A Novel Dialkylamino-Functionalized Chalcone, DML6, Inhibits Cervical Cancer Cell Proliferation, In Vitro, via Induction of Oxidative Stress, Intrinsic Apoptosis and Mitotic Catastrophe
Abstract
1. Introduction
2. Results and Discussion
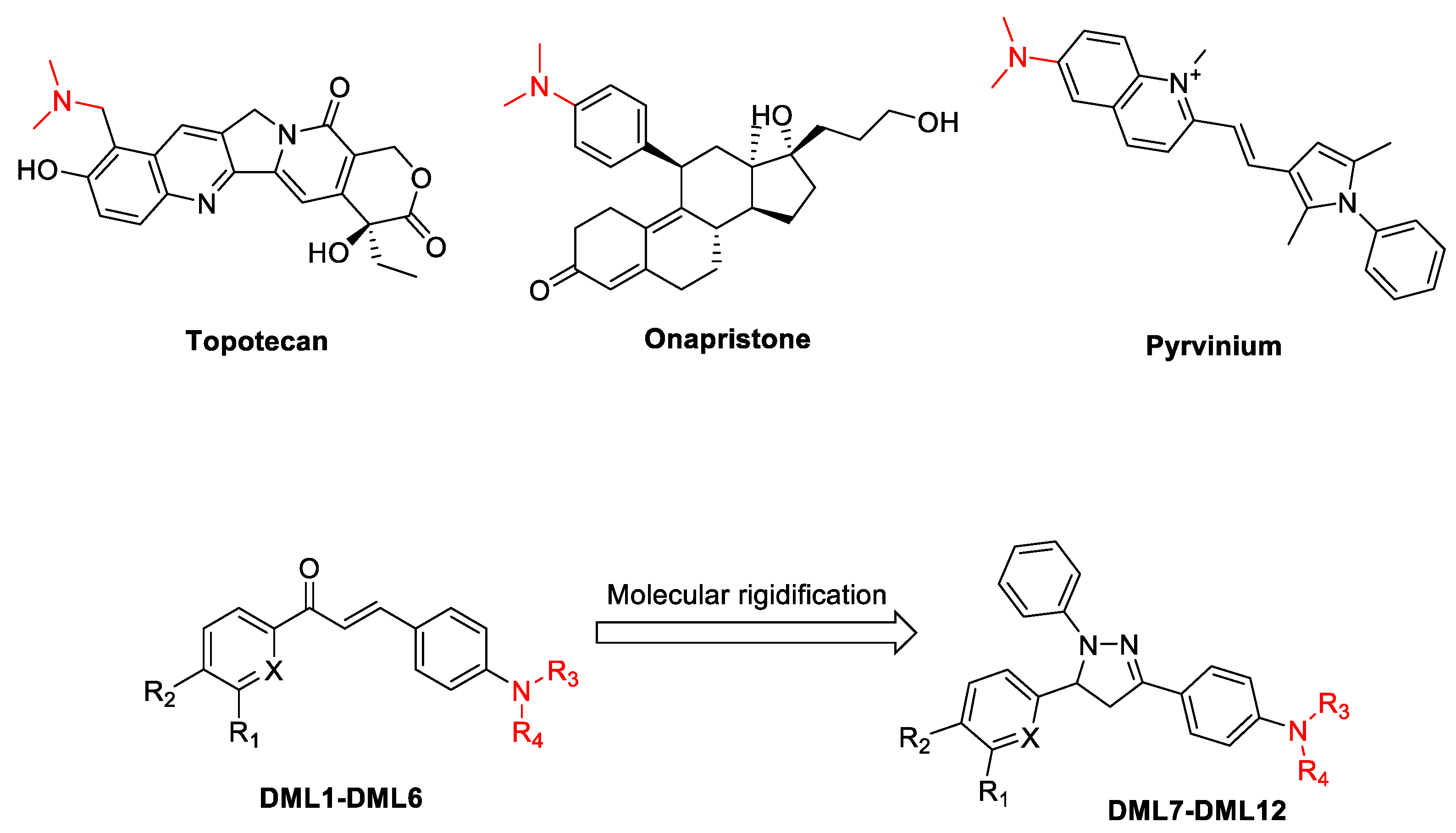
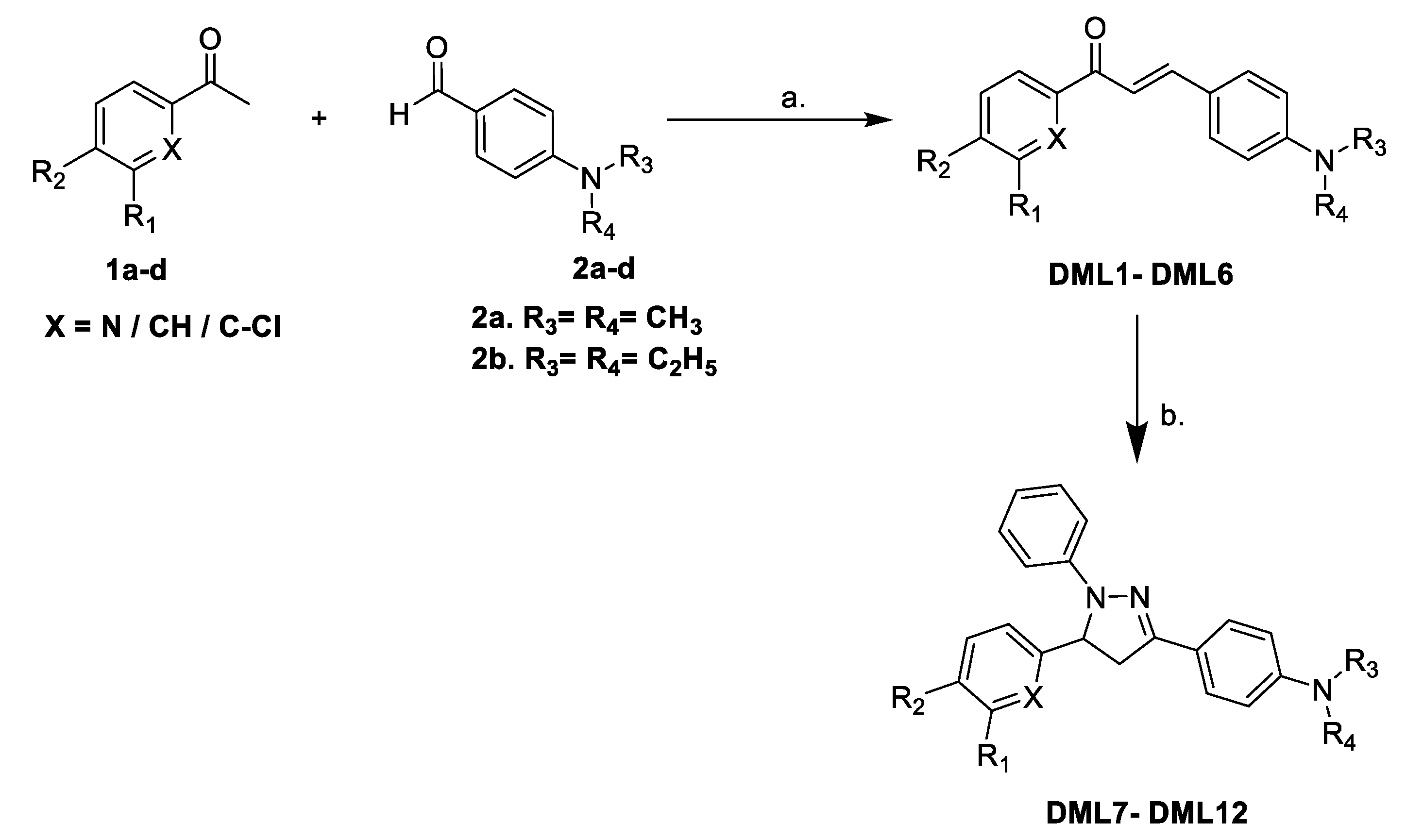
2.1. Chemistry
2.2. Structure–Activity Relationships for the Dialkylamine Substituted Chalcones and Their Corresponding Dihydropyrazoles, Based on Data Obtained Using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Cytotoxicity Assay
2.3. DML6 Antiproliferative Efficacy and Selectivity on Cervical Cancer Cell Lines
2.4. DML6 Induces Oxidative Stress in OV2008 Cells
2.5. DML6 Arrests the Cell Cycle of OV2008 at G2 Phase
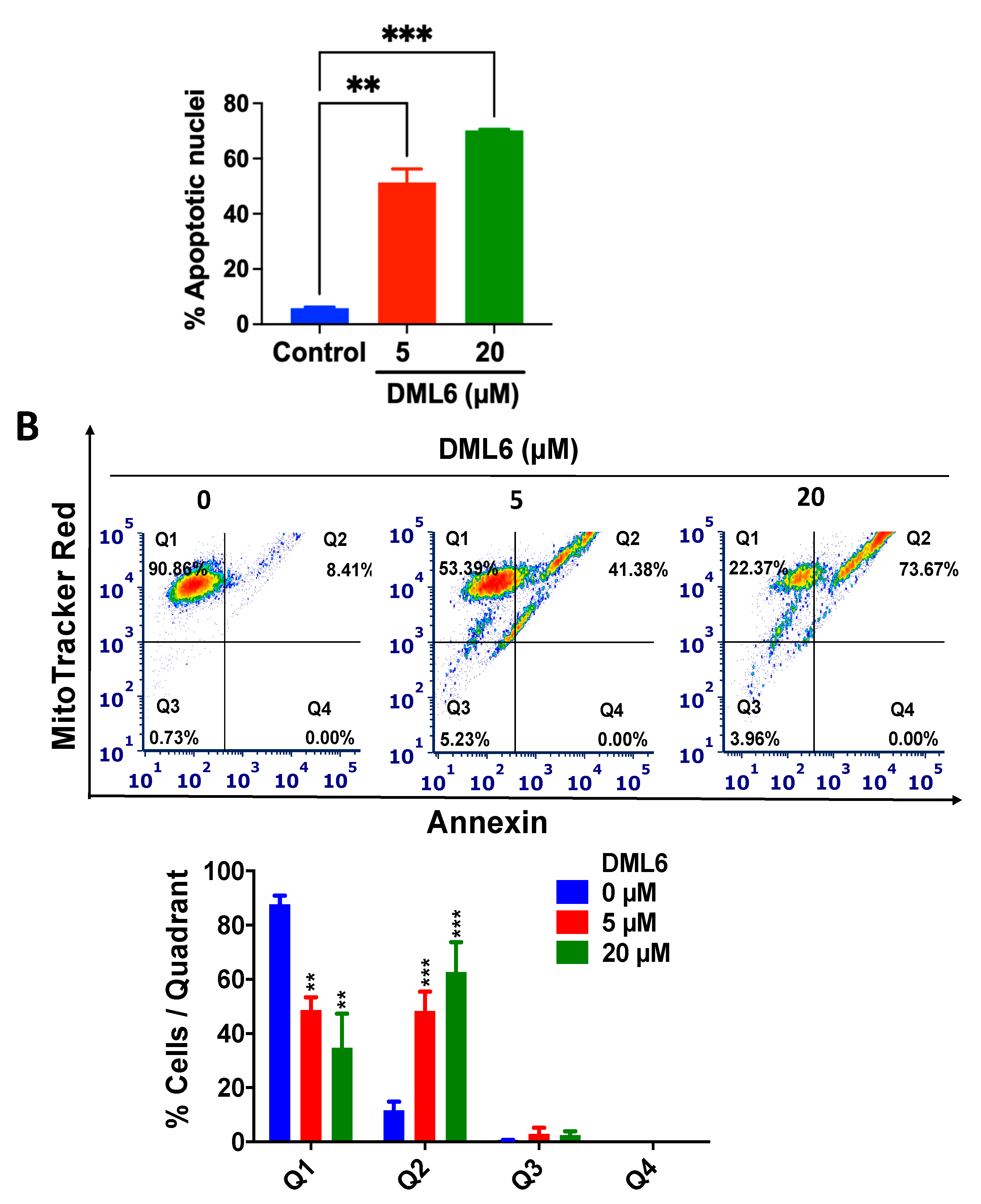
2.6. DML6 Induces Mitotic Catastrophe and Apoptosis in OV2008 Cells
2.7. DML6 Produces a Concentration-Dependent Increase in the Induction of Apoptosis by Activating the Intrinsic Apoptotic Pathway
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of Dialkylamino Substituted Chalcones (DML1–DML6)
3.1.2. General Procedure for the Synthesis of Dialkylamino Substituted 1,3,5-Triphenyl-4,5-dihydro-1H-pyrazole (DML7–DML12)
3.2. Biological Studies
3.2.1. Cell Lines and Cell Culture
3.2.2. MTT Assay
3.2.3. Time-Dependent Cytotoxicity Assays
IncuCyte™ Live-Cell Morphology Study
IncuCyte™ Cytotox Green Assay
3.2.4. Time-Dependent Apoptosis Induction Study
3.2.5. Cell Lysis and Western Blot Analysis
3.2.6. Nuclear Staining Using Hoechst 33258 Dye
3.2.7. Cell Cycle Analysis
3.2.8. Apoptosis and Mitochondrial Membrane Potential Analysis
3.2.9. Reactive Oxygen Species (ROS) Detection
3.2.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human Papillomavirus (HPV) and Cervical Cancer. 2020. Available online: https://www.who.int/en/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer (accessed on 24 June 2021).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef]
- Dunne, E.F.; Unger, E.R.; Sternberg, M.; McQuillan, G.; Swan, D.C.; Patel, S.S.; Markowitz, L.E. Prevalence of HPV infection among females in the United States. JAMA 2007, 297, 813–819. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts and Figures. 2021. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf (accessed on 24 June 2021).
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L. A review of human carcinogens–Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Serrano, B.; Brotons, M.; Bosch, F.X.; Bruni, L. Epidemiology and burden of HPV-related disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Kundu, R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front. Microbiol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Landoni, F.; Maneo, A.; Colombo, A.; Placa, F.; Milani, R.; Perego, P.; Favini, G.; Ferri, L.; Mangioni, C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997, 350, 535–540. [Google Scholar] [CrossRef]
- Rotman, M.; Sedlis, A.; Piedmonte, M.R.; Bundy, B.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: Follow-up of a gynecologic oncology group study. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 169–176. [Google Scholar] [CrossRef]
- Delgado, G.; Bundy, B.; Zaino, R.; Sevin, B.-U.; Creasman, W.T.; Major, F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. Gynecol. Oncol. 1990, 38, 352–357. [Google Scholar] [CrossRef]
- Josefson, D. Adding chemotherapy improves survival in cervical cancer. BMJ Clin. Res. Ed. 1999, 318, 623. [Google Scholar] [CrossRef][Green Version]
- Zhu, H.; Luo, H.; Zhang, W.; Shen, Z.; Hu, X.; Zhu, X. Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des. Devel. Ther. 2016, 10, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Madden, E.C.; Gorman, A.M.; Logue, S.E.; Samali, A. Tumour Cell Secretome in Chemoresistance and Tumour Recurrence. Trends Cancer 2020, 6, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dai, G.; Yang, J.; Wu, W.; Zhang, W. Cervical Cancer Cell Growth, Drug Resistance, and Epithelial-Mesenchymal Transition Are Suppressed by y-Secretase Inhibitor RO4929097. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 4046–4053. [Google Scholar] [CrossRef]
- Leonard, G.D.; Fojo, T.; Bates, S.E. The role of ABC transporters in clinical practice. Oncologist 2003, 8, 411–424. [Google Scholar] [CrossRef]
- Wagner, W.; Kania, K.D.; Blauz, A.; Ciszewski, W.M. The lactate receptor (HCAR1/GPR81) contributes to doxorubicin chemoresistance via ABCB1 transporter up-regulation in human cervical cancer HeLa cells. J. Physiol. Pharm. 2017, 68, 555–564. [Google Scholar]
- Karthikeyan, C.; Moorthy, S.H.N.; Ramasamy, S.; Vanam, U.; Manivannan, E.; Karunagaran, D.; Trivedi, P. Advances in chalcones with anticancer activities. Recent. Pat. Anticancer Drug Discov. 2015, 10, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Huang, G.; Xiao, J. Chalcone hybrids as potential anticancer agents: Current development, mechanism of action, and structure-activity relationship. Med. Res. Rev. 2020, 40, 2049–2084. [Google Scholar] [CrossRef]
- Mohamed, M.F.A.; Abuo-Rahma, G.E.-D.A. Molecular targets and anticancer activity of quinoline–chalcone hybrids: Literature review. RSC Adv. 2020, 10, 31139–31155. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Alam, R.; Alam, M.A.; Panda, A.K.; Rahisuddin. Design, Synthesis, and Cytotoxicity Evaluation of 3-(5-(3-(aryl)-1-phenyl-1H-pyrazol-4-yl)-1-phenyl-4,5-dihydro-1H-pyrazol-3-yl)pyridine and 5-(3-(aryl)-1-phenyl-1H-pyrazol-4-yl)-3-(pyridin-3-yl)-4,5-dihydropyrazole-1-carbaldehyde Derivatives as Potential Anticancer Agents. J. Heterocycl. Chem. 2017, 54, 1812–1821. [Google Scholar] [CrossRef]
- Alex, J.M.; Kumar, R. 4,5-Dihydro-1H-pyrazole: An indispensable scaffold. J. Enzym. Inhib. Med. Chem. 2014, 29, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hu, Y.; Yang, Y.-S.; Zhang, F.; Zhang, Y.-B.; Wang, X.-L.; Tang, J.-F.; Zhong, W.-Q.; Zhu, H.-L. Design, modification and 3D QSAR studies of novel naphthalin-containing pyrazoline derivatives with/without thiourea skeleton as anticancer agents. Bioorganic Med. Chem. 2013, 21, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Fang, L.; Gou, S.; Cheng, L. Design and synthesis of dimethylaminomethyl-substituted curcumin derivatives/analogues: Potent antitumor and antioxidant activity, improved stability and aqueous solubility compared with curcumin. Bioorg. Med. Chem. Lett. 2013, 23, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- D’anneo, A.; Carlisi, D.; Lauricella, M.; Puleio, R.; Martinez, R.; Di Bella, S.; Di Marco, P.; Emanuele, S.; Di Fiore, R.; Guercio, A. Parthenolide generates reactive oxygen species and autophagy in MDA-MB231 cells. A soluble parthenolide analogue inhibits tumour growth and metastasis in a xenograft model of breast cancer. Cell Death Dis. 2013, 4, e891. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, K.M. Chemistry and Biology of Camptothecin and its Derivatives. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 643–682. [Google Scholar] [CrossRef]
- Lim, M.; Otto-Duessel, M.; He, M.; Su, L.; Nguyen, D.; Chin, E.; Alliston, T.; Jones, J.O. Ligand-Independent and Tissue-Selective Androgen Receptor Inhibition by Pyrvinium. ACS Chem. Biol. 2014, 9, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, U.; Hess-Stumpp, H.; Cleve, A.; Neef, G.; Schwede, W.; Hoffmann, J.; Fritzemeier, K.-H.; Chwalisz, K. Synthesis and Biological Activity of a Novel, Highly Potent Progesterone Receptor Antagonist. J. Med. Chem. 2000, 43, 5010–5016. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Singh, S.P.; Häder, D.P.; Sinha, R.P. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2’,7’-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem. Biophys. Res. Commun. 2010, 397, 603–607. [Google Scholar] [CrossRef]
- Ziegler, U.; Groscurth, P. Morphological features of cell death. Physiology 2004, 19, 124–128. [Google Scholar] [CrossRef]
- Castedo, M.; Perfettini, J.-L.; Roumier, T.; Andreau, K.; Medema, R.; Kroemer, G. Cell death by mitotic catastrophe: A molecular definition. Oncogene 2004, 23, 2825–2837. [Google Scholar] [CrossRef]
- Kops, G.J.; Weaver, B.A.; Cleveland, D.W. On the road to cancer: Aneuploidy and the mitotic checkpoint. Nat. Rev. Cancer 2005, 5, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.Y.; Gorbsky, G.J. Spindle checkpoint function and cellular sensitivity to antimitotic drugs. Mol. Cancer Ther. 2006, 5, 2963–2969. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.-D.; Broude, E.V.; Dokmanovic, M.; Zhu, H.; Ruth, A.; Xuan, Y.; Kandel, E.S.; Lausch, E.; Christov, K.; Roninson, I.B. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 1999, 59, 3761–3767. [Google Scholar] [PubMed]
- Vakifahmetoglu, H.; Olsson, M.; Zhivotovsky, B. Death through a tragedy: Mitotic catastrophe. Cell Death Differ. 2008, 15, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Shibata, A.; Oike, T.; Nakano, T. One-step protocol for evaluation of the mode of radiation-induced clonogenic cell death by fluorescence microscopy. J. Vis. Exp. JoVE 2017, 128, 56338. [Google Scholar] [CrossRef] [PubMed]
- Castedo, M.; Kroemer, G. Mitotic catastrophe: A special case of apoptosis. J. Soc. Biol. 2004, 198, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C. Cytochrome c: Can’t live with it—Can’t live without it. Cell 1997, 91, 559–562. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Bouchier-Hayes, L.; Green, D.R. Mitochondrial outer membrane permeabilization during apoptosis: The innocent bystander scenario. Cell Death Differ. 2006, 13, 1396–1402. [Google Scholar] [CrossRef]
- Mariño, G.; Kroemer, G. Mechanisms of apoptotic phosphatidylserine exposure. Cell Res. 2013, 23, 1247–1248. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603. [Google Scholar] [CrossRef] [PubMed]
- Lizarbe, M.A.; Barrasa, J.I.; Olmo, N.; Gavilanes, F.; Turnay, J. Annexin-phospholipid interactions. Functional implications. Int. J. Mol. Sci. 2013, 14, 2652–2683. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef]
- Prokhorova, E.A.; Egorshina, A.Y.; Zhivotovsky, B.; Kopeina, G.S. The DNA-damage response and nuclear events as regulators of nonapoptotic forms of cell death. Oncogene 2020, 39, 1–16. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Dewson, G.; Kluck, R.M. Mechanisms by which Bak and Bax permeabilise mitochondria during apoptosis. J. Cell Sci. 2009, 122, 2801–2808. [Google Scholar] [CrossRef]
- Shamas-Din, A.; Kale, J.; Leber, B.; Andrews, D.W. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008714. [Google Scholar] [CrossRef]
- Lomonosova, E.; Chinnadurai, G. BH3-only proteins in apoptosis and beyond: An overview. Oncogene 2008, 27 (Suppl. 1), S2–S19. [Google Scholar] [CrossRef]
- Westphal, D.; Dewson, G.; Czabotar, P.E.; Kluck, R.M. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta BBA Mol. Cell Res. 2011, 1813, 521–531. [Google Scholar] [CrossRef]
- Takahashi, Y.; Karbowski, M.; Yamaguchi, H.; Kazi, A.; Wu, J.; Sebti, S.M.; Youle, R.J.; Wang, H.-G. Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis. Mol. Cell. Biol. 2005, 25, 9369–9382. [Google Scholar] [CrossRef] [PubMed]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb. Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef]
- International Cell Line Authentication Committee. Register of Misidentified Cell Lines. Available online: https://iclac.org/databases/cross-contaminations/ (accessed on 24 June 2021).
- Korch, C.; Spillman, M.A.; Jackson, T.A.; Jacobsen, B.M.; Murphy, S.K.; Lessey, B.A.; Jordan, V.C.; Bradford, A.P. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecol. Oncol. 2012, 127, 241–248. [Google Scholar] [CrossRef]
- Kniss, D.A.; Summerfield, T.L. Discovery of HeLa Cell Contamination in HES Cells: Call for Cell Line Authentication in Reproductive Biology Research. Reprod. Sci. Thousand Oaks Calif. 2014, 21, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Capes-Davis, A.; Theodosopoulos, G.; Atkin, I.; Drexler, H.G.; Kohara, A.; MacLeod, R.A.; Masters, J.R.; Nakamura, Y.; Reid, Y.A.; Reddel, R.R. Check your cultures! A list of cross-contaminated or misidentified cell lines. Int. J. Cancer 2010, 127, 1–8. [Google Scholar] [CrossRef]
- Manivannan, E.; Amawi, H.; Hussein, N.; Karthikeyan, C.; Fetcenko, A.; Narayana Moorthy, N.S.H.; Trivedi, P.; Tiwari, A.K. Design and discovery of silybin analogues as antiproliferative compounds using a ring disjunctive—Based, natural product lead optimization approach. Eur. J. Med. Chem. 2017, 133, 365–378. [Google Scholar] [CrossRef]
- Hussein, N.; Amawi, H.; Karthikeyan, C.; Hall, F.S.; Mittal, R.; Trivedi, P.; Ashby, C.R.; Tiwari, A.K. The dopamine D3 receptor antagonists PG01037, NGB2904, SB277011A, and U99194 reverse ABCG2 transporter-mediated drug resistance in cancer cell lines. Cancer Lett. 2017, 396, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Tukaramrao, D.B.; Malla, S.; Saraiya, S.; Hanely, R.A.; Ray, A.; Kumari, S.; Raman, D.; Tiwari, A.K. A Novel Thienopyrimidine Analog, TPH104, Mediates Immunogenic Cell Death in Triple-Negative Breast Cancer Cells. Cancers 2021, 13, 1954. [Google Scholar] [CrossRef]
- Amawi, H.; Hussein, N.A.; Ashby Jr, C.R.; Alnafisah, R.; Sanglard, L.M.; Manivannan, E.; Karthikeyan, C.; Trivedi, P.; Eisenmann, K.M.; Robey, R.W. Bax/tubulin/epithelial-mesenchymal pathways determine the efficacy of silybin analog HM015k in colorectal cancer cell growth and metastasis. Front. Pharmacol. 2018, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, C.; Amawi, H.; Viana, A.G.; Sanglard, L.; Hussein, N.; Saddler, M.; Ashby, C.R.; Moorthy, N.H.N.; Trivedi, P.; Tiwari, A.K. lH-Pyrazolo [3, 4-b] quinolin-3-amine derivatives inhibit growth of colon cancer cells via apoptosis and sub G1 cell cycle arrest. Bioorg. Med. Chem. Lett. 2018, 28, 2244–2249. [Google Scholar] [CrossRef]
- Amawi, H.; Hussein, N.; Boddu, S.H.; Karthikeyan, C.; Williams, F.E.; Ashby, C.R.; Raman, D.; Trivedi, P.; Tiwari, A.K. Novel thienopyrimidine derivative, RP-010, induces β-catenin fragmentation and is efficacious against prostate cancer cells. Cancers 2019, 11, 711. [Google Scholar] [CrossRef]
- Al-Oudat, B.A.; Ramapuram, H.; Malla, S.; Audat, S.A.; Hussein, N.; Len, J.M.; Kumari, S.; Bedi, M.F.; Ashby, C.R.; Tiwari, A.K. Novel Chrysin-De-Allyl PAC-1 Hybrid Analogues as Anticancer Compounds: Design, Synthesis, and Biological Evaluation. Molecules 2020, 25, 3063. [Google Scholar] [CrossRef] [PubMed]
- Amawi, H.; Karthikeyan, C.; Pathak, R.; Hussein, N.; Christman, R.; Robey, R.; Ashby, C.R.; Trivedi, P.; Malhotra, A.; Tiwari, A.K. Thienopyrimidine derivatives exert their anticancer efficacy via apoptosis induction, oxidative stress and mitotic catastrophe. Eur. J. Med. Chem. 2017, 138, 1053–1065. [Google Scholar] [CrossRef] [PubMed]





| Comp Code | Substitution | Molecular Formula | Molecular Weight | Yield % | ||||
|---|---|---|---|---|---|---|---|---|
| X | R1 | R2 | R3 | R4 | ||||
| DML1 | N | H | H | CH3 | CH3 | C16H16N2O | 252.32 | 84 |
| DML2 | N | H | H | C2H5 | C2H5 | C18H20N2O | 280.37 | 82 |
| DML3 | CH | H | OCH3 | CH3 | CH3 | C18H19NO2 | 281.36 | 86 |
| DML4 | CH | OCH3 | OCH3 | CH3 | CH3 | C19H21NO3 | 311.38 | 84 |
| DML5 | CH | H | OCH3 | C2H5 | C2H5 | C20H23NO2 | 309.41 | 85 |
| DML6 | C-Cl | H | Cl | CH3 | CH3 | C17H15Cl2NO | 320.21 | 81 |
| DML7 | N | H | H | CH3 | CH3 | C22H22N4 | 342.45 | 79 |
| DML8 | N | H | H | C2H5 | C2H5 | C24H26N4 | 370.50 | 80 |
| DML9 | CH | H | OCH3 | CH3 | CH3 | C24H25N3O | 371.48 | 74 |
| DML10 | CH | OCH3 | OCH3 | CH3 | CH3 | C25H27N3O2 | 401.51 | 75 |
| DML11 | CH | H | OCH3 | C2H5 | C2H5 | C26H29N3O | 399.54 | 78 |
| DML12 | C-Cl | H | Cl | CH3 | CH3 | C23H21Cl2N3 | 410.34 | 76 |
| Comp Code | IC50 ± SD (µM) | ||||||
|---|---|---|---|---|---|---|---|
| Kidney | Breast | Colon | Lung | Prostate | Cervical | ||
| HEK293 | MDA-MB- 231 | LOVO | A549 | DU145 | OV2008 | ||
| DML1 | >100 | >100 | >100 | >100 | >100 | >100 | |
| DML2 | >100 | >100 | >100 | >100 | >100 | >100 | |
| DML3 | >100 | >100 | >100 | >100 | >100 | >100 | |
| DML4 | 68.5 ± 2.1 | 26.2 ± 23.9 | 45 ± 22.6 | 66.3 ± 4.6 | 22.5 ± 4.9 | 13.8 ± 7 | |
| DML5 | >100 | >100 | 94 ± 8.4 | >100 | >100 | 79.3 ± 29.2 | |
| DML6 | 70 ± 42.4 | 91.9 ± 11.5 | 65 ± 49.5 | 84.9 ± 21.4 | 96.9 ± 4.4 | 7.8 ± 0 | |
| DML7 | >100 | >100 | >100 | >100 | >100 | >100 | |
| DML8 | >100 | >100 | >100 | >100 | >100 | >100 | |
| DML9 | >100 | >100 | >100 | >100 | >100 | >100 | |
| DML10 | >100 | >100 | >100 | >100 | >100 | >100 | |
| DML11 | >100 | >100 | >100 | >100 | >100 | >100 | |
| DML12 | >100 | >100 | >100 | >100 | >100 | >100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Len, J.M.; Hussein, N.; Malla, S.; Mcintosh, K.; Patidar, R.; Elangovan, M.; Chandrabose, K.; Moorthy, N.S.H.N.; Pandey, M.; Raman, D.; et al. A Novel Dialkylamino-Functionalized Chalcone, DML6, Inhibits Cervical Cancer Cell Proliferation, In Vitro, via Induction of Oxidative Stress, Intrinsic Apoptosis and Mitotic Catastrophe. Molecules 2021, 26, 4214. https://doi.org/10.3390/molecules26144214
Len JM, Hussein N, Malla S, Mcintosh K, Patidar R, Elangovan M, Chandrabose K, Moorthy NSHN, Pandey M, Raman D, et al. A Novel Dialkylamino-Functionalized Chalcone, DML6, Inhibits Cervical Cancer Cell Proliferation, In Vitro, via Induction of Oxidative Stress, Intrinsic Apoptosis and Mitotic Catastrophe. Molecules. 2021; 26(14):4214. https://doi.org/10.3390/molecules26144214
Chicago/Turabian StyleLen, Jenna M., Noor Hussein, Saloni Malla, Kyle Mcintosh, Rahul Patidar, Manivannan Elangovan, Karthikeyan Chandrabose, N. S. Hari Narayana Moorthy, Manoj Pandey, Dayanidhi Raman, and et al. 2021. "A Novel Dialkylamino-Functionalized Chalcone, DML6, Inhibits Cervical Cancer Cell Proliferation, In Vitro, via Induction of Oxidative Stress, Intrinsic Apoptosis and Mitotic Catastrophe" Molecules 26, no. 14: 4214. https://doi.org/10.3390/molecules26144214
APA StyleLen, J. M., Hussein, N., Malla, S., Mcintosh, K., Patidar, R., Elangovan, M., Chandrabose, K., Moorthy, N. S. H. N., Pandey, M., Raman, D., Trivedi, P., & Tiwari, A. K. (2021). A Novel Dialkylamino-Functionalized Chalcone, DML6, Inhibits Cervical Cancer Cell Proliferation, In Vitro, via Induction of Oxidative Stress, Intrinsic Apoptosis and Mitotic Catastrophe. Molecules, 26(14), 4214. https://doi.org/10.3390/molecules26144214







