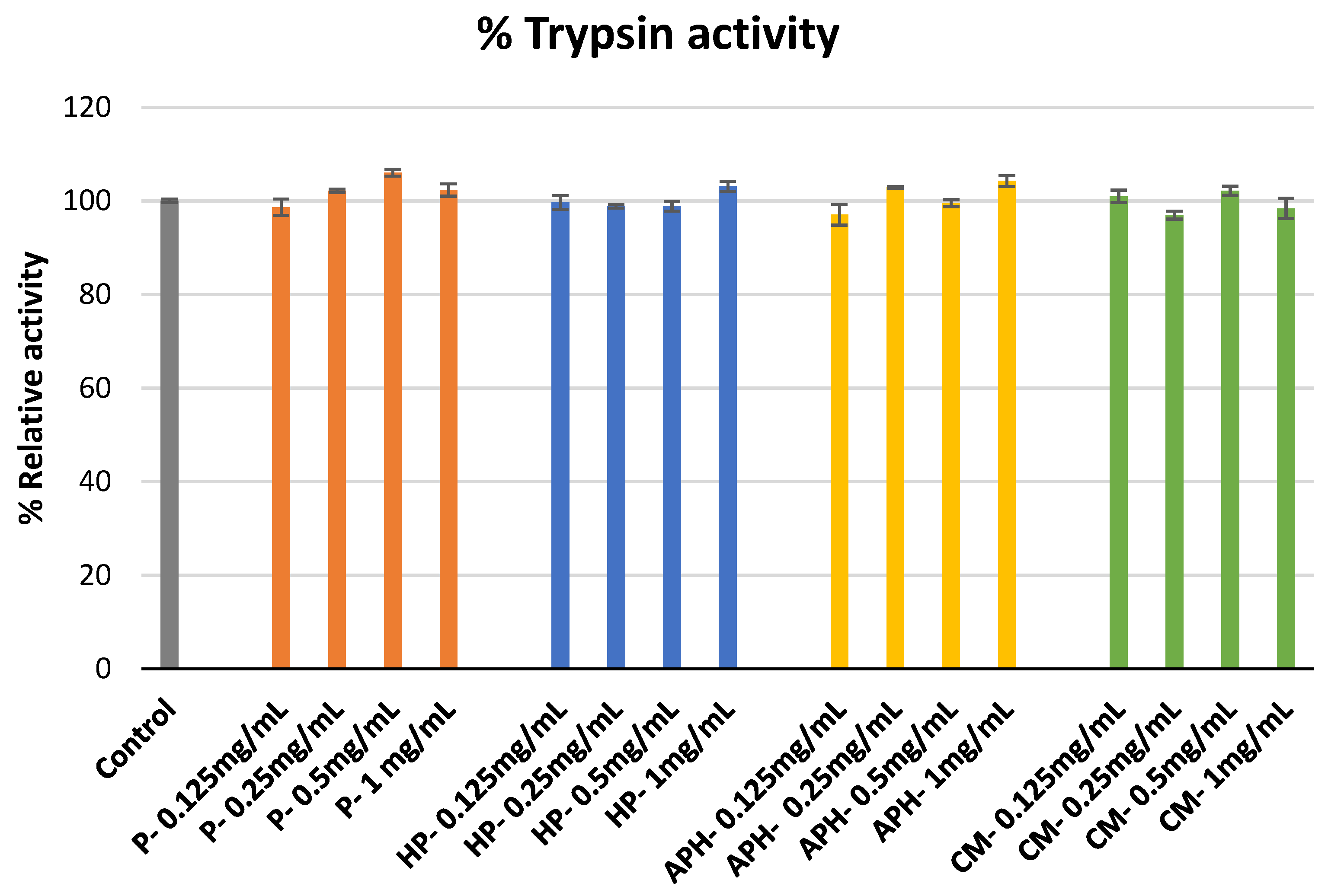
2.2. Proteinase (Trysin) Inhibitory Assay
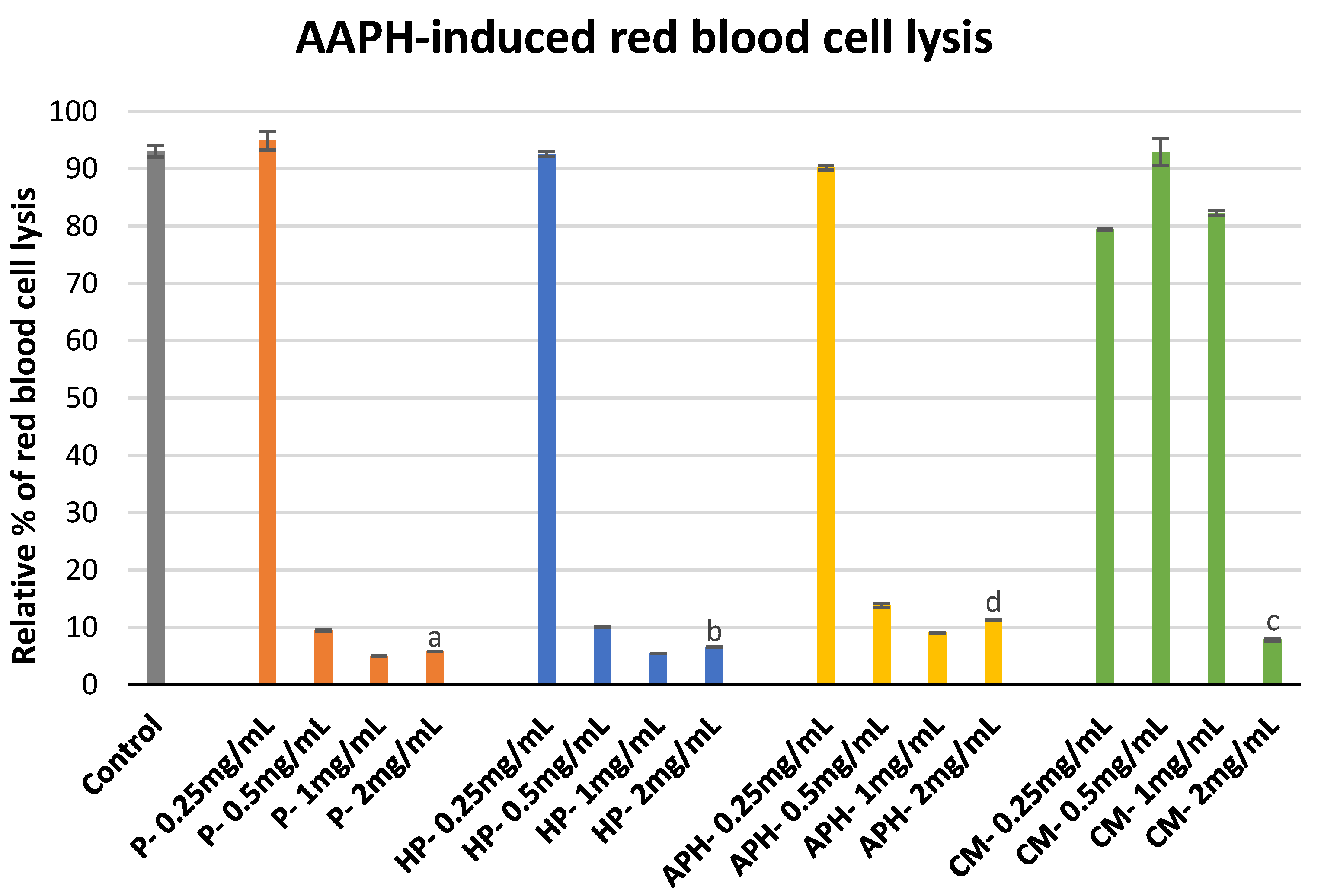
2.3. Erythrocyte Membrane Stability Assay
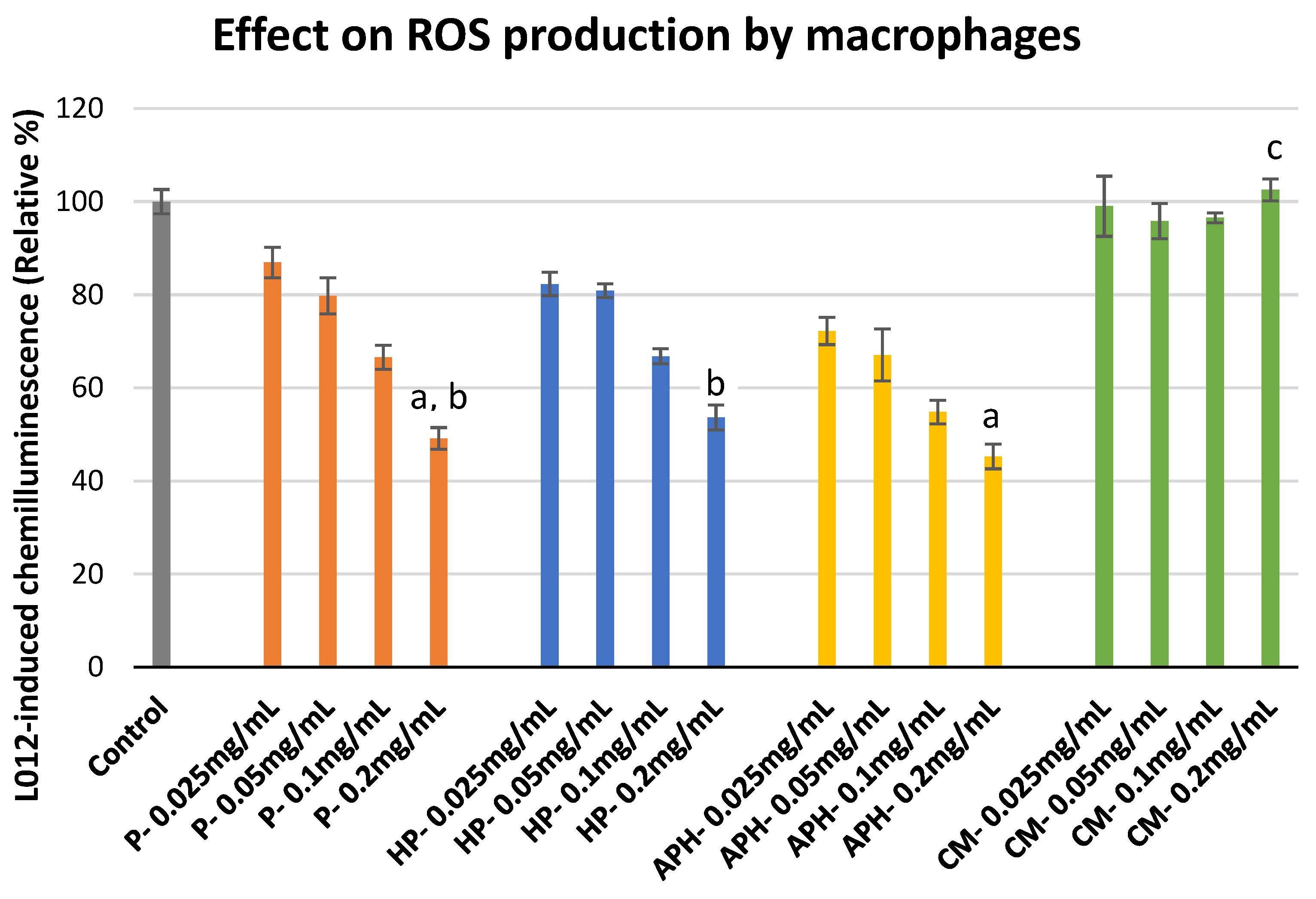
2.4. Reactive Oxygen Species (ROS) Production by Macrophages
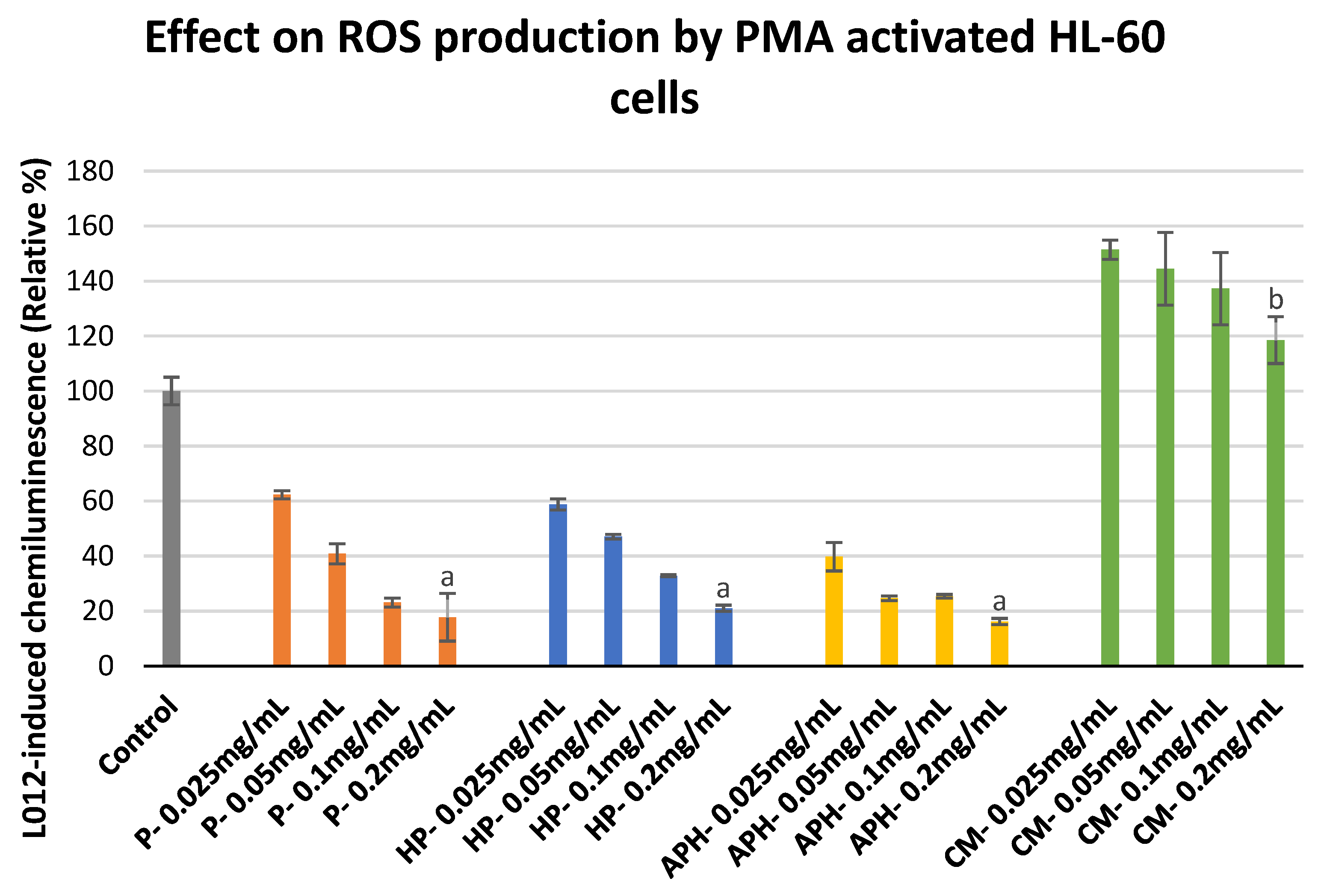
2.5. ROS Production by PMA Activated HL-60 Cells
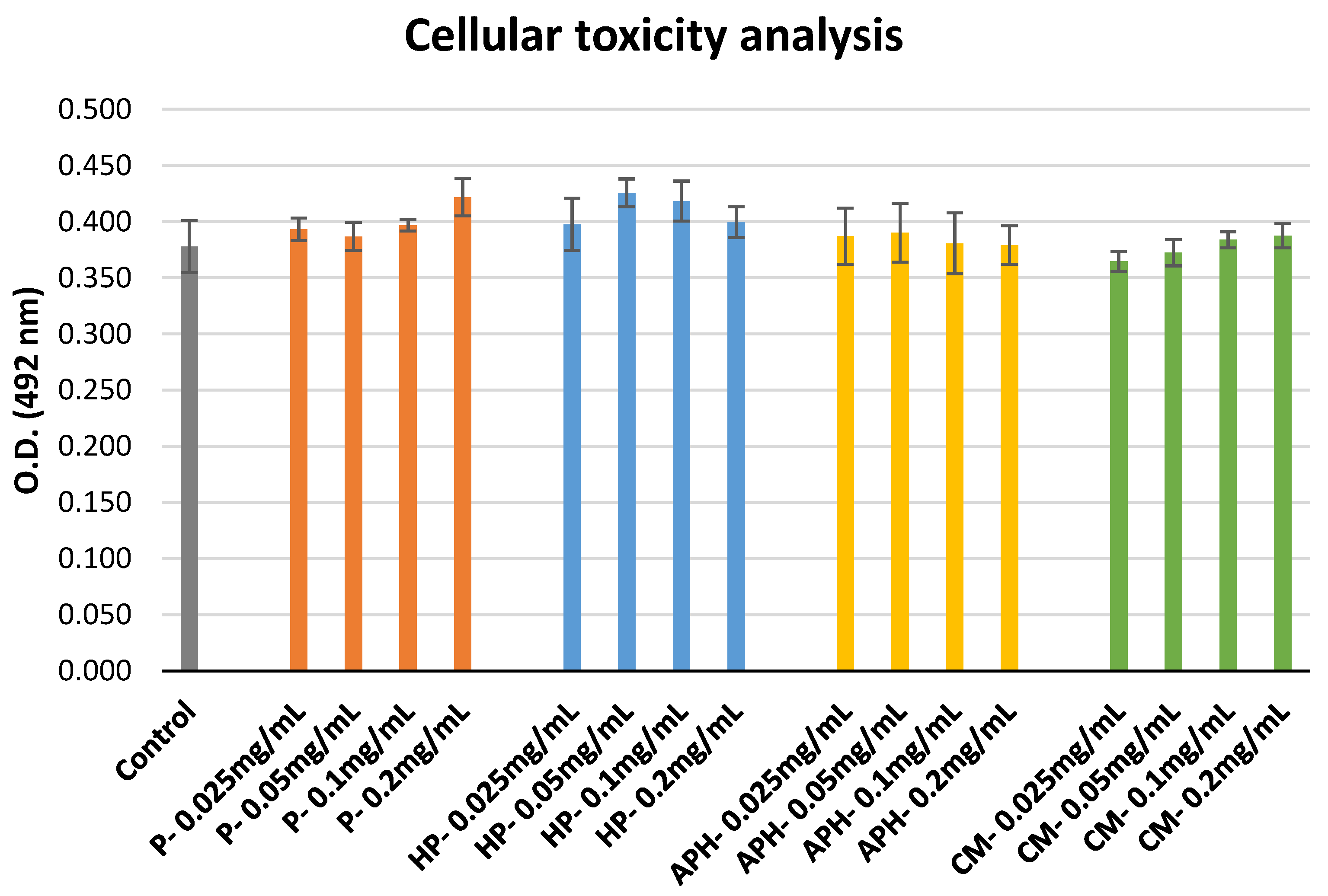
2.6. Metabolic Activity of HL-60 Cells
3. Discussion
3.1. Glucosamine Content
3.2. Proteinase Inhibition
3.3. Erythrocyte Membrane Stability
3.4. ROS Production by Macrophages
3.5. ROS Production by HL-60 Cells
3.6. Cellular Metabolic Activity
4. Materials and Methods
4.1. Reagents
4.2. Raw Materials
4.3. Glucosamine Content
4.4. Proteinase Inhibitory Assay
4.5. Erythrocyte Membrane Stability Assay
4.6. Cellular ROS Production
4.6.1. ROS Production by Macrophages
4.6.2. ROS Production by PMA Activated HL-60 Cells
4.7. Metabolic Acitivty of HL-60 Cells
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Acuff, H.L.; Dainton, A.N.; Dhakal, J.; Kiprotich, S.; Aldrich, G. Sustainability and Pet Food: Is There a Role for Veterinarians? Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Smetana, S.; Schmitt, E.; Mathys, A. Sustainable Use of Hermetia Illucens Insect Biomass for Feed and Food: Attributional and Consequential Life Cycle Assessment. Resour. Conserv. Recycl. 2019, 144, 285–296. [Google Scholar] [CrossRef]
- Terrey, D.; James, J.; Tankovski, I.; Dalim, M.; van Spankeren, M.; Chakraborty, A.; Schmitt, E.; Paul, A. Palatability Enhancement Potential of Hermetia Illucens Larvae Protein Hydrolysate in Litopenaeus vannamei Diets. Molecules 2021, 26, 1582. [Google Scholar] [CrossRef]
- Freel, T.A.; McComb, A.; Koutsos, E.A. Digestibility and Safety of Dry Black Soldier Fly Larvae Meal and Black Soldier Fly Larvae Oil in Dogs. J. Anim. Sci. 2021, 99, 3. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Ariëns, R.M.C.; America, A.H.P.; Paul, A.; Veldkamp, T.; Mes, J.J.; Wichers, H.J.; Govers, C. Clostridium perfringens Suppressing Activity in Black Soldier Fly Protein Preparations. LWT 2021, 149, 111806. [Google Scholar] [CrossRef]
- Veldkamp, T.; Dong, L.; Paul, A.; Govers, C. Bioactive Properties of Insect Products for Monogastric Animals—A Review. J. Insects Food Feed 2021, 1–14. [Google Scholar] [CrossRef]
- International Platform of Insect for Food and Feed (IPIFF). IPIFF Publications. Available online: https://ipiff.org/wp-content/uploads/2020/05/IPIFF-RegulatoryBrochure-update07-2020-1.pdf (accessed on 9 July 2021).
- Alexander, P.; Berri, A.; Moran, D.; Reay, D.; Rounsevell, M.D.A. The Global Environmental Paw Print of Pet Food. Glob. Environ. Chang. 2020, 65, 102153. [Google Scholar] [CrossRef]
- Chan, M.M.; Tapia Rico, G. The “Pet Effect” in Cancer Patients: Risks and Benefits of Human-Pet Interaction. Crit. Rev. Oncol. Hematol. 2019, 143, 56–61. [Google Scholar] [CrossRef]
- Comblain, F.; Serisier, S.; Barthelemy, N.; Balligand, M.; Henrotin, Y. Review of Dietary Supplements for the Management of Osteoarthritis in Dogs in Studies from 2004 to 2014. J. Vet. Pharmacol. Ther. 2016, 39, 1–15. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Lotz, M.K.; Stoltz, J.F. Osteoarthritis, Inflammation and Degradation: A Continuum (Vol. 70); IOS Press: Amsterdam, The Netherlands, 2007; pp. 3–299. [Google Scholar]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and Therapeutic Implications of Cellular Senescence in Osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.A.; McLaughlin, R.M.; Budsberg, S.C. Nonsurgical Management of Osteoarthritis in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 1449–1470. [Google Scholar] [CrossRef]
- Mouithys-Mickalad, A.; Schmitt, E.; Dalim, M.; Franck, T.; Tome, N.M.; van Spankeren, M.; Serteyn, D.; Paul, A. Black Soldier Fly (Hermetia Illucens) Larvae Protein Derivatives: Potential to Promote Animal Health. Animals 2020, 10, 941. [Google Scholar] [CrossRef]
- D’Altilio, M.; Peal, A.; Alvey, M.; Simms, C.; Curtsinger, A.; Gupta, R.C.; Canerdy, T.D.; Goad, J.T.; Bagchi, M.; Bagchi, D. Therapeutic Efficacy and Safety of Undenatured Type II Collagen Singly or in Combination with Glucosamine and Chondroitin in Arthritic Dogs. Toxicol. Mech. Methods 2007, 17, 189–196. [Google Scholar] [CrossRef]
- Yora. The Most Sustainable Dog Food in the World, Made in the UK. Available online: https://www.yorapetfoods.com/yora-pet-foods (accessed on 20 May 2021).
- Bhathal, A.; Spryszak, M.; Louizos, C.; Frankel, G. Glucosamine and Chondroitin Use in Canines for Osteoarthritis: A Review. Open Vet. J. 2017, 7, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Dodge, G.R.; Regatte, R.R.; Noyszewski, E.A.; Hall, J.O.; Sharma, A.V.; Callaway, D.A.; Reddy, R. The Fate of Oral Glucosamine Traced by 13C Labeling in the Dog. Cartilage. 2011, 2, 279–285. [Google Scholar] [CrossRef]
- Martel-Pelletier, J. Pathophysiology of Osteoarthritis. Osteoarthr. Cartil. 1999, 7, 371–373. [Google Scholar] [CrossRef][Green Version]
- Martel-Pelletier, J.; Tardif, G.; Fernandes, J.; Pelletier, J.-P. Metalloproteases and Their Modulation as Treatment in Osteoarthritis. In Principles of Molecular Rheumatology; Current Molecular Medicine; Tsokos, G.C., Ed.; Humana Press: Totowa, NJ, USA, 2000; pp. 499–513. ISBN 978-1-59259-018-6. [Google Scholar]
- Rawlings, N.D.; Barrett, A.J. Families of serine peptidases. In Methods in Enzymology; Proteolytic Enzymes: Serine and Cysteine Peptidases; Academic Press: Cambridge, MA, USA, 1994; Volume 244, pp. 19–61. [Google Scholar]
- Dozier, W.A.; Hess, J.B. Soybean Meal Quality and Analytical Techniques. Soybean Nutr. 2011, 112–124. [Google Scholar] [CrossRef]
- Hanada, M.; Takahashi, M.; Furuhashi, H.; Koyama, H.; Matsuyama, Y. Elevated Erythrocyte Sedimentation Rate and High-Sensitivity C-Reactive Protein in Osteoarthritis of the Knee: Relationship with Clinical Findings and Radiographic Severity. Ann. Clin. Biochem. 2016, 53, 548–553. [Google Scholar] [CrossRef]
- Bao, N.; Zhou, L.; Cong, Y.; Guo, T.; Fan, W.; Chang, Z.; Zhao, J. Free Fatty Acids Are Responsible for the Hidden Blood Loss in Total Hip and Knee Arthroplasty. Med. Hypotheses 2013, 81, 104–107. [Google Scholar] [CrossRef]
- Sogi, Y.; Yabe, Y.; Hagiwara, Y.; Tsuchiya, M.; Onoda, Y.; Sekiguchi, T.; Itaya, N.; Yoshida, S.; Yano, T.; Suzuki, K.; et al. Joint Hemorrhage Accelerates Cartilage Degeneration in a Rat Immobilized Knee Model. BMC Musculoskelet. Disord. 2020, 21, 761. [Google Scholar] [CrossRef]
- Fuentes-Lemus, E.; Dorta, E.; Escobar, E.; Aspée, A.; Pino, E.; Abasq, M.L.; Speisky, H.; Silva, E.; Lissi, E.; Davies, M.J.; et al. Oxidation of Free, Peptide and Protein Tryptophan Residues Mediated by AAPH-Derived Free Radicals: Role of Alkoxyl and Peroxyl Radicals. RSC Adv. 2016, 6, 57948–57955. [Google Scholar] [CrossRef]
- Karimi, G.; Hassanzadeh, M.; Mehri, S. Protective Effect of Rosmarinus Officinalis, L. Essential Oil against Free Radical-Induced Erythrocyte Lysis. Iran. J. Pharm. Sci. 2005, 1, 231–236. [Google Scholar]
- López-Alarcón, C.; Rocco, C.; Lissi, E.; Carrasco, C.; Squella, J.A.; Nuñez-Vergara, L.; Speisky, H. Reaction of 5-Aminosalicylic Acid with Peroxyl Radicals: Protection and Recovery by Ascorbic Acid and Amino Acids. Pharm. Res. 2005, 22, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, S.C.M.; Kruisbergen, N.N.L.; Walgreen, B.; Helsen, M.M.A.; Slöetjes, A.W.; Cremers, N.A.J.; Koenders, M.I.; van de Loo, F.A.J.; Roth, J.; Vogl, T.; et al. The Role of NOX2-Derived Reactive Oxygen Species in Collagenase-Induced Osteoarthritis. Osteoarthr. Cartil. 2018, 26, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Padgett, L.E.; Broniowska, K.A.; Hansen, P.A.; Corbett, J.A.; Tse, H.M. The Role of Reactive Oxygen Species and Proinflammatory Cytokines in Type 1 Diabetes Pathogenesis. Ann. N. Y. Acad. Sci. 2013, 1281, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Jahandideh, F.; Wu, J. Food-Derived Bioactive Peptides on Inflammation and Oxidative Stress. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Boly, R.; Franck, T.; Kohnen, S.; Lompo, M.; Guissou, I.P.; Dubois, J.; Serteyn, D.; Mouithys-Mickalad, A. Evaluation of Antiradical and Anti-Inflammatory Activities of Ethyl Acetate and Butanolic Subfractions of Agelanthus Dodoneifolius (DC.) Polhill & Wiens (Loranthaceae) Using Equine Myeloperoxidase and Both PMA-Activated Neutrophils and HL-60 Cells. Evid. Based Complement. Altern. Med. ECAM 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Eurofins Food Testing NL. Available online: https://www.eurofins.com/contact-us/worldwide-interactive-map/the-netherlands/eurofins-food-testing-nl/ (accessed on 18 May 2021).
- Murugesan, S.; Venkateswaran, M.R.; Jayabal, S.; Periyasamy, S. Evaluation of the Antioxidant and Anti-Arthritic Potential of Zingiber Officinale Rosc. by in Vitro and in Silico Analysis. S. Afr. J. Bot. 2020, 130, 45–53. [Google Scholar] [CrossRef]
- Ielciu, I.; Mouithys-Mickalad, A.; Franck, T.; Angenot, L.; Ledoux, A.; Păltinean, R.; Cieckiewicz, E.; Etienne, D.; Tits, M.; Crişan, G.; et al. Flavonoid Composition, Cellular Antioxidant Activity and (Myelo)Peroxidase Inhibition of a Bryonia Alba, L. (Cucurbitaceae) Leaves Extract. J. Pharm. Pharmacol. 2019, 71, 230–239. [Google Scholar] [CrossRef]
| Sample | Glucosamine Content (%) |
|---|---|
| Pasteurized minced meat of BSFL | 0.5 ± 0.2 a |
| Hydrolyzed and pasteurized minced meat of BSFL | 0.5 ± 0.2 a |
| Hydrolysate of water-soluble BSFL proteins | 0.4 ± 0.0 a |
| Chicken meal | 0.4 ± 0.0 a |
| Sample | Moisture Content (g/kg) | Crude Protein Content (g/kg) | Crude Fat Content (g/kg) |
|---|---|---|---|
| Pasteurized minced meat of BSFL | 700.0 | 120.0 | 122.5 |
| Hydrolyzed and pasteurized minced meat of BSFL | 700.0 | 120.0 | 122.5 |
| Hydrolysate of water-soluble BSFL proteins | 55.0 | 455.0 | 35.0 |
| Chicken meal | 60.0 | 700.0 | 120.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouithys-Mickalad, A.; Tome, N.M.; Boogaard, T.; Chakraborty, A.; Serteyn, D.; Aarts, K.; Paul, A. Unlocking the Real Potential of Black Soldier Fly (Hermetia illucens) Larvae Protein Derivatives in Pet Diets. Molecules 2021, 26, 4216. https://doi.org/10.3390/molecules26144216
Mouithys-Mickalad A, Tome NM, Boogaard T, Chakraborty A, Serteyn D, Aarts K, Paul A. Unlocking the Real Potential of Black Soldier Fly (Hermetia illucens) Larvae Protein Derivatives in Pet Diets. Molecules. 2021; 26(14):4216. https://doi.org/10.3390/molecules26144216
Chicago/Turabian StyleMouithys-Mickalad, Ange, Nuria Martin Tome, Thomas Boogaard, Arpita Chakraborty, Didier Serteyn, Kees Aarts, and Aman Paul. 2021. "Unlocking the Real Potential of Black Soldier Fly (Hermetia illucens) Larvae Protein Derivatives in Pet Diets" Molecules 26, no. 14: 4216. https://doi.org/10.3390/molecules26144216
APA StyleMouithys-Mickalad, A., Tome, N. M., Boogaard, T., Chakraborty, A., Serteyn, D., Aarts, K., & Paul, A. (2021). Unlocking the Real Potential of Black Soldier Fly (Hermetia illucens) Larvae Protein Derivatives in Pet Diets. Molecules, 26(14), 4216. https://doi.org/10.3390/molecules26144216






