Abstract
A number of plants used in folk medicine in Thailand and Eastern Asia are attracting interest due to the high bioactivities of their extracts. The aim of this study was to screen the edible leaf extracts of 20 plants found in Thailand and investigate the potential neuroprotective effects of the most bioactive sample. The total phenol and flavonoid content and 2,2-diphenyl-1-picrylhydrazyl radical-scavenging activity were determined for all 20 leaf extracts. Based on these assays, Glochidion littorale leaf extract (GLE), which showed a high value in all tested parameters, was used in further experiments to evaluate its effects on neurodegeneration in Caenorhabditis elegans. GLE treatment ameliorated H2O2-induced oxidative stress by attenuating the accumulation of reactive oxygen species and protected the worms against 1-methyl-4-phenylpyridinium-induced neurodegeneration. The neuroprotective effects observed may be associated with the activation of the transcription factor DAF-16. The characterization of this extract by LC-MS identified several phenolic compounds, including myricetin, coumestrin, chlorogenic acid, and hesperidin, which may play a key role in neuroprotection. This study reports the novel neuroprotective activity of GLE, which may be used to develop treatments for neurodegenerative diseases such as Parkinson’s syndrome.
1. Introduction
Neurodegenerative disorders including Alzheimer’s disease and Parkinson’s disease (PD) pose major health and financial concerns to global health care organizations [1]. Although the human lifespan has increased in the last few decades in industrialized countries, the prevalence of age-related diseases has also increased. The incidence of late-onset disorders such as neurological disruptions is expected to increase rapidly over the next few decades. Therefore, it is crucial to encourage studies and perform clinical trials on compounds that may have the potential to cure, prevent, or at least delay the onset of neurodegenerative diseases [2]. One of the characteristic features of PD is the progressive loss of dopaminergic (DA) neurons in the substantia nigra [3]. In PD pathogenesis, increased production of reactive oxygen species (ROS) plays a key role in the loss of DA cells [4]. Therefore, the reduction in oxidative stress is considered a promising therapeutic approach in PD treatment [5]. The 1-methyl-4-phenylpyridinium (MPP+), which inhibits mitochondrial complex I activity, can induce PD-like symptoms in humans and animal models [6].
The use of Caenorhabditis elegans as an in vivo model provides certain advantages in the study of PD [7]. The nematode is simple, inexpensive, and has a short life cycle. It supports studies involving large-scale analyses. Moreover, the neuronal network of C. elegans has been mapped completely. It contains 8 DA neurons and PD-related homologous genes [8]. Neurodegeneration, which mimics parkinsonian symptoms, can be induced in C. elegans via treatment with neurotoxins such as MPP+ [9].
Natural antioxidant compounds represent attractive sources for developing drugs to treat neurodegenerative diseases due to their neuroprotective effects in animal models and low toxicity [3]. Polyphenols are known to be among the most abundant antioxidants in the human diet [10]. It has also been established that oxidative processes are involved in many pathologies, including neurodegeneration, cancer, diabetes, cardiovascular and anti-inflammatory diseases. Hence, finding polyphenols exhibiting antioxidant properties from natural sources could contribute toward preventing or treating those pathologies. This study focused on extracts from the edible leaves of plants found in Thailand. Most varieties cultivated widely in northern and southern Thailand have been used as folk medicine against general injuries and diseases; however, there are few reports concerning their neuroprotective effects.
In this study, we first screened the extracts of edible leaves from 20 plants cultivated in Thailand and assessed their phenolic and flavonoid contents and their 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging activity. The effects of Glochidion littorale leaf extract (GLE), which showed a high value in all tested parameters, were evaluated on C. elegans with neurodegeneration. Furthermore, the potential pathways involved in the neuroprotective effect of GLE were examined, along with the identification of the main components in GLE.
2. Results
2.1. Screening of Thai Plant Leaves
Crude extracts of edible leaves from plants cultivated in Thailand were prepared by ultrasonication. The leaf extracts of 20 plants were screened for their phenolic and flavonoid contents and antioxidant activity by DPPH radical-scavenging assay. Few of the tested samples, such as Glochidion sphaerogynum and Mentha piperita, were found to possess high radical-scavenging activity with low phenolic and flavonoid content, whereas certain samples, such as Clinacanthus nutans and Ocimum × citriodorum, exhibited the opposite trend (Table 1). The leaf extract of G. littorale showed high DPPH radical-scavenging activity as well as high phenolic and flavonoid content. Therefore, the bioactivities associated with G. littorale were further investigated.

Table 1.
Properties of the plants investigated in this study.
2.2. GLE Enhanced Resistance against Oxidative Stress via DAF-16 in C. Elegans
The effect of GLE on the survival of N2 worms under oxidative stress was investigated. Treatment with H2O2 (5 mM) induced 75% death in the control group, whereas co-treatment with 50 µg/mL and higher concentrations of GLE was associated with a high survival rate (Figure 1A). Among the tested concentrations of GLE, 100 µg/mL and 200 µg/mL were associated with the highest survival rates (82.0% and 88.2%, respectively). Therefore, these two concentrations were used in subsequent experiments. To evaluate the antioxidant effect of GLE in vivo, the intracellular ROS levels were measured in wild-type nematodes using 2’,7’-dichlorodihydrofluorescein diacetate (H2DCF-DA), a well-known fluorescence probe for detecting intracellular ROS production. Significant decreases in the fluorescence intensities in the GLE-treated groups were observed compared to that in the untreated group (Figure 1B), confirming the antioxidant property of GLE.
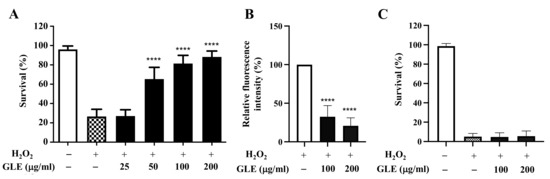
Figure 1.
Effect of Glochidion litorale leaf extract (GLE) on stress resistance in wild-type and daf-16 mutant Caenorhabditis elegans. (A) Effect of GLE against H2O2-induced toxicity in wild-type worms. (B) Intracellular reactive oxygen species (ROS) contents in wild-type worms. (C) Effect of GLE against H2O2-induced toxicity in daf-16 mutant worms. Experiments were performed in triplicate. Data are presented as mean ± standard deviation (SD). **** p < 0.0001 compared to H2O2-treated worms.
As the transcription factor DAF-16 is known to play a key role in regulating oxidative stress [11], it was hypothesized that GLE may target DAF-16. The C. elegans strain CF1038, which is a DAF-16 loss-of-function mutant strain, was used to determine the survival rate of worms treated with and without GLE. In H2O2-induced oxidative stress, GLE treatment did not increase the survival rate of transgenic worms (Figure 1C).
2.3. GLE Treatment Reduced the Lethality of MPP+-Induced DA Neurotoxicity via DAF-16 in C. Elegans
C. elegans possesses 8 DA neurons [8]. Selective degeneration of these DA neurons was evaluated after exposure to MPP+. The treatment of wild-type N2 worms with 0.75 mM MPP+ resulted in a remarkable decrease in survival (Figure 2). However, co-treatment with GLE significantly increased the survival of the worms. The effect of GLE treatment on daf-16 mutant worms was investigated. As shown in Figure 3 and Table 2, GLE treatment did not increase the survival of these worms after exposure to MPP+ compared to that in the control group. These results suggest that DAF-16 may be required for mediating the neuroprotective effect of GLE in C. elegans. Next, a DAF-2 loss-of-function mutant strain, CB1370, was used to determine whether DAF-2 was involved in the observed neuroprotective effects. As shown in Figure 4 and Table 3, the median and maximum survival significantly increased in daf-2 mutant worms treated with GLE.
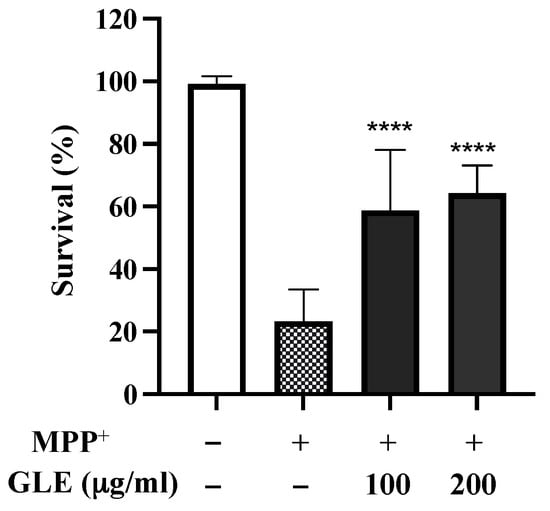
Figure 2.
Effect of GLE on 1-methyl-4-phenylpyridinium ion (MPP+)-induced neurotoxicity in N2 C. elegans. The effects of GLE (100 and 200 µg/mL) on MPP+-induced toxicity were evaluated. Experiments were performed in triplicate. Data are presented as mean ± SD. **** p < 0.0001 compared to MPP+-treated worms.
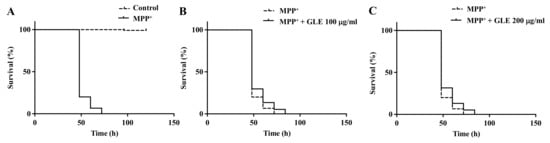
Figure 3.
Effect of GLE on MPP+-induced neurotoxicity in daf-16 mutant C. elegans. (A) Lifespan curve of worms in the presence or absence of MPP+. (B) Lifespan curve of worms with MPP+-induced toxicity treated with 100 µg/mL GLE. (C) Lifespan curve of worms with MPP+-induced toxicity treated with 200 µg/mL GLE. Each experiment was repeated independently at least thrice, and one of the representative data is shown.

Table 2.
Survival of daf-16 mutant C. elegans treated with MPP+.
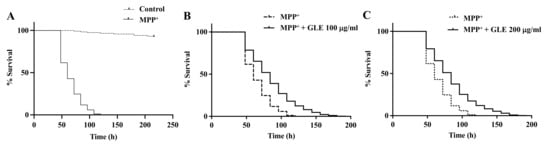
Figure 4.
Effect of GLE on MPP+-induced neurotoxicity in daf-2 mutant C. elegans. (A) Lifespan curve of worms in the presence or absence of MPP+. (B) Lifespan curve of worms with MPP+-induced toxicity treated with 100 µg/mL GLE. (C) Lifespan curve of worms with MPP+-induced toxicity treated with 200 µg/mL GLE. Each experiment was repeated independently at least thrice, and one of the representative data is shown.

Table 3.
Survival of daf-2 mutant C. elegans treated with MPP+.
2.4. Effects of GLE on DAF-16 Localization
It has been demonstrated that DAF-16 activation is regulated by its nuclear accumulation [12]. Subsequently, we investigated whether GLE could induce the nuclear accumulation of DAF-16 in a transgenic strain TJ356 that expresses a DAF-16::GFP fusion protein. Results showed that after 48 h of incubation with 100 µg/mL GLE, the green fluorescence intensity of DAF-16 in the nucleus increased significantly compared to that in the untreated group (Figure 5).
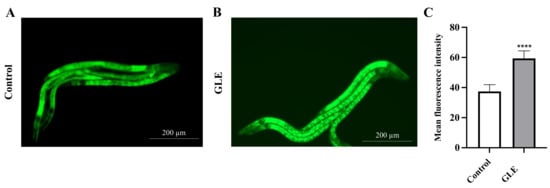
Figure 5.
Effect of GLE on DAF-16 localization. (A) Untreated worms. (B) Worms treated with 100 µg/mL GLE. (C) Quantification of DAF-16::GFP nuclear accumulation in GLE and GLE-free conditions. The scale bar shows 200 µm. Each experiment was repeated independently at least thrice. Significant differences were analyzed using the t-test method; **** p < 0.0001 as compared with control.
2.5. Phytochemical Characterization in GLE
LC-MS was conducted for profiling the phytochemicals in GLE, and its results are presented in Figure 6. The chromatographic peaks were identified by comparing the MS data with databases based on the search of m/z values of molecular ion peaks in the positive mode [M + H]+. Consequently, myricetin, coumestrin, chlorogenic acid, and hesperidin were detected as the major compounds (Table 4).
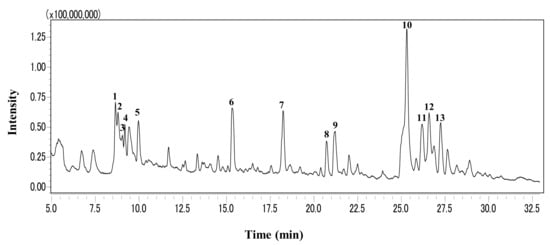
Figure 6.
LC-MS profile of GLE. The total ion chromatogram was obtained by a triple quadrupole mass spectrometer operated in the positive electrospray ionization mode.

Table 4.
Compounds identified from the chromatogram of GLE.
3. Discussion
Plant extracts are a rich source of natural bioactive compounds. Many studies have evaluated plant extracts used in Southeast Asian countries, including Thailand, where these extracts are components of folk medicine [13,14]. In this study, the extracts of 20 edible plant leaves from Thailand were screened, and G. littorale was selected for further investigation because it showed high phenol content, flavonoid content, and radical-scavenging activity. Several studies have investigated various species of the genus Glochidion [15,16,17,18,19]; however, there are few studies concerning the functional properties and constituents of G. littorale. Our data showed that GLE protected C. elegans against H2O2-induced oxidative stress by reducing intracellular ROS accumulation. This might have been due to the high content of phenolic compounds such as flavonoids, which are known to possess strong antioxidant activity [20]. These findings are similar to those obtained by Duangjan et al. (2019), who showed that G. zeylanicum leaf extracts can protect C. elegans against oxidative stress [21]. The insulin/insulin-like signaling (IIS) pathway regulates growth, stress responsiveness, and longevity in C. elegans [22,23]. We found that daf-16 null mutant C. elegans treated with GLE were susceptible to oxidative stress. This result suggests that the antioxidant effect of GLE in reducing oxidative stress in nematodes is possibly involved in not only radical-scavenging activity but also the regulation of the DAF-16 transcription factor.
The protective effects of GLE against MPP+-induced toxicity in C. elegans were examined. DA neurons in nematodes take up MPP+ mainly via high-affinity DA transporters, which is similarly observed in mammals. The accumulation of MPP+ inside the neurons inactivates the mitochondrial complex I of the respiratory chain and induces cell death [24,25,26,27]. GLE treatment was found to significantly reduce the lethality associated with MPP+ treatment in wild-type worms. The IIS pathway is modulated by insulin-like peptides through the DAF-2 receptor in C. elegans [28]. Under normal conditions, the IIS pathway inhibits the phosphorylation of DAF-16 and prevents its nuclear translocation. In daf-2 null mutants, the GLE-treated group survived longer than the control group. In contrast, no difference in survival was observed between the control group and the GLE-treated group containing daf-16 null mutant worms. It is known that downregulated DAF-2 signaling facilitates the entry of DAF-16 into the nucleus, where it can upregulate the expression of target genes and control stress resistance and longevity [29]. This may explain why daf-2 mutant worms treated with GLE showed a relatively higher survival. Furthermore, an increased nuclear accumulation of DAF-16 in worms treated with GLE was observed using transgenic TJ356 DAF-16::GFPC. elegans. Cumulatively, these results indicated that GLE might have exhibited its neuroprotective effects via activation of DAF-16.
LC-MS profiling led to the identification of 11 phytochemical compounds in GLE. Myricetin identified in the main peak of GLE is a flavonoid widely found in many plants and is well known to exhibit protective effects against oxidative stress. A previous study has demonstrated that myricetin extended the lifespan of C. elegans by diminishing stress-induced ROS accumulation and the pro-longevity effects of myricetin were dependent on DAF-16 [30]. Chlorogenic acid has also been reported to exhibit pro-longevity effects via the attenuation of oxidative stress in C. elegans [31]. Considering these findings, the neuroprotective effects of GLE were mainly induced by flavonoids such as myricetin, and GLE might be a suitable candidate for the management of neurodegenerative diseases.
In conclusion, our study demonstrated that GLE possessed strong antioxidant activity, which reduced oxidative stress in C. elegans. The extract also showed neuroprotective activity against MPP+-induced neurotoxicity in C. elegans. Various experiments performed using different transgenic worms suggested the possible involvement of the DAF-16 transcription factor in the observed neuroprotection. The high content of phenolic compounds, including flavonoids present in GLE, may be responsible for the observed stress resistance and neuroprotective properties. Further studies should identify the target genes involved in the neuroprotection mechanism.
4. Materials and Methods
4.1. Materials
The leaves of 20 different plants (Table 1) were obtained from a local market in Chiang Rai, Thailand. All reagents were of analytical grade. DPPH and H2DCF-DA were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were obtained from Wako Pure Chemical Industries (Osaka, Japan).
4.2. Preparation of Leaf Extracts
Leaf samples were frozen in liquid nitrogen, and then powdered samples (5 g) were mixed in 100 mL of distilled water at 45 °C for 30 min, following sonication using a Branson SLPe Sonifier (Branson, North Billerica, MA, USA) at 35 kHz. The extract was filtered and freeze-dried to obtain a powdered sample.
4.3. Total Phenolic Contents
The Folin-Ciocalteu method was used to determine the total phenolic content. Briefly, 11.4 μL of the extract (1 mg/mL) was mixed with 227.3 μL of 2% (w/v) Na2CO3 solution, and then the mixture was allowed to stand at room temperature for 2 min. After addition of 11.4 μL of 10% (v/v) Folin-Ciocalteu reagent. The incubation in the dark was conducted for 30 min. Subsequently, the absorbance was measured at 750 nm using a microplate reader (Nivo 3F Multimode Plate Reader, PerkinElmer, Waltham, MA, USA). Gallic acid was used as a standard for the calibration curve. The total phenolic content was expressed as gallic acid equivalents (mg gallic acid equivalent/g of plant extract).
4.4. Total Flavonoid Contents
The aluminum chloride colorimetric method was used to measure the total flavonoid content. Briefly, 25 μL of the extract (2 mg/mL) was mixed with 7.5 μL of 5% (w/v) NaNO2 solution and 152.5 μL of distilled water. After 6 min, 15 μL of 10% (w/v) AlCl3 solution was added and allowed to stand for 5 min. Then, 50 μL of 1 M NaOH solution was added to the mixture. Subsequently, the mixture was incubated in the dark for 15 min, and the absorbance was measured at 510 nm using a microplate reader. The total flavonoid content was calculated by generating a calibration curve using quercetin as a standard, and the results were expressed as quercetin equivalent (mg quercetin equivalent/g of plant extract).
4.5. Free Radical-Scavenging Activity
The capacity to scavenge free radicals was assessed using DPPH assays [32]. Briefly, 100 μL of the extract (1 mg/mL) were mixed with 100 μL of DPPH solution. After 30 min, the absorbance was measured at 517 nm using a microplate reader. The results were expressed as a percentage of inhibition of the DPPH radicals.
4.6. C. Elegans Maintenance
Wild-type N2, CF1038 (daf-16(mu86) I), CB1370 (daf-2(e1370) III), and TJ356 (zIs356 [daf-16p::daf-16a/b::GFP + rol-6(su1006)]) strains and their diet, Escherichia coli OP50, were obtained from the Caenorhabditis Genetics Center (Minneapolis, MN, USA). According to the standard protocols, N2, CF1038, and TJ356 strains and CF 1370 strain were maintained at 20 and 15 °C, respectively, on nematode growth medium (NGM) agar plates containing heat inactivated E. coli OP50 [33]. S-complete solution was prepared according to previously described literature [34].
4.7. Oxidative Stress Assays
Oxidative stress was induced by treating wild-type (N2) and daf-16 mutant (CF1038) worms with H2O2. L1 larvae were added to 96-well plates at an average of 15 nematodes per well in a 40 μL solution containing E. coli OP50. Five mM of H2O2 solution and the tested GLE dissolved in S-complete solution were added to achieve a final volume of 50 μL per well. L1 larvae were incubated for 48 h with H2O2 alone or in the presence of various concentrations of GLE, and worm viability was visually inspected under a stereomicroscope. The results from the H2O2-treated groups were normalized and expressed as a percentage of normal controls. The results were obtained from three independent experiments (100–160 worms/treatment in each experiment).
4.8. Intracellular ROS Levels
Intracellular ROS levels were determined using the H2DCF-DA probe. L1 larvae of wild-type N2 worms were treated with 5 mM H2O2 and GLE at different concentrations in S-solution for 48 h in black 96-well plates; each well comprised a minimum of 100 worms. Worms were subsequently incubated with 25 μM H2DCF-DA in the dark at 20 °C for 1 h. After incubation, the fluorescence intensity was measured at wavelengths of 485/530 nm using a Powerscan HT microplate reader (DS Pharma Biomedical, Osaka, Japan).
4.9. Neurotoxicity Assay
Neurotoxicity was induced by treating wild-type (N2) and transgenic (CF1038 and CB1370) worms with MPP+. L1 larvae were added to 96-well plates (15 worms/well) in a 40 μL solution containing E. coli OP50. Worms were then incubated with 50 μL of 0.75 mM MPP+ alone or in the presence of different concentrations of the tested sample for 48 h. After incubation, worm viability was visually inspected under a stereomicroscope. Live worms were counted every 12 h post treatment until no live worms remained. The results of the MPP+-treated groups were normalized and expressed as a percentage of the normal controls. The results were obtained from three independent experiments (80–130 worms/treatment in each experiment).
4.10. Nuclear Localization of DAF-16
Transgenic C. elegans TJ356, which expresses a DAF-16-GFP fusion protein, was used to examine the intracellular distribution of DAF-16. L1 stage nematodes were treated with GLE for 48 h at 20 °C. The worms were then transferred to a 2% agarose pad on a glass slide and anesthetized by adding one drop (approximately 20 μL) of 25 μM sodium azide to the agarose pad. The expression of GFP was examined via fluorescence microscopy (EVOS fl; Advanced Microscopy Group, Bothell, WA, USA). The mean fluorescence intensity of DAF-16 in the nuclei was analyzed using Image J software (National Institutes of Health, Bethesda, MD, USA).
4.11. Phytochemical Profiling Using LC-MS
The leaf extract was analyzed using the LCMS-8040 (Shimadzu). Mass spectra were acquired over a range of m/z 50–1000 using the Q3 scan mode. The solution was injected onto an Inertsil ODS-3 (250 × 2.1 mm, 5 µm, GL Sciences, Tokyo Japan) at a column temperature at 40 °C using a gradient of (A) 0.1% formic acid and (B) acetonitrile/water (80/20) containing 0.1% formic acid. The following gradient with a flow rate of 0.2 mL/min was used: 0–100% B (0–45 min), 100% B (45–50 min), and 0% B (50–60 min). Compounds were putatively identified by matching the experimental m/z values to the library of theoretical calculated m/z values in databases, including the Human Metabolome Database and the METLIN database.
4.12. Statistical Analysis
Data were expressed as the mean ± standard deviation for each group. The significant difference between the two groups was assessed using the t-test, whereas the difference between three and more groups was assessed using one-way ANOVA, followed by Tukey’s post-hoc comparison test. Statistical significance was set at p < 0.001 and p < 0.0001. For lifespan assays, C. elegans survival was plotted using Kaplan–Meier survival curves and analyzed via log-rank tests using GraphPad Prism software (version 9.01; GraphPad Software, San Diego, CA, USA).
Author Contributions
Conceptualization, S.R., D.A.V., and S.K.; investigation, A.F.B., Y.Z., M.N., S.R., H.-Y.P., D.A.V., and K.S.; writing—original draft preparation, A.F.B.; writing—review and editing, S.K.; supervision, S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available by the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Pohl, F.; Lin, P.K.T. The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-l.; Yao, X.-l.; Liu, Z.; Zhang, H.; Li, W.; Li, Z.; Wang, G.-L.; Pang, J.; Lin, Y.; Xu, Z. Protective effects of xyloketal B against MPP+-induced neurotoxicity in Caenorhabditis elegans and PC12 cells. Brain Res. 2010, 1332, 110–119. [Google Scholar] [CrossRef]
- Trimmer, P.A.; Bennett, J.P., Jr. The cybrid model of sporadic Parkinson’s disease. Exp. Neurol. 2009, 218, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Cheon, S.-M.; Jang, I.; Lee, M.H.; Kim, D.K.; Jeon, H.; Cha, D.S. Sorbus alnifolia protects dopaminergic neurodegeneration in Caenorhabditis elegans. Pharm. Biol. 2016, 55, 481–486. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, N.; Ferger, B. Neurochemical findings in the MPTP model of Parkinson’s disease. J. Neural Transm. 2001, 108, 1263–1282. [Google Scholar] [CrossRef] [PubMed]
- Harrington, A.; Yacoubian, T.A.; Slone, S.R.; Caldwell, K.; Caldwell, G. Functional Analysis of VPS41-Mediated Neuroprotection in Caenorhabditis elegans and Mammalian Models of Parkinson’s Disease. J. Neurosci. 2012, 32, 2142–2153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, R.-H.; Harn, H.-J.; Liu, S.-P.; Chen, C.-S.; Chang, W.-L.; Chen, Y.-M.; Huang, J.-E.; Li, R.-J.; Tsai, S.-Y.; Hung, H.-S.; et al. n-Butylidenephthalide Protects against Dopaminergic Neuron Degeneration and α-Synuclein Accumulation in Caenorhabditis elegans Models of Parkinson’s Disease. PLOS ONE 2014, 9, e85305. [Google Scholar] [CrossRef] [Green Version]
- Jadiya, P.; Khan, A.; Sammi, S.R.; Kaur, S.; Mir, S.S.; Nazir, A. Anti-Parkinsonian effects of Bacopa monnieri: Insights from transgenic and pharmacological Caenorhabditis elegans models of Parkinson’s disease. Biochem. Biophys. Res. Commun. 2011, 413, 605–610. [Google Scholar] [CrossRef]
- Andrade, J.M.D.M.; Fasolo, D. Polyphenol Antioxidants from Natural Sources and Contribution to Health Promotion. In Polyphenols in Human Health and Disease; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 253–265. [Google Scholar]
- Hsu, A.-L.; Murphy, C.T.; Kenyon, C. Regulation of Aging and Age-Related Disease by DAF-16 and Heat-Shock Factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef] [Green Version]
- Henderson, S.T.; Johnson, T.E. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 2001, 11, 1975–1980. [Google Scholar] [CrossRef] [Green Version]
- Hutadilok-Towatana, N.; Chaiyamutti, P.; Panthong, K.; Mahabusarakam, W.; Rukachaisirikul, V. Antioxidative and Free Radical Scavenging Activities of Some Plants Used in Thai Folk Medicine. Pharm. Biol. 2006, 44, 221–228. [Google Scholar] [CrossRef]
- Stewart, P.; Boonsiri, P.; Puthong, S.; Rojpibulstit, P. Antioxidant activity and ultrastructural changes in gastric cancer cell lines induced by Northeastern Thai edible folk plant extracts. BMC Complement. Altern. Med. 2013, 13, 60. [Google Scholar] [CrossRef] [Green Version]
- Hui, W.; Lee, W.; Ng, K.; Chan, C. The occurrence of triterpenoids and steroids in three Glochidion species. Phytochemistry 1970, 9, 1099–1102. [Google Scholar] [CrossRef]
- Takeda, Y.; Mima, C.; Masuda, T.; Hirata, E.; Takushi, A.; Otsuka, H. Glochidioboside, a glucoside of (7S,8R)-dihydrodehydrodiconiferyl alcohol from leaves of glochidion obovatum. Phytochemistry 1998, 49, 2137–2139. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Gao, K. Chemical constituents from Glochidion wrightii Benth. Biochem. Syst. Ecol. 2012, 45, 7–11. [Google Scholar] [CrossRef]
- Tian, J.-M.; Fu, X.-Y.; Zhang, Q.; He, H.-P.; Gao, J.-M.; Hao, X.-J. Chemical constituents from Glochidion assamicum. Biochem. Syst. Ecol. 2013, 48, 288–292. [Google Scholar] [CrossRef]
- Kongkachuichai, R.; Charoensiri, R.; Yakoh, K.; Kringkasemsee, A.; Insung, P. Nutrients value and antioxidant content of indigenous vegetables from Southern Thailand. Food Chem. 2015, 173, 838–846. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Duangjan, C.; Rangsinth, P.; Gu, X.; Zhang, S.; Wink, M.; Tencomnao, T. Glochidion zeylanicum leaf extracts exhibit lifespan extending and oxidative stress resistance properties in Caenorhabditis elegans via DAF-16/FoxO and SKN-1/Nrf-2 signaling pathways. Phytomedicine 2019, 64, 153061. [Google Scholar] [CrossRef]
- Jensen, V.L.; Gallo, M.; Riddle, D.L. Targets of DAF-16 involved in Caenorhabditis elegans adult longevity and dauer formation. Exp. Gerontol. 2006, 41, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Daitoku, H.; Fukamizu, A. FOXO Transcription Factors in the Regulatory Networks of Longevity. J. Biochem. 2007, 141, 769–774. [Google Scholar] [CrossRef]
- Lakso, M.; Vartiainen, S.; Moilanen, A.-M.; Sirviö, J.; Thomas, J.H.; Nass, R.; Blakely, R.D.; Wong, G. Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human α-synuclein. J. Neurochem. 2004, 86, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nass, R.; Hall, D.H.; Miller, D.M.; Blakely, R.D. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 3264–3269. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-M.; Pu, P.; Le, W.-D. ATP depletion is the major cause of MPP+ induced dopamine neuronal death and worm lethality in α-synuclein transgenic C. elegans. Neurosci. Bull. 2007, 23, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Braungart, E.; Gerlach, M.; Riederer, P.; Baumeister, R.; Hoener, M. Caenorhabditis elegans MPP+ Model of Parkinson’s Disease for High-Throughput Drug Screenings. Neurodegener. Dis. 2004, 1, 175–183. [Google Scholar] [CrossRef]
- Panowski, S.H.; Dillin, A. Signals of youth: Endocrine regulation of aging in Caenorhabditis elegans. Trends Endocrinol. Metab. 2009, 20, 259–264. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Tissenbaum, H.A. Reproduction and longevity: Secrets revealed by C. elegans. Trends Cell Biol. 2007, 17, 65–71. [Google Scholar] [CrossRef]
- Büchter, C.; Ackermann, D.; Havermann, S.; Honnen, S.; Chovolou, Y.; Fritz, G.; Kampkötter, A.; Wätjen, W. Myricetin-Mediated Lifespan Extension in Caenorhabditis elegans Is Modulated by DAF. Int. J. Mol. Sci. 2013, 14, 11895–11914. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.Q.; Huang, X.B.; Xing, T.K.; Ding, A.J.; Wu, G.S.; Luo, H.R. Chlorogenic Acid Extends the Lifespan of Caenorhabditis elegans via Insulin/IGF-1 Signaling Pathway. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 464–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nat. Cell Biol. 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. WormBook 2006, 2, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amrit, F.R.G.; Ratnappan, R.; Keith, S.A.; Ghazi, A. The C. elegans lifespan assay toolkit. Methods 2014, 68, 465–475. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).