Review of Functional and Pharmacological Activities of Berries
Abstract
:1. Introduction
2. Data Collection
3. Composition
3.1. Nutrient Composition
3.2. Phenolic Composition
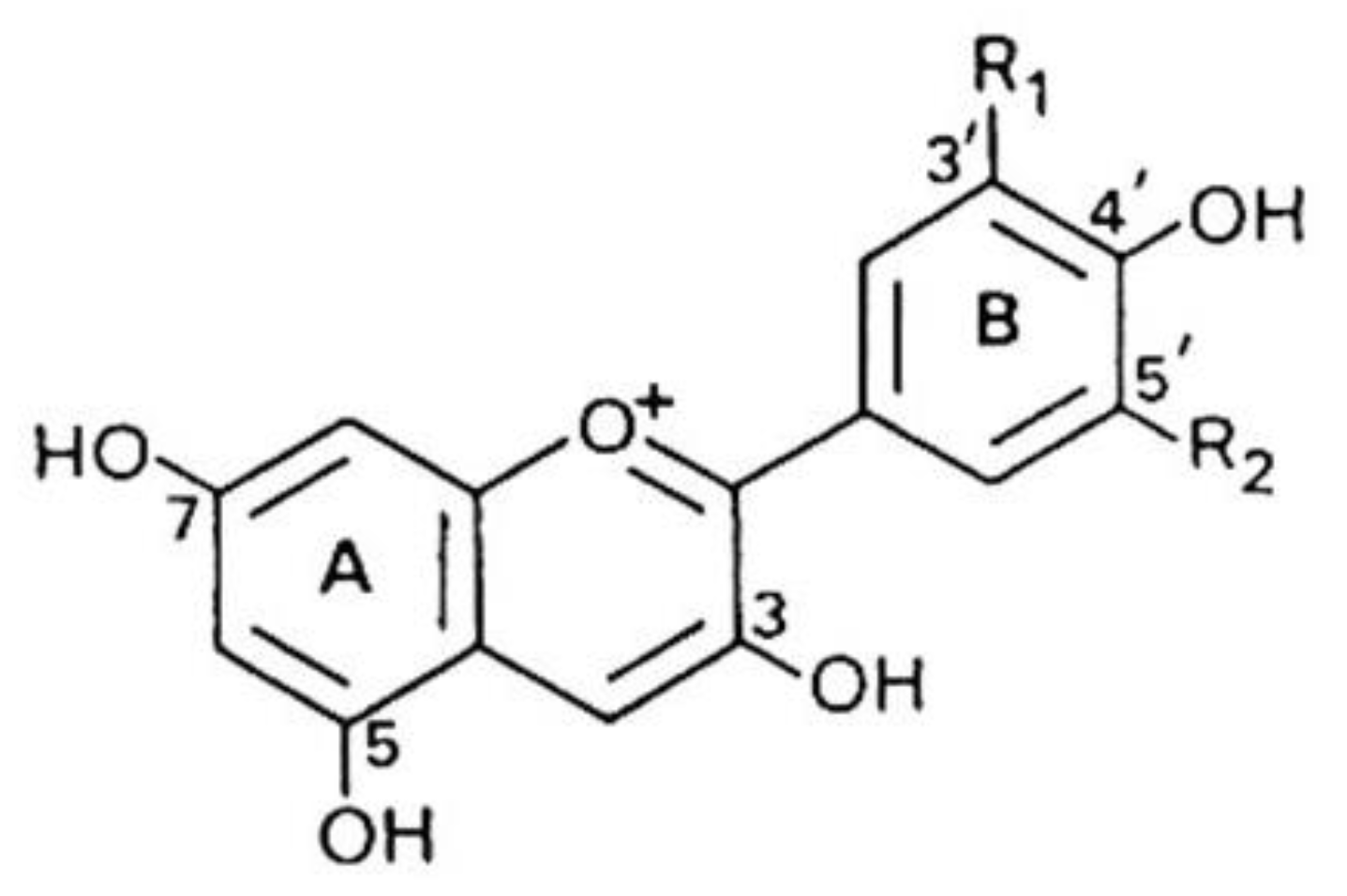
3.2.1. Anthocyanins
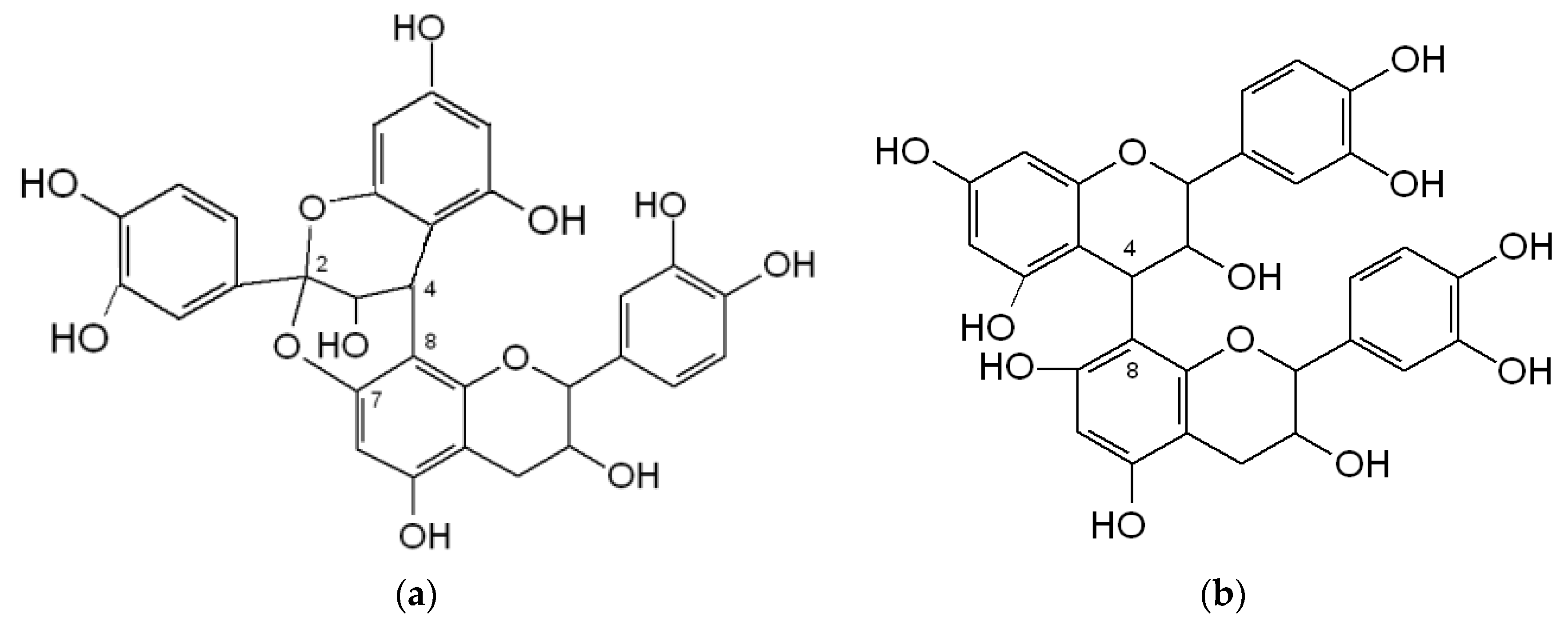
3.2.2. Proanthocynidins (PACs)
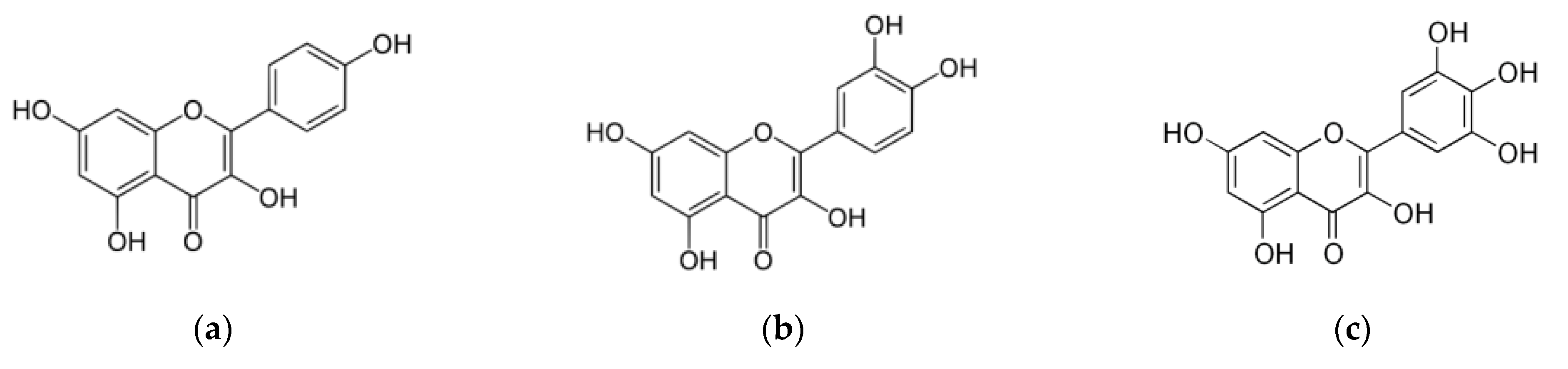
3.2.3. Flavonols
3.2.4. Phenolic Acid
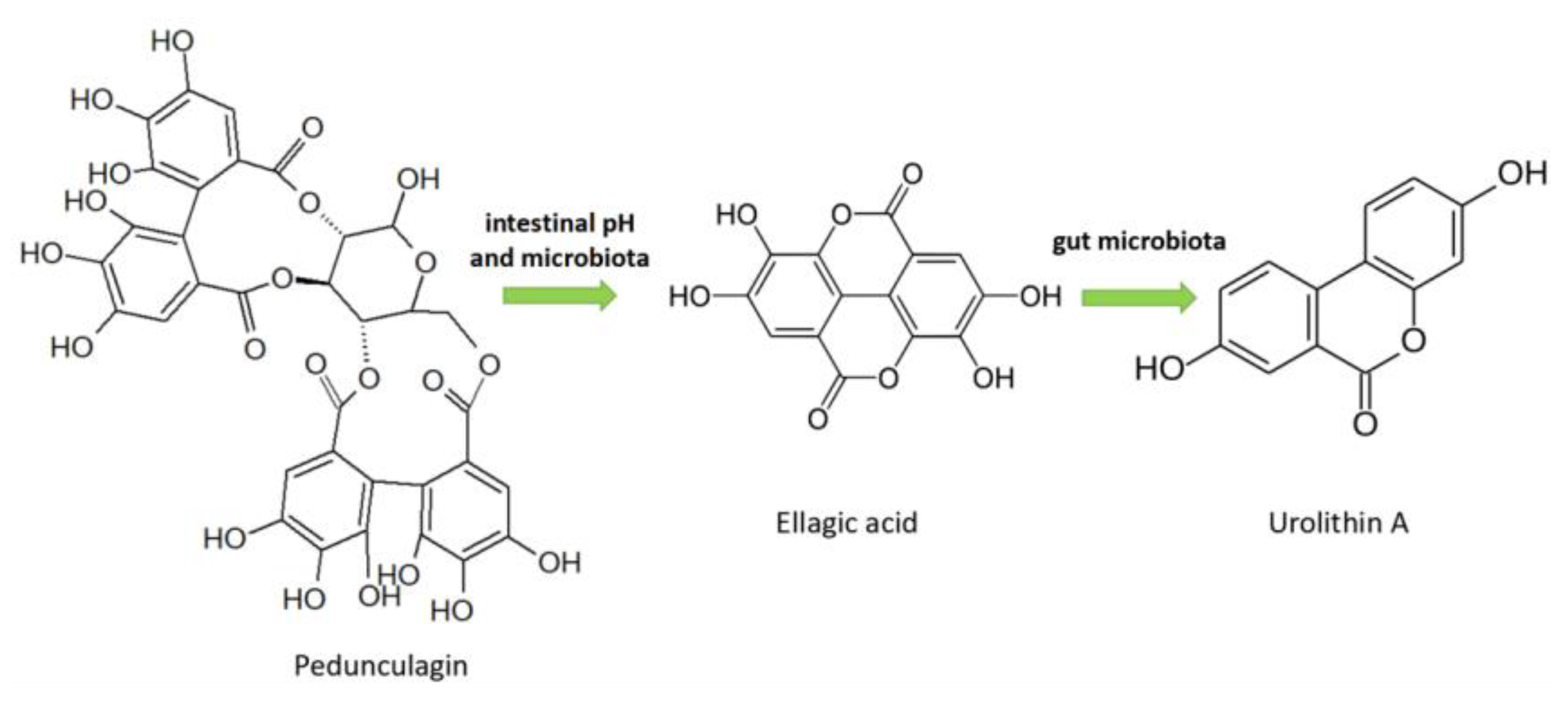
3.2.5. Ellagitannins
3.2.6. Stilbenes
4. Bioavailability
4.1. Anthocyanins
4.2. Proanthocyanidins
4.3. Flavonols
4.4. Phenolic Acids
4.5. Ellagitannins
5. Oxidative Stress Suppression
6. Antimicrobial Properties
7. Anticancer Properties
8. Diabetes
9. Obesity
10. Cardiovascular Disease
11. Blood Pressure
12. Neuroprotection
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Raimundo, A.F.; Félix, F.; Andrade, R.; García-Conesa, M.T.; González-Sarrías, A.; Gilsa-Lopes, J.; do Ó, D.; Raimundo, A.; Ribeiro, R.; Rodriguez-Mateos, A.; et al. Combined effect of interventions with pure or enriched mixtures of (poly)phenols and anti-diabetic medication in type 2 diabetes management: A meta-analysis of randomized controlled human trials. Eur. J. Nutr. 2020, 59, 1329–1343. [Google Scholar] [CrossRef]
- García-Conesa, M.T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andrés-Lacueva, C.; Pascual-Teresa, S.; Mena, P.; Ristic, A.K.; Kroon, P.A.; et al. Meta-analysis of the effects of foods and derived products containing ellagitannins and anthocyanins on cardiometabolic biomarkers: Analysis of factors influencing variability of the individual responses. Int. J. Mol. Sci. 2018, 19, 694. [Google Scholar] [CrossRef] [Green Version]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [Green Version]
- Pan, P.; Skaer, C.W.; Stirdivant, S.M.; Young, M.R.; Stoner, G.D.; Lechner, J.F.; Huang, Y.W.; Wang, L.S. Beneficial regulation of metabolic profiles by black raspberries in human colorectal cancer patients. Cancer Prev. Res. 2015, 8, 743–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.S.; Burke, C.A.; Hasson, H.; Kuo, C.T.; Molmenti, C.L.S.; Seguin, C.; Liu, P.; Huang, T.H.; Frankel, W.L.; Stoner, G.D. A phase Ib study of the effects of black raspberries on rectal polyps in patients with familial adenomatous polyposis. Cancer Prev. Res. 2014, 7, 666–674. [Google Scholar] [CrossRef] [Green Version]
- Molan, A.L.; Liu, Z.; Plimmer, G. Evaluation of the effect of blackcurrant products on gut microbiota and on markers of risk for colon cancer in humans. Phytother. Res. 2014, 28, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Giampieri, F.; Cianciosi, D.; Ansary, J.; Chen, X.; Zhang, D.; Gila, E.; Forbes-Hernández, T. The roles of strawberry and honey phytochemicals on human health: A possible clue on the molecular mechanisms involved in the prevention of oxidative stress and inflammation. Phytomedicine 2021, 86, 153170. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Mezzetti, B.; Quiles, J.L.; Bompadre, S.; Battino, M. Protective effect of strawberry extract against inflammatory stress induced in human dermal fibroblasts. Molecules 2017, 22, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeram, N.P. Emerging research supporting the positive effects of berries on human health and disease prevention. J. Agric. Food Chem. 2012, 60, 5685–5686. [Google Scholar] [CrossRef]
- Haytowitz, D.B.; Pehrsson, P.R. USDA’s National Food and Nutrient Analysis Program (NFNAP) produces high-quality data for USDA food composition databases: Two decades of collaboration. Food Chem. 2018, 238, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Skupien, K.; Oszmiański, J. Comparison of six cultivars of strawberries (Fragaria x ananassa Duch.) grown in northwest Poland. Eur. Food Res. Technol. 2004, 219, 66–70. [Google Scholar] [CrossRef]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- da Silva Pinto, M.; Lajolo, F.M.; Genovese, M.I. Bioactive compounds and quantification of total ellagic acid in strawberries (Fragaria × ananassa Duch.). Food Chem. 2008, 107, 1629–1635. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, I.M.; Meyer, A.S.; Frankel, E.N. Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation. J. Agric. Food Chem. 2008, 46, 4107–4112. [Google Scholar] [CrossRef]
- Cervantes, L.; Martínez-Ferri, E.; Soria, C.; Ariza, M.T. Bioavailability of phenolic compounds in strawberry, raspberry and blueberry: Insights for breeding programs. Food Biosci. 2020, 37, 100680. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid glycosides and antioxidant capacity of various blackberry, blueberry and red grape genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Li, D.; Li, B.; Ma, Y.; Sun, X.; Lin, Y.; Meng, X. Polyphenols, anthocyanins, and flavonoids contents and the antioxidant capacity of various cultivars of highbush and half-high blueberries. J. Food Compos. Anal. 2017, 62, 84–93. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.). Food Chem. 2017, 217, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Stretch, A.W. Antioxidant capacity in cranberry is influenced by cultivar and storage temperature. J. Agric. Food Chem. 2001, 49, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Narwojsz, A.; Tańska, M.; Mazur, B.; Borowska, E.J. Fruit physical features, phenolic compounds profile and inhibition activities of cranberry cultivars (Vaccinium macrocarpon) compared to wild-grown cranberry (Vaccinium oxycoccus). Plant Foods Hum. Nutr. 2019, 74, 300–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grace, M.H.; Massey, A.R.; Mbeunkui, F.; Yousef, G.G.; Lila, M.A. Comparison of health-relevant flavonoids in commonly consumed cranberry products. J. Food Sci. 2012, 77, H176–H183. [Google Scholar] [CrossRef]
- Anttonen, M.J.; Karjalainen, R.O. Environmental and genetic variation of phenolic compounds in red raspberry. J. Food Compos. Anal. 2005, 18, 759–769. [Google Scholar] [CrossRef]
- Wada, L.; Ou, B. Antioxidant activity and phenolic content of Oregon caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef]
- Gansch, H.; Weber, C.A.; Lee, C.Y. Antioxidant capacity and phenolic phytochemicals in black raspberries. Red 2009, 17, 20–23. [Google Scholar]
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Bordonaba, J.G.; Terry, L.A. Biochemical profiling and chemometric analysis of seventeen UK-grown black currant cultivars. J. Agric. Food Chem. 2008, 56, 7422–7430. [Google Scholar] [CrossRef] [PubMed]
- Kahkonen, M.P.; Heinamaki, J.; Ollilainen, V.; Heinonen, M. Berry anthocyanins: Isolation, identification, and antioxidant activities. J. Sci. Food Agric. 2003, 83, 1403–1411. [Google Scholar] [CrossRef]
- Gündeşli, M.A.; Korkmaz, N.; Okatan, V. Polyphenol content and antioxidant capacity of berries: A review. Int. J. Agric. For. Life Sci. 2019, 3, 350–361. [Google Scholar]
- Djurić, M.; Maškovic, P.; Murtić, S.; Veljković, B.; Ćurčić, S.; Paunović, G.; Rakočević, L.B. Quantitation of ellagic acid in blackberries. Hem. Ind. 2014, 68, 241–245. [Google Scholar] [CrossRef] [Green Version]
- Vrhovsek, U.; Giongo, L.; Mattivi, F.; Viola, R. A survey of ellagitannin content in raspberry and blackberry cultivars grown in Trentino (Italy). Eur. Food Res. Technol. 2008, 226, 817–824. [Google Scholar] [CrossRef]
- Peña-Sanhueza, D.; Inostroza-Blancheteau, C.; Ribera-Fonseca, A.; Reyes-Díaz, M. Anthocyanins in berries and their potential use in human health. In Superfood and Functional Food—The Development of Superfoods and Their Roles as Medicine; Shiomi, N., Waisundara, V., Eds.; IntechOpen: Temuco, Chile, 2017; pp. 155–172. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Hellstrom, J.K.; Torronen, A.R.; Mattila, P.H. Proanthocyanidins in common food products of plant origin. J. Agric. Food Chem. 2009, 57, 7899–7906. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, X.; Yu, H.; Tang, H. Changes of total anthocyanins and proanthocyanidins in the developing blackberry fruits. Int. J. ChemTech Res. 2012, 4, 129–137. [Google Scholar]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronis, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Ruiz, A.; Bustamante, L.; Vergara, C.; von Baer, D.; Hermosín-Gutiérrez, I.; Obando, L.; Mardones, C. Hydroxycinnamic acids and flavonols in native edible berries of South Patagonia. Food Chem. 2015, 167, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Seruga, M.; Novak, I.; Medvidovic-Kosanovic, M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch. Lebensm. Rundsch. 2007, 103, 369–377. [Google Scholar]
- Vvedenskaya, I.O.; Rosen, R.T.; Guido, J.E.; Russell, D.J.; Mills, K.A.; Vorsa, N. Characterization of flavonols in cranberry (Vaccinium macrocarpon) powder. J. Agric. Food Chem. 2004, 52, 188–195. [Google Scholar] [CrossRef]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid content of US fruits, vegetables, and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef]
- Govers, C.; Berkel Kasikci, M.; van der Sluis, A.A.; Mes, J.J. Review of the health effects of berries and their phytochemicals on the digestive and immune systems. Nutr. Rev. 2018, 76, 29–46. [Google Scholar] [CrossRef]
- Feresin, R.G.; Huang, J.; Klarich, D.S.; Zhao, Y.; Pourafshar, S.; Arjmandi, B.H.; Salazar, G. Blackberry, raspberry and black raspberry polyphenol extracts attenuate angiotensin II-induced senescence in vascular smooth muscle cells. Food Funct. 2016, 7, 4175–4187. [Google Scholar] [CrossRef]
- Häkkinen, S.H.; Kärenlampi, S.O.; Mykkänen, H.M.; Törrönen, A.R. Influence of domestic processing and storage on flavonol contents in berries. J. Agric. Food Chem. 2000, 48, 2960–2965. [Google Scholar] [CrossRef]
- Paunović, S.M.; Mašković, P.; Nikolić, M.; Miletić, R. Bioactive compounds and antimicrobial activity of black currant (Ribes nigrum L.) berries and leaves extract obtained by different soil management system. Sci. Hortic. 2017, 222, 69–75. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The blackberry fruit: A review on its composition and chemistry, metabolism and bioavailability, and health benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Red raspberries and their bioactive polyphenols: Cardiometabolic and neuronal health links. Adv. Nutr. 2016, 7, 44–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häkkinen, S.; Heinonen, M.; Kärenlampi, S.; Mykkänen, H.; Ruuskanen, J.; Törrönen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999, 32, 345–353. [Google Scholar] [CrossRef]
- Neto, C.C. Cranberry and its phytochemicals: A review of in vitro anticancer studies. J. Nutr. 2007, 137, 186S–193S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit Rev. Food Sci Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Hannum, S.M. Potential impact of strawberries on human health: A review of the science. Crit. Rev. Food Sci. Nutr. 2004, 44, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimiä, R.; Seppänen-Laakso, T.; Kankainen, M.; Maukonen, J.; Törrönen, R.; Kolehmainen, M.; Leppänen, T.; Moilanen, E.; Nohynek, L.; Aura, A.M.; et al. Effects of ellagitannin-rich berries on blood lipids, gut microbiota, and urolithin production in human subjects with symptoms of metabolic syndrome. Mol. Nutr. Food Res. 2013, 57, 2258–2263. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Vrhovsek, U.; Rossoni, G.; Colombo, E.; Brunelli, C.; Brembati, L.; Trivulzio, S.; Gasperotti, M.; Mattivi, F.; Bosisio, E.; et al. Ellagitannins from Rubus berries for the control of gastric inflammation: In vitro and in vivo studies. PLoS ONE 2013, 8, e71762. [Google Scholar] [CrossRef] [Green Version]
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How much wine do you have to drink to stay healthy? Adv. Nutr. 2016, 7, 706–718. [Google Scholar] [CrossRef] [Green Version]
- Ehala, S.; Vaher, M.; Kaljurand, M. Characterization of phenolic profiles of Northern European berries by capillary electrophoresis and determination of their antioxidant activity. J. Agric. Food Chem. 2005, 53, 6484–6490. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Németh, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestine epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef]
- Talavera, S.; Felgines, C.; Texier, O.; Besson, C.; Manach, C.; Lamaison, J.L.; Rêmêsy, C. Anthocyanins are efficiently absorbed from the small intestine in rats. J. Nutr. 2004, 134, 2275–2279. [Google Scholar] [CrossRef]
- Passamonti, S.; Vrhovsek, U.; Mattivi, F. The interaction of anthocyanins with bilitranslocase. Biochem. Biophys. Res. Commun. 2002, 296, 631–636. [Google Scholar] [CrossRef]
- Stoner, G.D.; Sardo, C.; Apseloff, G.; Mullet, D.; Wargo, W.; Pound, V.; Singh, A.; Sanders, J.; Aziz, R.; Casto, B.; et al. Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. J. Clin. Pharmacol. 2005, 45, 1153–1164. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015, 89, 758–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, I.; de Freitas, V.; Reis, C.; Mateus, N. A new approach on the gastric absorption of anthocyanins. Food Funct. 2012, 3, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Felgines, C.; Talavera, S.; Gonthier, M.P.; Texier, O.; Scalbert, A.; Lamaison, J.L.; Remesy, C. Strawberry anthocyanins are recovered in urine as glucuro- and sulfoconjugates in humans. J. Nutr. 2003, 133, 1296–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felgines, C.; Talavera, S.; Texier, O.; Gil-Izquierdo, A.; Lamaison, J.L.; Remesy, C. Blackberry anthocyanins are mainly recovered from urine as methylated and glucuronidated conjugates in humans. J. Agric. Food Chem. 2005, 53, 7721–7727. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Redgate, E.L.; McGhie, T.K.; Hurst, R.D. In vitro studies of modulation of pathogenic and probiotic bacterial proliferation and adhesion to intestinal cells by blackcurrant juices. J. Funct. Foods 2014, 8, 35–44. [Google Scholar] [CrossRef]
- Bò, C.D.; Ciappellano, S.; Klimis-Zacas, D.; Martini, D.; Gardana, C.; Riso, P.; Porrini, M. Anthocyanin absorption, metabolism, and distribution from a wild blueberry-enriched diet (Vaccinium angustifolium) is affected by diet duration in the Sprague-Dawley rat. J. Agric. Food Chem. 2010, 58, 2491–2497. [Google Scholar] [CrossRef]
- Gonzalez-Barrio, R.; Edwards, C.A.; Crozier, A. Colonic catabolism of ellagitannins, ellagic acid, and raspberry anthocyanins: In vivo and in vitro studies. Drug Metab. Dispos. 2011, 39, 1680–1688. [Google Scholar] [CrossRef]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Gu, L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Erlund, I.; Freese, R.; Marniemi, J.; Hakala, P.; Alfthan, G. Bioavailability of quercetin from berries and the diet. Nutr. Cancer 2006, 54, 13–17. [Google Scholar] [CrossRef]
- Wang, C.; Zuo, Y.; Vinson, J.A.; Deng, Y. Absorption and excretion of cranberry-derived phenolics in humans. Food Chem. 2012, 132, 1420–1428. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Xiao, J. Polyphenols: Absorption, bioavailability, and metabolomics. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Vienna, Austria, 2018; pp. 45–67. [Google Scholar]
- Wu, X.; Pittman, H.E., III; Hager, T.; Hager, A.; Howard, L.; Prior, R.L. Phenolic acids in black raspberry and in the gastrointestinal tract of pigs following ingestion of black raspberry. Mol. Nutr. Food Res. 2009, 53, S76–S84. [Google Scholar] [CrossRef]
- Khanal, R.; Howard, L.R.; Prior, R.L. Urinary excretion of phenolic acids in rats fed cranberry, blueberry, or black raspberry powder. J. Agric. Food Chem. 2014, 62, 3987–3996. [Google Scholar] [CrossRef]
- Beattie, J.; Crozier, A.; Duthie, G.G. Potential health benefits of berries. Curr. Nutr. Food Sci. 2005, 1, 71–86. [Google Scholar] [CrossRef]
- Borges, G.; Roowi, S.; Rouanet, J.M.; Duthie, G.G.; Lean, M.E.; Crozier, A. The bioavailability of raspberry anthocyanins and ellagitannins in rats. Mol. Nutr. Food Res. 2007, 51, 714–725. [Google Scholar] [CrossRef]
- Gu, J.; Thomas-Ahner, J.M.; Riedl, K.M.; Bailey, M.T.; Vodovotz, Y.; Schwartz, S.J.; Clinton, S.K. Dietary black raspberries impact the colonic microbiome and phytochemical metabolites in mice. Mol. Nutr. Food Res. 2019, 63, 1800636. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Barrio, R.; Cerdá, B.; López-Bote, C.; Rey, A.I.; Tomás-Barberán, F.A. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J. Agric. Food Chem. 2007, 55, 10476–10485. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Lee, R.; Heber, D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta 2004, 348, 63–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zhang, H.; Xu, Z.; Wu, M.; Xia, W.; Zhang, W. Chimonanthus praecox extract/cyclodextrin inclusion complexes: Selective inclusion, enhancement of antioxidant activity and thermal stability. Ind. Crops Prod. 2017, 95, 60–65. [Google Scholar] [CrossRef]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Enhanced dissolution and bioavailability of grapefruit flavonoid Naringenin by solid dispersion utilizing fourth generation carrier. Drug Dev. Ind. Pharm. 2015, 41, 772–779. [Google Scholar] [CrossRef]
- Zeng, C.; Jiang, W.; Wang, X.C.; Tan, M.E.; Xing, J.G. Total flavonoids extract from Dracocephalum moldavica Composite phospholipid liposomes: Preparation, in vitro drug release and permeability of Caco-2 cell. J. Pharm. Phytochem. 2016, 5, 30. [Google Scholar]
- Yang, D.; Wang, X.Y.; Lee, J.H. Effects of flavonoids on physical and oxidative stability of soybean oil O/W emulsions. Food Sci. Biotechnol. 2015, 24, 851–858. [Google Scholar] [CrossRef]
- Tomas-Navarro, M.; Vallejo, F.; Borrego, F.; Tomás-Barberán, F.A. Encapsulation and micronization effectively improve orange beverage flavanone bioavailability in humans. J. Agric. Food Chem. 2014, 62, 9458–9462. [Google Scholar] [CrossRef] [PubMed]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; de Vos, C.H.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Romandini, S.; Mazzoni, L.; Giampieri, F.; Tulipani, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Locorotondo, N.; D’Alessandro, M.; Mezzetti, B.; Bompadre, S.; et al. Effects of an acute strawberry (Fragaria × ananassa) consumption on the plasma antioxidant status of healthy subjects. J. Berry Res. 2013, 3, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Henning, S.M.; Seeram, N.P.; Zhang, Y.; Li, L.; Gao, K.; Lee, R.P.; Wang, D.C.; Zerlin, A.; Karp, H.; Thames, G.; et al. Strawberry consumption is associated with increased antioxidant capacity in serum. J. Med. Food 2010, 13, 116–122. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Dekanski, D.; Ristić, S.; Radonjić, N.V.; Petronijević, N.D.; Giampieri, F.; Astolfi, P.; González-Paramás, A.M.; Santos-Buelga, C.; Tulipani, S.; et al. Strawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increase. PLoS ONE 2011, 6, e25878. [Google Scholar] [CrossRef] [Green Version]
- Dvaranauskait, A.; Venskutonis, P.R.; Labokas, J. Radical scavenging activity of raspberry (Rubus idaeus L.) fruit extracts. Acta Aliment. 2006, 35, 73–83. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compost. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Beekwilder, J.; Hall, R.D.; de Vos, C.H. Identification and dietary relevance of antioxidants from raspberry. BioFactors 2005, 23, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Tulio, A.Z.; Reese, R.N.; Wyzgoski, F.J.; Rtnaldi, P.L.; Fu, R.; Scheerens, J.C.; Miller, A.R. Cyanidin 3-rutinoside and cyanidin 3-xylo-sylrutinoside as primary phenolic antioxidants in black raspberry. J. Agric. Food Chem. 2008, 56, 1880–1888. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Haytowitz, D.B.; Bhagwat, S. USDA database for the oxygen radical absorbance capacity (ORAC) of selected foods, Release 2. USDA 2010, 3, 10–48. [Google Scholar]
- Routray, W.; Orsat, V. Blueberries and their anthocyanins: Factors affecting biosynthesis and properties. Compr. Rev. Food Sci. F. 2011, 10, 303–320. [Google Scholar] [CrossRef]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Youdim, K.A.; McDonald, J.; Kalt, W.; Joseph, J.A. Potential role of dietary flavonoids in reducing microvascular endothelium vulnerability to oxidative and inflammatory insults. J. Nutr. Biochem. 2002, 13, 282–288. [Google Scholar] [CrossRef]
- Bunea, A.; Rugină, D.; Sconţa, Z.; Pop, R.M.; Pintea, A.; Socaciu, C.; Tăbăran, F.; Grootaert, C.; Struijs, K.; VanCamp, J. Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochemistry 2013, 95, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Diaconeasa, Z.; Leopold, L.; Rugină, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef] [Green Version]
- Mazza, G.; Kay, C.D.; Cottrell, T.; Holub, B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 2002, 50, 7731–7737. [Google Scholar] [CrossRef]
- Blando, F.; Calabriso, N.; Berland, H.; Maiorano, G.; Gerardi, C.; Carluccio, M.A.; Andersen, O.M. Radical scavenging and anti-inflammatory activities of representative anthocyanin groupings from pigment-rich fruits and vegetables. Int. J. Mol. Sci. 2018, 19, 169. [Google Scholar] [CrossRef] [Green Version]
- Nour, V.; Stampar, F.; Veberic, R.; Jakopic, J. Anthocyanins profile, total phenolics and antioxidant activity of black currant ethanolic extracts as influenced by genotype and ethanol concentration. Food Chem. 2013, 141, 961–966. [Google Scholar] [CrossRef]
- Lee, S.G.; Vance, T.M.; Nam, T.G.; Kim, D.O.; Koo, S.I.; Chun, O.K. Contribution of anthocyanin composition to total antioxidant capacity of berries. Plant. Foods Hum. Nutr. 2015, 70, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Cortez, R.E.; Gonzalez de Mejia, E. Blackcurrants (Ribes nigrum): A Review on chemistry, processing, and health benefits. J. Food Sci. 2019, 84, 2387–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, D.L.; Blumberg, J.B. Cranberries (Vaccinium macrocarpon) and cardiovascular disease risk factors. Nutr. Rev. 2007, 65, 490–502. [Google Scholar] [CrossRef]
- Basu, A.; Betts, N.M.; Ortiz, J.; Simmons, B.; Wu, M.; Lyons, T.J. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr. Res. 2011, 31, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Hollman, P.C.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 141, 989S–1009S. [Google Scholar] [CrossRef] [Green Version]
- Vieira, D.R.; Amaral, F.M.; Maciel, M.C.; Nascimento, F.R.; Libério, S.A.; Rodrigues, V.P. Plant species used in dental diseases: Ethnopharmacology aspects and antimicrobial activity evaluation. J. Ethnopharmacol. 2014, 155, 1441–1449. [Google Scholar] [CrossRef]
- Yen, C.H.; Chiu, H.F.; Huang, S.Y.; Lu, Y.Y.; Han, Y.C.; Shen, Y.C.; Venkatakrishnan, K.; Wang, C.K. Beneficial effect of Burdock complex on asymptomatic Helicobacter pylori-infected subjects: A randomized, double-blind placebo-controlled clinical trial. Helicobacter 2018, 23, e12469. [Google Scholar] [CrossRef]
- Lengsfeld, C.; Deters, A.; Faller, G.; Hensel, A.D. High molecular weight polysaccharides from black currant seeds inhibit adhesion of Helicobacter pylori to human gastric mucosa. Planta Med. 2004, 70, 620–626. [Google Scholar] [CrossRef]
- Nohynek, L.J.; Alakomi, H.L.; Kähkönen, M.P.; Heinonen, M.; Helander, I.M.; Oksman-Caldentey, K.; Puupponen-Pimiä, R.H. Berry phenolics: Antimicrobial properties and mechanisms of action against severe human pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Alakomi, H.L.; Oksman-Caldentey, K.M. Bioactive berry compounds-novel tools against human pathogens. Appl. Microbiol. Biotechnol. 2005, 67, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.B. Cranberry proanthocyanidins and the maintenance of urinary tract health. Crit. Rev. Food Sci. Nutr. 2002, 42, 273–278. [Google Scholar] [CrossRef]
- Shmuely, H.; Burger, O.; Neeman, I.; Yahav, J.; Samra, Z.; Niv, Y.; Sharon, N.; Weiss, E.; Athamna, A.; Tabak, M.; et al. Susceptibility of Helicobacter pylori isolates to the antiadhesion activity of a high-molecular weight constituent of cranberry. Diagn. Microbiol. Infect. Dis. 2004, 50, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Ma, J.L.; Guo, Y.; Liu, W.D.; Li, M.; Zhang, L.F.; Zhang, Y.; Zhou, T.; Zhang, J.Y.; Gao, H.E.; et al. Suppression of Helicobacter pylori infection by daily cranberry intake: A double-blind, randomized, placebo-controlled trial. J. Gastroenterol. Hepatol. 2021, 36, 927–935. [Google Scholar] [CrossRef]
- Wang, L.S.; Echeveste, C.E.; Yu, J.; Huang, Y.W.; Lechner, J.; Mei, L.; Sanvanson, P.; Yearsley, M.; Wang, C.K.; Stoner, G.D. Can Natural Products Suppress Resistant Helicobacter pylori to Fight against Gastric Diseases in Humans? eFood 2020, 1, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Howell, A.B. Potential of cranberry for suppressing Helicobacter pylori, a risk factor for gastric cancer. J. Berry Res. 2020, 10, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.; Gautam, L.K.; Kaur, I.R. Effect of oral cranberry extract (standardized proanthocyanidin-A) in patients with recurrent UTI by pathogenic E. coli: A randomized placebo-controlled clinical research study. Int. Urol. Nephrol. 2016, 48, 1379–1386. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y.R.; Howell, A.B.; Vorsa, N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry 2000, 54, 173–181. [Google Scholar] [CrossRef]
- Joshi, S.S.; Howell, A.B.; D’Souza, D.H. Antiviral effects of blueberry proanthocyanidins against Aichi virus. Food Microbiol. 2019, 82, 202–208. [Google Scholar] [CrossRef]
- Weiss, E.I.; Kozlovsky, A.; Steinberg, D.; Lev-Dor, R.; Bar Ness Greenstein, R.; Feldman, M.; Sharon, N.; Ofek, I. A high molecular mass cranberry constituent reduces mutans streptococci level in saliva and inhibits in vitro adhesion to hydroxyapatite. FEMS Microbiol. Lett. 2004, 232, 89–92. [Google Scholar] [CrossRef]
- Paturi, G.; Mandimika, T.; Butts, C.A.; Zhu, S.; Roy, N.C.; McNabb, W.C.; Ansell, J. Influence of dietary blueberry and broccoli on cecal microbiota activity and colon morphology in mdr1a−/− mice, a model of inflammatory bowel diseases. Nutrition 2012, 28, 324–330. [Google Scholar] [CrossRef]
- Molan, A.L.; Lila, M.A.; Mawson, J.; De, S. In vitro and in vivo evaluation of the prebiotic activity of water-soluble blueberry extracts. World J. Microbiol. Biotechnol. 2009, 25, 1243–1249. [Google Scholar] [CrossRef]
- Castro, D.; Teodoro, A. Anticancer Properties of Bioactive Compounds of Berry Fruits—A Review. Br. J. Med. Med. Res. 2015, 6, 771–794. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [Green Version]
- Sosa, V.; Moline, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Somasagara, R.R.; Hegde, M.; Chiruvella, K.K.; Musini, A.; Choudhary, B.; Raghavan, S.C. Extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS ONE 2012, 7, e47021. [Google Scholar] [CrossRef] [Green Version]
- Shi, N.; Clinton, S.K.; Liu, Z.; Wang, Y.; Riedl, K.M.; Schwartz, S.J.; Zhang, X.; Pan, Z.; Chen, T. Strawberry phytochemicals inhibit azoxymethane/dextran sodium sulfate-induced colorectal carcinogenesis in Crj: CD-1 mice. Nutrients 2015, 7, 1696–1715. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Seeram, N.P.; Lee, R.; Feng, L.; Heber, D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J. Agric. Food Chem. 2008, 56, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yan, F.; Qian, J.; Guo, M.; Zhang, H.; Tang, X.; Chen, F.; Stoner, G.D.; Wang, X. Randomized phase II trial of lyophilized strawberries in patients with dysplastic precancerous lesions of the esophagus. Cancer Prev. Res. 2012, 5, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef] [PubMed]
- Coates, E.M.; Popa, G.; Gill, C.I.; McCann, M.J.; McDougall, G.J.; Stewart, D.; Rowland, I. Colon-available raspberry polyphenols exhibit anti-cancer effects on in vitro models of colon cancer. J. Carcinog. 2007, 6, 4. [Google Scholar] [CrossRef]
- Wang, L.S.; Hecht, S.S.; Carmella, S.G.; Yu, N.; Larue, B.; Henry, C.; McIntyre, C.; Rocha, C.; Lechner, J.F.; Stoner, G.D. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev. Res. 2009, 2, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Harris, G.K.; Gupta, A.; Nines, R.G.; Kresty, L.A.; Habib, S.G.; Frankel, W.L.; LaPerle, K.; Gallaher, D.D.; Schwartz, S.J.; Stoner, G.D. Effects of lyophilized black raspberries on azoxy-methane-induced colon cancer and 8-hydroxy-2-deoxyguanosine levels in the Fischer 344 rat. Nutr. Cancer 2001, 40, 125–133. [Google Scholar] [CrossRef]
- Wang, L.S.; Dombkowski, A.A.; Seguin, C.; Rocha, C.; Cukovic, D.; Mukundan, A.; Henry, C.; Stoner, G.D. Mechanistic basis for the chemopreventive effects of black raspberries at a late stage of rat esophageal carcinogenesis. Mol. Carcinog. 2011, 50, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Stoner, G.D.; Wang, L.S.; Seguin, C.; Rocha, C.; Stoner, K.; Chiu, S.; Kinghorn, A.D. Multiple berry types prevent N-nitrosomethylbenzylamine-induced esophageal cancer in rats. Pharm. Res. 2010, 27, 1138–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallery, S.R.; Zwick, J.C.; Pei, P.; Tong, M.; Larsen, P.E.; Shumway, B.S.; Stoner, G.D. Topical application of a bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein in human premalignant oral lesions. Cancer Res. 2008, 68, 4945–4957. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Garavello, W.; Talamini, R.; Negri, E.; Bosetti, C.; Dal Maso, L.; Lagiou, P.; Tavani, A.; Polesel, J.; Barzan, L.; et al. Flavonoids and the risk of oral and pharyngeal cancer: A case-control study from Italy. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1621–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresty, L.A.; Frankel, W.L.; Hammond, C.D.; Baird, M.E.; Mele, J.M.; Stoner, G.D.; Fromkes, J.J. Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: Interim results show berries modulate markers of oxidative stress in Barrett’s esophagus patients. Nutr. Cancer 2006, 54, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Arnold, M.; Huang, Y.W.; Sardo, C.; Seguin, C.; Martin, E.; Huang, T.H.; Riedl, K.; Schwartz, S.; Frankel, W.; et al. Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: A phase I pilot study. Clin. Cancer Res. 2011, 17, 598–610. [Google Scholar] [CrossRef] [Green Version]
- Kresty, L.A.; Weh, K.M.; Zeyzus-Johns, B.; Perez, L.N.; Howell, A.B. Cranberry proanthocyanidins inhibit esophageal adenocarcinoma in vitro and in vivo through pleiotropic cell death induction and PI3K/AKT/mTOR inactivation. Oncotarget 2015, 6, 33438. [Google Scholar] [CrossRef] [Green Version]
- Weh, K.M.; Clarke, J.; Kresty, L.A. Cranberries and cancer: An update of preclinical studies evaluating the cancer inhibitory potential of cranberry and cranberry derived constituents. Antioxidants 2016, 5, 27. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [Green Version]
- Jeyabalan, J.; Aqil, F.; Munagala, R.; Annamalai, L.; Vadhanam, M.V.; Gupta, R.C. Chemopreventive and therapeutic activity of dietary blueberry against estrogen-mediated breast cancer. J. Agric. Food Chem. 2014, 62, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Hecht, S.; Carmella, S.; Seguin, C.; Rocha, C.; Yu, N.; Stoner, K.; Chiu, S.; Stoner, G. Berry ellagitannins may not be sufficient for prevention of tumors in the rodent esophagus. J. Agric. Food Chem. 2010, 58, 3992–3995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duthie, S.J. Berry phytochemicals, genomic stability and cancer: Evidence for chemoprotection at several stages in the carcinogenic process. Mol. Nutr. Food Res. 2007, 51, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Agullo, G.; Gamet, L.; Besson, C.; Demigné, C.; Rémésy, C. Quercetin exerts a preferential cytotoxic effect on active dividing colon carcinoma HT29 and Caco-2 cells. Cancer Lett. 1994, 87, 55–63. [Google Scholar] [CrossRef]
- Kellogg, J.; Wang, J.; Flint, C.; Ribnicky, D.; Kuhn, P.; De Mejia, E.G.; Raskin, I.; Lila, M.A. Alaskan wild berry resources and human health under the cloud of climate change. J. Agric. Food Chem. 2010, 58, 3884–3900. [Google Scholar] [CrossRef] [Green Version]
- McDougall, G.J.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef]
- Grussu, D.; Stewart, D.; McDougall, G.J. Berry polyphenols inhibit α-amylase in vitro: Identifying active components in rowanberry and raspberry. J. Agric. Food Chem. 2011, 59, 2324–2331. [Google Scholar] [CrossRef]
- Tsuda, T. Regulation of adipocyte function by anthocyanins; possibility of preventing the metabolic syndrome. J. Agric. Food Chem. 2008, 56, 642–646. [Google Scholar] [CrossRef]
- Chao, C.Y.; Mong, M.C.; Chan, K.C.; Yin, M.C. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J. Agric. Food Chem. 2005, 53, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Ward, L.; Brown, L. Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2013, 52, 559–568. [Google Scholar] [CrossRef]
- Törrönen, R.; Kolehmainen, M.; Sarkkinen, E.; Poutanen, K.; Mykkänen, H.; Niskanen, L. Berries reduce postprandial insulin responses to wheat and rye breads in healthy women. J. Nutr. 2013, 143, 430–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvano, A.; Izuora, K.; Oh, E.C.; Ebersole, J.L.; Lyons, T.J.; Basu, A. Dietary berries, insulin resistance and type 2 diabetes: An overview of human feeding trials. Food Funct. 2019, 10, 6227–6243. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Pinto, M.; de Carvalho, J.E.; Lajolo, F.M.; Genovese, M.I.; Shetty, K. Evaluation of antiproliferative, anti-type 2 diabetes, and antihypertension potentials of ellagitannins from strawberries (Fragaria × ananassa Duch.) using in vitro models. J. Med. Food 2010, 13, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, Y.; Ozeki, A.; Tani, T.; Tsuda, T. Blackcurrant extract ameliorates hyperglycemia in type 2 diabetic mice in association with increased basal secretion of glucagon-like peptide-1 and activation of AMP-activated protein kinase. J. Nutr. Sci. Vitaminol. 2018, 64, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Elks, C.M.; Terrebonne, J.D.; Ingram, D.K.; Stephens, J.M. Blueberries improve glucose tolerance without altering body composition in obese postmenopausal mice. Obesity 2015, 23, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, T. Possible abilities of dietary factors to prevent and treat diabetes via the stimulation of glucagon-like peptide-1 secretion. Mol. Nutr. Food Res. 2015, 59, 1264–1273. [Google Scholar] [CrossRef]
- Wilson, T.; Luebke, J.L.; Morcomb, E.F.; Carrell, E.J.; Leveranz, M.C.; Kobs, L.; Schmidt, T.P.; Limburg, P.J.; Vorsa, N.; Singh, A.P. Glycemic responses to sweetened dried and raw cranberries in humans with type 2 diabetes. J. Food Sci. 2010, 75, H218–H223. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Meyers, S.L.; Singh, A.P.; Limburg, P.J.; Vorsa, N. Favorable glycemic response of type 2 diabetics to low-calorie cranberry juice. J. Food Sci. 2008, 73, H241–H245. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.; Ndou, T.; Hughey, C.A.; Straut, C.; Howell, A.; Dai, Z.; Kaletunc, G. Inhibition of α-amylase and glucoamylase by tannins extracted from cocoa, pomegranates, cranberries, and grapes. J. Agric. Food Chem. 2013, 61, 1477–1486. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Wang, W.; Khoo, C.; Taylor, J.; Gu, L. Cranberry phytochemicals inhibit glycation of human hemoglobin and serum albumin by scavenging reactive carbonyls. Food Funct. 2011, 2, 475–482. [Google Scholar] [CrossRef]
- Paquette, M.; Larqué, A.S.M.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonné, S.; Marette, A.; Jacques, H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: A parallel, double-blind, controlled and randomised clinical trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef] [Green Version]
- Novotny, J.A.; Baer, D.J.; Khoo, C.; Gebauer, S.K.; Charron, C.S. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults. J. Nutr. 2015, 145, 1185–1193. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Park, H.J.; Kang, S.N.; Jang, S.H.; Lee, S.J.; Ko, Y.G.; Kim, G.S.; Cho, J.H. Blueberry peel extracts inhibit adipogenesis in 3T3-L1 cells and reduce high-fat diet-induced obesity. PLoS ONE 2013, 8, e69925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-β-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef]
- Prior, R.L.; Wilkes, S.; Rogers, T.; Khanal, R.C.; Wu, X.; Howard, L.R. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 2010, 58, 3970–3976. [Google Scholar] [CrossRef]
- Seymour, E.M.; Tanone, I.I.; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A.; Kondoleon, M.G.; Kaufman, P.B.; Bolling, S.F. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J. Med. Food 2011, 14, 1511–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zunino, S.J.; Parelman, M.A.; Freytag, T.L.; Stephensen, C.B.; Kelley, D.S.; Mackey, B.E.; Woodhouse, L.R.; Bonnel, E.L. Effects of dietary strawberry powder on blood lipids and inflammatory markers in obese human subjects. Br. J. Nutr. 2012, 108, 900–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, A.; Kurien, B.T.; Tran, H.; Maher, J.; Schell, J.; Masek, E.; Schell, J.; Masek, E.; Barrett, J.R.; Lyons, T.J.; et al. Strawberries decrease circulating levels of tumor necrosis factor and lipid peroxides in obese adults with knee osteoarthritis. Food Funct. 2018, 9, 6218–6226. [Google Scholar] [CrossRef]
- Fernandes, I.; Marques, F.; de Freitas, V.; Mateus, N. Antioxidant and antiproliferative properties of methylated metabolites of anthocyanins. Food Chem. 2013, 141, 2923–2933. [Google Scholar] [CrossRef]
- Liu, W.; Lu, X.; He, G.; Gao, X.; Li, M.; Wu, J.; Li, Z.; Wu, J.; Wang, J.; Luo, C. Cytosolic protection against ultraviolet induced DNA damage by blueberry anthocyanins and anthocyanidins in hepatocarcinoma HepG2 cells. Biotechnol. Lett. 2013, 35, 491–498. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, Y.; Li, C.; Sui, Z.; Min, W. Effect of blueberry anthocyanins malvidin and glycosides on the antioxidant properties in endothelial cells. Oxidative Med. Cell. Longev. 2016, 2016, e1591803. [Google Scholar] [CrossRef] [Green Version]
- Wightman, J.D.; Heuberger, R.A. Effect of grape and other berries on cardiovascular health. J. Sci. Food Agric. 2015, 95, 1584–1597. [Google Scholar] [CrossRef]
- Lin, L.; Li, J.; Lv, H.; Ma, Y.; Qian, Y. Effect of Lycium ruthenicum anthocyanins on atherosclerosis in mice. Zhongguo Zhong Yao Za Zhi 2012, 37, 1460–1466. [Google Scholar]
- Rodriguez-Mateos, A.; Istas, G.; Boschek, L.; Feliciano, R.P.; Mills, C.E.; Boby, C.; Gomez-Alonso, S.; Milenkovic, D.; Heiss, C. Circulating anthocyanin metabolites mediate vascular benefits of blueberries: Insights from randomized controlled trials, metabolomics, and nutrigenomics. J. Gerontol. Ser. A 2019, 74, 967–976. [Google Scholar] [CrossRef]
- Wang, C.K.; Fu, H.Y.; Chiang, M. Cardiovascular disease prevention of cranberry vinegar. Nutr. Sci. 2007, 32, 129–132. [Google Scholar]
- Rodriguez-Mateos, A.; Feliciano, R.P.; Boeres, A.; Weber, T.; Dos Santos, C.N.; Ventura, M.R.; Heiss, C. Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study. Mol. Nutr. Food Res. 2016, 60, 2130–2140. [Google Scholar] [CrossRef] [Green Version]
- Radovanović, B.C.; Anđelković, S.M.; Radovanović, A.B.; Anđelković, M.Z. Antioxidant and antimicrobial activity of polyphenol extracts from wild berry fruits grown in southeast Serbia. Trop. J. Pharm. Res. 2013, 12, 813–819. [Google Scholar] [CrossRef]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Riso, P.; Klimis-Zacas, D.; Del Bo, C.; Martini, D.; Campolo, J.; Vendrame, S.; Møller, P.; Loft, S.; De Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2013, 52, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.L.; Zahradka, P.; Taylor, C.G. Efficacy of flavonoids in the management of high blood pressure. Nutr. Rev. 2015, 73, 799–822. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Klimis-Zacas, D. Potential factors influencing the effects of anthocyanins on blood pressure regulation in humans: A review. Nutrients 2019, 11, 1431. [Google Scholar] [CrossRef] [Green Version]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Edwards, V.C.; Willems, M.E.T. Cardiovascular function during supine rest in endurance-trained males with New Zealand blackcurrant: A dose–response study. Eur. J. Appl. Physiol. 2017, 117, 247–254. [Google Scholar] [CrossRef]
- Jeong, H.S.; Kim, S.; Hong, S.J.; Choi, S.C.; Choi, J.H.; Kim, J.H.; Park, C.Y.; Cho, J.Y.; Lee, T.B.; Kwon, J.W.; et al. Black raspberry extract increased circulating endothelial progenitor cells and improved arterial stiffness in patients with metabolic syndrome: A randomized controlled trial. J. Med. Food 2016, 19, 346–352. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, J.; Lu, W.; Wang, X.; Han, Z.; Qiu, C. Effects of blueberry supplementation on blood pressure: A systematic review and meta-analysis of randomized clinical trials. J. Hum. Hypertens. 2017, 31, 165–171. [Google Scholar] [CrossRef]
- Whyte, A.R.; Cheng, N.; Fromentin, E.; Williams, C.M. A randomized, double-blinded, placebo-controlled study to compare the safety and efficacy of low dose enhanced wild blueberry powder and wild blueberry extract (ThinkBlue™) in maintenance of episodic and working memory in older adults. Nutrients 2018, 10, 660. [Google Scholar] [CrossRef] [Green Version]
- Ramassamy, C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: A review of their intracellular targets. Eur. J. Pharmacol. 2006, 545, 51–64. [Google Scholar] [CrossRef]
- Van Bulck, M.; Sierra-Magro, A.; Alarcon-Gil, J.; Perez-Castillo, A.; Morales-Garcia, J.A. Novel Approaches for the Treatment of Alzheimer’s and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 719. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.F.; Pogačnik, L. Polyphenols from food and natural products: Neuroprotection and safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, S.L.; Silva, V.D.; Dos Santos Souza, C.; Santos, C.C.; Paris, I.; Munoz, P.; Segura-Aguilar, J. Impact of Plant-Derived Flavonoids on Neurodegenerative Diseases. Neurotox. Res. 2016, 30, 41–52. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Gordon, S.L.; Brennan, R.M.; Stewart, D. Anthocyanin-flavanol adducts from blackcurrant (Ribes nigrum L.). J. Agric. Food Chem. 2005, 53, 7878–7885. [Google Scholar] [CrossRef]
- Ghosh, D.; McGhie, T.K.; Zhang, J.; Adaim, A.; Skinner, M. Effects of anthocyanins and other phenolics of boysenberry and blackcurrant as inhibitors of oxidative stress and damage to cellular DNA in SH-SY5Y and HL-60 cells. J. Sci. Food Agric. 2006, 86, 678–686. [Google Scholar] [CrossRef]
- Watson, A.W.; Haskell-Ramsay, C.F.; Kennedy, D.O.; Cooney, J.M.; Trower, T.; Scheepens, A. Acute supplementation with blackcurrant extracts modulates cognitive functioning and inhibits monoamine oxidase-B in healthy young adults. J. Funct. Foods 2015, 17, 524–539. [Google Scholar] [CrossRef] [Green Version]
- Watson, A.W.; Okello, E.J.; Brooker, H.J.; Lester, S.; McDougall, G.J.; Wesnes, K.A. The impact of blackcurrant juice on attention, mood and brain wave spectral activity in young healthy volunteers. Nutr. Neurosci. 2019, 22, 596–606. [Google Scholar] [CrossRef] [Green Version]
- Shukitt-Hale, B.; Cheng, V.; Joseph, J.A. Effects of blackberries on motor and cognitive function in aged rats. Nutr. Neurosci. 2009, 12, 135–140. [Google Scholar] [CrossRef]
- Debom, G.; Gazal, M.; Soares, M.S.P.; do Couto, C.A.T.; Mattos, B.; Lencina, C.; Kaster, M.P.; Ghisleni, G.C.; Tavares, R.; Braganhol, E.; et al. Preventive effects of blueberry extract on behavioral and biochemical dysfunctions in rats submitted to a model of manic behavior induced by ketamine. Brain Res. Bull. 2016, 127, 260–269. [Google Scholar] [CrossRef]
- Yao, Y.; Vieira, A. Protective activities of Vaccinium antioxidants with potential relevance to mitochondrial dysfunction and neurotoxicity. Neurotoxicology 2007, 28, 93–100. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Galli, R.L.; Meterko, V.; Carey, A.; Bielinski, D.F.; McGhie, T.; Joseph, J.A. Dietary supplementation with fruit polyphenolics ameliorates age-related deficits in behavior and neuronal markers of inflammation and oxidative stress. Age 2005, 27, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Dreiseitel, A.; Schreier, P.; Oehme, A.; Locher, S.; Rogler, G.; Piberger, H.; Hajak, G.; Sand, P.G. Inhibition of proteasome activity by anthocyanins and anthocyanidins. Biochem. Biophys. Res. Commun. 2008, 372, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Johnson, S.L.; Liu, W.; DaSilva, N.A.; Meschwitz, S.; Dain, J.A.; Seeram, N.P. Evaluation of polyphenol anthocyanin-enriched extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry for free radical scavenging, reactive carbonyl species trapping, anti-glycation, anti-β-amyloid aggregation, and microglial neuroprotective effects. Int. J. Mol. Sci. 2018, 19, 461. [Google Scholar]
- Farbood, Y.; Sarkaki, A.; Dianat, M.; Khodadadi, A.; Haddad, M.K.; Mashhadizadeh, S. Ellagic acid prevents cognitive and hippocampal long-term potentiation deficits and brain inflammation in rat with traumatic brain injury. Life Sci. 2015, 124, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Jo, M.G.; Badshah, H.; Kim, M.O. Anthocyanins protect against LPS-induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem. Int. 2016, 100, 1–10. [Google Scholar] [CrossRef]
- Shah, S.A.; Amin, F.U.; Khan, M.; Abid, M.N.; Rehman, S.U.; Kim, T.H.; Kim, M.W.; Kim, M.O. Anthocyanins abrogate glutamate-induced AMPK activation, oxidative stress, neuroinflammation, and neurodegeneration in postnatal rat brain. J. Neuroinflamm. 2016, 13, 286. [Google Scholar] [CrossRef] [Green Version]
- Meireles, M.; Marques, C.; Norberto, S.; Fernandes, I.; Mateus, N.; Rendeiro, C.; Spencer, J.P.; Faria, A.; Calhau, C. The impact of chronic blackberry intake on the neuroinflammatory status of rats fed a standard or high-fat diet. J. Nutr. Biochem. 2015, 26, 1166–1173. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Huang, I.-M.; Hwang, L.S.; Ho, C.-T.; Li, S.; Lo, C.-Y. Anthocyanins in blackcurrant effectively prevent the formation of advanced glycation end products by trapping methylglyoxal. J. Funct. Foods 2014, 8, 259–268. [Google Scholar] [CrossRef]
| Nutrient | Strawberry | Blackberry | Raspberry | Cranberry | Blueberry | Blackcurrant |
|---|---|---|---|---|---|---|
| Water (g) | 90.95 | 88.15 | 85.75 | 87.32 | 84.21 | 83.95 |
| Energy (kcal) | 32 | 43 | 52 | 46 | 57 | 56 |
| Protein (g) | 0.67 | 1.39 | 1.2 | 0.46 | 0.74 | 1.4 |
| Total lipid (fat) (g) | 0.3 | 0.49 | 0.65 | 0.13 | 0.33 | 0.2 |
| Carbohydrate (g) | 7.68 | 9.61 | 11.94 | 11.97 | 14.49 | 13.8 |
| Fiber, total dietary (g) | 2 | 5.3 | 6.5 | 3.6 | 2.4 | 4.3 |
| Sugars, total (g) | 4.89 | 4.88 | 4.42 | 4.27 | 9.96 | 7.37 |
| Calcium, Ca (mg) | 16 | 29 | 25 | 8 | 6 | 33 |
| Iron, Fe (mg) | 0.41 | 0.62 | 0.69 | 0.23 | 0.28 | 1 |
| Magnesium, Mg (mg) | 13 | 20 | 22 | 6 | 6 | 13 |
| Phosphorus, P (mg) | 24 | 22 | 29 | 11 | 12 | 44 |
| Potassium, K (mg) | 153 | 162 | 151 | 80 | 77 | 275 |
| Sodium, Na (mg) | 1 | 1 | 1 | 2 | 1 | 1 |
| Zinc, Zn (mg) | 0.14 | 0.53 | 0.42 | 0.09 | 0.16 | 0.23 |
| Copper, Cu (mg) | 0.048 | 0.165 | 0.09 | 0.056 | 0.057 | 0.107 |
| Selenium, Se (µg) | 0.4 | 0.4 | 0.2 | 0.1 | 0.1 | 0.6 |
| Vitamin C (mg) | 58.8 | 21 | 26.2 | 14 | 9.7 | 41 |
| Thiamin (mg) | 0.024 | 0.02 | 0.032 | 0.012 | 0.037 | 0.04 |
| Riboflavin (mg) | 0.022 | 0.026 | 0.038 | 0.02 | 0.041 | 0.05 |
| Niacin (mg) | 0.386 | 0.646 | 0.598 | 0.101 | 0.418 | 0.1 |
| Vitamin B6 (mg) | 0.047 | 0.03 | 0.055 | 0.057 | 0.052 | 0.07 |
| Folate, total (µg) | 24 | 25 | 21 | 1 | 6 | 8 |
| Vitamin A (µg) | 1 | 11 | 2 | 3 | 3 | 2 |
| Carotene, beta (µg) | 7 | 128 | 12 | 38 | 32 | 25 |
| Carotene, alpha (µg) | 0 | 0 | 16 | 0 | 0 | 0 |
| Lutein + zeaxanthin (µg) | 26 | 118 | 136 | 91 | 80 | 47 |
| Vitamin E (mg) | 0.29 | 1.17 | 0.87 | 1.32 | 0.57 | 0.1 |
| Vitamin K (phylloquinone) (µg) | 2.2 | 19.8 | 7.8 | 5 | 19.3 | 11 |
| Berries | Phenolics | Anthocyanins Contents |
|---|---|---|
| Strawberry (Fragaria ananassa) | 317.2–443.4 [13] | 32.6–52.4 [14] |
| 209.0–318.0 [15] | 21.2–41.7 [16] | |
| 264.0–324.0 [17] | 32.0–36.0 [18] | |
| Blackberry (Rubus fructicosus) | 411.0–459.0 [17] | 245.0–300.5 [16] |
| 417.8–555.2 [19] | 114.4–241.5 [20] | |
| 472.0–678.0 [21] | 110.5–122.7 [19] | |
| 143.0–211.0 [21] | ||
| Blueberry (Vaccinium corymbosum) | 181.1–390.5 [22] | 93.1–235.4 [22] |
| 261.9–585.3 [19] | 94.5–301.0 [23] | |
| 154.7–398.0 [23] | 308.9–464.3 [16] | |
| 212.7–460.4 [24] | 143.5–822.7 [20] | |
| 314.0–382.0 [17] | 35.5–129.9 [19] | |
| Cranberry (Vaccinium macrocarpon) | 120.0–176.5 [25] | 19.8–65.6 [25] |
| 163.4–315.9 [26] | 111.5–168.5 [16] | |
| 68.4–87.0 [27] | ||
| Raspberry (Rubus idaeous) | 192.0–359.0 [28] | 62.0–68.0 [29] |
| 505.0–529.0 [29] | 19.0–51.0 [28] | |
| 305.5–378.5 [30] | 39.4–53.9 [14] | |
| 305.8–503.9 [29] | 72.4–111.8 [16] | |
| 295.0–310.0 [17] | 41.8–86.2 [31] | |
| 68.0–80.0 [18] | ||
| Black Raspberry (Rubus occidentalis) | 489.3–875.3 [30] | 318.6–332.4 [31] |
| 970.0–990.0 [29] | 585.0–593.0 [29] | |
| 699.2–730.2 [31] | 464.0–627.0 [21] | |
| 890.0–1079.0 [21] | ||
| Blackcurrant (Ribes nigrum) | 498.0–1342.0 [21] | 128.0–411.0 [21] |
| 817.0–1042.0 [32] | 361.0–591.0 [16] | |
| 233.4–237.8 [33] |
| Berries | Total Ellagic Acid After Hydrolysis | Free Ellagic Acid |
|---|---|---|
| Strawberry (Fragaria ananassa) | 25.0–56.4 [13] | 2.1–28.8 [13] |
| 19.3–48.3 [15] | 0.6–2.6 [15] | |
| 71.4–78.5 [14] | 0.7–4.3 [14] | |
| Blackberry (Rubus fructicosus) | 30.0–33.8 [19] | ND 1 |
| 10.6–51.5 [34] | ||
| 35.7–54.7 [35] | ||
| Blueberry (Vaccinium corymbosum) | 0.8–6.7 [19] | ND 1 |
| Cranberry (Vaccinium macrocarpon) | ND 1 | ND 1 |
| Raspberry (Rubus idaeous) | 260.0–326.2 [14] | 3.7–4.7 [14] |
| 83.9–210.4 [31] | 2.0–5.5 [31] | |
| 61.2–117.4 [36] | ||
| 38.0–118.0 [28] | ||
| Black Raspberry (Rubus occidentalis) | 234.2–258.4 [31] | 3.7–3.9 [31] |
| Blackcurrant (Ribes nigrum) | ND 1 | ND 1 |
| Berries | Proanthocyanidins |
|---|---|
| Strawberry (Fragaria ananassa) | 15.0–183.0 [42] |
| 34.2–57.0 [39] | |
| 120.1–169.9 [43] | |
| Blackberry (Rubus fructicosus) | 5.0–46.0 [42] |
| 9.5–44.0 [43] | |
| Blueberry (Vaccinium corymbosum) | 311.0–335.0 [42] |
| 296.0–330.0 [39] | |
| 318.0–346.0 [43] | |
| Cranberry (Vaccinium macrocarpon) | 646.5–691.3 [27] |
| 343.0–494.0 [43] | |
| 399.0–412.0 [39] | |
| Raspberry (Rubus idaeous) | 76.9–80.6 [39] |
| Black Raspberry (Rubus occidentalis) | 3.0–74.0 [42] |
| Blackcurrant (Ribes nigrum) | 105.0–255.0 [42] |
| 120.6–165.8 [44] | |
| 114.8–180.8 [43] |
| Berries | Flavonols |
|---|---|
| Strawberry (Fragaria ananassa) | 1.8–5.6 [15] |
| 1.8–6.2 [13] | |
| 0.8–1.6 [18] | |
| 1.2–1.5 [46] | |
| Blackberry (Rubus fructicosus) | 10.2–16.0 [20] |
| 8.9–11.0 [19] | |
| Blueberry (Vaccinium corymbosum) | 15.0–17.0 [18] |
| 17.2–32.7 [20] | |
| 19.4–23.8 [19] | |
| 17.0–19.0 [47] | |
| Cranberry (Vaccinium macrocarpon) | 11.0–25.0 [48] |
| 15.7–26.3 [46] | |
| 18.4–36.0 [49] | |
| Raspberry (Rubus idaeous) | 0.9–2.0 [49] |
| 0.6–0.8 [46] | |
| 0.3–0.4 [47] | |
| Black Raspberry (Rubus occidentalis) | 10.3–19.0 [50,51] |
| Blackcurrant (Ribes nigrum) | 12.5–15.0 [52] |
| 8.8–11.5 [46] |
| Berry, Raw | ORAC Value, μmol TE/100 g |
|---|---|
| Strawberry | 2154–8384 [104] |
| Red Raspberry | 3748–5792 [104] 2220–2580 [29] |
| Blackberry | 4686–7610 [104] 4160–7880 [21] |
| Blueberry | 2746–9245 [104] 2627–6747 [24] |
| Blackcurrant | 5010–10,144 [104] 4450–9200 [21] |
| Cranberry | 8596–9679 [104] |
| Black raspberry | 7470–7970 [29] 10,030–14,600 [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golovinskaia, O.; Wang, C.-K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. https://doi.org/10.3390/molecules26133904
Golovinskaia O, Wang C-K. Review of Functional and Pharmacological Activities of Berries. Molecules. 2021; 26(13):3904. https://doi.org/10.3390/molecules26133904
Chicago/Turabian StyleGolovinskaia, Oksana, and Chin-Kun Wang. 2021. "Review of Functional and Pharmacological Activities of Berries" Molecules 26, no. 13: 3904. https://doi.org/10.3390/molecules26133904
APA StyleGolovinskaia, O., & Wang, C.-K. (2021). Review of Functional and Pharmacological Activities of Berries. Molecules, 26(13), 3904. https://doi.org/10.3390/molecules26133904







