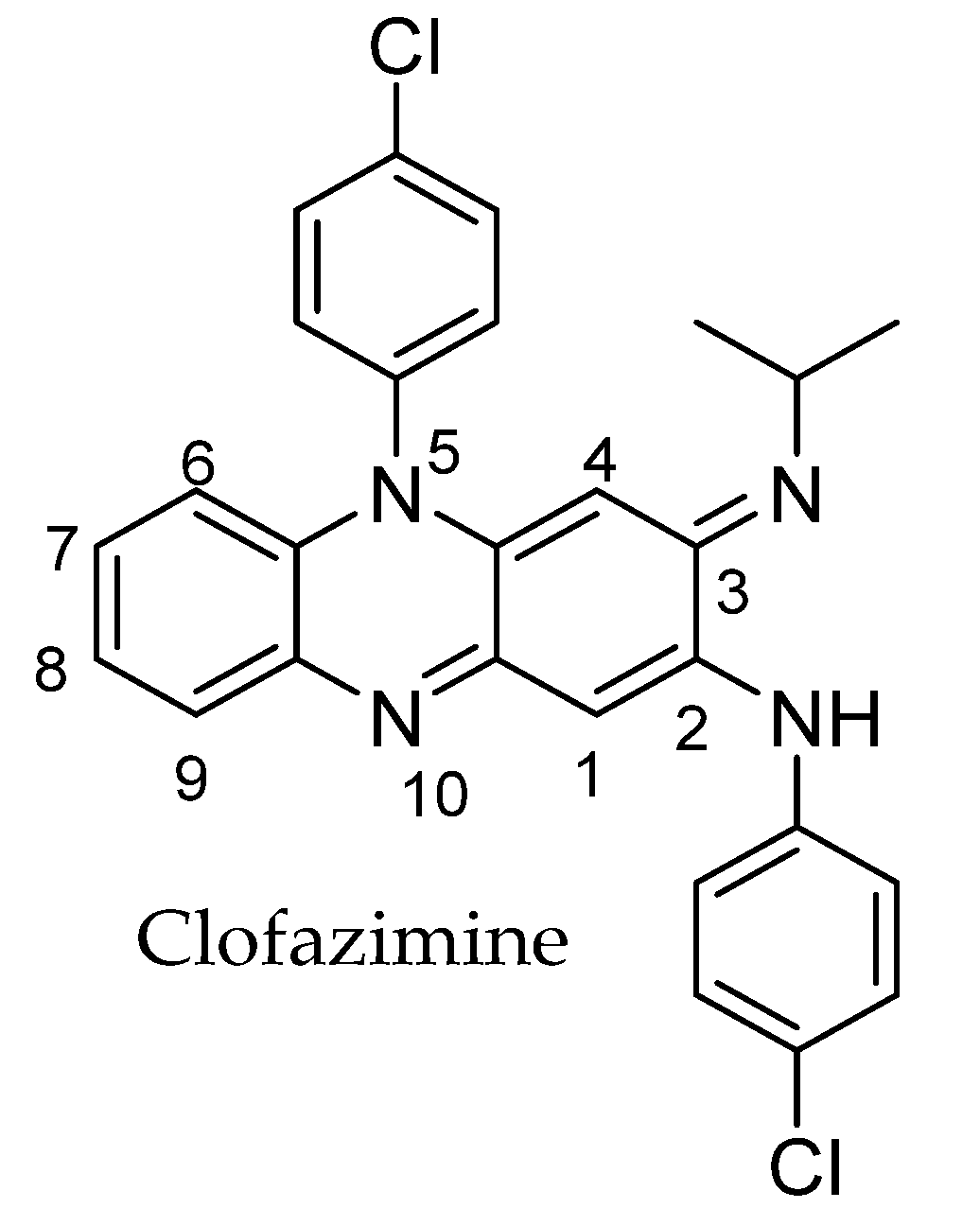
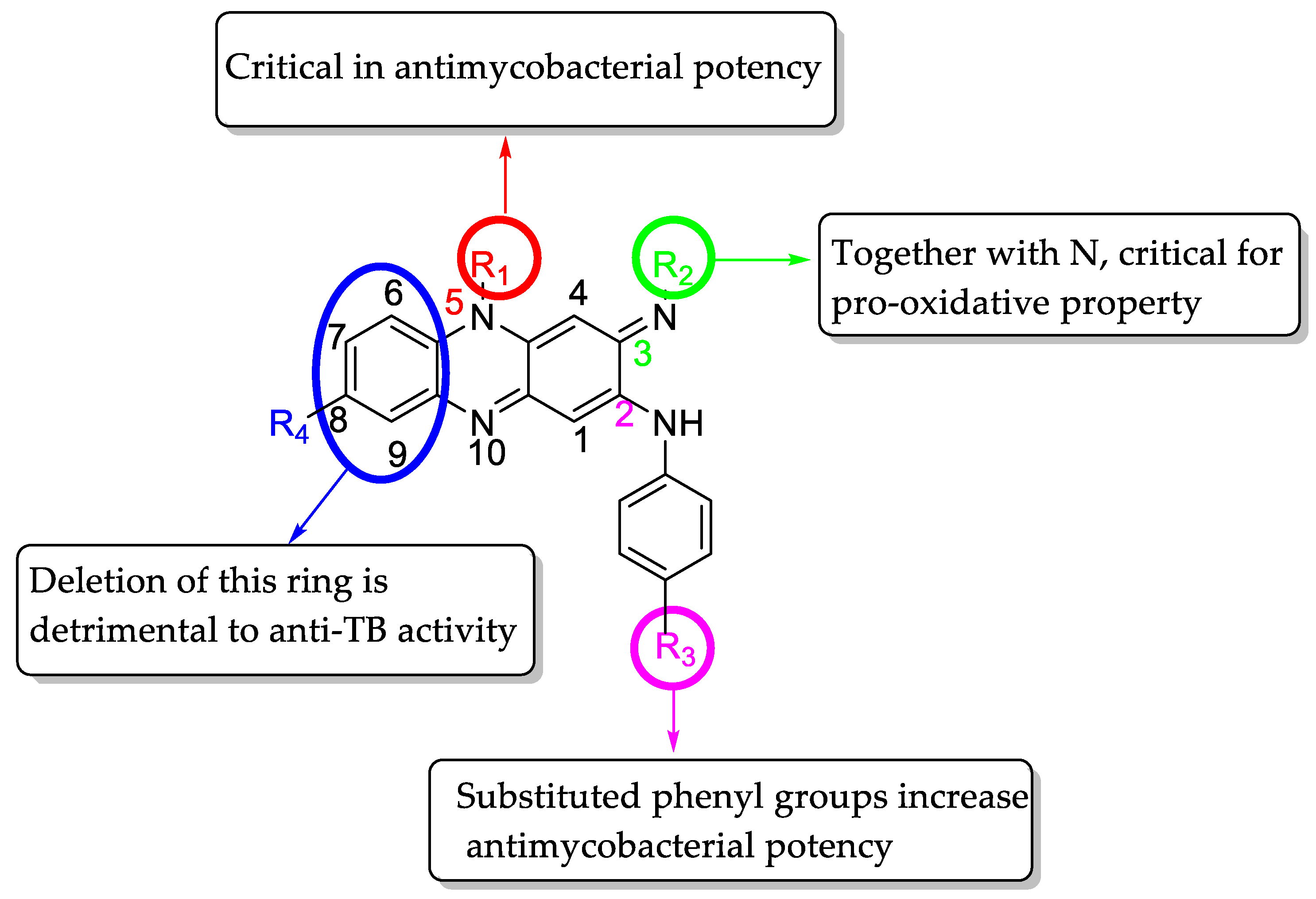
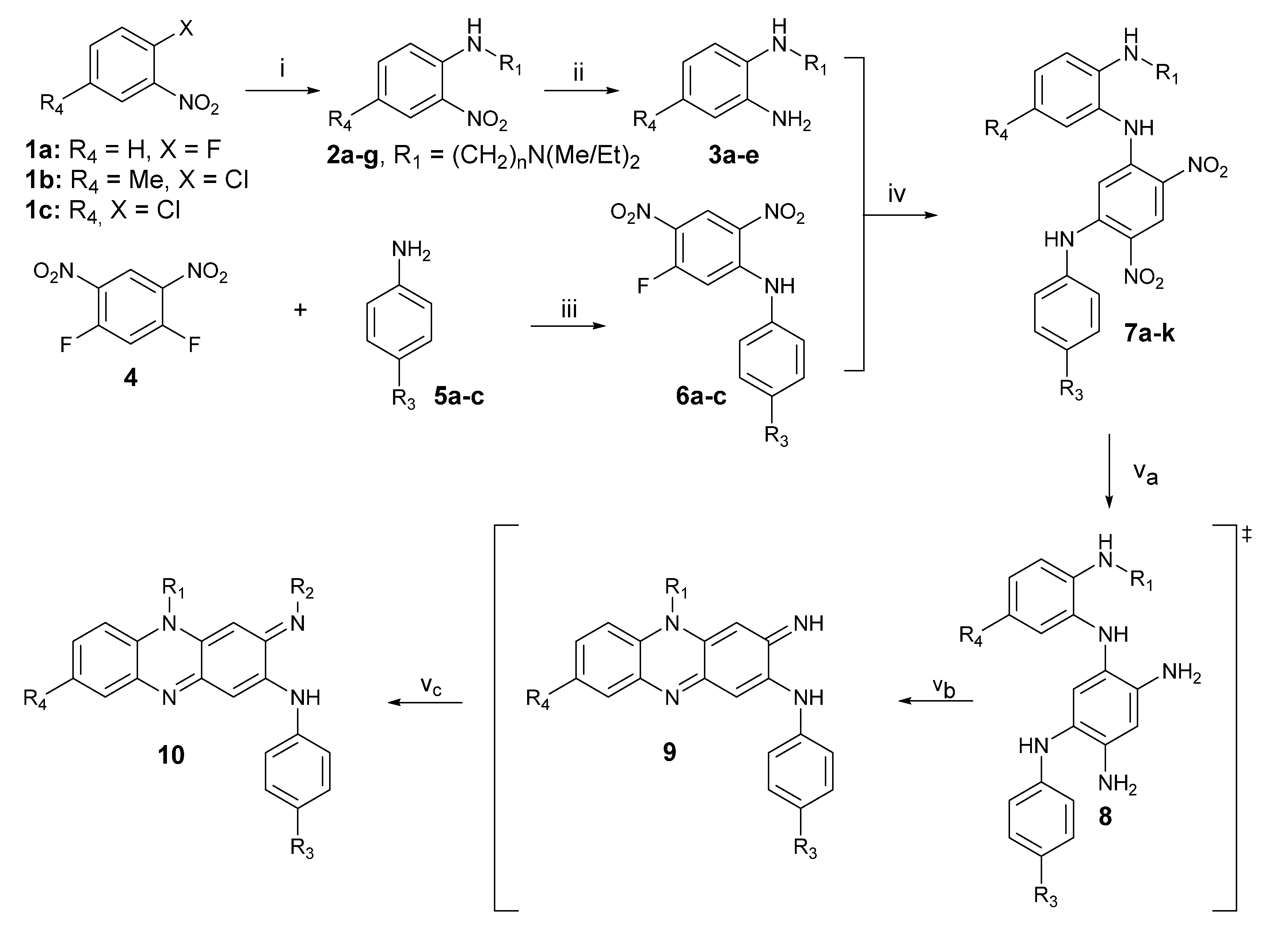
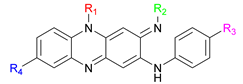
General Procedure for Final Compounds 10a–p
The chosen dinitro compound 7 (3 mmol) and 5% palladium on carbon (3 mmol) in 2:3 (v/v) methanol:THF was stirred at room temperature under 275 kPa hydrogen atmosphere for 12 h in a steel pressure vessel. The orange solution turned maroon after the completion. The catalyst was filtered off through a celite pad, and the filtrate was concentrated under reduced pressure. The gummy maroon residue was dissolved in methanol and stirred at room temperature under pure oxygen (99.5%) atmosphere (345 kPa) for 12 h. The mixture was concentrated under reduced pressure to give a dark red/black product. The crude residue was purified on silica using 5–15% methanol:chloroform as eluent and used immediately without characterization. The material obtained was divided among a choice of amines (isobutylamine, cyclohexylamine, or isopentylamine) (25 eq.) in dioxane (3–4 mL) and glacial acetic acid (0.2 eq.) and heated at 110 °C in a sealed or closed tube for 48 h. After cooling to room temperature, the material was concentration in vacuo, and the residue obtained was purified by column chromatography on silica gel [2–5% (v/v) methanol:chloroform as eluent]. Further purification using preparative TLC was required using the same eluent to ensure adequate purity of the isolated materials.
(E)-5-[2-(Dimethylamino)ethyl]-3-cyclohexylimino-2-phenylamino-3,5-dihydrophenazine (10a). Dark red solid (20.4 mg, 20.4%); Rf 0.46 [10% (v/v) methanol: chloroform]; mp. 94–96 °C (ethanol). 1H NMR (400 MHz, CD3OD): δ 7.33 (dd, J = 7.93, 1.03 Hz, 1H, H 9), 7.27 (dd, J = 8.51, 7.78 Hz, 2H, H 3’), 7.21–7.17 (m, H 2’), 7.15 (d, J = 7.70 Hz, 1H, H 6), 7.11 (d, J = 8.48 Hz, 1H, H 7), 6.99 (dt, J = 13.1, 7.3 Hz, 2H, H 8), 6.53 (s, 1H, H 1), 5.74 (s, 1H, H 4), 3.91 (br s, 2H, NCH2CH2), 3.48–3.36 (m, 1H, =NCH), 2.46–2.35 (m, 2H, CH2NMe2), 2.26 (s, 6H, NMe2), 1.93–1.75, 1.75–1.63, 1.49–1.34, and 1.33–1.24 [4 × m, 10H, NCH(C5H10)]; 13C NMR (101 MHz, CD3OD): δ 152.4 (C 3), 151.7 (C-10a), 145.1 (C 2), 141.4 (C 1’), 136.7 (C 9a), 134.7 (C-4a), 131.5 (C 5a), 130.6 (C 3’), 129.7 (C 4’), 128.9 (C 9), 124.7 (C 7), 124.2 (C 8), 122.4 (C 2’), 114.0 (C 6), 99.9 (C 1), 89.0 (C 4), 59.3 (=NCH), 54.8 (CH2NMe2), 46.1 (NMe2), 44.8 (NCH2), 35.0 [CH(CH2)2], 27.2 [CH2(CH2)2], 26.3 [CH2(CH2)2]; HRMS (ESI-TOF+): m/z calculated for C28H34N5: 440.2814; found: 440.2810 (MH+). IR (cm−1): 3209 (N-H), 2923 (Ar-C-H), 1508 (C=N), 1463 (Ar-C=C), 1241 (C-N).
(E)-5-[3-(Diethylamino)propyl]-3-isobutylimino-2-phenylamino-3,5-dihydrophenazine (10b). Dark red solid (12.5 mg, 37%); Rf 0.42 [20% (v/v) methanol: chloroform]; mp. 102–104 °C (ethanol). 1H NMR (400 MHz, CD3OD): δ 8.19 (br d, J = 8.79 Hz, 1H, H 6), 8.03 (dd, J = 8.35, 1.42 Hz, 1H, H 9), 7.89 (ddd, J = 8.76, 7.10, 1.51 Hz, 1H, H 7), 7.70 (ddd, J = 8.18, 7.10, 0.93 Hz, 1H, H 8), 7.50–7.45 (m, 2H, H 3′), 7.41–7.37 (m, 2H, H 2′), 7.29 (s, 1H, H 1), 7.23 (ddt, J = 7.30, 7.23, 1.17 Hz, 1H, H 4′), 6.77 (s, 1H, H 4), 4.93 (br t, J = 7.62 Hz, 2H, NCH2CH2), 3.54 (d, J = 7.13 Hz, 2H, NCH2CH), 2.88 (t, J = 7.08 Hz, 2H, CH2NEt2), 2.73 [q, J = 7.16 Hz, 4H, N(CH2CH3)2], 2.29 (dquin, J = 13.49, 6.78 Hz, 1H, CHMe2), 2.17 (dt, J = 15.11, 7.43 Hz, 2H, -CH2-), 1.16 (d, J = 6.64 Hz, 6H, CHMe2) and 1.15 [t, J = 7.13 Hz, 6H, N(CH2CH3)2]; 13C NMR (101 MHz, CD3OD): 154.4 (C 3), 147.2 (C 10a), 142.6 (C 2), 141.3 (C 1′), 140.4 (C 9a), 135.5 (C 4a), 133.7 (C 7), 131.5 (C 9), 131.0 (C 3′), 130.7 (C 5a), 128.5 (C 8), 126.2 (C 4′), 123.7 (C 2′), 117.2 (C 6), 107.7 (C 1), 90.2 (C 4), 53.9 (NCH2CH), 50.6 (CH2NEt2), 48.3 [N(CH2CH3)2], 47.6 (NCH2CH2), 29.2 (CHMe2), 25.7 (-CH2-), 21.2 (CHMe2) and 11.6 [N(CH2CH3)2]. HRMS (ESI-TOF+): m/z calculated for C29H38N5: 456.3127; found: 456.3114 (MH+). IR (cm−1): 3381 (N-H), 2980 (Ar-C-H), 1509 (C=N), 1461 (Ar-C=C), 1250 (C-N).
(E)-5-[2-(Dimethylamino)ethyl]-3-cyclohexylimino-2-(4-methylphenyl)amino-3,5-dihydrophenazine (10c). Maroon solid (38 mg, 12% yield); Rf 0.39 [10% (v/v) methanol: chloroform]; mp. 110–112 °C (ethanol). 1H NMR (400 MHz, CD3OD): δ 7.39 (dd, J = 7.91, 1.37 Hz, 1H, H 6), 7.19 (br d, J = 7.70 Hz, 1H, H 9), 7.24 (ddd, J = 8.40, 7.03, 1.37 Hz, 1H, H 7), 7.12 (d, J = 8.49 Hz, 2H, H 2’), 7.10 (d, J = 8.89 Hz, 2H, H 3’), 7.04 (ddd, J = 8.01, 6.93, 1.07 Hz, 1H, H 8), 6.53 (s, 1H, H 1), 5.82 (s, 1H, H 4), 4.00 (br t, J = 7.30 Hz, 2H, NCH2CH2), 3.50 (br td, 1H, =NCH), 2.50 (br t, J = 7.81 Hz, 2H, CH2NMe2), 2.34 (s, 6H, NMe2), 2.29 (3H, s, C-4’ Me), 1.93–1.75, 1.75–1.63, and 1.53–1.35 [10H, 3 × m, NCH(C5H10)]; 13C NMR (101 MHz, CD3OD): δ 152.4 (C 3), 151.6 (C-10a), 145.4 (C 2), 138.6 (C 9a), 136.8 (C 1’), 134.6 (C 4’), 134.0 (C 4a), 131.3 (C 7), 131.1 (C 3’), 129.6 (C 9), 128.8 (C 5a), 124.2 (C 8), 122.6 (C 2’), 113.9 (C 6), 99.5 (C 1), 89.0 (C 4), 59.2 (=NCH), 54.8 (CH2NMe2), 46.1 (NMe2), 44.8 (NCH2), 35.0 [CH(CH2)2], 27.2 [CH2(CH2)2], 26.3 (C-4’ Me), 21.1 [CH2(CH2)2]; HRMS (ESI-TOF+): m/z calculated for C29H36N5: 454.2971; found: 454.2956 (MH+); IR (cm−1): 3292 (N-H), 2920 (Ar-C-H), 1517 (C=N) and 1465 (Ar-C=C), 1240 (C-N).
(E)-5-[3-(Dimethylamino)propyl]-3-isobutylimino-2-(4-methylphenyl)amino-3,5-dihydrophenazine (10d). Dark maroon crystals (49 mg; 13%); Rf 0.30 [20% (v/v) methanol:chloroform]; mp. > 230 °C (ethanol). 1H NMR (600 MHz, CD3OD): δ 8.25 (br d, J = 8.30 Hz, 1H, H 6), 8.23 (d, J = 7.90, Hz, 1H, H 9), 8.08 (t, J = 7.52 Hz, 1H, H 7), 7.90 (t, J = 7.50 Hz, 1H, H 8), 7.66 (d, J = 8.25 Hz, 2H, H 3′), 7.40 (d, J = 8.40 Hz, 2H, H 2′), 7.40 (s, 1H, H 1), 6.88 (s, 1H, H 4), 5.03 (br t, J = 7.63 Hz, 2H, NCH2CH2), 3.80 (d, J = 7.04 Hz, 2H, NCH2CH), 2.93 (t, J = 6.90 Hz, 2H, CH2NMe2), 2.77 (s, 6H, NMe2), 2.71 (s, C-4′ 3H, Me), 2.58 (quin, J = 7.04 Hz, CHMe2), 2.44 (dt, J = 15.26, 7.34 Hz, 2H, -CH2-) and 1.52 (d, J = 6.75 Hz, 6H, CHMe2); 13C NMR (151 MHz, CD3OD): 154.4 (C 3), 147.0 (C 10a), 142.8 (C 2), 140.7 (C 9a), 138.4 (C 1′), 136.6 (C 4′), 135.6 (C 4a), 133.8 (C 7), 131.7 (C 9), 131.6 (C 3′), 130.6 (C 5a), 128.8 (C 8), 124.3 (C 2′), 117.4 (C 6), 107.4 (C 1), 90.3 (C 4), 57.1 (CH2NMe2), 53.4 (NCH2CH), 47.5 (NMe2), 45.6 (NCH2CH2), 29.1 (CHMe2), 26.1 (-CH2-), 21.2 (C-4′ Me) and 21.1 (CHMe2); HRMS (ESI-TOF+): m/z calculated for C28H36N5: 442.2971; found: 442.2892 (MH+). IR (cm−1): 3108 (N-H), 2954 (Ar-C-H), 1512 (C=N), 1459 (Ar-C=C), 1239 (C-N).
(E)-5-[3-(Diethylamino)propyl]-3-isobutylimino-2-(4-methylphenyl)amino-3,5-dihydrophenazine (10e). Dark red solid (22.4 mg, 5.8%); Rf 0.38 [10% (v/v) methanol:chloroform]; mp. 80–82 °C (ethanol). 1H NMR (400 MHz, CD3OD): δ 8.11 (br d, J = 8.89 Hz, 1H, H 6), 7.99 (br d, J = 8.30 Hz, 1H, H 9), 7.82 (br t, 1H, J = 7.80 Hz, H 7), 7.64 (br t, J = 7.80 Hz, 1H, H 8), 7.27 (q, J = 8.89 Hz, 4H, H 3′, H 2′), 7.17 (s, 1H, H 1), 6.68 (s, 1H, H 4), 4.84 (br t, J = 8.30 Hz, 2H, NCH2CH2), 3.49 (d, J = 7.03 Hz, 2H, NCH2CH), 2.76 (t, J = 6.93 Hz, 2H, CH2NEt2), 2.64 [q, J = 7.13 Hz, 4H, N(CH2CH3)2], 2.38 (s, 3H, C-4′ Me), 2.25 (dquin, J = 13.54, 6.75 Hz, 1H, CHMe2), 2.15–2.06 (m, J = 8.50, 2H, -CH2-), 1.13 [d, J = 7.18 Hz, 6H, N(CH2CH3)2], and 1.10 (t, J = 6.64 Hz, 6H, CHMe2); 13C NMR (101 MHz, CD3OD): 154.2 (C 3), 147.9 (C 10a), 143.9 (C 2), 140.1 (C 9a), 138.5 (C 1′), 136.3 (C 4′), 135.3 (C 4a), 133.1 (C 7), 131.2 (C 9), 131.5 (C 3′), 130.7 (C 5a), 128.0 (C 8), 124.0 (C 2′), 116.9 (C 6), 107.0 (C 1), 90.4 (C 4), 54.0 (NCH2CH), 51.0 (CH2NEt2), 48.3 [N(CH2CH3)2], 47.5 (NCH2CH2), 29.5 (CHMe2), 26.1 (-CH2-), 21.4 (CHMe2), 21.3 (C-4′ Me) and 11.9 [N(CH2CH3)2]; HRMS (ESI-TOF+): m/z calculated for C30H40N5: 470.3284; found: 470.3291 (MH+); IR (cm−1): 3367 (N-H), 2980 (Ar-C-H), 1517 (C=N), 1464 (Ar-C=C), 1250 (C-N).
(E)-5-[3-(Diethylamino)propyl]-3-cyclohexylimino-2-(4-methylphenyl)amino-3,5-dihydrophenazine (10f). Dark red solid (35.9 mg, 9.7%); Rf 0.36 [20% (v/v) methanol:chloroform]; mp. 108–110 °C (ethanol). 1H NMR (400 MHz, CD3OD): δ 8.19 (br d, J = 8.79 Hz, 1H, H 6), 8.04 (dd, J = 8.40, 1.37 Hz, 1H, H 9), 7.87 (ddd, J = 8.69, 7.13, 1.57 Hz, 1H, H 7), 7.70 (ddd, J = 8.30, 7.13, 0.88 Hz, 1H, H 8), 7.29 (d, J = 8.01 Hz, 2H, H 3’), 7.26 (d, J = 9.18 Hz, 2H, H 2’), 7.22 (s, 1H, H 1), 6.81 (s, 1H, H 4), 4.98–4.88 (m, 2H, NCH2), 4.07 (br t, J = 7.81 Hz, 1H, =NCH), 2.81 (t, J = 7.13 Hz, 2H, CH2NEt2), 2.67 [q, J = 7.10 Hz, 4H, N(CH2CH3)2], 2.38 (s, 3H, C-4’ Me), 2.23–2.17 (m, 2H, -CH2-), 1.82–1.18 [4 × m, 10H, NCH(C5H10)], and 1.10 [t, J = 7.23 Hz, 6H, N(CH2CH3)2]; 13C NMR (101 MHz, CD3OD): δ 152.8 (C 3), 147.3 (C-10a), 143.2 (C 2), 140.5 (C 9a), 138.4 (C 1’), 136.6 (C 4’), 135.5 (C 4a), 133.4 (C 7), 131.6 (C 3’), 131.5 (C 9), 130.6 (C 5a), 128.5 (C 8), 124.3 (C 2’), 117.2 (C 6), 106.8 (C 1), 90.1 (C 4), 55.3 (=NCH), 50.9 (CH2NEt2), 48.3 [N(CH2CH3)2], 47.6 (NCH2), 33.1 [CH(CH2)2], 26.7 (-CH2-), 26.2 [CH2(CH2)2], 21.1 (C-4’ Me) and 11.8 [N(CH2CH3)2]; HRMS (ESI-TOF+): m/z calculated for C32H42N5: 496.3440; found: 496.3460 (MH+). IR (cm−1): 3367 (N-H), 2980 (Ar-C-H), 1511 (C=N), 1462 (Ar-C=C), 1250 (C-N).
(E)-5-[2-(Dimethylamino)ethyl]-3-isobutylimino-2-(4-ethoxyphenyl)amino-3,5-dihydrophenazine (10g). Dark red solid (193 mg, 47%); Rf 0.32 [10% (v/v) methanol:chloroform]; mp. 208–210 °C (ethanol). 1H NMR (400 MHz, CD3OD): δ 8.09 (br d, J = 8.98 Hz, 1H, H 6), 8.04 (br d, J = 7.81 Hz, 1H, H 9), 7.83 (dd, J = 8.49, 6.93 Hz, 1H, H 7), 7.65 (t, J = 7.42 Hz, 1H, H 8), 7.29 (d, J = 8.79 Hz, H 2’), 7.09 (d, J = 8.89 Hz, 2H, H 3’), 7.03 (s, 1H, H 1), 6.87 (s, 1H, H 4), 4.90 (dd, J = 7.42, 6.54 Hz, 2H, NCH2CH2), 4.09 (q, J = 6.97 Hz, 2H, OCH2CH3), 3.52 (d, J = 7.0 Hz, 2H, NCH2CH), 2.89 (t, J = 7.37 Hz, 2H, CH2NMe2), 2.46 (s, NMe2), 2.26 (sep, J = 6.63 Hz, 1H, CHMe2), 1.43 (t, J = 6.98 Hz, 3H, OCH2CH3), 1.14 (d, J = 6.64 Hz, 6H, CHMe2); 13C NMR (101 MHz, CD3OD): δ 158.5 (C 4’), 154.1 (C 3), 147.2 (C 10a), 143.5 (C 2), 140.4 (C 9a), 135.5 (C 4a), 133.3 (C 1’), 133.1 (C 7), 131.3 (C 9), 130.4 (C 5a), 128.5 (C 8), 126.4 (C 2’), 116.9 (C 3’), 116.8 (C 6), 105.7 (C 1), 90.3 (C 4), 64.9 (OCH2CH3), 56.6 (CH2NMe2), 53.5 (NCH2CH), 47.9 (NCH2CH2), 46.1 (NMe2), 28.9 (CHMe2), 20.9 (CHMe2) and 15.2 (OCH2CH3); HRMS (ESI-TOF+): m/z calculated for C28H36N5O: 458.2920; found: 458.2891 (MH+); IR (cm−1): 3128 (N-H), 2954 (Ar-C-H), 1507 (C=N), 1464 (Ar-C=C), 1230 (C-N).
(E)-5-[2-(Dimethylamino)ethyl]-3-cyclohexylimino-2-(4-ethoxyphenyl)amino-3,5-dihydrophenazine (10h). Dark red solid (45 mg, 14%); Rf 0.42 [10% (v/v) methanol:chloroform]; mp. 222–224 °C (ethanol). 1H NMR (400 MHz, CD3OD): δ 8.04–7.96 (m, 2H, H 6, H-9), 7.82 (dd, J = 11.5, 4.2 Hz, 1H, H 7), 7.65 (br t, J = 7.45 Hz, 1H, H 8), 7.30–7.25 (m, 2H, H 2’), 7.04 (s, 1H, H 1), 7.02 (d, J = 8.80 Hz, 2H, H 3’), 6.83 (s, 1H, H 4), 4.85 (obscured, 2H, NCH2), 4.08 (q, J = 6.85 Hz, 2H, OCH2CH3), 3.92 (m, 2H, =NCH), 2.85 (t, J = 7.63 Hz, 2H, CH2NMe2), 2.46 (s, 6H, NMe2), 2.23–2.15, 1.96–1.85, 1.83–1.75 and 1.62–1.55 [4 × m, 10H, NCH(C5H10)], 1.40 (t, J = 6.90 Hz, 3H, OCH2CH3); 13C NMR (101 MHz, CD3OD): δ 158.6 (C 4’), 152.7 (C 3), 147.7 (C 10a), 144.1 (C 2), 140.2 (C 9a), 135.6 (C 4a), 133.3 (C 7), 133.2 (C 1’), 131.1 (C 9), 130.6 (C 5a), 128.2 (C 8), 126.6 (C 2’), 117.0 (C 3’), 116.7 (C 6), 105.1 (C 1), 90.4 (C 4), 65.1 (OCH2CH3), 56.8 (=NCH), 55.8 (CH2NMe2), 47.7 (NCH2), 46.3 (NMe2), 33.3 [CH(CH2)2], 26.7 and 26.2 [CH2(CH2)2] and 15.3 (OCH2CH3); HRMS (ESI-TOF+): m/z calculated for C30H38N5O: 484.3076; found: 484.3048 (MH+).
(E)-5-[3-(Dimethylamino)propyl]-3-isobutylimino-2-(4-ethoxyphenyl)amino-3,5-dihydrophenazine (10i). Dark red solid (41.0 mg, 15%); Rf 0.40 [20% (v/v) methanol:chloroform]; mp. 185–187 °C (ethanol). 1H NMR (400 MHz, CD3OD): δ 8.21 (d, J = 8.79 Hz, 1H, H 6), 8.05 (d, J = 8.40 Hz, 1H, H 9), 7.84 (ddd, J = 9.00, 7.19, 1.27 Hz, 1H, H 7), 7.72 (t, J = 7.70 Hz, 1H, H 8), 7.30 (d, J = 8.89 Hz, 2H, H 2’), 7.11 (s, 1H, H 1), 7.03 (d, J = 8.98 Hz, 2H, H 3’), 6.87 (s, 1H, H 4), 4.98 (t, J = 7.80 Hz, 2H, NCH2CH2), 4.08 (q, J = 6.93 Hz, 2H, OCH2CH3), 3.55 (d, J = 7.03 Hz, 2H, NCH2CH), 2.61 (t, J = 6.83 Hz, 2H, CH2NMe2), 2.34 (s, 6H, NMe2), 2.27 (sep, J = 6.77 Hz, 1H, CHMe2), 2.18 (br t, J = 6.74 Hz, 2H, -CH2-), 1.41 (t, J = 6.98 Hz, 3H, OCH2CH3), 1.15 (d, J = 6.64 Hz, 6H, CHMe2); 13C NMR (101 MHz, CD3OD): δ 158.7 (C 4’), 154.1 (C 3), 147.2 (C 10a), 143.7 (C 2), 140.6 (C 9a), 135.5 (C 4a), 133.5 (C 1’), 133.3 (C 7), 131.5 (C 9), 130.5 (C 5a), 128.7 (C 8), 126.6 (C 3’), 117.3 (C 6), 117.0 (C 2’), 106.0 (C 1), 90.3 (C 4), 65.1 (OCH2CH3), 57.3 (CH2NMe2), 53.6 (NCH2CH), 47.6 (NCH2CH2), 46.0 (NMe2), 29.1 (CHMe2), 26.4 (-CH2-), 21.1 (CHMe2), and 15.3 (OCH2CH3); HRMS (ESI-TOF+): m/z calculated for C29H38N5O: 472.3076; found: 472.3048 (MH+); IR (cm−1): 3085 (N-H), 2955 (Ar-C-H), 1502 (C=N), 1462 (Ar-C=C), 1232 (C-N).
(E)-5-[3-(Diethylamino)propyl]-3-isobutylimino-2-(4-ethoxyphenyl)amino-3,5-dihydrophenazine (10j). Dark purple solid (22.1 mg, 6.2%); Rf 0.37 [20% (v/v) methanol:chloroform]; mp. 205–207 °C (ethanol). 1H NMR (400 MHz, CD3OD): δ 7.98 (d, J = 8.79 Hz, 1H, H 6), 7.84 (dd, J = 8.24, 1.28 Hz, 1H, H 9), 7.69 (ddd, J = 8.63, 7.17, 1.40 Hz, 1H, H 7), 7.52 (ddd, J = 8.20, 6.95, 0.70 Hz, 1H, H 8), 7.16 (d, J = 9.03 Hz, 2H, H 2’), 6.91 (s, 1H, H 1), 6.88 (d, J = 8.91 Hz, 2H, H 3’), 6.55 (s, 1H, H 4), 4.71 (t, J = 7.80 Hz, 2H, NCH2CH2), 3.99 (q, J = 7.00 Hz, 2H, OCH2CH3), 3.39 (d, J = 7.08 Hz, 2H, NCH2CH), 2.66 (t, J = 6.96 Hz, 2H, CH2NEt2), 2.55 [q, J = 7.12 Hz, 4H, N(CH2CH3)2], 2.14 (sep, J = 6.76 Hz, 1H, CHMe2), 2.03–1.94 (m, 2H, -CH2-), 1.29 (t, J = 7.02 Hz, 3H, OCH2CH3), 1.01 (d, J = 6.59 Hz, 6H, CHMe2), and 0.97 [t, J = 7.14 Hz, 6H, N(CH2CH3)2]; 13C NMR (101 MHz, CD3OD): δ 158.5 (C 4’), 154.0 (C 3), 148.0 (C 10a), 144.2 (C 2), 139.9 (C 9a), 135.5 (C 4a), 133.4 (C 1’), 132.7 (C 7), 130.9 (C 9), 130.6 (C 5a), 127.8 (C 8), 126.3 (C 3’), 116.9 (C 2’), 116.8 (C 6), 104.6 (C 1), 89.9 (C 4), 65.1 (OCH2CH3), 54.7 (CH2NEt2), 50.9 (NCH2CH), 48.3 [N(CH2CH3)2], 47.4 (NCH2CH2), 29.5 (CHMe2), 25.8 (-CH2-), 21.2 (CHMe2), 15.3 (OCH2CH3) and 11.9 [N(CH2CH3)2]; HRMS (ESI-TOF+): m/z calculated for C31H42N5O: 500.3389; found: 500.3391 (MH+); IR (cm−1): 3365 (N-H), 2980 (Ar-C-H), 1509 (C=N), 1462 (Ar-C=C), 1233 (C-N).
(E)-5-[3-(Diethylamino)propyl]-3-isopentylimino-2-(4-ethoxyphenyl)amino-3,5-dihydrophenazine (10k). Dark purple solid (19.1 mg, 6.2%) after crystallization; Rf 0.39 [20% (v/v) methanol:chloroform]; mp. 198–200 °C (ethanol). 1H NMR (400 MHz, CD3OD): δ 8.17 (d, J = 8.79 Hz, 1H, H 6), 8.02 (dd, J = 8.30, 1.37 Hz, 1H, H 9), 7.85 (ddd, J = 8.74, 7.13, 1.42 Hz, 1H, H 7), 7.69 (ddd, J = 8.30, 6.90, 0.70 Hz, 1H, H 8), 7.29 (d, J = 8.89 Hz, 2H, H 2’), 7.05 (d, J = 8.89 Hz, 2H, H 3’), 7.02 (s, 1H, H 1), 6.74 (s, 1H, H 4), 4.91 (dd, J = 8.40, 7.40 Hz, 2H, NCH2CH2), 4.07 (q, J = 7.03 Hz, 2H, OCH2CH3), 3.34–3.29 (m, 2H, =NCH2), 2.79 (t, J = 6.93 Hz, 2H, CH2NEt2), 2.66 [q, J = 7.13 Hz, 4H, N(CH2CH3)2], 1.95–1.81 (m, 3H, CH2CHMe2), 2.15 (quin, J = 7.13 Hz, 2H, -CH2-), 1.42 (t, J = 6.98 Hz, 3H, OCH2CH3), 1.11 (d, J = 7.2 Hz, 6H, CHMe2) and 1.08 [d, J = 6.3 Hz, 6H, N(CH2CH3)2]; 13C NMR (101 MHz, CD3OD): δ 158.6 (C 4’), 153.8 (C 3), 147.5 (C 10a), 143.8 (C 2), 140.4 (C 9a), 135.3 (C 4a), 133.3 (C 1’), 133.1 (C 7), 131.3 (C 9), 130.5 (C 5a), 128.4 (C 8), 126.4 (C 3’), 117.1 (C 6), 116.9 (C 2’), 105.3 (C 1), 89.9 (C 4), 65.1 (OCH2CH3), 50.9 (CH2NEt2), 48.3 [N(CH2CH3)2], 47.4 (NCH2CH2), 44.9 (=NCH2), 38.3 (CH2CH), 27.6 (CHMe2), 25.9 (-CH2-), 23.1 (CHMe2), 15.3 (OCH2CH3) and 11.8 [N(CH2CH3)2]; HRMS (ESI-TOF+): m/z calculated for C32H44N5O: 514.3546; found: 514.3546 (MH+); IR (cm−1): 3381 (N-H), 2980 (Ar-C-H), 1507 (C=N), 1462 (Ar-C=C), 1253 (C-N).
(E)-5-[2-(Dimethylamino)ethyl]-3-isobutylimino-8-methyl-2-(4-methylphenyl)amino-3,5-dihydrophenazine (10l). Dark red solid (23.0 mg, 11%); Rf 0.37 [10% (v/v) methanol: chloroform]; mp. 148–150 °C (ethanol). 1H NMR (600 MHz, CDCl3 + CD3OD): δ 7.12 (br s, 1H, H 6), 7.08–7.04 (m, 3H, H 9, H-3’), 7.04–6.99 (m, 3H, H-7, H 2’), 6.46 (s, 1H, H 1), 5.68 (s, 1H, H 4), 3.96 (t, J = 7.19 Hz, 2H, NCH2CH2), 3.07 (d, J = 6.90 Hz, 2H, NCH2CH), 2.43 (t, J = 8.22 Hz, 2H, CH2NMe2), 2.25 (s, 6H, NMe2), 2.22 (s, 3H, C-8 Me), 2.18 (s, 3H, C-4’ Me), 1.92 (dquin, J = 13.41, 6.74 Hz, 1H, CHMe2), and 0.94 (d, J = 6.75 Hz, 6H, CHMe2), 13C NMR (151 MHz, CD3OD): δ 153.7 (C 3), 151.2 (C 10a), 144.8 (C 2), 138.7 (C 1’), 137.0 (C 4a), 134.6 (C 9a), 134.6 (C 5a), 134.0 (C 4’), 131.2 (C 8), 131.1 (C 3’), 129.2 (C 9), 128.7 (C 7), 122.5 (C 2’), 114.1 (C 6), 99.9 (C 1), 88.5 (C 4), 58.9 (NCH2CH), 54.6 (CH2NMe2), 46.0 (NMe2), 45.1 (NCH2), 31.1 (CHMe2), 21.6 (CHMe2), 21.1 (C-4’ Me) and 20.9 (C-8 Me); HRMS (ESI-TOF+): m/z calculated for C28H36N5: 442.2971; found: 442.2831 (MH+);IR (cm−1): 3221 (N-H), 2959 (Ar-C-H), 1518 (C=N) and 1464 (Ar-C=C), 1241 (C-N).
(E)-3-(Cyclohexylimino)-5-[2-(dimethylamino)ethyl]-8-methyl-N-p-tolyl-3,5-dihydrophenazin-2-amine (10m). Maroon solid (26.0 mg, 21%); Rf 0.50 [10% (v/v) methanol:chloroform]; mp. 155–157 °C (ethanol). 1H NMR (400 MHz, CD3OD): δ 7.44 (br d, J = 8.80 Hz, 1H, H 6), 7.38 (br s, 1H, H 9), 7.30 (br dd, J = 8.80, 1.60 Hz, 3H, H 7), 7.14 (d, J = 8.01 Hz, 2H, H 3′), 7.08 (d, J = 8.60 Hz, 2H, H 2’), 6.73 (s, H 1), 1H, 6.22 (s, 1H, H 4), 4.37 (br t, J = 7.03 Hz, 2H, NCH2), 3.68–3.57 (m, 1H, =NCH), 2.60 (t, J = 7.60 Hz, 2H, CH2NMe2), 2.31 (s, 6H, NMe2), 2.29 (s, 3H, C-8 Me), 2.26 (s, 3H, C-4’ Me), 2.05–1.91, 1.85–1.73, 1.71–1.58 and 1.50–1.31 [4 × m, 10H, NCH(C5H10)]; 13C NMR (101 MHz, CD3OD): δ 152.3 (C 3), 148.8 (C 10a), 143.7 (C 2), 138.7 (C 1’), 136.9 (C 4a), 135.5 (C 9a), 134.4 (C 5a), 133.3 (C 4’), 131.3 (C 8), 131.2 (C 3’), 129.4 (C 9), 128.8 (C 7), 123.4 (C 2’), 115.3 (C 6), 103.0 (br, C 1), 89.5 (C 4), 57.1 (=NCH), 56.0 (CH2NMe2), 46.6 (NCH2), 46.1 (NMe2), 33.9 [CH(CH2)2], 26.8 and 26.2 [CH2(CH2)2], 21.0 (C-4’ Me) and 20.98 (C-8 Me); HRMS (ESI-TOF+): m/z calculated for C30H39N5: 469.3205; found: 469.3041 (MH+). IR (cm−1): 3231 (N-H), 2922 (Ar-C-H), 1512 (C=N) and 1460 (Ar-C=C), 1242 (C-N).
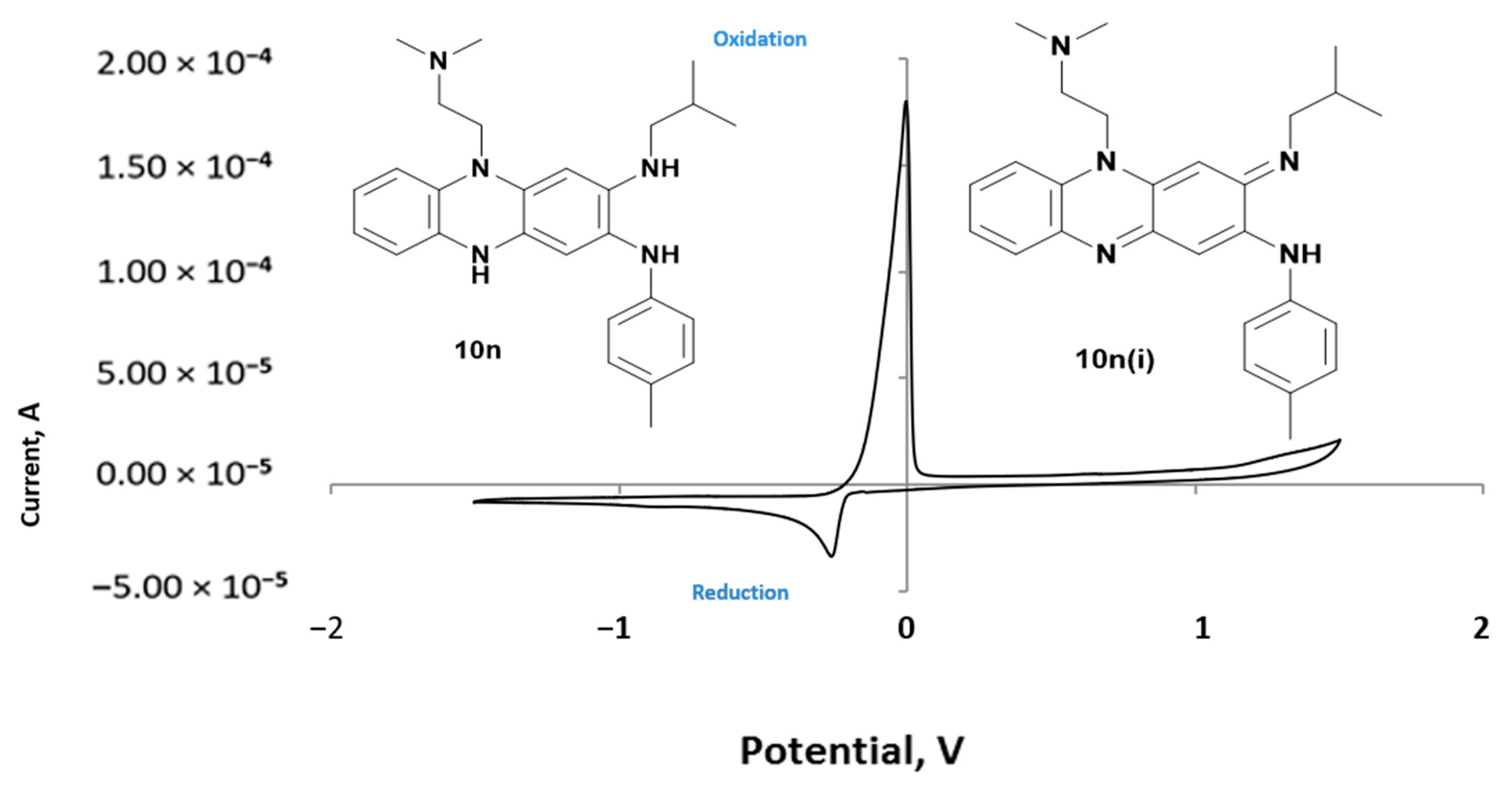
(E)-5-[2-(Dimethylamino)ethyl]-3-(isobutylimino)-N-p-tolyl-3,5-dihydrophenazin-2-amine (10n). Dark red solid (98 mg, 17% yield); Rf 0.50 [20% (v/v) methanol: chloroform]; mp. 140–142 °C (ethanol). 1H NMR (600 MHz, CD3OD3): δ 8.14 (d, J = 8.8 Hz, 1 H, H-6), 8.09 (d, J = 8.3 Hz, 1 H, H-7), 7.93 (t, J = 7.9 Hz, 1 H, H-9), 7.74 (t, J = 7.6 Hz, 1-H, H-8), 7.47 (d, J = 7.9 Hz, 2 H, H-3′), 7.38 (d, J = 7.7 Hz, 2 H, 2′), 7.37 (s, 1 H, H-1), 6.93 (s, 1 H, H-4), 5.05 (t, J = 7.3 Hz, 2 H, NCH2), 3.58 (s, 3 H, Ph-CH3) 3.56 (d, J = 7.2 Hz, 2 H, NCH2CH), 2.98–2.92 (m, 2 H, CH2NEt2), 2.46 (s, 2.28, 6H, CH2NMe2), (t, J = 13.6, 6.7 Hz, 1 H, CHMe2), 1.14 (d, J = 6.6 Hz, 6 H, CHMe2). 13C NMR (151 MHz, CD3OD3): δ 153.2 (C 3), 149.9 (C-10a), 143.8 (C 2), 137.4 (C 1’), 137.2 (C-4a), 133.7 (C-9a), 133.4 (C-5a), 130.1 (C 3), 129.8 (C 7), 129.4 (C 9), 128.8 (C 8), 124.0 (C-4’), 122.6 (C 2’), 112.9 (C 6), 100.2 (C 1), 87.6 (C 4), 56.8 (CH2NMe2), 54.6 (NCH2CH), 46.1 [N(CH3)2], 45.1 (C 1’), 29.6 (CHMe2), 21.2 (CHMe2), 21.1 (PhCH3); HRMS (ESI-TOF+): m/z calculated for C27H34N5: 428.2814; found: 428.2823 (M+H)+. IR (cm−1): 3301 (N-H), 2975 (Ar-C-H), 1510 (C=N) and 1465 (Ar-C=C), 1238 (C-N).
(E)-5-[2-(Dimethylamino)ethyl]-3-(isobutylimino)-N-phenyl-3,5-dihydrophenazin-2-amine (10o). Dark maroon tacky compound (6.40 mg, 25%); Rf 0.39 [10% (v/v) methanol: chloroform]; mp. 119–121 °C (ethanol). 1H NMR (400 MHz, CDCl3):δ 7.72 (d, J = 7.80 Hz, 1 H, H-6), 7.40–7.32 (m, 6 H, H-7, H-9, H-2’, H-3’), 7.07 (t, J = 7.22 Hz, 1 H, H 8), 6.92 (s, 1 H, H-1), 6.06 (s, 1 H, H-4), 4.29 (br s, 2 H, H 1’), 3.32 (d, J = 7.24 Hz, 2 H, NCH2CH), 2.72 (t, J = 7.80 Hz, 2-H, CH2NMe2), 2.43 [s, 6-H, N(CH3)2], 2.15 (br s, 1-H, CHMe2), 1.07 (d, J = 6.63 Hz, 6-H N(CH2CH3)2). 13C NMR (101 MHz, CDCl3): δ 153.1 (C 3), 150.3 (C 10a), 143.5 (C 2), 140.1 (C 1’), 137.0 (C 4a), 133.4 (C 9a), 131.1 (C 5a), 130.1 (C 7), 129.5 (C 9), 129.1 (C 3’), 129.0 (C 8), 122.2 (C 2’, C 4’), 112.8 (C 6), 100.2 (C 1), 87.6 (C 4), 57.6 (CH2NEt2), 54.5 (C 2), 46.1 [N(CH3)2], 45.0 (C 1’), 29.9 (CHMe2), 20.9 (CHMe2). HRMS (ESI-TOF+): m/z calculated for C27H33N5: 428.2382; found: 428.2378 (M+H)+. IR (cm−1): 3289 (N-H), 2950 (Ar-C-H), 1513 (C=N), 1464 (Ar-C=C), 1239 (C-N).
(E)-3-(Cyclohexylimino)-5-[(3-dimethylamino)propyl]-N-p-tolyl-3,5-dihydrophenazin-2-amine (10p). A red solid (97.4 mg, 26%); Rf 0.43 [20% (v/v) methanol:chloroform]; mp. 212–215 °C (ethanol). 1H NMR (400 MHz, CD3OD): δ 7.65 (d, J = 7.50 Hz, 2 H, H 6, H 9), 7.50 (t, J = 8.30 Hz, 1 H, H 7), 7.32 (t, J = 7.60 Hz, 1 H, H 8), 7.22 (q, J = 8.44 Hz, 4 H, H 2’’, H 3’’), 6.81 (s, 1 H, H 1), 6.24 (s, 1 H, H 4), 4.45–4.26 (m, 2 H, H 1’), 3.83–3.63 (m, 1 H, H 1*), 2.52 (t, J = 7.04 Hz, 2 H, H 3’), 2.35 (s, 3 H, PhCH3), 2.31 [s, 6 H, N(CH3)2], 2.07–1.94 (m, 4 H, H 2’, H 2*), 1.90 (br s, 2 H, H 2*), 1.77 (br d, J = 12.7Hz, 2 H, H 4*), 1.61–1.45 (m, 4 H, H 3*). 13C NMR (151 MHz, CD3OD): δ 152.6 (C 3), 150.3 (C 10a), 145.0 (C 2), 138.6 (C 1’’), 138.0 (C 4a), 135.4 (C 9a), 134.7 (C 5a), 131.3 (C 3’’), 131.2 (C 7), 130.8 (C 4’’), 129.6 (C 9), 125.6 (C 8), 123.4 (C 2’’), 115.3 (C 6), 101.7 (C 1), 89.3 (C 4), 65.6 (C 1*), 57.7 (C 3’), 46.0 [N(CH3)2], 45.7 (C 1’), 34.4 (C 2*), 27.0 (C 4*), 26.3 (C 2’), 25.2 (C 3*), 21.1 (PhCH3). HRMS (ESI-TOF+): m/z calculated for C30H38N5: 468.3726; found: 468.3102 (M+H)+. IR (cm−1): 3253 (N-H), 2922 (Ar-C-H), 1514 (C=N), 1459 (Ar-C=C), 1210 (C-N).