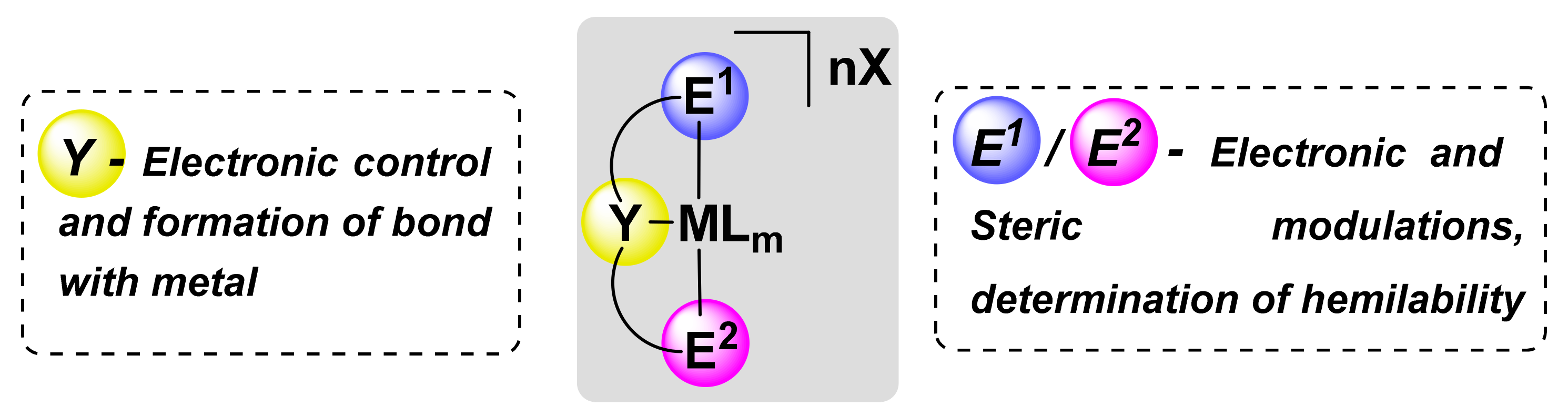
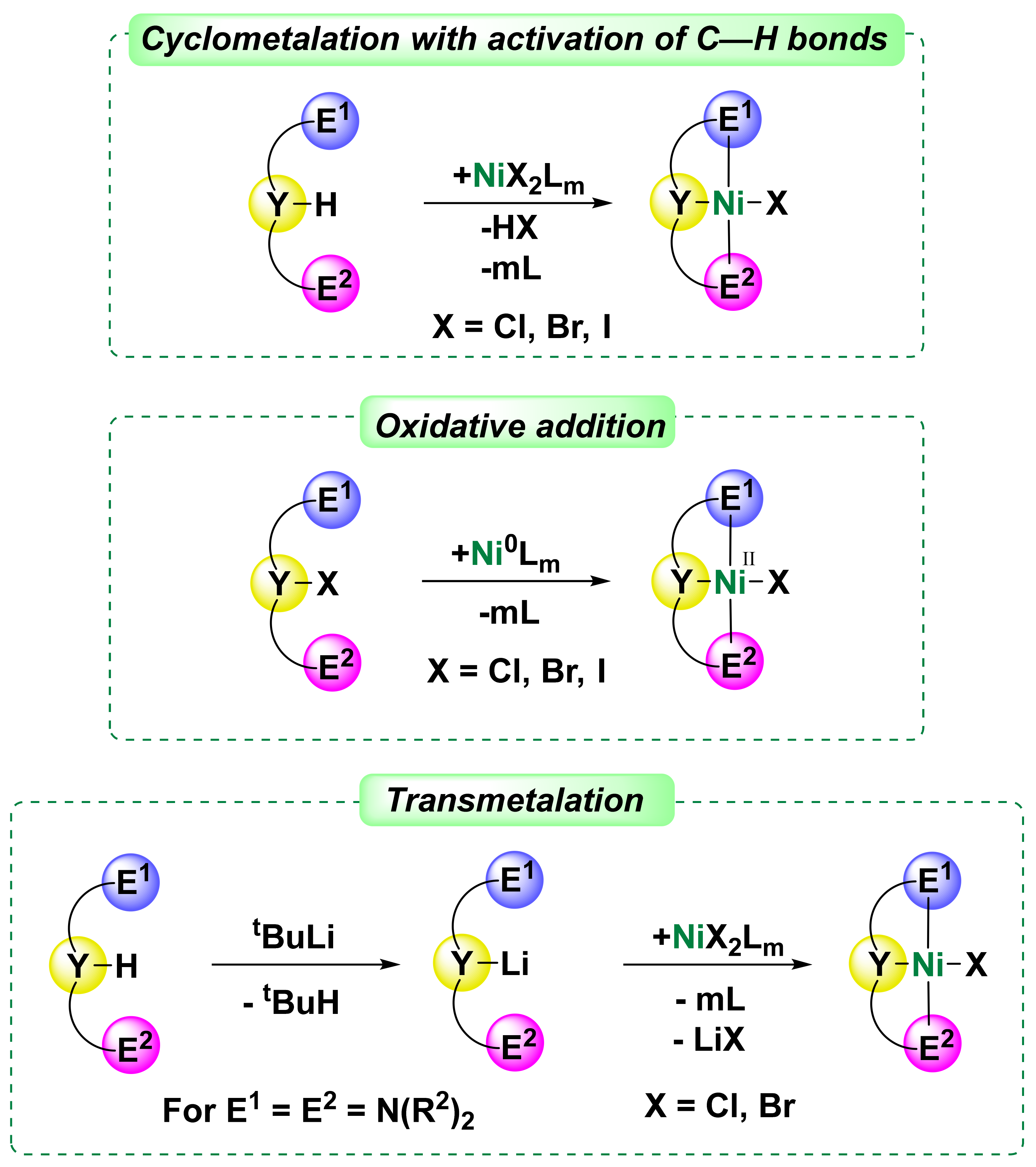
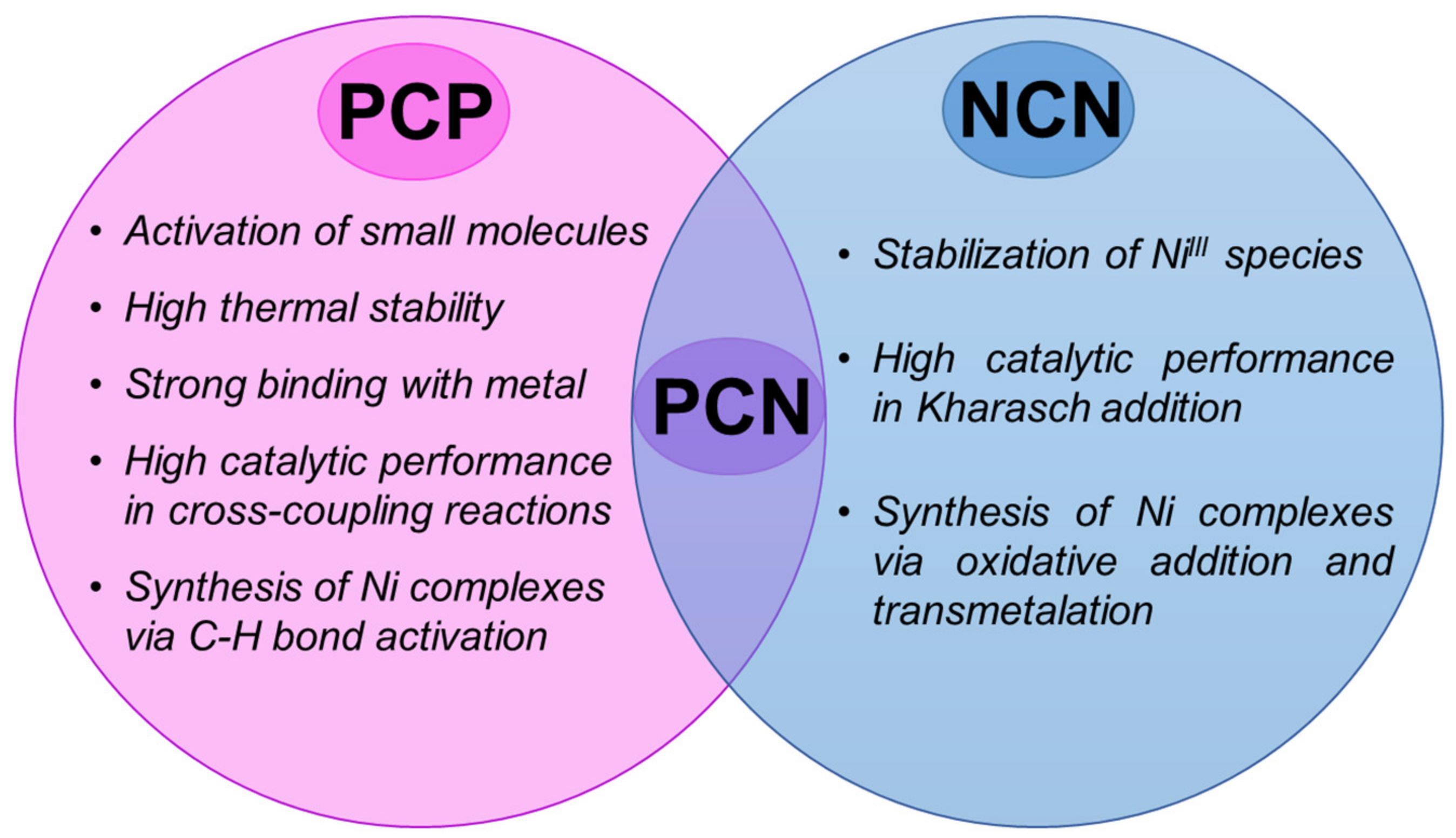
1. Introduction
The control of the properties of metal complexes by varying the nature of the ligand to obtain highly efficient catalytic systems and materials with useful properties is one of the main tasks of modern organometallic and inorganic chemistry. Fine-tuning of the steric and electronic parameters of the formed metal complexes can be realized by chelation by binding the ligand to the metal through two or more bonds, which leads to a dramatic change in the properties of metal complexes such as stability and reactivity [
1,
2].
The introduction of a direct metal–carbon bond in chelate systems leads to the formation of an organometallic complex with a metal cycle, providing additional stabilization of this bond [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19]. Pincer ligands are tridentate and typically bind to a metal in a meridional fashion, forming complexes of general formula [M(E
1YE
2)L
m]X
n (
Figure 1), where E
1 and E
2 are typically a neutral two-electron donor group (such as amine or phosphine group), while Y is usually represented by an anionic carbon atom of a 2,6-disubstituted aryl moiety or, less often, pyridine group; X and L are counterions and auxiliary ligands, respectively; M is metal [
20]. Pincer complexes play an important role in organometallic chemistry mainly due to their versatility, arising from the numerous possibilities to adjust the ligand environment, as well as due to their high thermal stability, which is mainly attributed to the rigidity of the pincer framework [
2]. In particular, their use as homogeneous catalysts for organic transformations has increased dramatically in recent years [
1,
3,
4,
12,
13,
14,
15]. The high catalytic activity of such bis-cyclometallic complexes is associated with the easy tunability of their properties [
8]. PCP-type nickel complexes have been reported as the first example of nickel pincer systems [
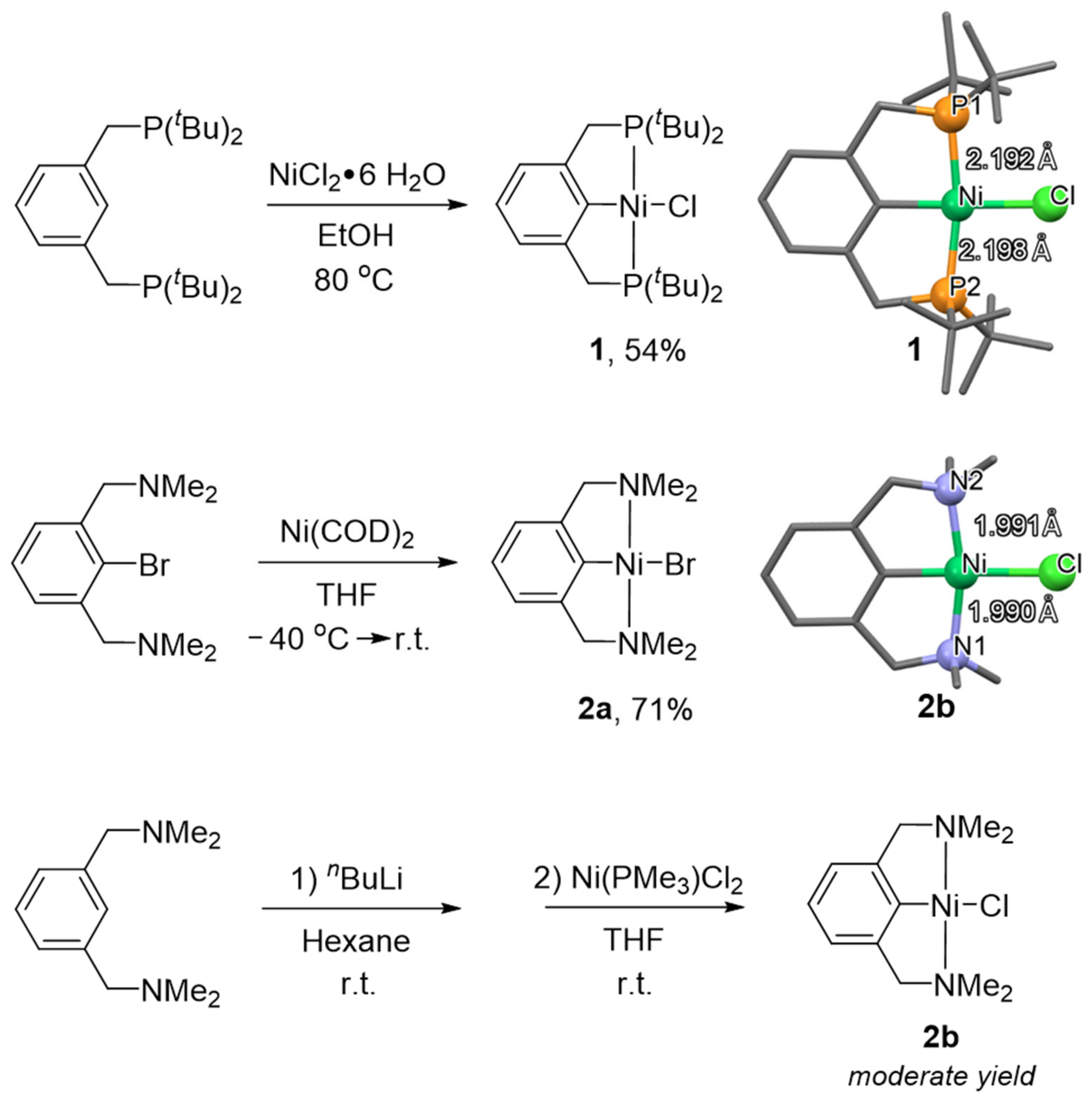
21], followed by NCN pincer nickel complexes [
22,
23]; this success promoted the development of the chemistry of pincer ligands as a whole. These two types of ligands show different influence of the properties of the formed nickel complexes. For example, the use of an NCN pincer ligand enabled the stabilization of nickel (III) complexes, while PCP pincer nickel complexes engaged in the activation of small molecules. These early pincer ligands were symmetric with respect to ligand “arms” [
21,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34].
However an increasing interest in the development of nickel complexes based on unsymmetrical phosphorus containing pincer ligands (PCN, POCN, PNN, PCO, PCS, etc.) is observed in modern literature [
35,
36,
37,
38,
39,
40,
41,
42,
43,
44].
Thus, metal complexes based on pincer ligands have established themselves as highly effective catalysts for such processes as the formation of a new carbon–carbon bond (Heck, Suzuki–Miyaura reactions), hydrogenation of aryl and alkyl ketones, alkane dehydrogenation, hydrosilylation, oligo- and polymerization of unsaturated hydrocarbons and others [
8]; moreover, unsymmetrical pincer complexes showed higher catalytic activity than their symmetrical analogues [
45,
46,
47,
48]. Thus, Milshtein and colleagues described the better catalytic activity of unsymmetrical ruthenium pincer complexes for the dehydrogenation of primary alcohols to esters and hydrogen [
49] and hydrogenation of esters to alcohols [
40,
50]. Gebbink, Szabó et al. showed higher TOF values for the unsymmetrical palladium PCS complex compared to PCP and SCS complexes in aldol reactions [
44]. Huang and colleagues reported that the benzoquinoline-based iridium PCN complex catalyzes the alkene hydrogenation reaction more efficiently than the PCP counterpart [
51]. Shubina et al. demonstrated the superior catalytic activity of the PCN pincer complex of iridium containing a pyrazole N-donor compared to the PCP complex in the dehydrogenation of amine boranes [
52].
The chemistry of pincer compounds, including their catalytic use, is described in detail in a number of works [
3,
13,
14,
15,
28,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63]. Most of them are devoted to the complexes based on metals such as ruthenium, rhodium, iridium, palladium and platinum. However, in modern literature, there is an increasing interest in the development of catalysts based on non-precious metals. For example, nickel, which is a more affordable low-cost and high-natural-abundance analogue of platinum and palladium, has attracted increasing attention in the catalytic chemistry of transition metals in recent years [
1,
64,
65,
66,
67,
68,
69,
70,
71,
72,
73]. Thus, this mini-review is devoted to the recent advances in the chemistry of unsymmetrical phosphorus-based pincer nickel complexes, including the ligand design, the synthesis of nickel complexes and their catalytic applications.
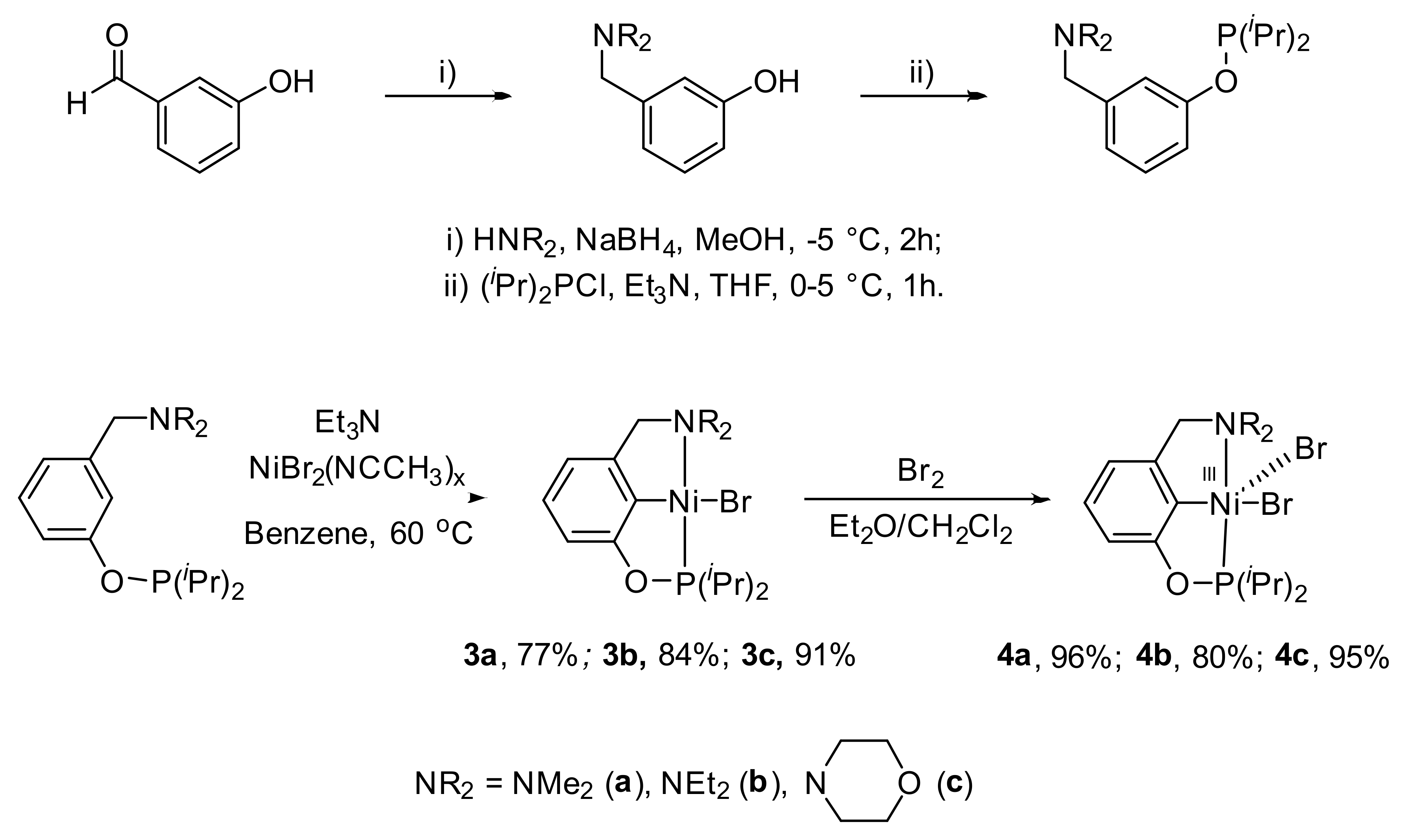
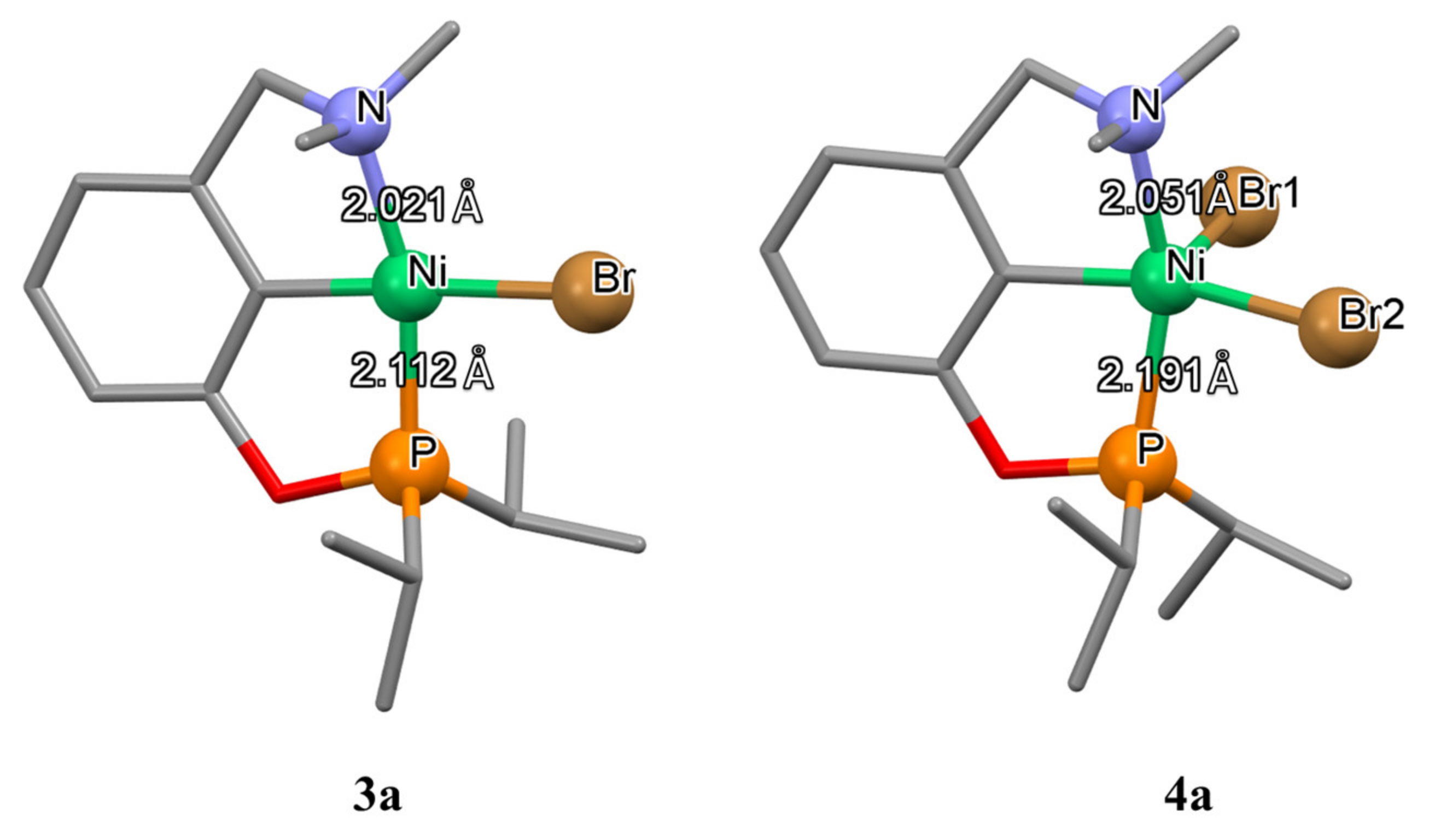
3. POCN Complexes
Unsymmetrical nickel pincer complexes are less studied, and one of the first examples was represented by the POCN-type phosphinite amine ligands (complexes
3a–
3c in
Scheme 3; see
Table A1 in
Appendix A for selected bond lengths (Å) of unsymmetrical nickel pincer complexes presented in this paper) described by Zargarian and Miller [
78,
79,
80,
81]. It is interesting to note that, unlike their symmetric NCN analogues, these complexes can be obtained by direct metalation with activation of the C-H bond, and, unlike PCP analogues, they can be oxidized with bromine to form stable nickel (III) complexes (complexes
4a–
4c in
Scheme 3). Thus, POCN nickel complexes combine the unique properties of both classes of ligands. However, the synthesis of unsymmetrical pincer ligands is more complex and requires multiple steps (
Scheme 3).
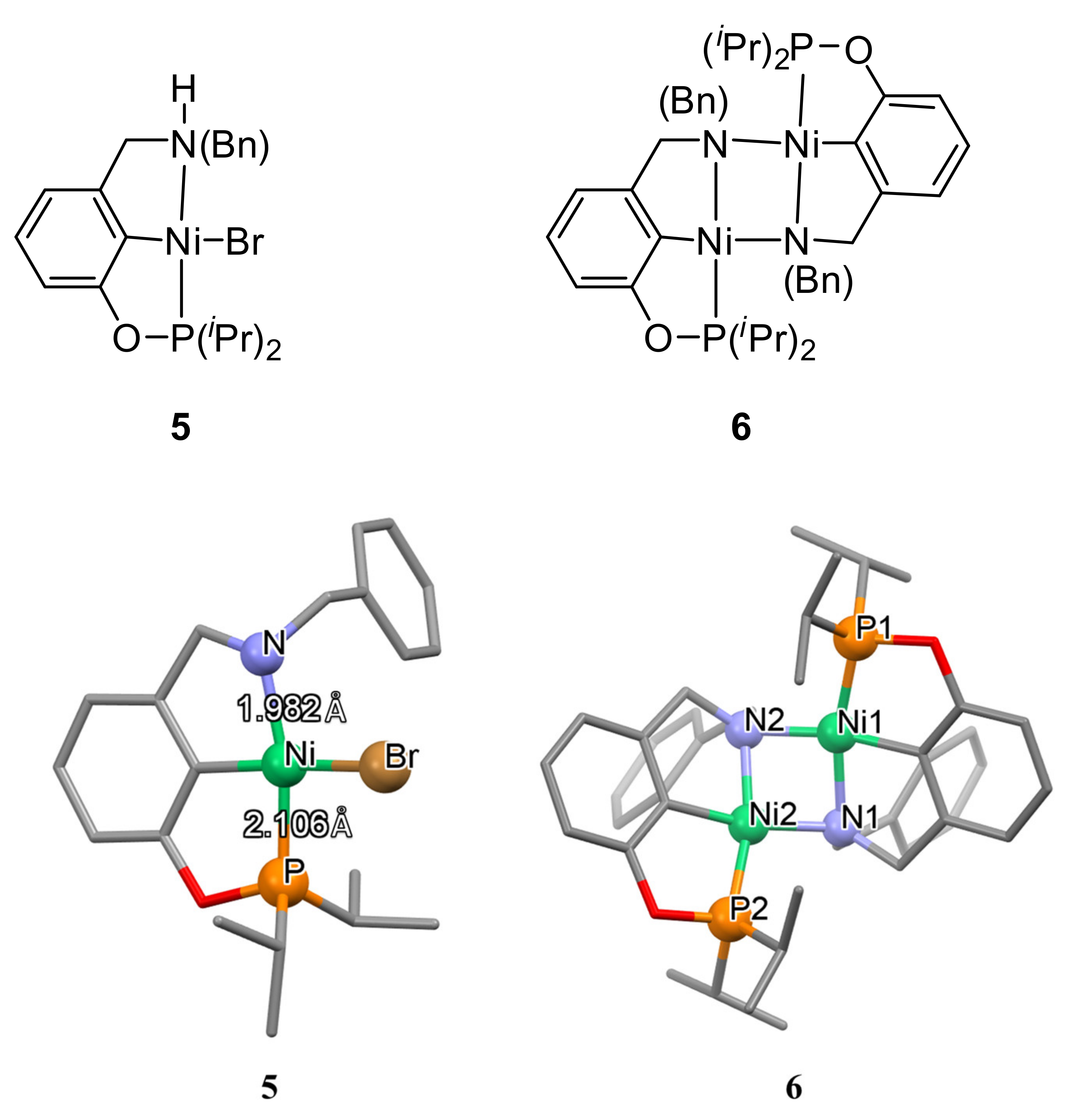
The presence of bulky substituents at the phosphorus atom can increase the stability of the resulting nickel complexes and allow their catalytically active forms to be isolated. Therefore, the same authors obtained a complex containing a secondary amine in its structure (complex
5 in
Figure 3). This complex is capable of dimerization with the formation of binuclear complex
6, which turned out to be an active catalyst in the processes of functionalization of acrylonitrile in alcoholic media [
79].
Since then, many other nickel complexes containing unsymmetrical POCN ligands have been described [
82], including various substituted analogues of complex
3a, such as compound
7 (
Figure 4) [
80]. Optically active species are also known, for example, a complex containing the imidazole fragment (
8 and
9) (
Figure 4), which is successfully used in the asymmetric Suzuki–Miyaura cross-coupling reaction [
83,
84,
85,
86], as well as complex
10 [
87], containing diisopropylphosphinite and imine fragments, which can be oxidized with bromine or
N-bromosuccinimide to complex
11 (
Figure 4). It is interesting to note that complex
12 also obtained by Zargarian and co-workers, containing the diphenylphosphinite fragment, undergoes two-electron oxidation of the phosphinite fragment with the formation of complex
13 upon interaction with bromine (
Figure 4). Thus, we can conclude that the choice of the P-substituent is very important for the stabilization of Ni (III) particles [
87].
4. PCN Complexes
Phosphorus-based complexes such as PCN-type systems are particularly intriguing since, unlike their symmetric NCN analogues, their nickel complexes can be obtained by direct cyclometalation with activation of the C-H bond, and, unlike PCP analogues, they can stabilize nickel (III) species. Thus, PCN nickel complexes combine the unique properties of both classes of N- and P-based ligands [
88,
89,
90,
91,
92,
93,
94]. In such species, the difference in the trans effect between the two different donor arms provides the hemilability of the ligand since the group with the weaker trans effect (N) is more likely to dissociate from the metal center, which leads to a vacant coordination site at the metal center and thus can allow for effective coordination and transformation of substrate molecules in the homogeneous catalysis conditions [
95].
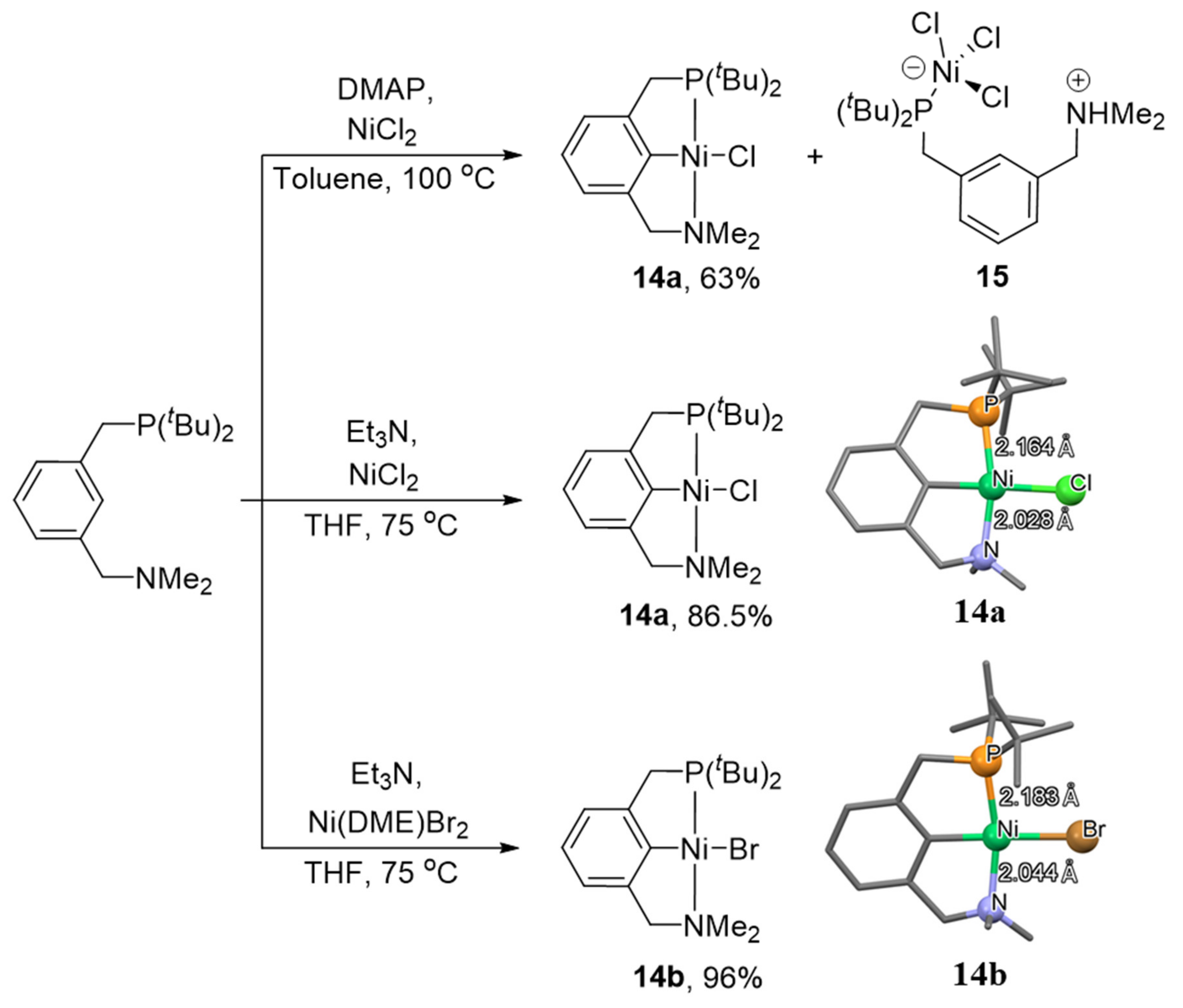
Recently, Moussa and co-authors reported the synthesis of PCN complexes of nickel with ditertbutylphosphine and dimethylamine fragments (
Scheme 4) [
96]. It is interesting to note that the cyclometalation of this PCN ligand with anhydrous nickel chloride in toluene in the presence of 4-dimethylaminopyridine (DMAP) leads to the formation of complex
14a with 63% yield, while this reaction in THF in the presence of triethylamine allows complex
14a to be obtained in 86.5% yield. Such a low yield of compound
14a in the first case is associated with the formation of a paramagnetic complex
15 as a by-product. Triethylamine is a stronger base than DMAP, which avoids protonation of the amine fragment of the PCN ligand with acid (HCl), formed during the reaction. The bromide analogue
14b was obtained by a similar method with a yield of 96%.
Interestingly, the P-Ni bond in
14a is significantly shorter, than the corresponding bond in its symmetrical PCP analogue (complex
1); at the same time, the N-Ni bond is found to be much longer than in symmetrical NCN complex
2b (
Table 1). These differences are due to the strong trans influence of the phosphine moiety, and they determine hemilability.
On the cyclic voltammograms of complexes
14a and
14b in dichloromethane, irreversible oxidation peaks at E
1/2 = 0.837 and 0.797 V for
14a and
14b, respectively, are observed [
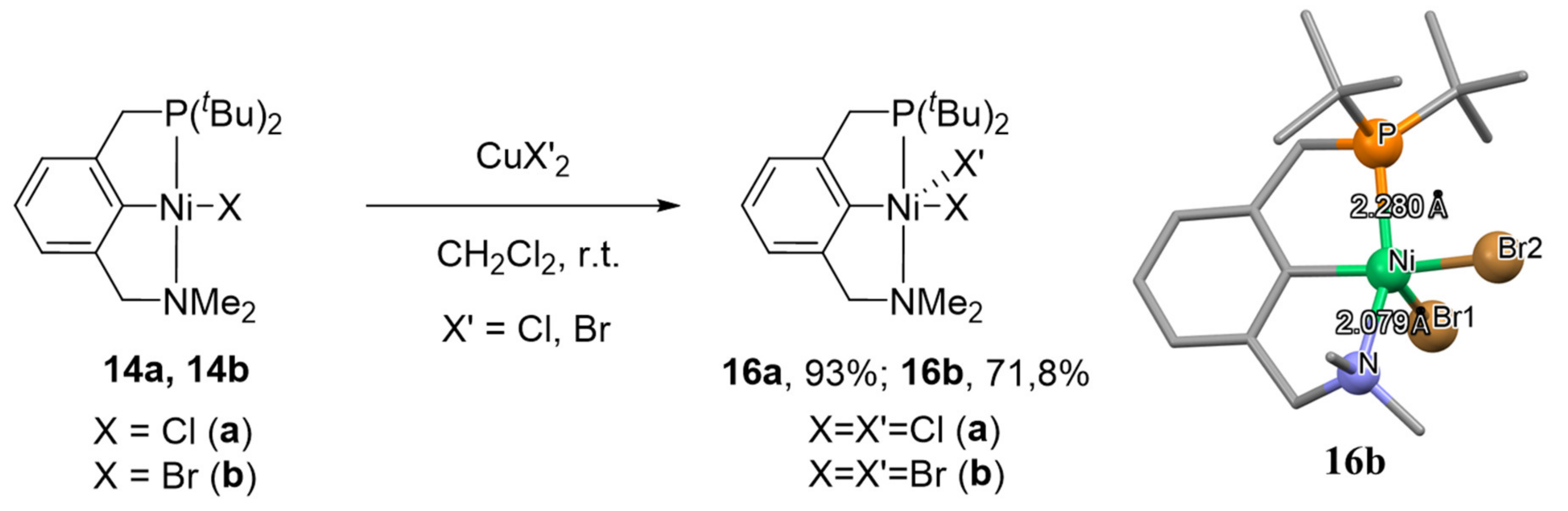
96]. These oxidation potentials are lower than the corresponding values for complexes of nickel with POCN ligands (around 1.0 V), which is associated with a higher donor ability of PCN ligand. Thus, PCN nickel (III) complexes can be easily obtained by oxidation of the corresponding nickel (II) complexes. Indeed, the interaction of complexes
14a and
14b with anhydrous salts of CuX
2 (X = Cl, Br) in dichloromethane leads to the formation of the corresponding Ni(III) complexes
16a and
16b (
Scheme 5) [
96]. Both of the obtained complexes in the crystal display distorted square-pyramidal coordination geometries around nickel with a halide in the apical position.
It is well known that trivalent nickel complexes are important intermediates of the Kharasch addition reaction [
97]. Therefore, NCN nickel complexes are efficient for this process, while the aromatic PCP nickel pincer analogues are not suitable as catalysts in this reaction mainly because they do not produce the corresponding Ni(III) species. PCN complexes
14a and
14b were tested in this process, showing higher catalytic activity in the process of addition of CCl
4 to styrene than their POCN analogues; however, they turned out to be less active than NCN nickel complexes. Thus, the catalytic efficiency of nickel pincer complexes in the Kharasch addition reaction appears in the following order: NCN > POC
sp3OP > PCN > POCN [
78].
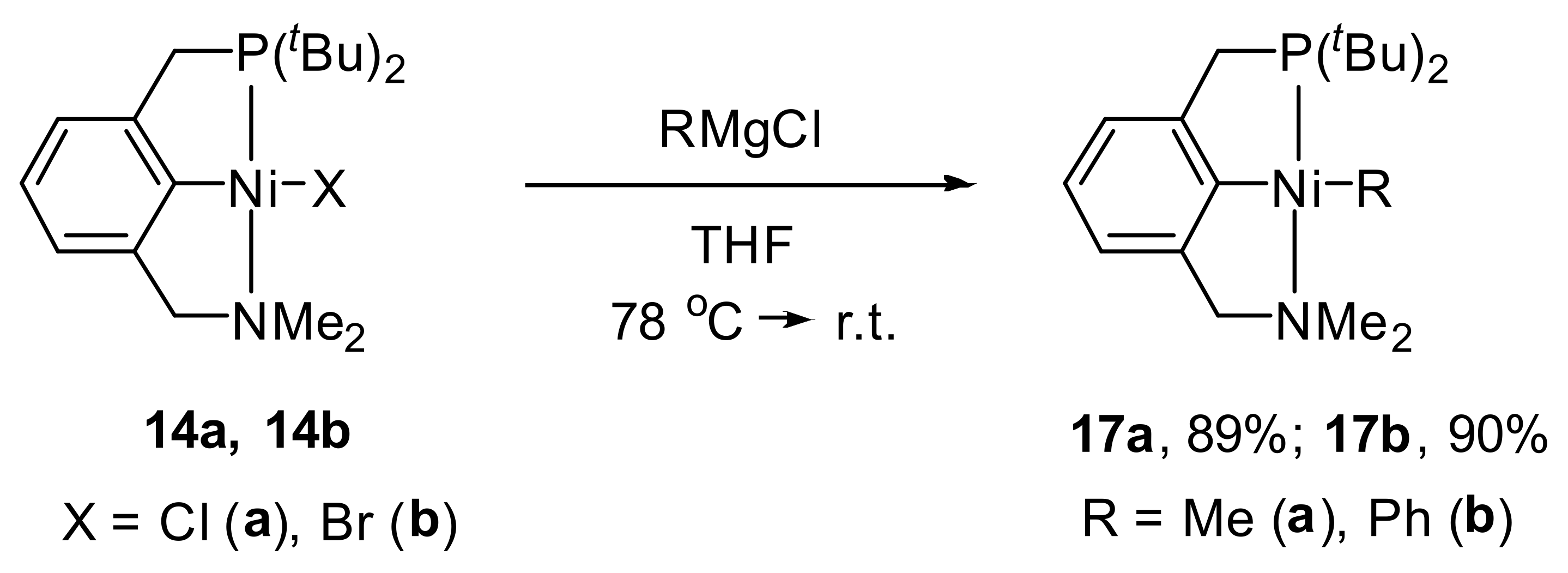
It is also worth noting that the interaction of
14a and
14b with Grignard reagents yielded the first examples of alkyl and aryl-nickel complexes based on an unsymmetrical aromatic pincer ligand
17a and
17b (
Scheme 6) [
96].
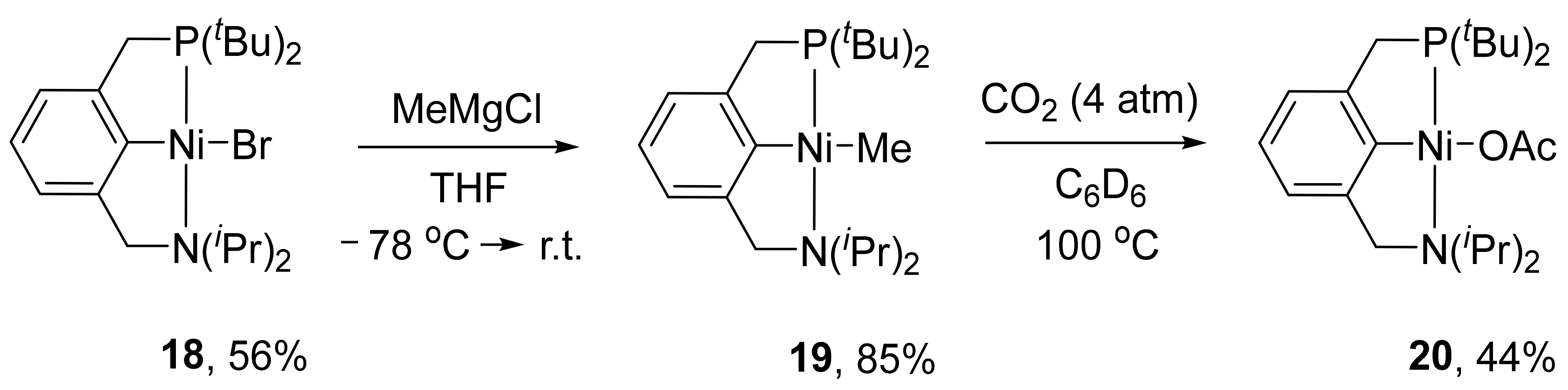
Later, Moussa and co-authors presented the synthesis of an unsymmetrical PCN pincer complex of nickel bearing tertbutyl groups on the phosphorus atom and isopropyl groups on the nitrogen donor atom (complex
18 in
Scheme 7) [
98]. The corresponding methyl complex
19 was also obtained from
18 through a transmetalation reaction with MeMgCl. Complex
19 demonstrated a high reactivity in a carboxylation of a Ni−C bond using CO
2 with the formation of acetate complex
20.
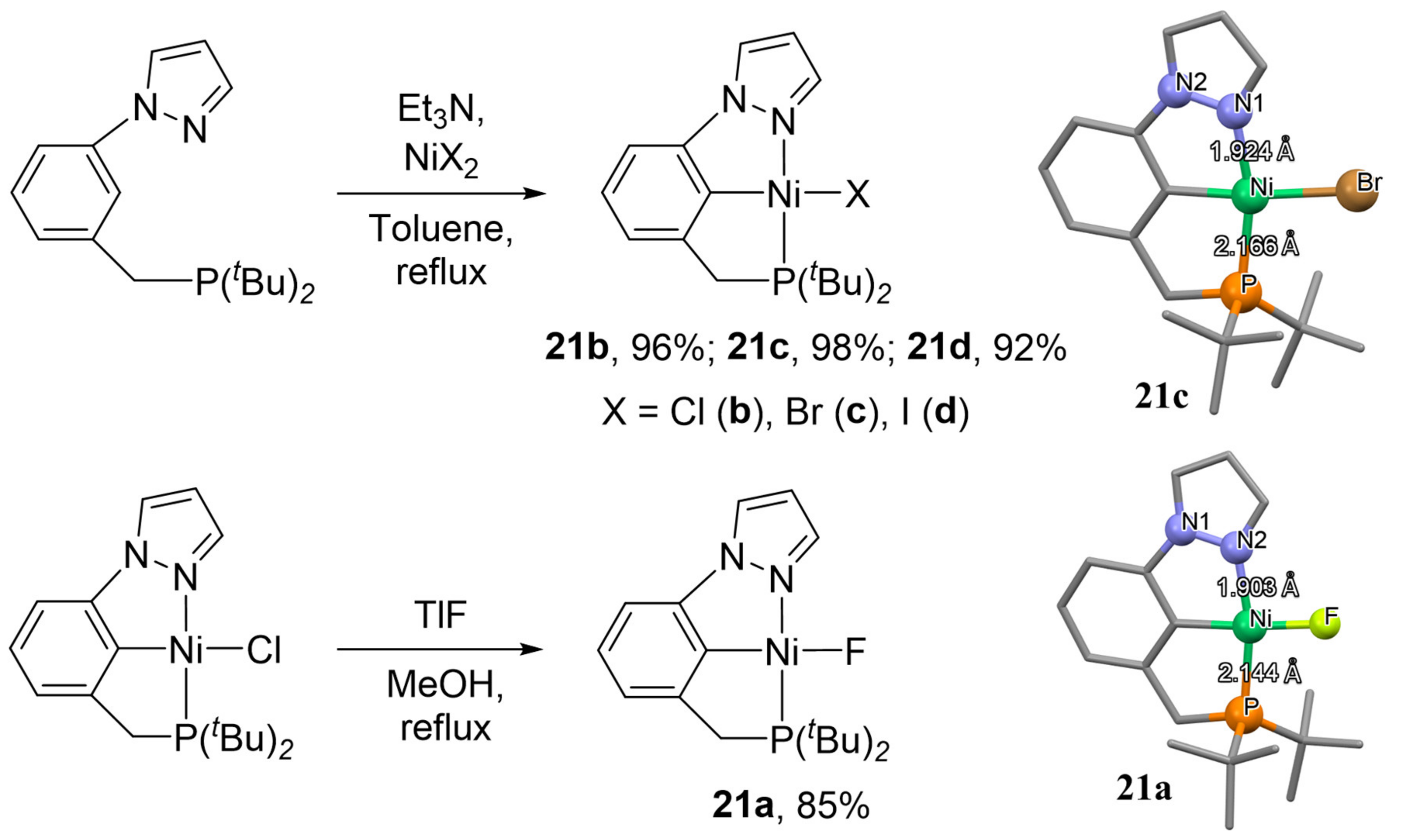
In 2019, Luconi et al. demonstrated a synthesis of PCN nickel complexes containing ditertbutylphosphine and pyrazolyl fragments and different halogens in the structure (F, Cl, Br, I; see
Scheme 8) [
88]. The electrochemical properties of the obtained complexes
21a-
21d were investigated using cyclic voltammetry and in situ EPR spectroelectrochemistry.
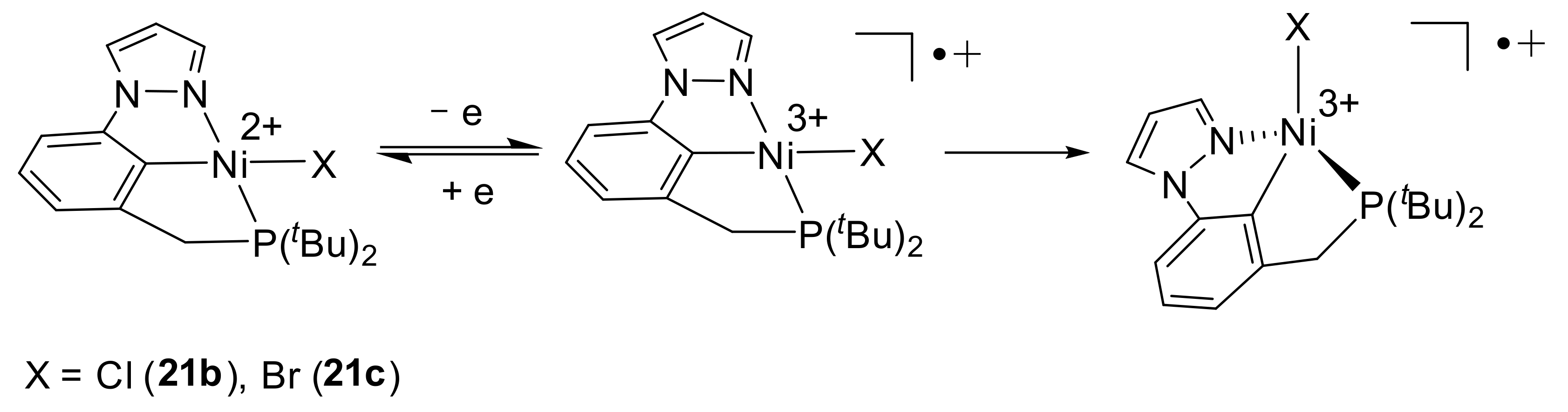
Authors found that the complexes exhibit different behaviors during electro-oxidation. Thus, complexes
21a and
21d during the electro-oxidation process (proceeding at E
1/2 = 0.44 and 0.29 V for
21a and
21d, respectively) form halogen-free species of nickel (III), while chloride and bromide complexes
21b and
21c (E
1/2 = 0.79 and 0.83 V, respectively) change the square-planar geometry of the molecule to a distorted tetrahedron, preserving the halogen atom to the coordination sphere of nickel (
Scheme 9), as evidenced by the hyperfine interactions on their EPR spectra [
88].
Authors have also found that the high nucleophilicity of the fluoride ligand in
21a leads to halogen bonding with the electrophilic iodine atom in iodopentafluorobenzene (C
6F
5I). However, the halogen-bonding capability is weaker than that of the symmetrical analogue (
tBuPCP)NiF [
tBuPCP = 2,6-C
6H
3(CH
2P
tBu
2)
2], and it is featured by a positive ΔS° value because of a higher degree of aggregation in solution [
88,
99].
Interestingly, the oxidation of complexes
21b and
21c with anhydrous CuX
2 salts (X = Cl, Br) did not lead to stable Ni(III) species, which were observed only in situ by EPR spectroscopy [
89]. The presence of the aromatic pyrazole ligand as a weaker sigma donor compared with an aliphatic -NR
2 group (complexes such
3a–
3c,
9,
14a and
14b) is detrimental for the stabilization of the electron-poorer Ni(III) complexes.
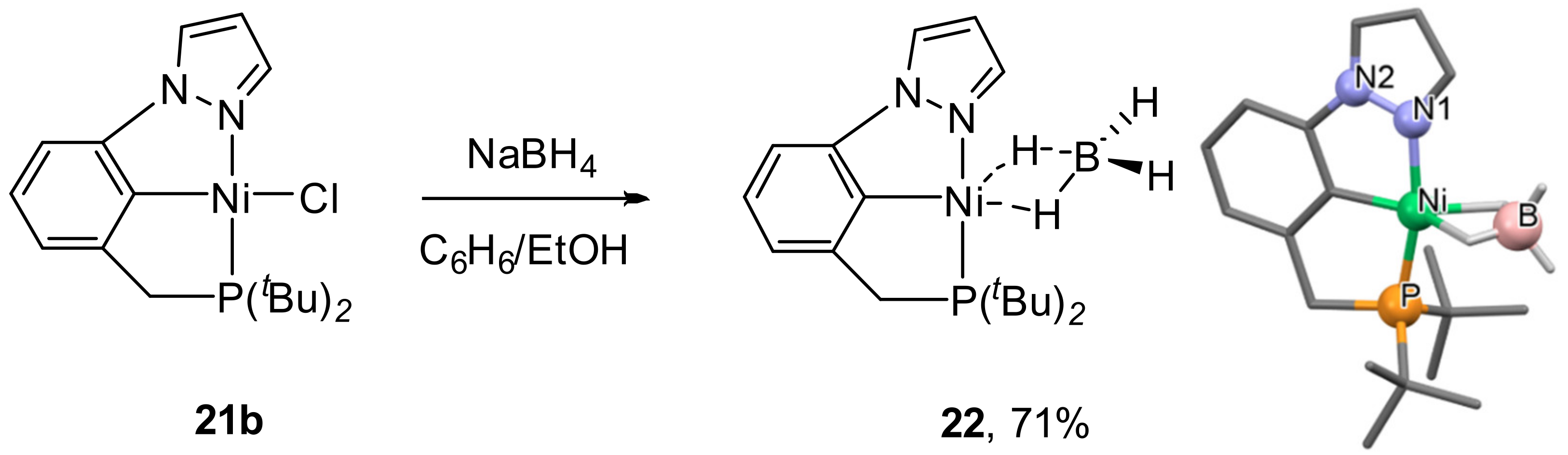
The interaction of chloride complex
21b with an excess of sodium borohydride led to the formation of the relatively robust borohydride complex
22 (
Scheme 10) [
89], which was isolated and characterized by X-ray diffraction. Interestingly, the reaction of symmetrical (
tBuPCP)NiCl with NaBH
4 generates (
tBuPCP)NiH as the isolable product with (
tBuPCP)Ni(BH
4) observed as intermediate in solution by NMR spectroscopy [
99] and only later was isolated by Enthaler and co-workers [
100].
The nickel complexes
21a–
21d preactivated by MMAO have shown moderate activity in the ethylene oligomerization process leading to even-numbered olefins, predominantly C
4-C
10 fractions. To the best of our knowledge, this is the first example of application of PCN nickel complexes on the ethylene oligomerization reaction [
101,
102,
103,
104,
105,
106].
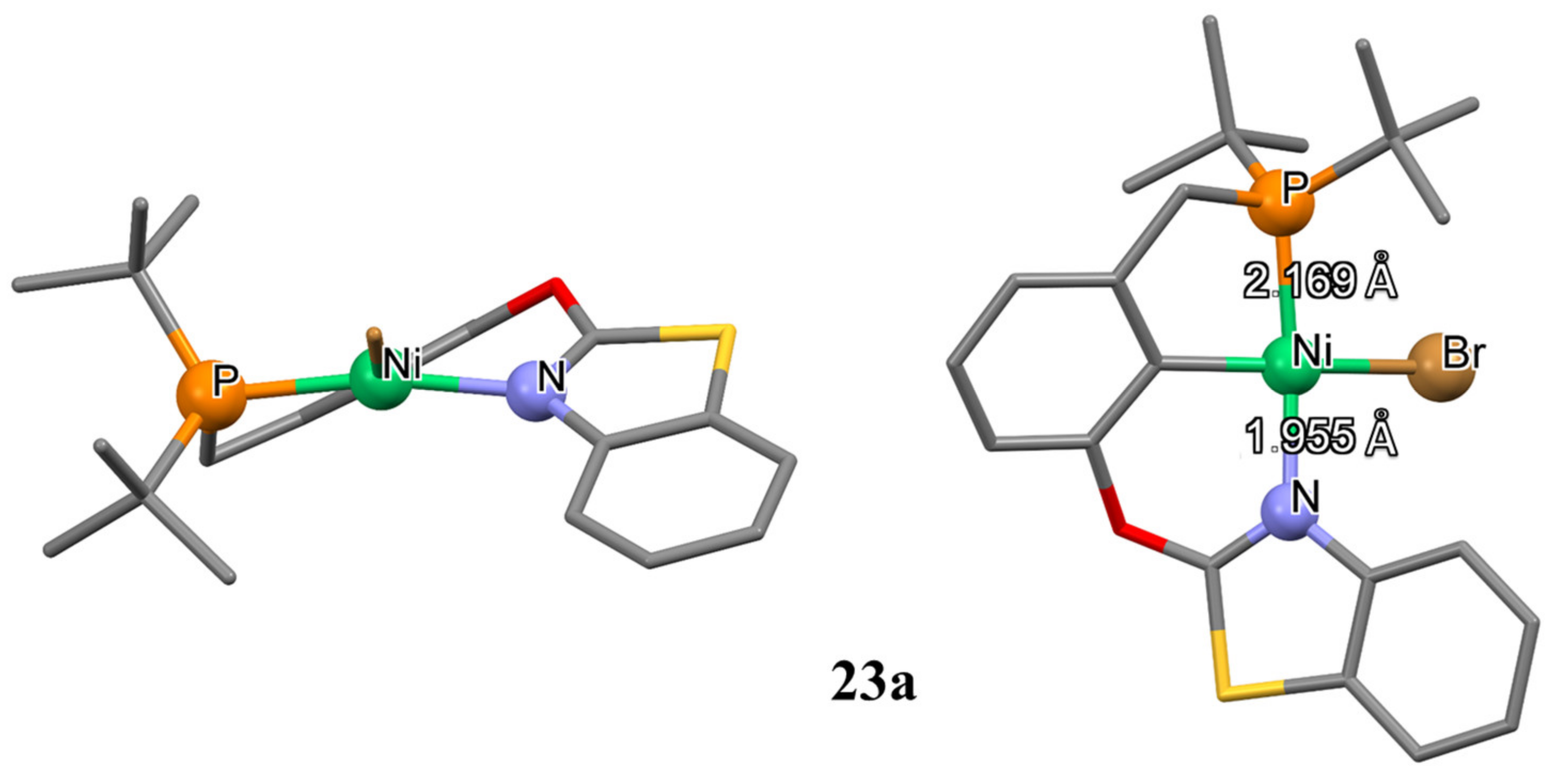
It also should be mentioned that Luconi and co-authors demonstrated the synthesis of unusual benzothiazole-based unsymmetrical pincer nickel complexes
23a,
23b and
24 (
Scheme 11) [
107]. The reaction of the corresponding benzothiazole-based pincer ligand with nickel dibromide in toluene in the presence of Et
3N as a base leads to the bromide complex
23a. Fluoride analogue
23b was obtained by treatment of
23a with silver fluoride in toluene (
Scheme 11). The ionic
aquo complex
24 was synthesized through bromide abstraction from
23a with a silver tetrafluoroborate in THF. The water molecule came from the AgBF
4 or from THF. The N atom in the thiazole ring is a stronger donor, therefore no sulfur coordination was ever observed. Interestingly, binding of ligand to a metal ion generates coordination rings of different sizes: a five-membered cycle on the phosphine arm and a six-membered cycle on the benzothiazole arm. Moreover, the oxo-bridge induces a distortion of the ligand, which is not lying on the P-Ni-N plane (
Figure 5).
Table 2 represents the comparison of P-Ni and N-Ni bond distances of analogous PCN nickel pincers with different N-donor groups (dimethylamine for
14a, pyrazole for
21c and benzothiazole for
23a). The corresponding distances of
21c and
23a are almost equal due to the similar basicity of N atom in the thiazole and pyrazole rings (
pKa of the conjugate acid 2.50 vs. 2.48 for thiazole and pyrazole, respectively), while a higher
pKa value of the dimethylamine (10.73) indicates stronger basicity. Since lower
pKa values of electron-poor pyrazole and benzothiazole groups indicate their weaker σ-donor ability, these groups more likely dissociate from the metal center, yielding more pronounced hemilability, which leads to higher catalytic activity of related complexes in various catalytic processes compared to dimethylamine-containing analogues.
Interestingly, complexes
23a and
23b preactivated by MMAO demonstrated high catalytic activity in ethylene oligomerization (up to 200 × 10
3 mol
C2H4∙mol
Ni−1∙h
−1) with the formation of even-numbered olefins (mainly C
4-C
10 fractions) as products. The comparison of their performance with the results obtained for more rigid pyrazole-based analogues
21a and
21c demonstrates a positive effect of the flexibility modification of the ligand [
108].
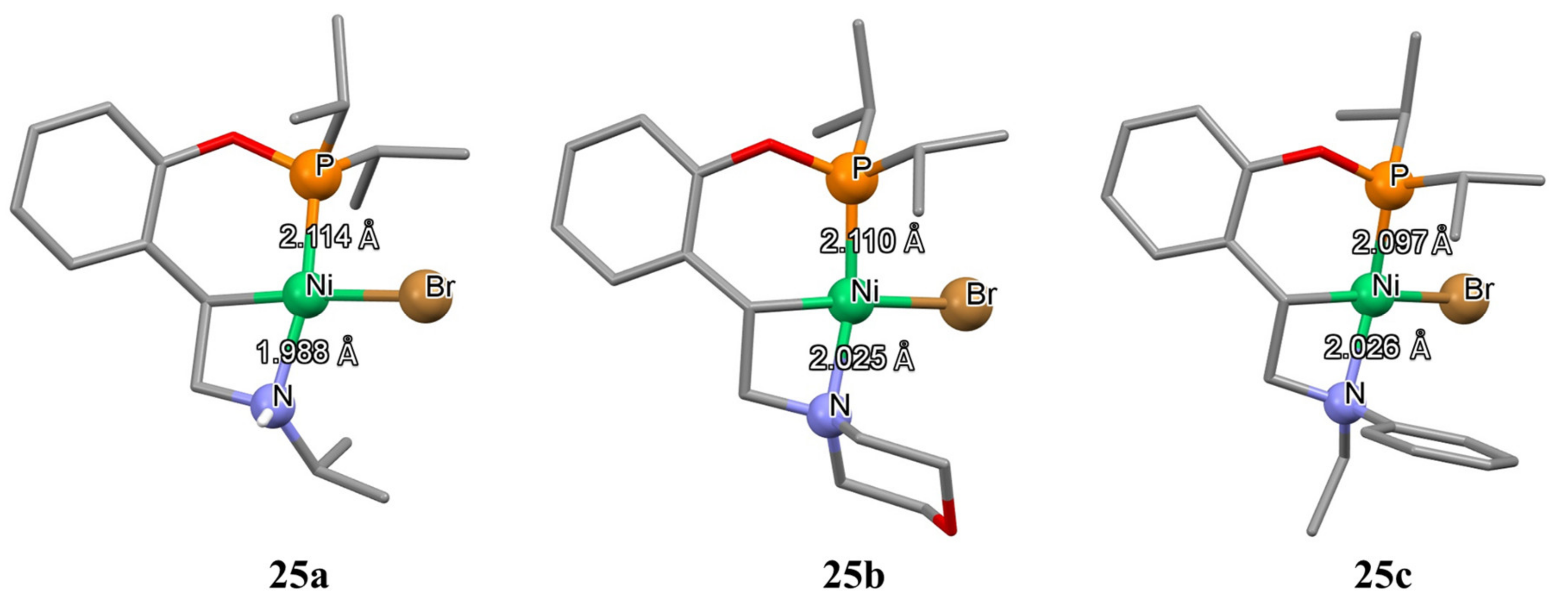
Recently, Zargarian and co-workers demonstrated the synthesis of unsymmetrical pincer Ni-C
sp3 complexes
25a–25f containing two metallacycles of different sizes: a six-membered cycle on the phosphine side and a four-membered cycle on the amine side (
Scheme 12). The synthetic methodology presented in this study is based on the reactivity of phosphinite derived from 2-vinylphenol. Starting phosphinite, nickel (II) precursor and a base (Et
3N) in THF at room temperature produce the target complexes. The solid-state structure of the complexes was established by XRD analyses, which revealed that the Ni-P bond is typically longer than the corresponding Ni-N bond (
Figure 6). Interestingly, authors observed isomerization of these complexes due to the hemilability of the N-Ni coordination bond, which may provide the basis for the future rational design of new active systems, since this hemilability generates a vacant coordination site, which is a prerequisite for the obtainment of an active catalyst. Moreover, CV experiments revealed that these complexes undergo facile one-electron oxidation due to the impact of a C
sp3−Ni moiety [
109].
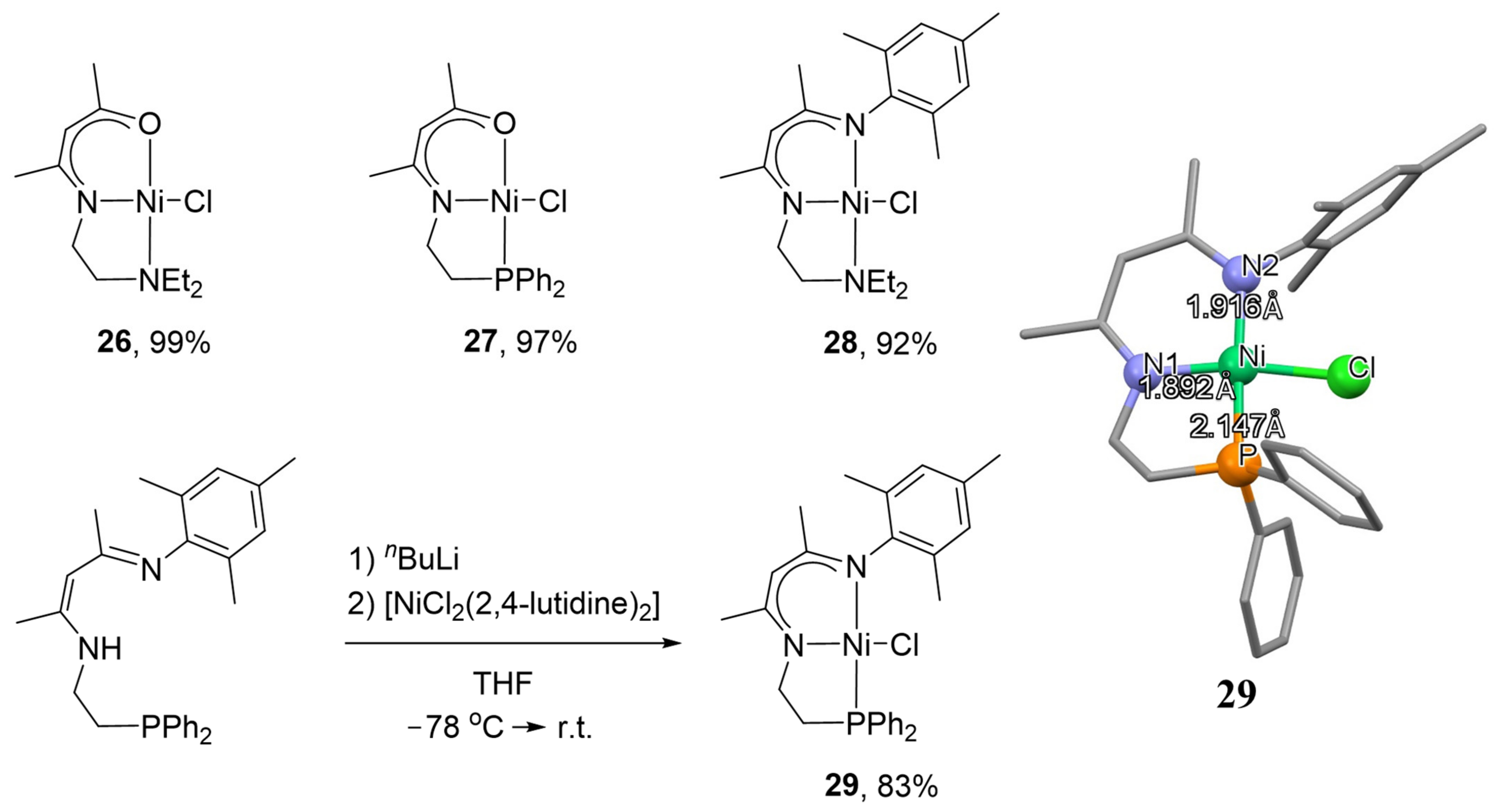
5. NNP Complexes
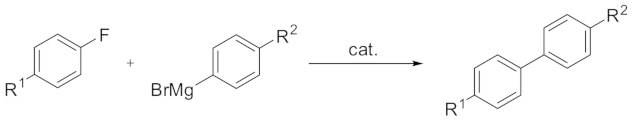
The other important class of phosphorus and nitrogen containing unsymmetrical pincer nickel systems is NNP complexes. The combination of pincer-type ligands with a nickel ion has attracted much attention to create effective cross-coupling catalysts in recent decades. Mostly, aryl chlorides, bromides and iodides are used as electrophiles in these processes [
110,
111], while the activation of aryl fluorides using nickel catalysts is still a challenge in synthetic chemistry [
112,
113]. In 2019, Yamaguchi and co-workers described the synthesis of β-aminoketonato- and β-diketiminato-based unsymmetrical pincer complexes
26–
28 (
Scheme 13), whose catalytic performances in the cross-coupling of aryl chlorides with aryl Grignard reagents [
114] and in the highly Markovnikov-selective hydroboration of vinylarenes using bis(pinacolato)diboron have been investigated [
115]. It was found that the presence of the phosphorus group in complex
27 brings to remarkable catalytic activity in the cross-coupling process over the phosphorus-free analogues, while the β-diketiminato framework increases the energy level of the HOMO of the complex
28, according to the DFT study, making the system electronically more favorable. However,
NNN complex
28 is not an effective catalyst for cross-coupling reactions. Later, in 2019, the working group combined these features introducing a phosphorus donor into the β-diketiminato framework to create a sterically and electronically favorable environment at the Ni center (complex
29), which was prepared by the reaction of the nickel (II) precursor [NiCl
2(2,4-lutidine)
2] with the lithiated NNP ligand and effectively facilitated the cross-coupling of aryl fluorides with aryl Grignard reagents (
Table 3) [
116].
The reaction was carried out with 1.0 mmol of fluoroarene and 1.2 mmol of arylmagnesium bromide in the presence of a Ni (II) complex (0.05 mmol) in THF (5 mL) at room temperature for 6 h [
116].
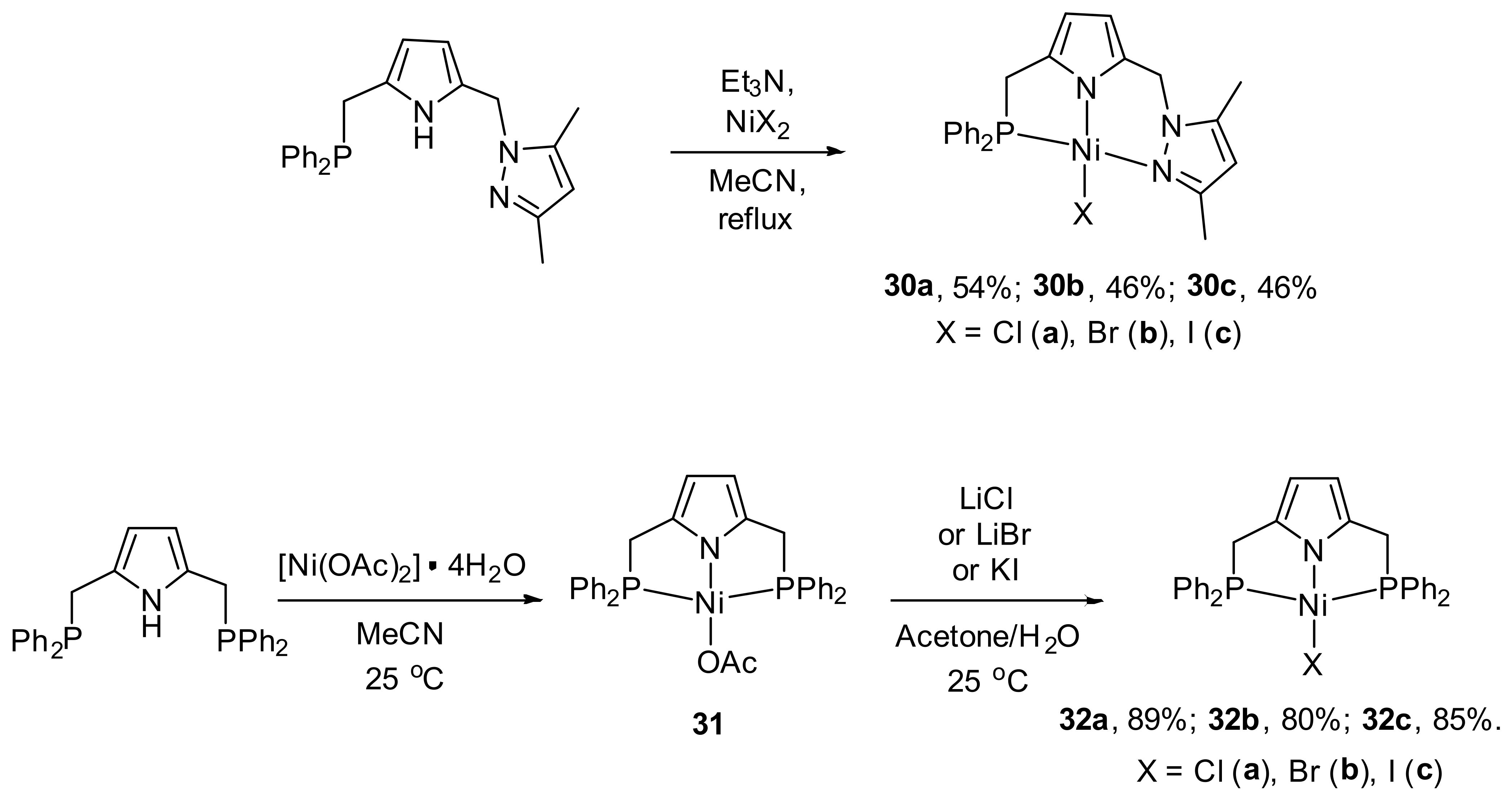
Shortly afterward, the synthesis of the first pyrrole-based unsymmetrical NNP pincer complexes of nickel (
30a–
30c) containing diphenylphosphine and pyrazole fragments was presented (
Scheme 14) [
117]. 1:1 reaction between NNP ligand and [NiCl
2(DME)] or NiX
2 (X = Br, I) in acetonitrile in the presence of NEt
3 under reflux conditions afforded the pincer nickel complexes
30a–
30c with good yields. The pyrrole-based pincers are well known to stabilize a variety of nickel complexes [
118,
119,
120]. Authors compared the efficiencies of complexes
30a–
30c in catalyzing norbornene to polynorbornene with efficiencies of their symmetrical analogues—PNP complexes
32a–
32c (
Scheme 14) [
121]—which were synthesized by the reaction between PNP pincer ligand and [Ni(OAc)
2]·4H
2O with the formation of
31, which further treated with an excess of LiCl or LiBr or KI in acetone/water at room temperature to give the corresponding halide ion substituted Ni complexes
32a–
32c.
Interestingly, according to X-ray diffraction analysis the Ni-P distance in
30b is 2.1613 Å, while for
30c, it is 2.1647 Å, which are shorter than the distances found in their symmetrical PNP analogues
32b and
32c, indicating the strong trans effect of the P atom in comparison to the N atom (
Figure 7). Moreover, the pyrrolide N−Ni distances in
30b and
30c (1.886 Å and 1.847 Å, correspondingly) are shorter than the amide N−Ni distances found in the analogous pincer nickel (II) complexes: 1.924 Å in [NiCl{N(SiMe
2CH
2PPh
2)
2}] [
122] and 1.895/1.912 Å in [NiX{N(
o-C
6H
4PPh
2)
2}] (X = Cl, Br) [
110], which indicate the strong bond formed by the pyrrolide N atom.
The authors carried out a systematic study of the polymerization of norbornene using symmetric (
32a–
32c) and unsymmetrical (
30a–
30c) pyrrole-based complexes. Nickel pincer NNP complexes in the presence of MMAO or EtAlCl
2 showed high yields and high activities (in the range of 10
7 g of PNB mol
−1 h
−1). Meanwhile, symmetric PNP complexes, on the contrary, turned out to be ineffective catalysts in this process, suggesting that with an increase in the number of N donors, the yield and activity of the catalytic system increase, following the order PNN > PNP (
Table 4).
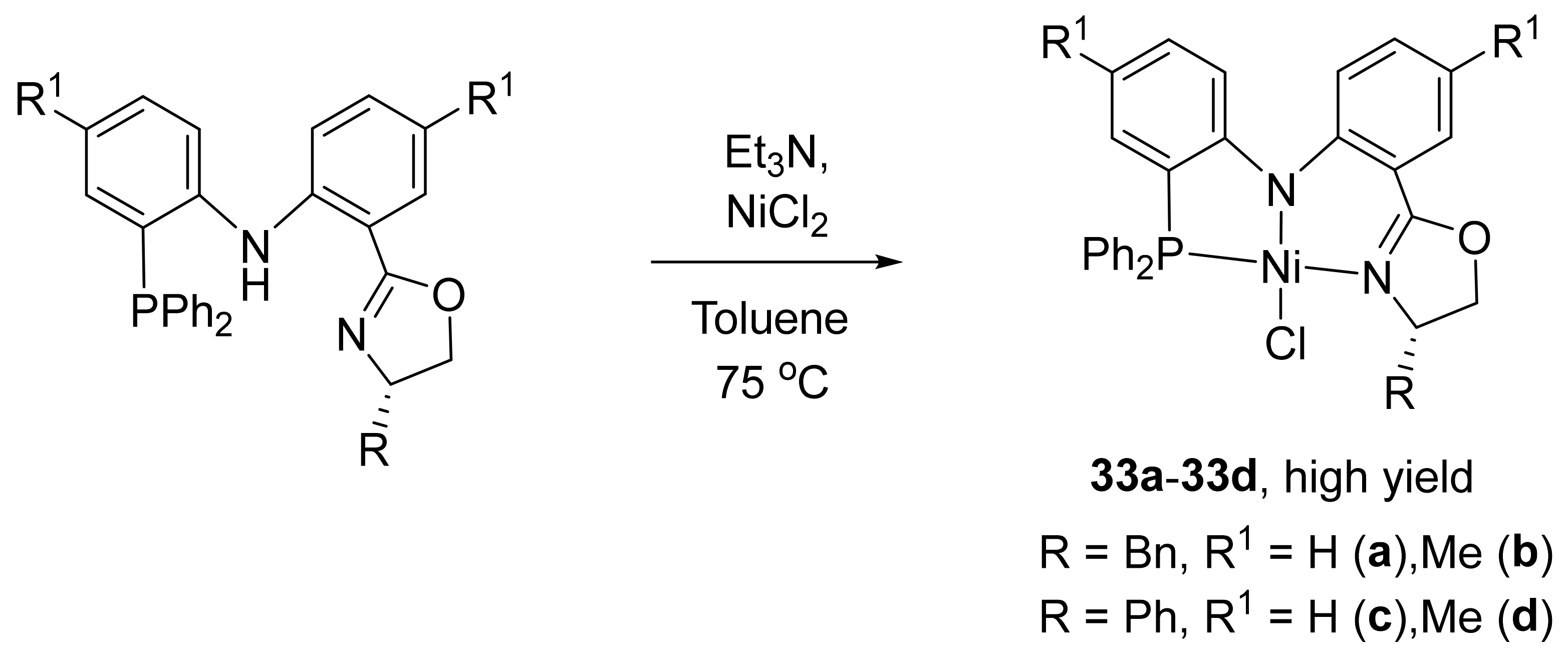
It is interesting to note that nickel complexes
33a–
33d with diarylamido-based unsymmetrical pincer ligands containing a chiral oxazoline ring (
Scheme 15) showed much lower activity during the catalytic polymerization of norbornene in the presence of MAO (in the range 0.12–1.5 × 10
5 g of PNB mol
−1 h
−1) [
123]. Meanwhile, the analogous palladium complexes exhibited relatively higher activities (in the range of 4 × 10
6 g of PNB mol
−1 h
−1). However, it has been demonstrated that the steric hindrance and electronic effect of the ligands have a significant influence on the complexes and the consequent catalytic properties (the activity increase, following the order
33c <
33b <
33d <
33a, see
Table 5).
Recently, Gardinier and Wang demonstrated synthesis of unsymmetrical PNN pincer nickel complexes
34a−
34c with pyrazolyl and diphenylphosphino donor groups (
Scheme 16) [
124]. These complexes were tested in hydrodehalogenation reactions of 1-bromooctane and different aryl halides in using sodium borohydride as a hydride source and as a base.
34a derivative showed the best catalytic performance that correlates with its electron donor properties [
125].
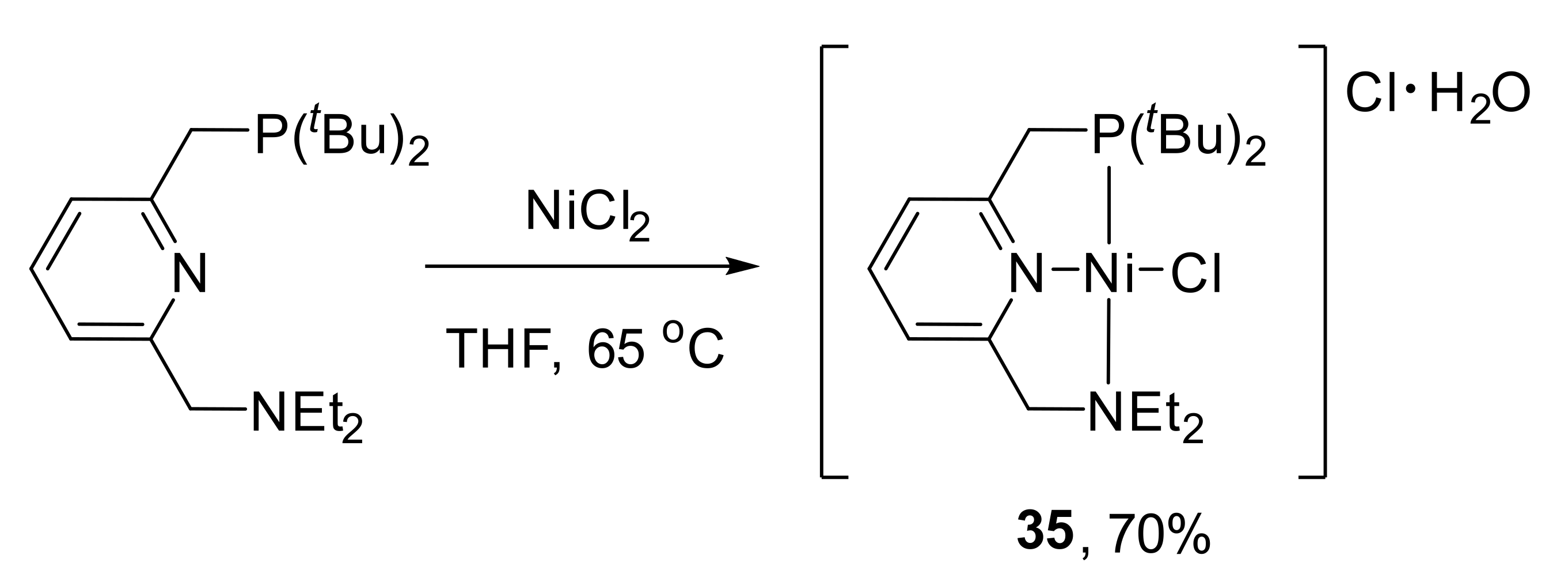
One of the latest published
cationic unsymmetrical pincer complexes of nickel was synthesized by the reaction of anhydrous NiCl
2 with 1.2 equivalents of the Milstein’s NNP ligand in THF (
Scheme 17) [
126,
127]. In
35, the Ni−N(pyridyl) distance (1.876 Å) is shorter than the Ni−N(amine) bond length (2.014 Å). The complex was tested as a catalyst precursor for the hydrogenation of CO
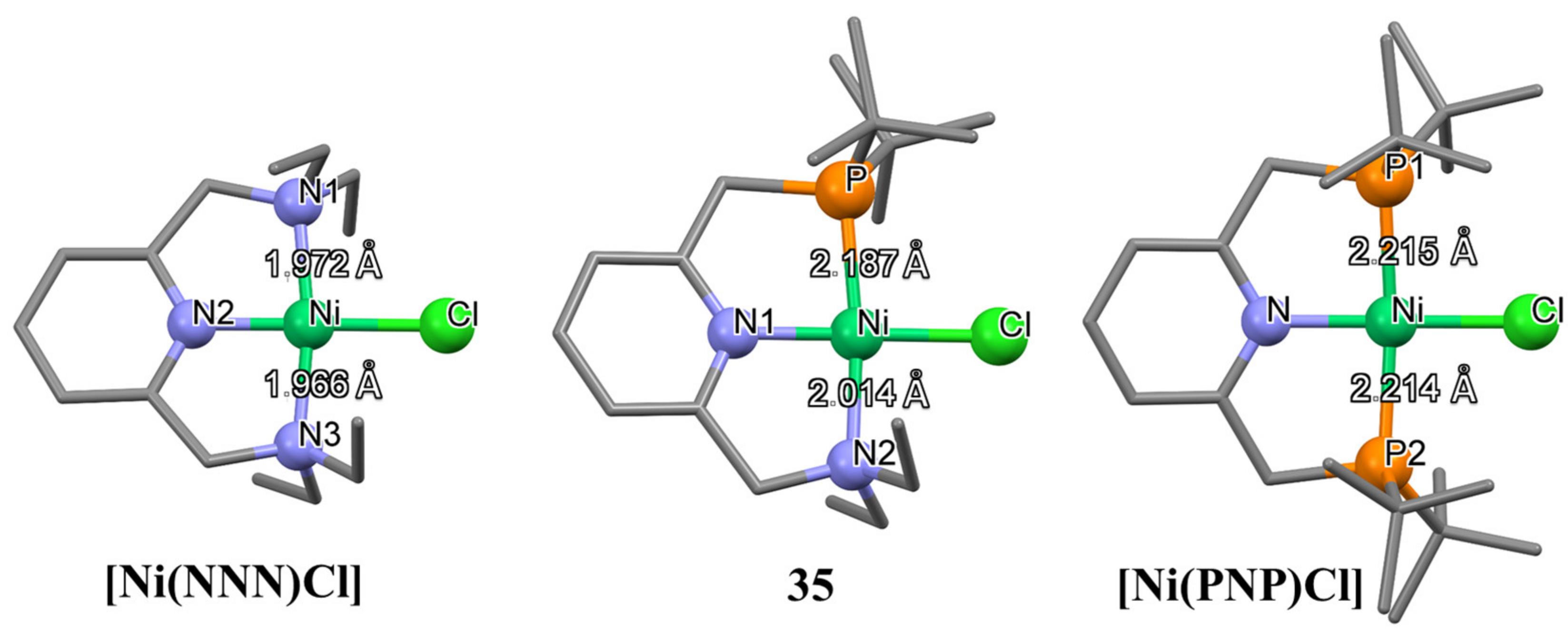
2 to formamide in anhydrous DMSO as a solvent; however, no catalytic activity was observed in the presence of morpholine or dimethylamine as substrates under the applied reaction conditions.
Analogously to
14a, the Ni-P bond in
35 is significantly shorter than the corresponding bond in its symmetrical PNP analogue [
128]; at the same time, the Ni-N(amine) bond of
35 is much longer than in the symmetrical NNN complex (
Figure 8) [
129]. These differences are due to the strong
trans influence of the phosphine moiety, and they determine hemilability (see
Table A1 in
Appendix A for selected bond lengths (Å) of unsymmetrical nickel pincer complexes presented in this paper).
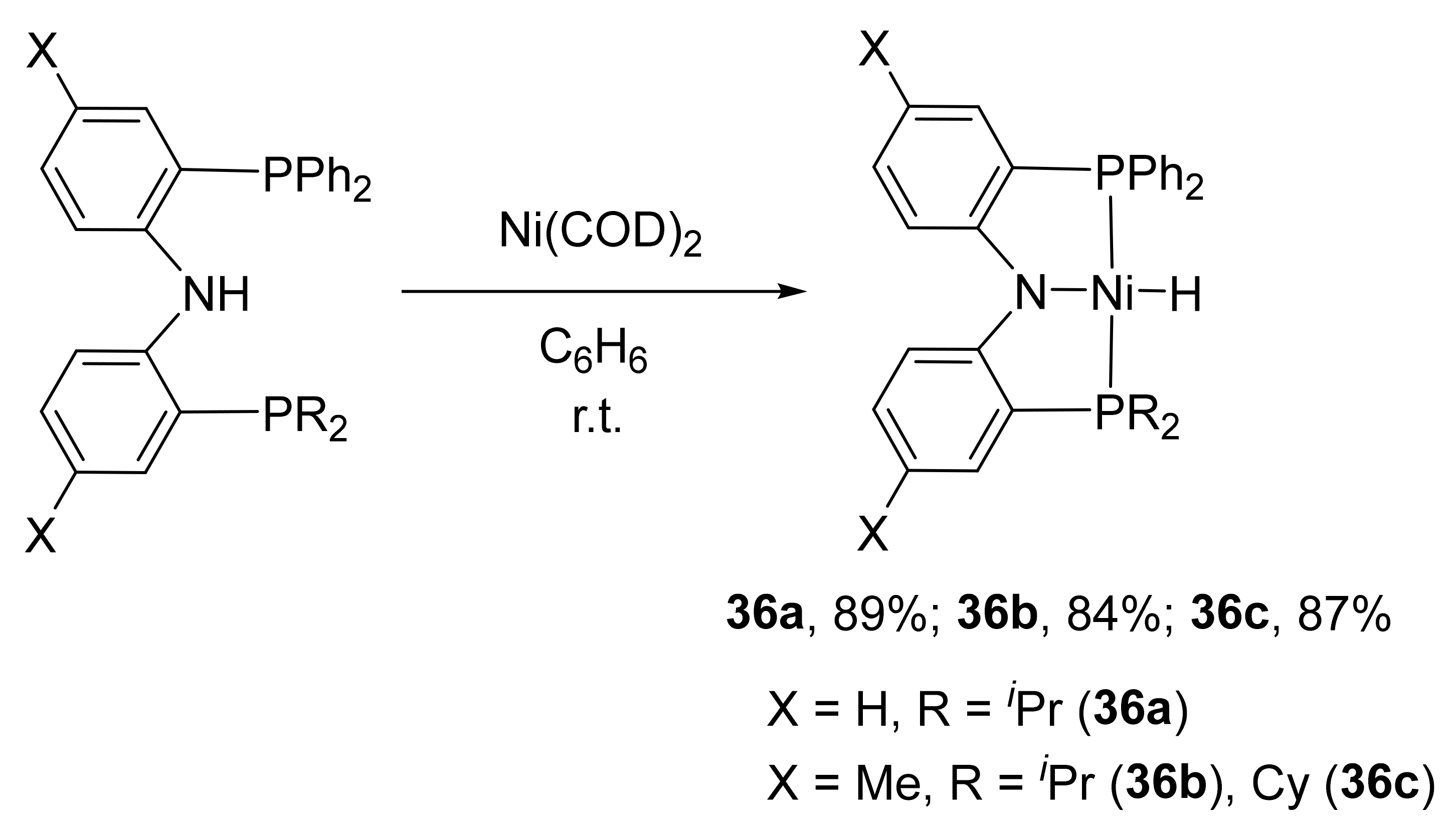
6. PNP’ Complexes
The unsymmetrical
RPNP
R′ pincer hydride nickel complexes (
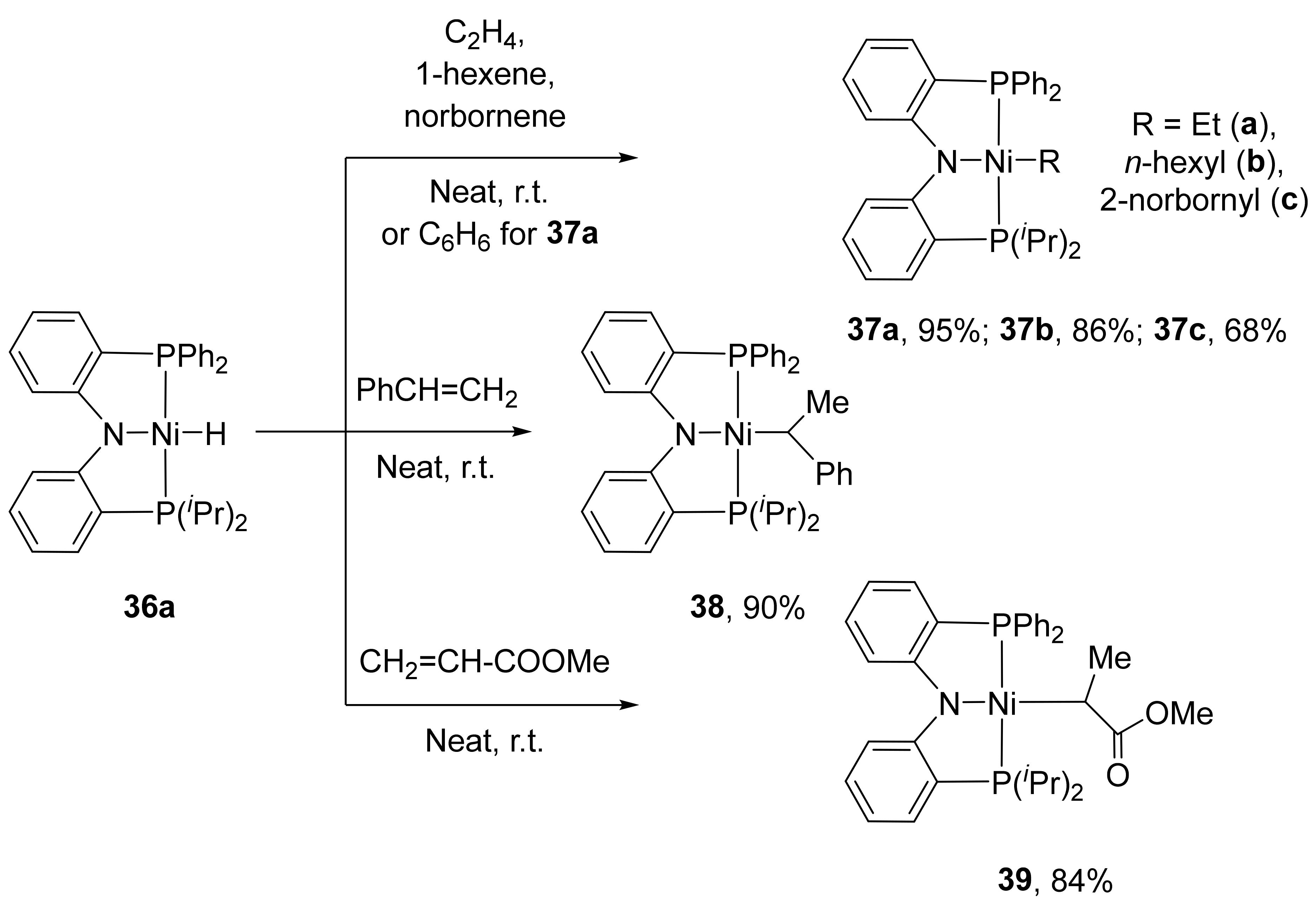
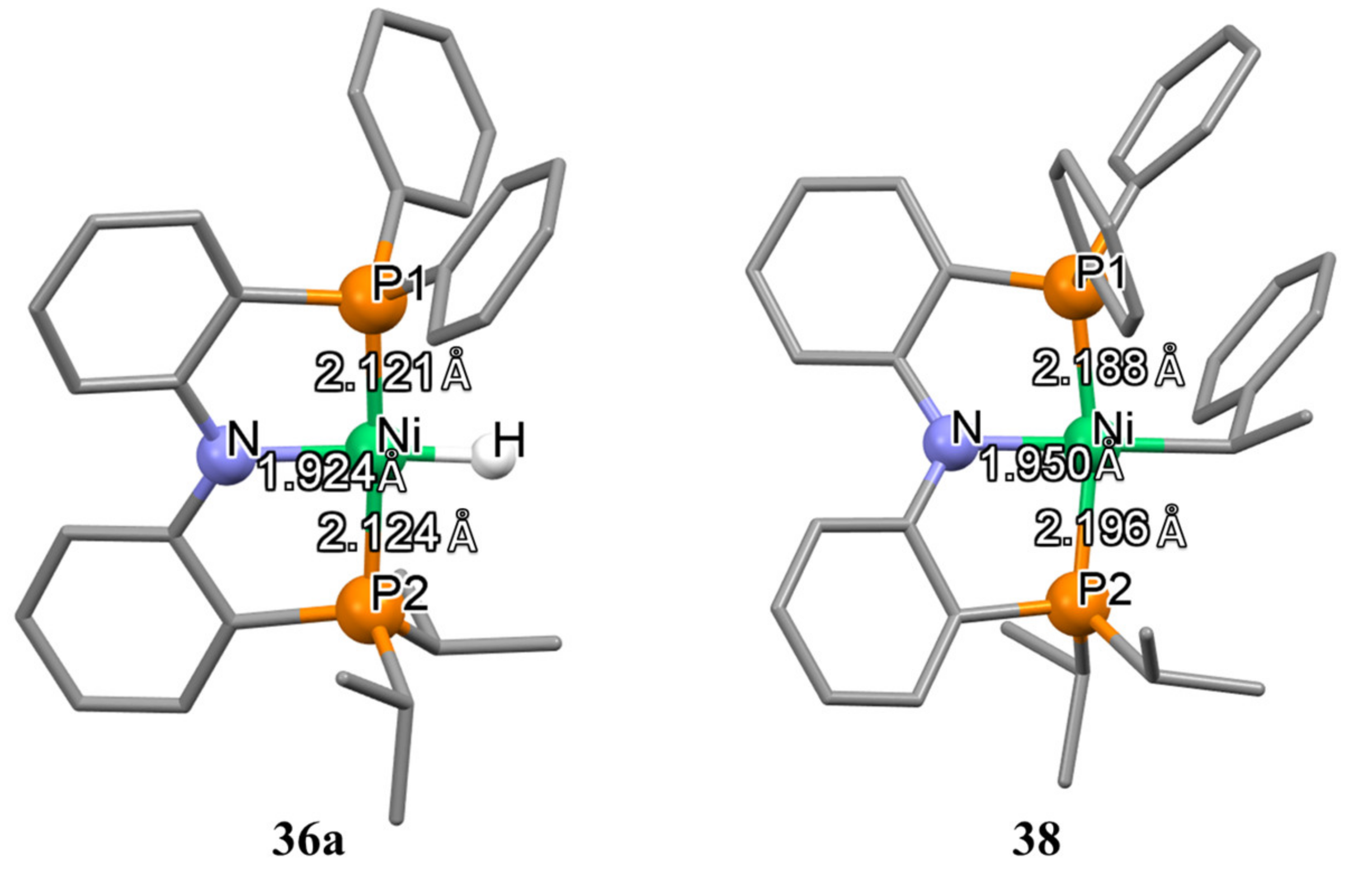
36a–
36c) containing various substituents at the phosphorus atom (R = Ph, R′ =
iPr, Cy) are also known in the literature. These complexes can be obtained by direct interaction of the corresponding
RPNP
R′ ligand with the zero-valent metal precursor Ni(COD)
2 with a yield of 84–89% (
Scheme 18) [
130,
131].
It was found that these complexes are capable of activating small molecules, such as O
2, CO, olefins and some others [
130,
131,
132,
133]. The reaction includes the insertion of the substrate into the Ni-H bond. For example, complex
36a reacts with olefins such as ethylene, 1-hexene, norbornene and styrene to generate complexes
37a–
37c,
38 as thermally stable solids (
Scheme 19) [
131]. In contrast to the selective 1,2-insertion found for 1-hexene, styrene inserts into the Ni-H bond of
36a in an exclusively 2,1-manner. In addition,
36a reacts with electronically activated olefins such as methyl acrylate affording product
39.
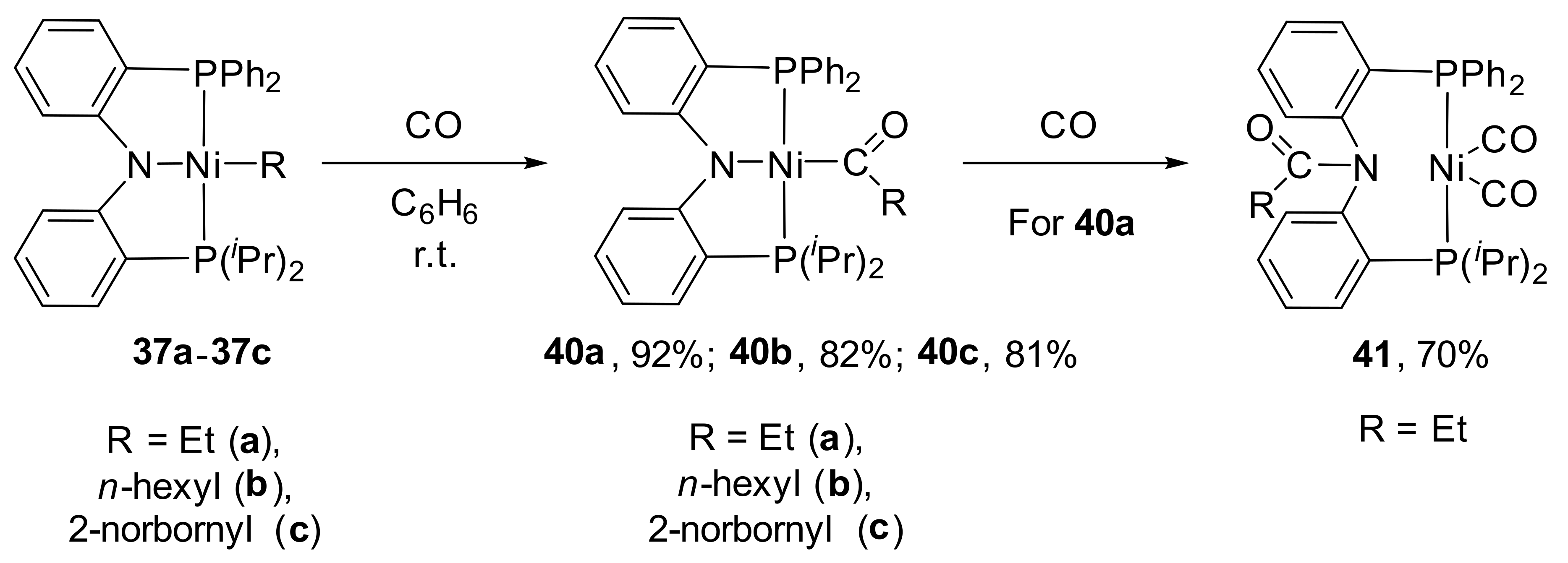
It should be noted that authors described the reactivity of obtained complexes
37a–
37c toward carbon monoxide. It was found, that migratory insertion of CO into the Ni-R bonds affords Ni(II)-acyl derivatives
40a–
40c (
Scheme 20) [
133].
Interestingly, further carbonylation of acyl compound 40a leads to generation of nickel zero complex 41 as a result of the C−N bond-forming reductive elimination whereas no reaction occurs for 40b and 36c under similar conditions. At the same time, in the presence of carbon monoxide, hydride complex 34 undergoes exclusively N−H bond-forming reductive elimination to generate zero-valent nickel dicarbonyl derivatives.
7. N-Heterocyclic Carbene Complexes
N-Heterocyclic carbenes (NHCs) are isolobal with electron-rich phosphines and they are often considered as phosphine ligand alternatives [
134]; therefore, unsymmetrical pincer nickel complexes bearing NHC groups are also discussed herein. These carbenes show low toxicity and strong σ-donating properties easily tunable by varying the substituents at the nitrogen atom and known to exert electronic and steric effects. Due to these unique properties, NHCs are a versatile and indispensable class of ligands applied in coordination chemistry and homogeneous catalysis by transition metal complexes. Consequently, NHC-based C donors have been commonly employed in symmetrical-type ligands. NHC-containing unsymmetrical pincer-type ligands have also been reported by adding different donors to the pincer architectures.
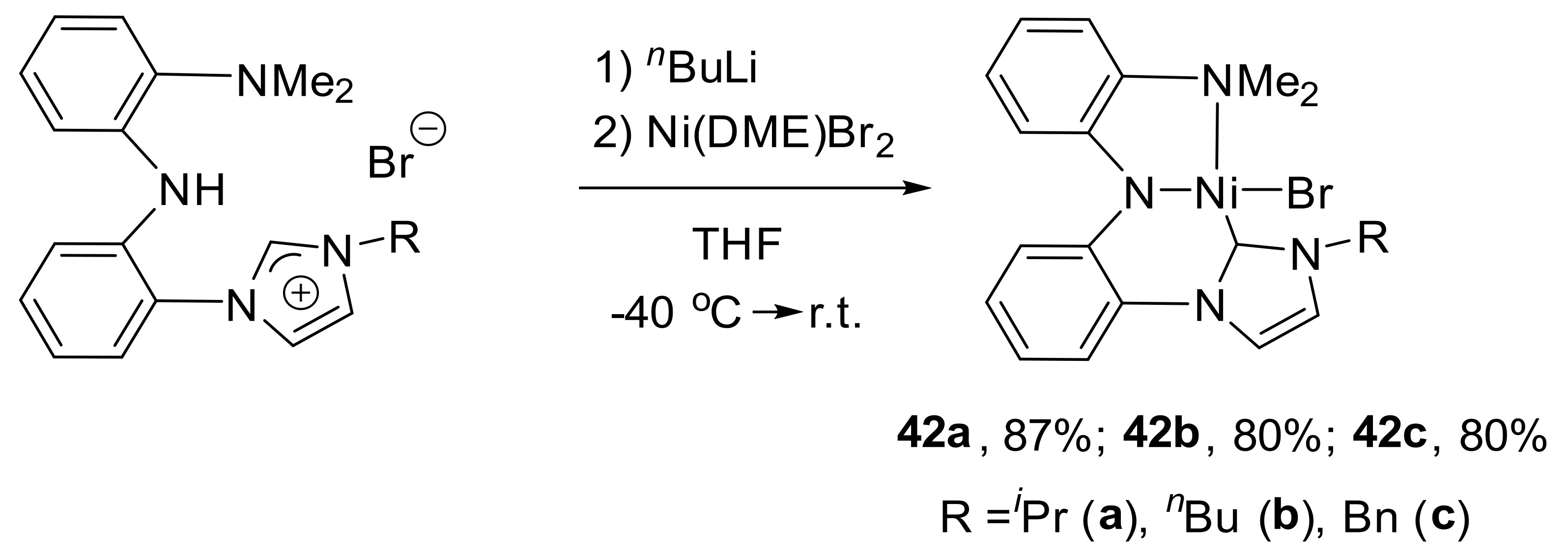
As a very good example of unsymmetrical pincer complexes of nickel with NHC-moiety, Sun and co-workers reported the preparation and characterization of the CNN-pincer complex
42a–
42c (
Scheme 21) [
135]. Complexes were synthesized via the lithiation of related pincer ligands and transmetalation by Ni(DME)Br
2 precursor.
Obtained complexes showed high catalytic activity in Kumada cross-coupling reactions of aryl chlorides or dichlorides with aryl Grignard reagents under mild conditions as well as in Sonogashira coupling reactions of aliphatic halides with terminal alkynes [
136].
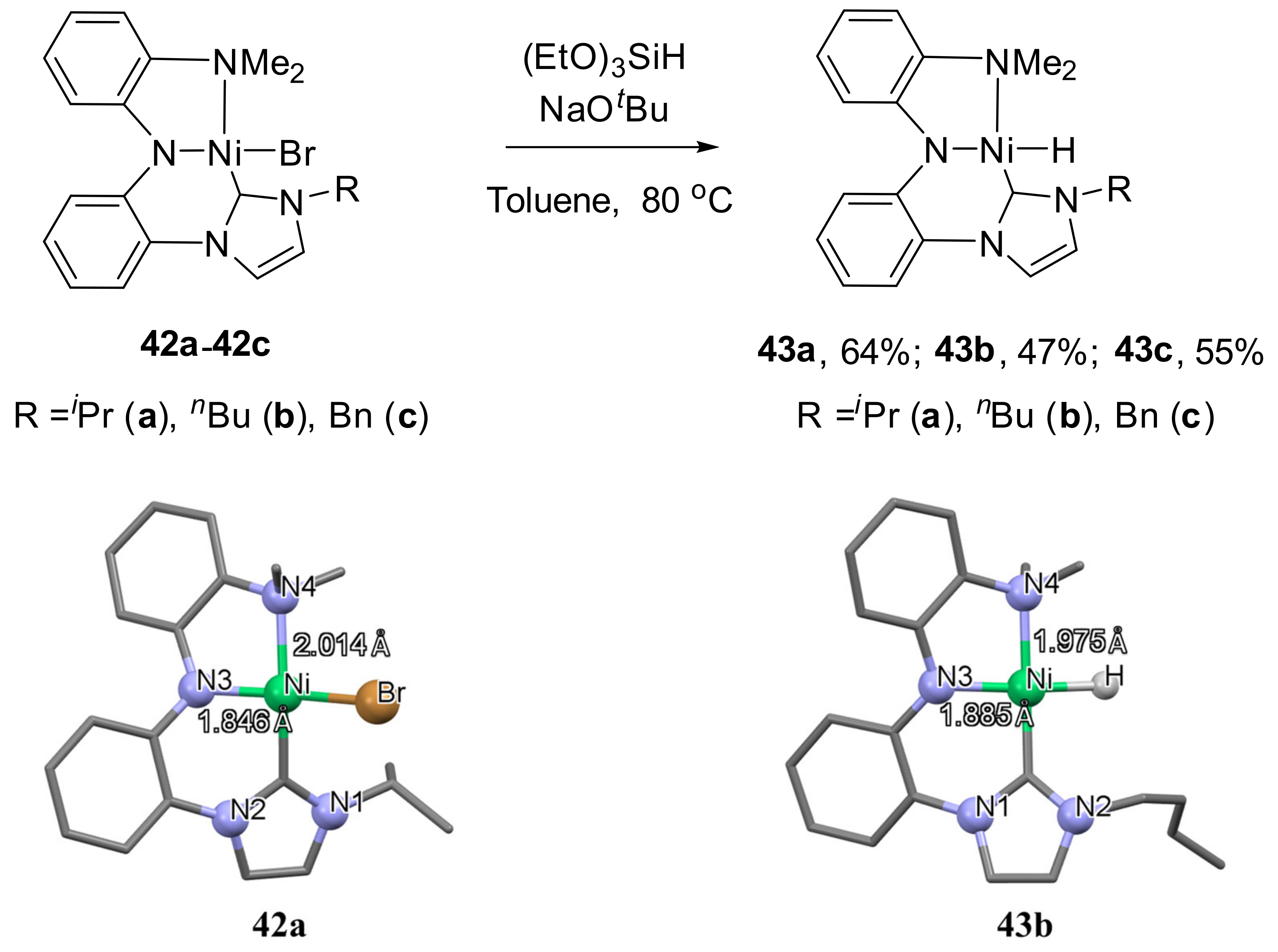
It has also been confirmed that the hydrogenation of nickel halides
42a–
42c in the presence of (EtO)
3SiH and NaO
tBu as a base leads to the formation of hydrido nickel complexes
43a–
43c (
Scheme 22) [
137]. According to the X-ray diffraction analysis of
42a and
43b, the Ni−N(amine) bond distance is remarkably longer than the Ni−N(amide) due to the strong trans influence of the carbon atom of the NHC coordination moiety. Complex
43a exhibited high catalytic activity in the hydrodehalogenation of organic halides.
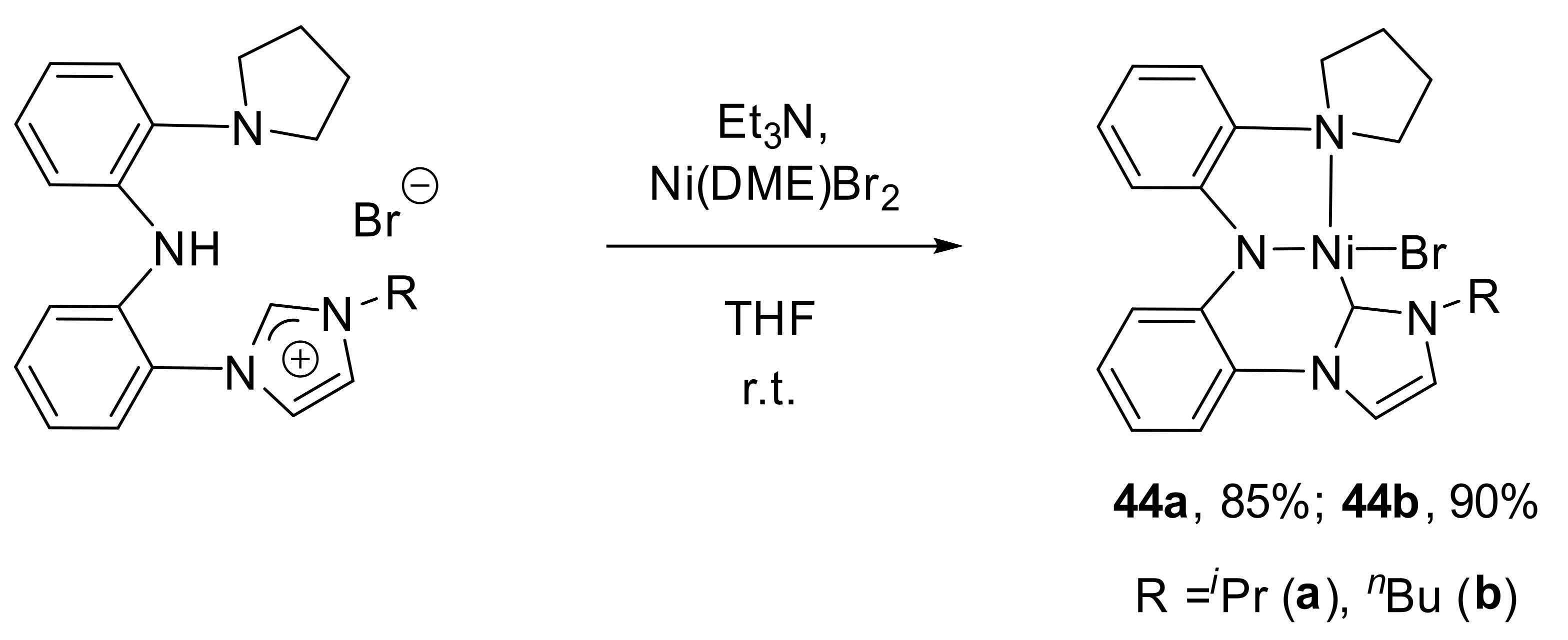
Later, authors introduced a pyrrolidine group, instead of a dimethyl amino group to the pincer ligand structure and synthesized complexes
44a and
44b by the direct cyclometalation of the corresponding ligand precursor in the presence of Et
3N (
Scheme 23) [
138]. Interestingly, the yields of
44a and
44b were higher (85 and 90%, respectively) than the yields of
42a and
42b (80–87%).
Complexes 42a, 42b, 44a and 44b were examined in transfer hydrogenation of ketones in the presence of a base (NaOtBu or KOtBu). The results show that the catalytic activity follows the order 44a > 42a > 44b > 42b for acetophenone hydrogenation. Authors considered that the strong electron-donating properties of the pyrrolidine and iso-propyl group in complex 44a made the metal center electron-rich. Authors also proposed the hydrido nickel complex to be the intermediate of this process, whose electron-rich property would increase the activity of the Ni-H bond. Thus, hydrido complex 43a was tested as the catalyst to explore the mechanism of the interaction. Indeed, it was found that complexes 42a, 42b, 44a and 44b were catalyst precursors, which generate the corresponding catalytically active hydrido complexes.
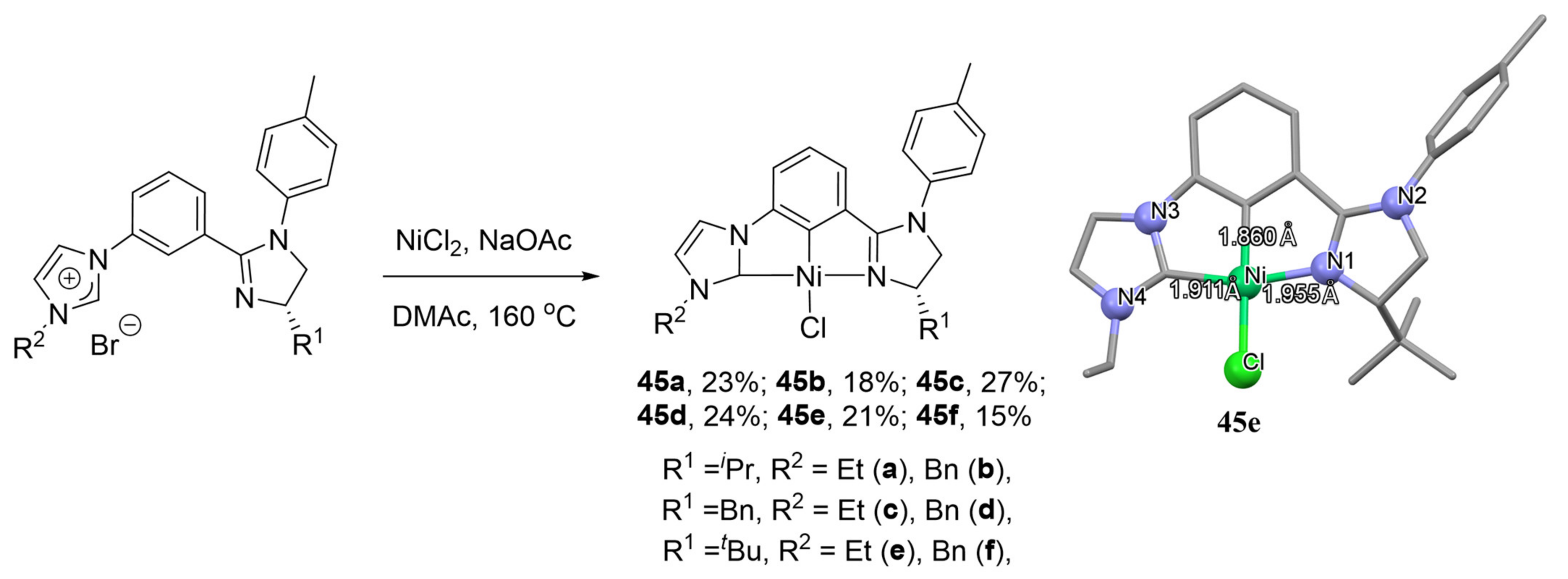
It is also interesting to note the synthesis of the NHC pincer complexes of nickel with both unsymmetrical and chiral properties. The cyclometalation was carried out via direct C−H bond activation in the presence of NaOAc to form corresponding complexes
45a–
45f (
Scheme 24) [
139]. However, the yields of
45a–
45f were in the range of 15–27%, and they did not find their place in catalysis yet. However, their palladium analogues showed moderate stereoselectivities in the asymmetric Friedel−Crafts alkylation.
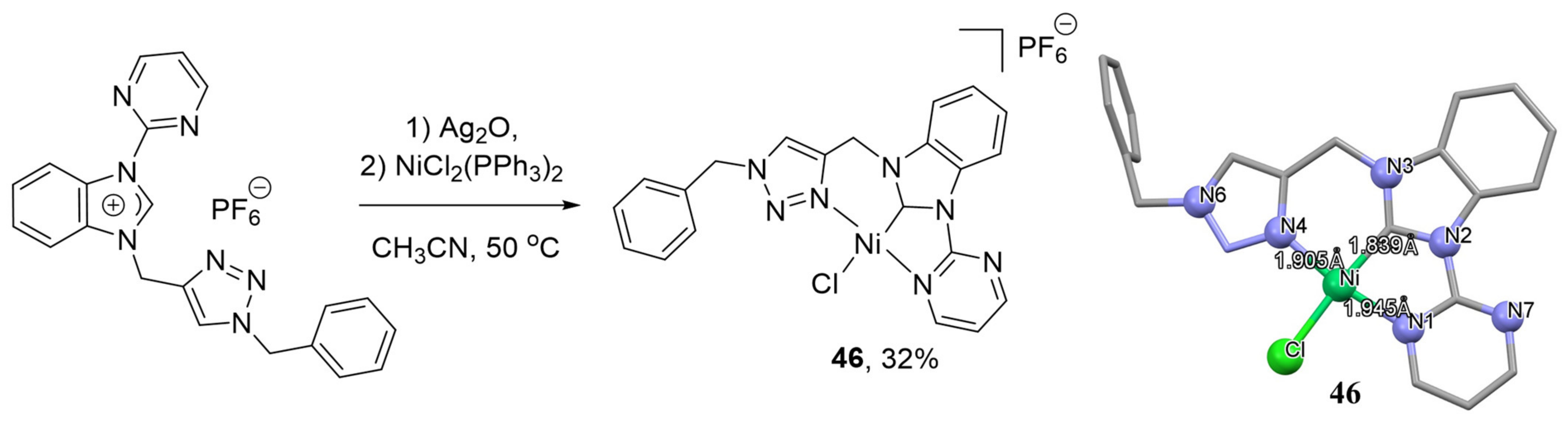
It is also worth mentioning the unsymmetrical nickel complex with NHC-triazole arms, synthesized by the reaction of a ligand precursor with Ag
2O with in situ formation of a silver NHC complex, which was transmetalated by [NiCl
2(PPh
3)
2] to provide desired complex
46 (
Scheme 25) with 32% yield [
140]. Complex
46 was found to be highly active in the Suzuki–Miyaura cross-coupling reactions of aryl bromides or aryl iodides with phenylboronic acid at 110 °C.
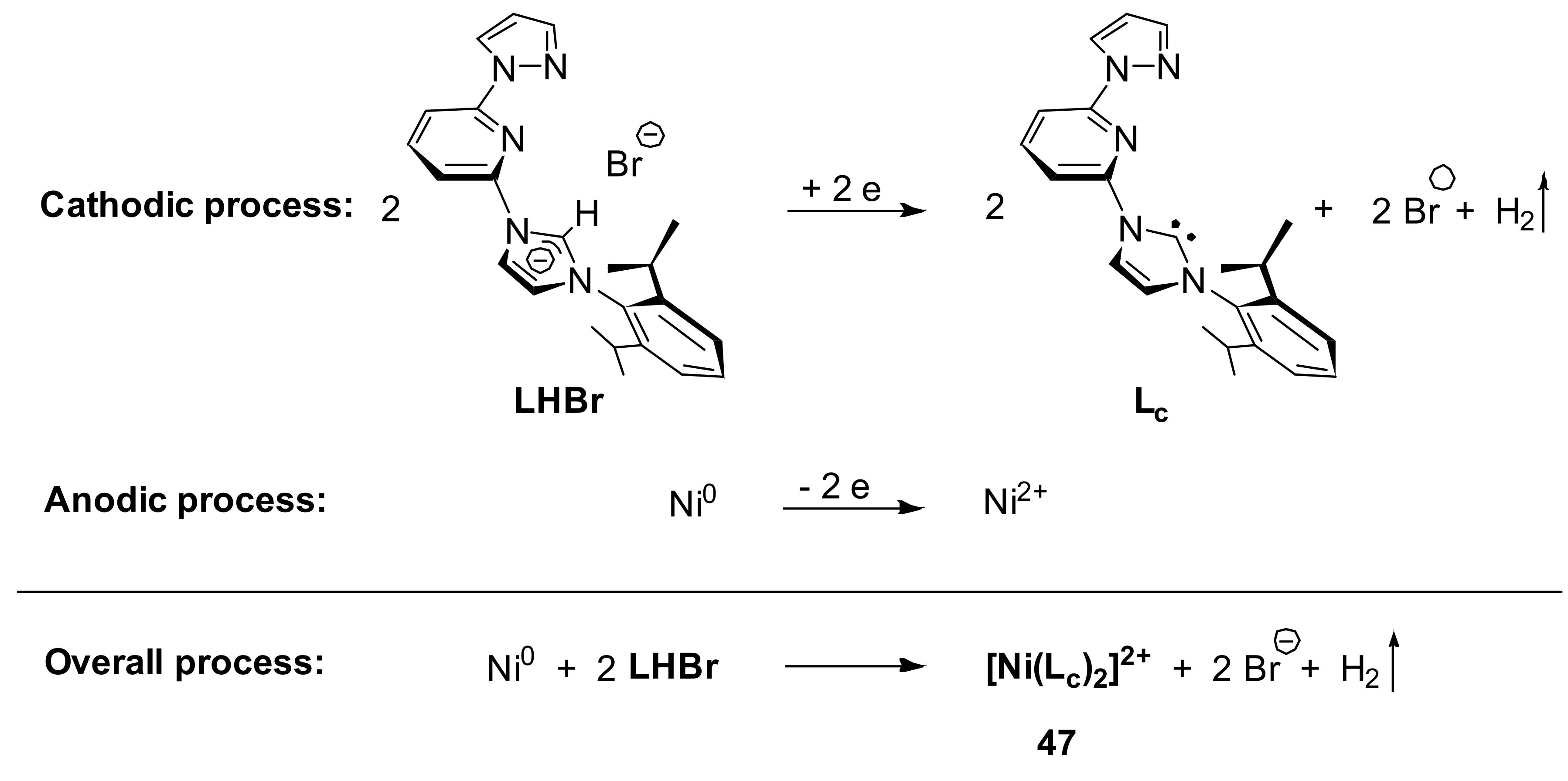
The electrochemical generation of NHC containing unsymmetrical pincer nickel complex
47 has been described by some of us (
Scheme 26) [
141,
142]. It was found that cathodic reduction of the imidazolium salt (LHBr in
Scheme 26) in the presence of nickel (II) ions leads to the formation of
N-heterocyclic carbene complexes of nickel (II) with the molecular hydrogen as the only byproduct. Interestingly, the imidazolium salt plays a role of both NHC source and electrolyte, while the source of nickel ions is the nickel plate.
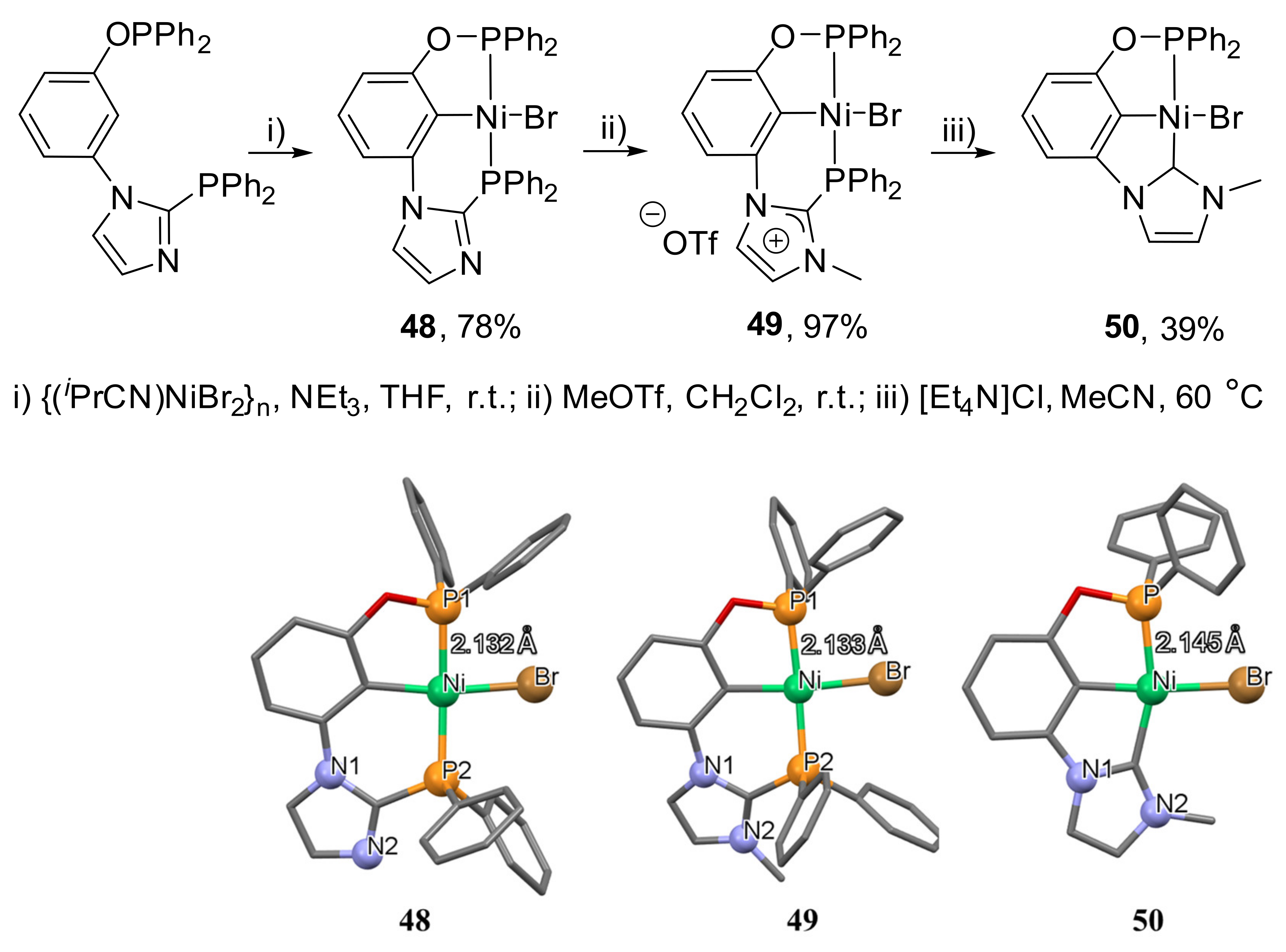
Vabre et al. demonstrated the synthesis of nickel complexes with PIMCOP, PIMIOCOP and NHCCOP ligands (
Scheme 27) [
143]. PIMCOP complex
48 was obtained by nickelation of an unsymmetrical meta-phenylene-based ligand bearing phosphinite and imidazolophosphine groups.
N-methylation of the latter generates a new PIMIOCOP complex
49 with NHC moiety, which can be converted subsequently into NHCCOP complex
50 (
Scheme 27). Interestingly, the Ni-P distance in
50 is much longer (2.145 Å) compared to the related Ni-P bond of
48 and
49 (2.132 and 2.133 Å). These indicate the much stronger trans influence of the NHC moiety compared to the phosphine group.
8. Other Types of Complexes
Other types of unsymmetrical pincer nickel complexes are also known in the literature. For example, phosphinito-thiophosphinito PSCOP complex
51 (
Scheme 28), which was obtained by cyclometalation of the related ligand by NiCl
2 [
144]. The X-ray diffraction analysis showed that the nickel center is located in a slightly distorted square-planar geometry. PSCOP complex
51 demonstrated a good catalytic activity and selectivity in C-S couplings of iodobenzene with different disulfides.
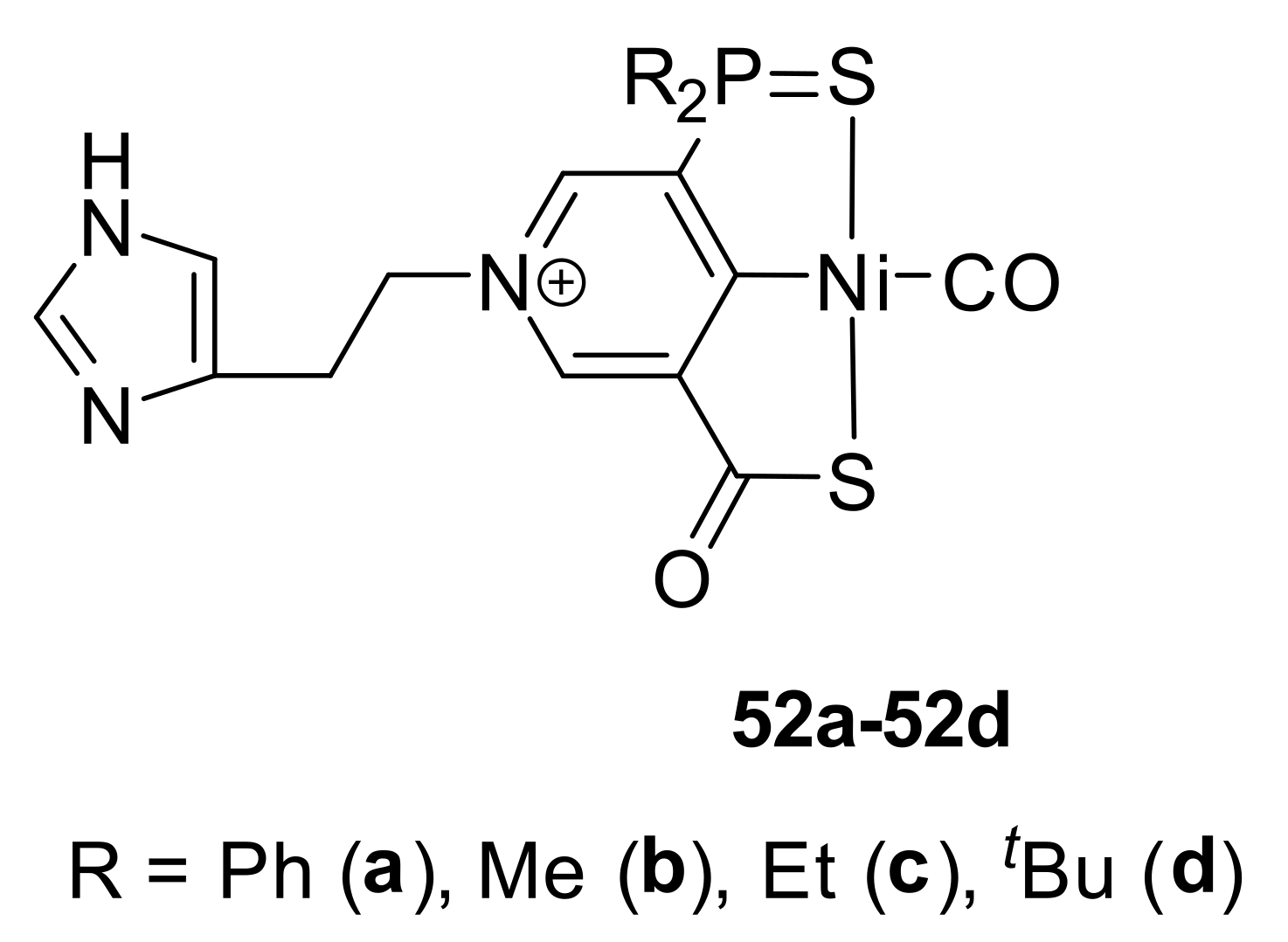
Other sulfur-containing nickel complexes are SCS-type pincers. Interestingly, SCS-ligand-based nickel pincer nucleotide (lactate racemase) is the first enzyme known to contain a pincer metal complex as a cofactor [
145]. This enzyme converts L-lactic acid into D-lactic acid. The mechanisms of this transformation have been studied using DFT calculations. The most probable pathway for proton-coupled hydride transfer involves the intermediate formation of an unsymmetrical complex [
146]. Inspired by the structures of the active site of lactate racemase, similar scorpion-like SCS nickel complexes were proposed, and their potentials for catalytic hydrogenation of CO
2 [
147] and for the asymmetric transfer hydrogenation of 1-acetonaphthone (the phosphorus-based complexes
52a–
52d in
Figure 9) [
148] were computationally predicted.
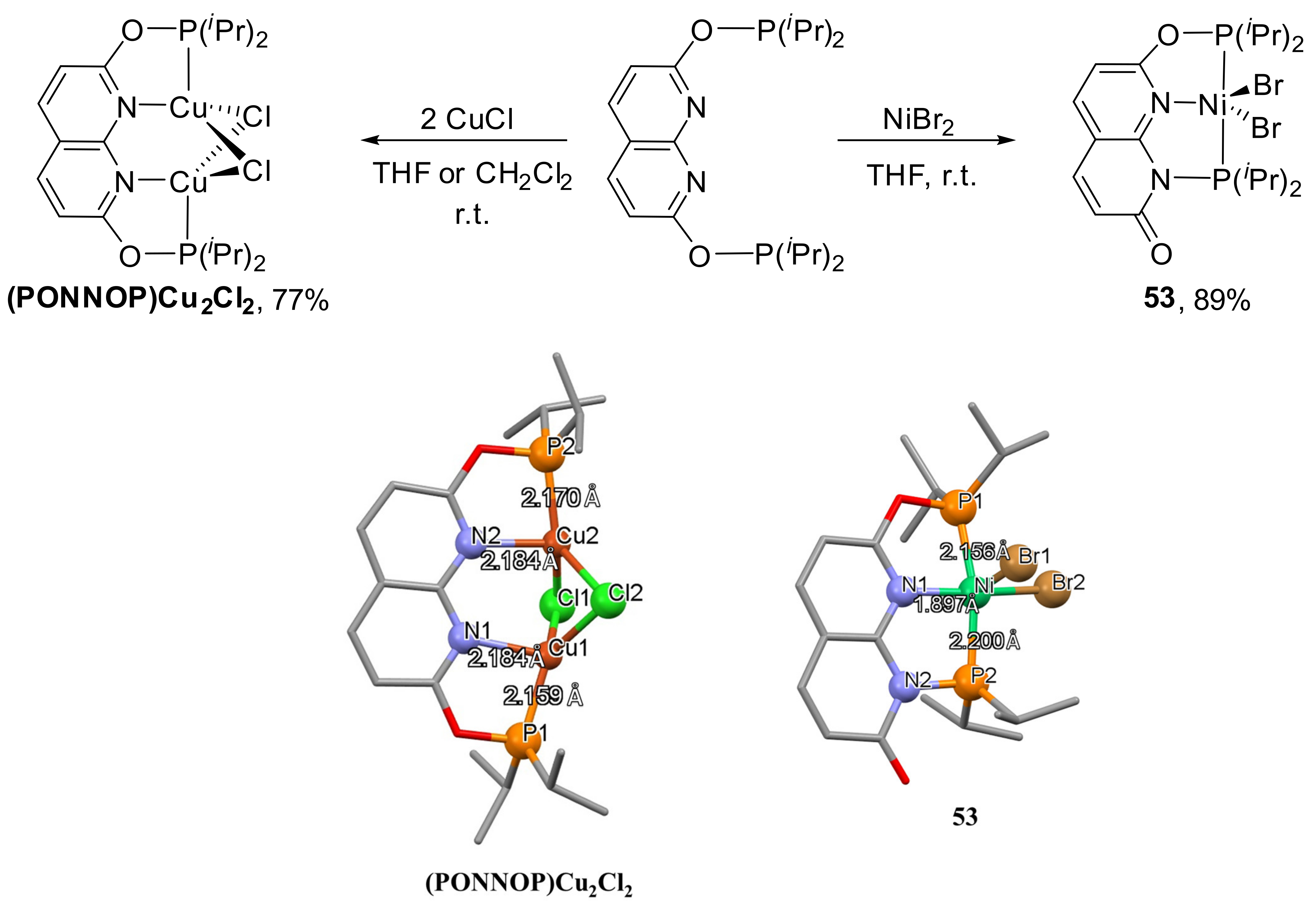
It is also interesting to mention the phosphinite PONNOP expanded pincer ligand, which undergoes an unexpected rearrangement in the presence of nickel (II) species to form an unusual PONNP complex
53 (
Scheme 29) [
149]. Interestingly, the reaction of the PONNOP ligand with copper chloride did not lead to the rearrangement but to a binuclear PONNOP copper complex. The performed DFT calculations confirmed that PONNOP and PONNP free ligand isomers are similar in energy and have a high barrier for their interconversion. However, authors concluded that the P atom, which migrates from O to N, is bound to the transition metal atom, which provides the additional stabilization of the transition state to enable the isomerization toward the more stable PONNP pincer motif.
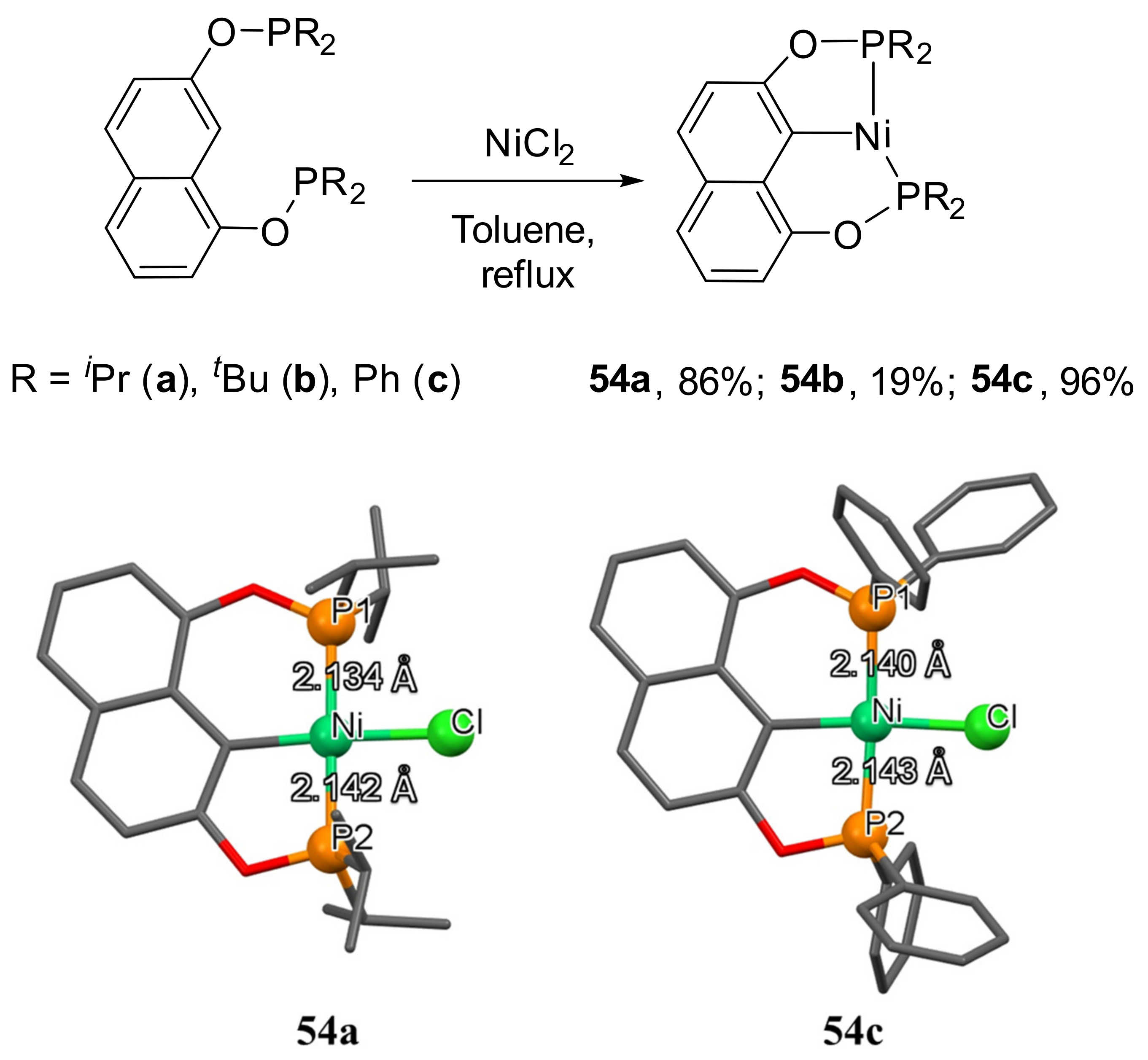
More recently, the synthesis of a series of unsymmetrical Ni(II)-POCOP pincer complexes (
54a–
54c) derived from 1,7-naphthalenediol were described (
Scheme 30) [
150]. The molecular structures of two complexes were determined by single-crystal X-ray diffraction analysis. Interestingly, the Ni-P distances were almost identical, despite the size of the metallocycle. For example, for
54a, the M-P
1 bond length was 2.1340 Å, while that for the M-P
2 distance was slightly longer (2.1417 Å). These complexes were examined as catalysts in Suzuki–Miyaura C-C cross-coupling reactions. However, the results are not as good as those obtained with their analogous POCOP pincer derivatives of 1,3-naphtalene diol [
151]. Complex
54c, bearing a more sterically demanding – PPh
2 group, was the most efficient catalyst of the series, reaching 53% yield of the biphenyl product.
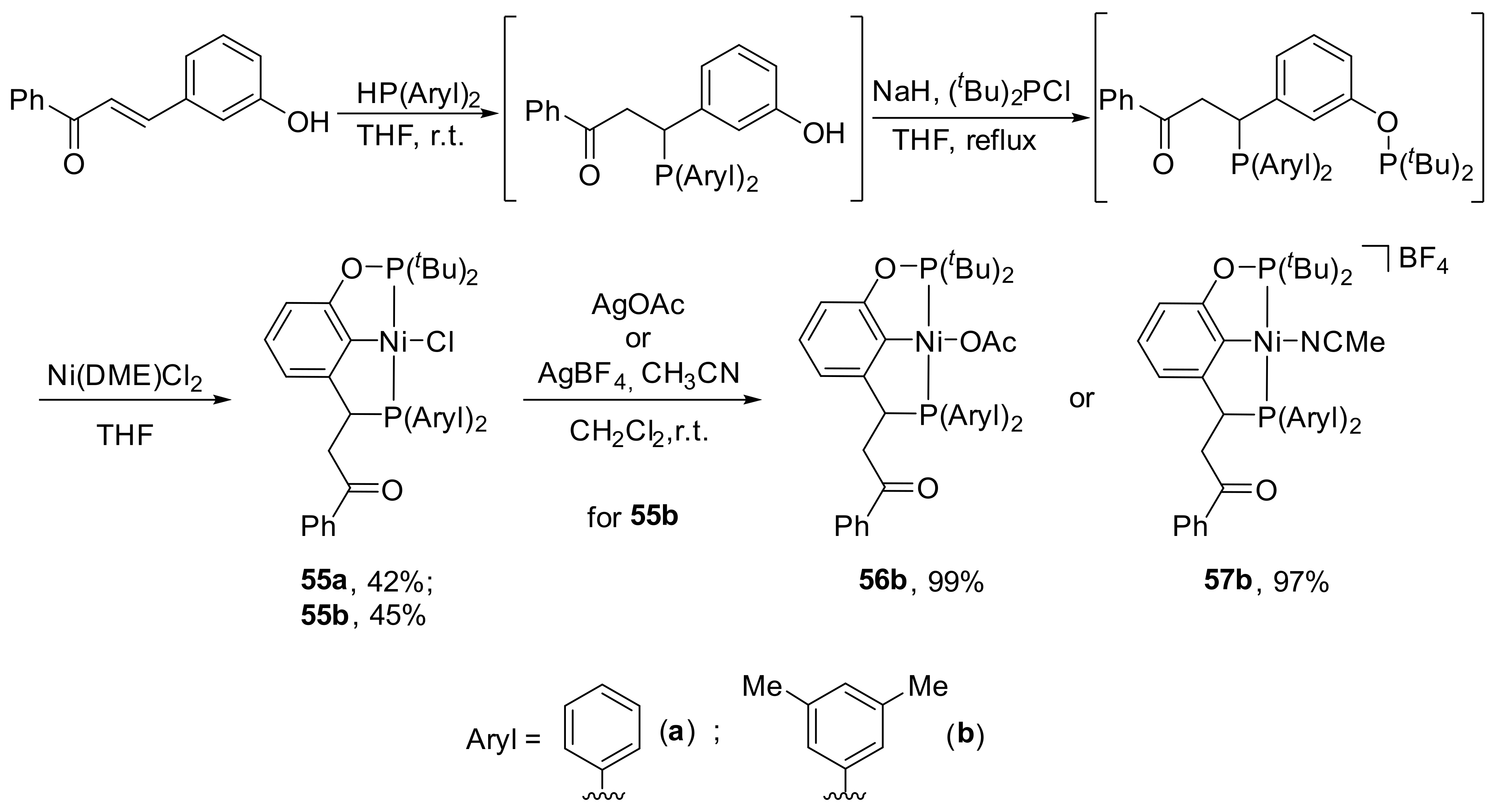
Very recently, Wang et al. demonstrated the synthesis of unsymmetrical PCP′ pincer nickel complexes bearing a phosphine-phosphinite ligand with a stereogenic carbon center [
152]. The corresponding enantiopure nickel complexes
55a and
55b were obtained via a convenient one-pot three-step reaction (
Scheme 31). Moreover, the authors showed the possibility of the replacement of the chloride ligand in
55b by the action of silver agents AgOAc or AgBF
4 with the formation of a neutral complex
56b or a cationic complex
57b (
Scheme 31). The crystal structures of complexes
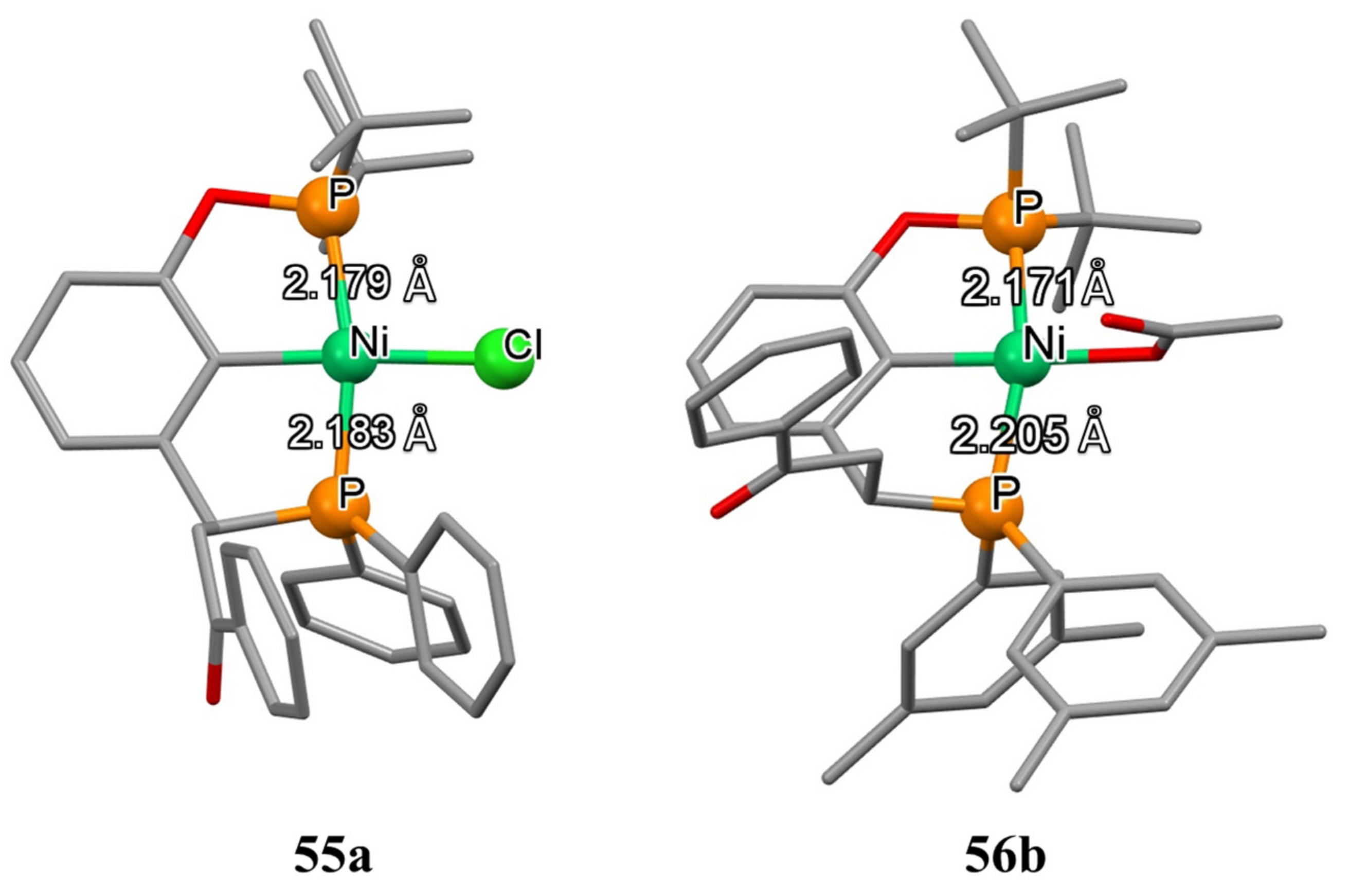
55a and
56b were determined by X-ray diffraction analysis (
Figure 10), which revealed that, in
55a, atoms of phosphorus lie on the same plane defined by the [PCP’Cl] atoms. Meanwhile, 3,5-methyl substituents and –OAc group in
56b lead to the distortion of the ligand in which the phosphine moiety is not located in the same plane [PCP’Cl].
Authors applied these newly developed nickel pincer complexes for the highly enantioselective catalytic synthesis of P-stereogenic secondary phosphine-boranes by asymmetric addition of primary phosphine to electron-deficient alkenes. The resulting products may be easily converted into useful chiral phosphine ligands for asymmetric transformations. Interestingly, the nickel-based catalysts demonstrated the improved enantio- and diastereoselectivity to 94% ee and 13:1 dr (for complex
55b, entry 2) compared to palladium-based analogues (59% ee with low dr). While Ni-OAc complex
56b exhibited similar catalytic activity compared to
55b (entry 3,
Table 6), in contrast, the cationic complex
57b did not catalyze the reaction at all (entry 4,
Table 6). Moreover, authors revealed that CH
2Cl
2 as a solvent and temperature of −40 °C is the best combination (entries 8–11,
Table 6) compared to other conditions (entries 5–7,
Table 6). Control experiments confirmed that both the catalyst and the base were necessary for product formation (entries 10 and 11,
Table 6).
According to the proposed by the authors catalytic cycle for 55b, catalyzed synthesis of secondary phosphine-boranes includes the formation of Ni-OAc (56b) complex by the ligand exchange reaction, followed by the transphosphination step between diphenylphosphine and formed complex, which leads to a nickel phosphide intermediate. Then, the nucleophilic phosphorus addition to the β-position of enone affords a nickel phosphine complex, which, finally, reacts with HOAc, formed at the transphosphination step, and releases the product as well as regenerates the active catalyst.
9. Conclusions
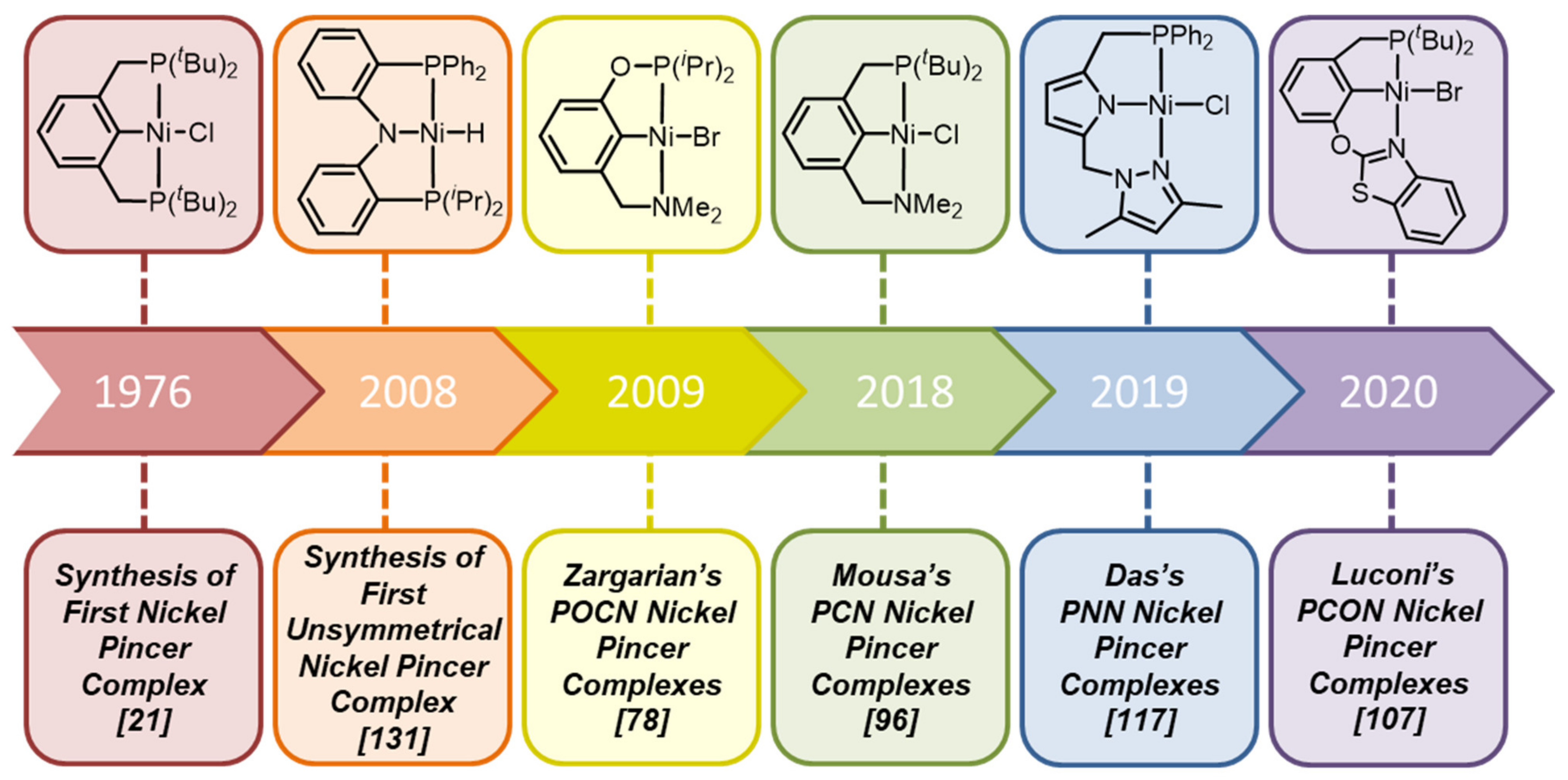
In summary, the chemistry of unsymmetrical P-based nickel pincer complexes is young and started its way only in 2008, which is 32 years later after the synthesis of the first symmetrical nickel pincer complex (
Figure 11). However, the number of publications in this area has increased dramatically in recent years. Research gains new unprecedented complexes with unexpected properties in the past few years. The development in this field seems to be very promising [
109,
124,
125,
153,
154].
As follows from the review, unlike their symmetric NCN analogues, unsymmetrical PCN complexes can be obtained by direct metalation with activation of the C-H bond, and, unlike PCP analogues, they can be oxidized with bromine to form stable nickel (III) complexes. Thus, unsymmetrical P- and N- based nickel complexes combine the unique properties of both classes of ligands. Moreover, POCN complexes turned out to be active catalysts in the processes of functionalization of acrylonitrile and asymmetric Suzuki–Miyaura cross-coupling reaction, while the catalytic efficiency of nickel pincer complexes in the Kharasch addition follows the order: NCN > POCsp3OP > PCN > POCN. The utilization of the PCN scaffold can be efficient for the ethylene oligomerization process. The catalytic efficiency of nickel pincer complexes in the cross-coupling of aryl fluorides with aryl Grignard reagents follows the order: NNP > ONP > ONN > NNN, demonstrating the importance of the presence of the P atom in ligand design. Meanwhile, the asymmetry in NNP scaffold causes higher catalytic activity in the polymerization of norbornene compared to symmetrical PNP analogues. Unsymmetrical PNP’ nickel hydrides react with olefins such as ethylene, 1-hexene and norbornene and styrene to generate corresponding Ni–R derivatives. Meanwhile, NHC-based analogues of P-based nickel pincers turned out to be active catalysts in Kumada, Sonogashira and Suzuki–Miyaura cross-coupling reactions, as well as in asymmetric Friedel−Crafts alkylation and transfer hydrogenation of ketones. Unsymmetrical PCP’ scaffold is particularly interesting since complexes based on it have been successfully applied for highly enantioselective catalytic synthesis of P-stereogenic secondary phosphine-boranes.
The steric and electronic effects of ligands have a significant effect on the catalytic properties of the complexes formed by them. Therefore, the design of the ligand frameworks and their combination with the metal play crucial roles in the development of highly active catalysts. In this context, the use of such donor atoms as phosphorus and nitrogen for the development of pincer ligands and nickel complexes, which have the unique properties in both stoichiometric and catalytic reactions, is a very promising task of modern organometallic chemistry. In such species, the difference in the trans effect between the two different donor arms provides the hemilability of the ligand since the group with the weaker trans effect (N) is more likely to dissociate from the metal center, which leads to a vacant coordination site at the metal center and thus can allow for effective coordination and transformation of substrate molecules in the homogeneous catalysis conditions. Thus, phosphorus- and nitrogen-based pincer nickel complexes demonstrated high activities in such processes as the formation of a new carbon–carbon bond (Heck, Suzuki–Miyaura reactions), hydrogenation of aryl and alkyl ketones, alkane dehydrogenation, carboxylation, hydrosilylation, oligo- and polymerization of unsaturated hydrocarbons and others; moreover, unsymmetrical pincer complexes showed higher catalytic activity than their symmetrical analogues. However, to improve the catalytic activity of the complexes bearing unsymmetrical pincer ligands, the strategies for regulating hemilability of coordination site and flexibility of ligand backbone need to be deeply explored.