Increasing Endocannabinoid Tone Alters Anxiety-Like and Stress Coping Behaviour in Female Rats Prenatally Exposed to Valproic Acid
Abstract
1. Introduction
2. Results
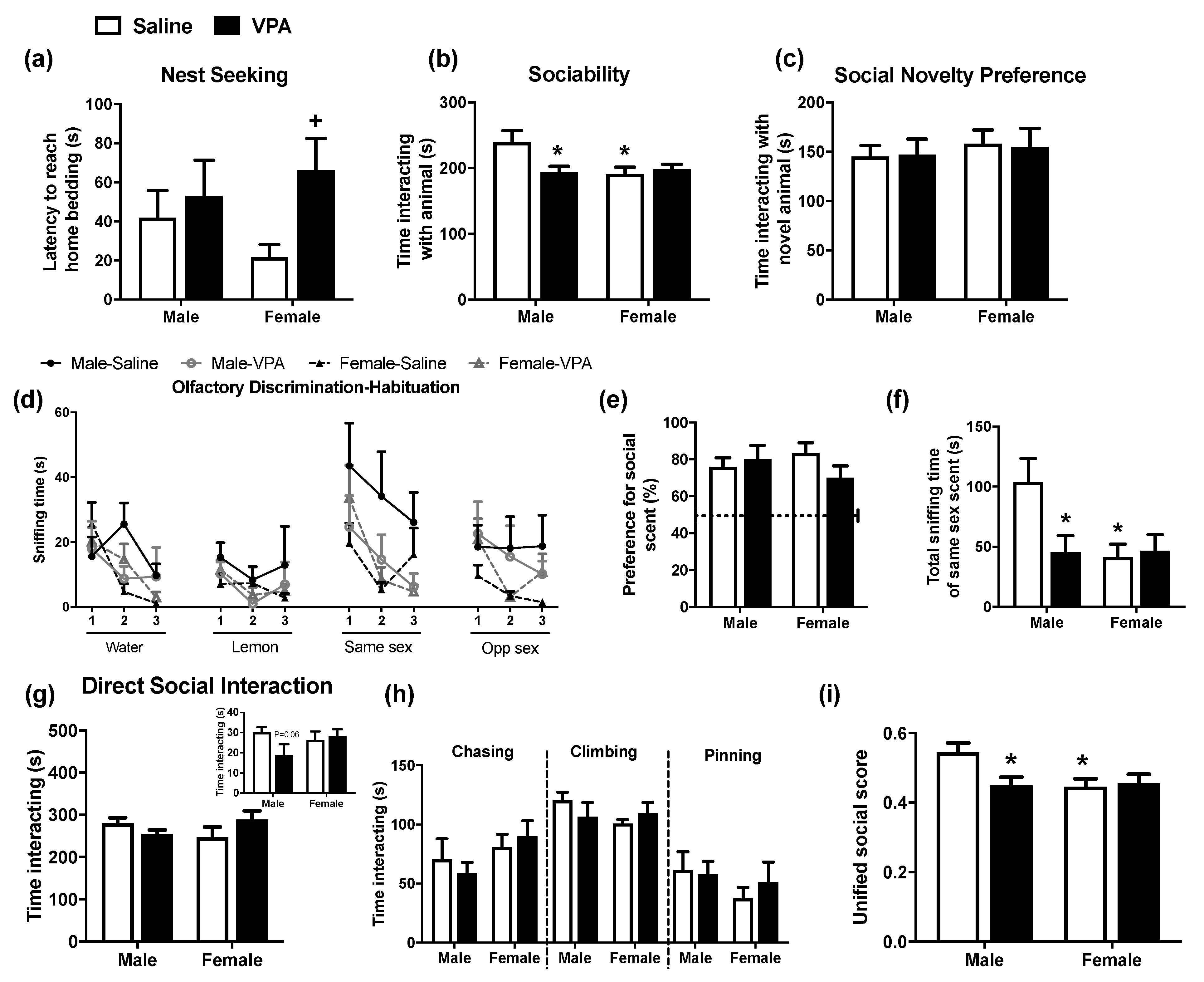
2.1. Prenatal VPA Exposure Induces Changes in Social Responding at Different Developmental Periods for Male and Female Rats
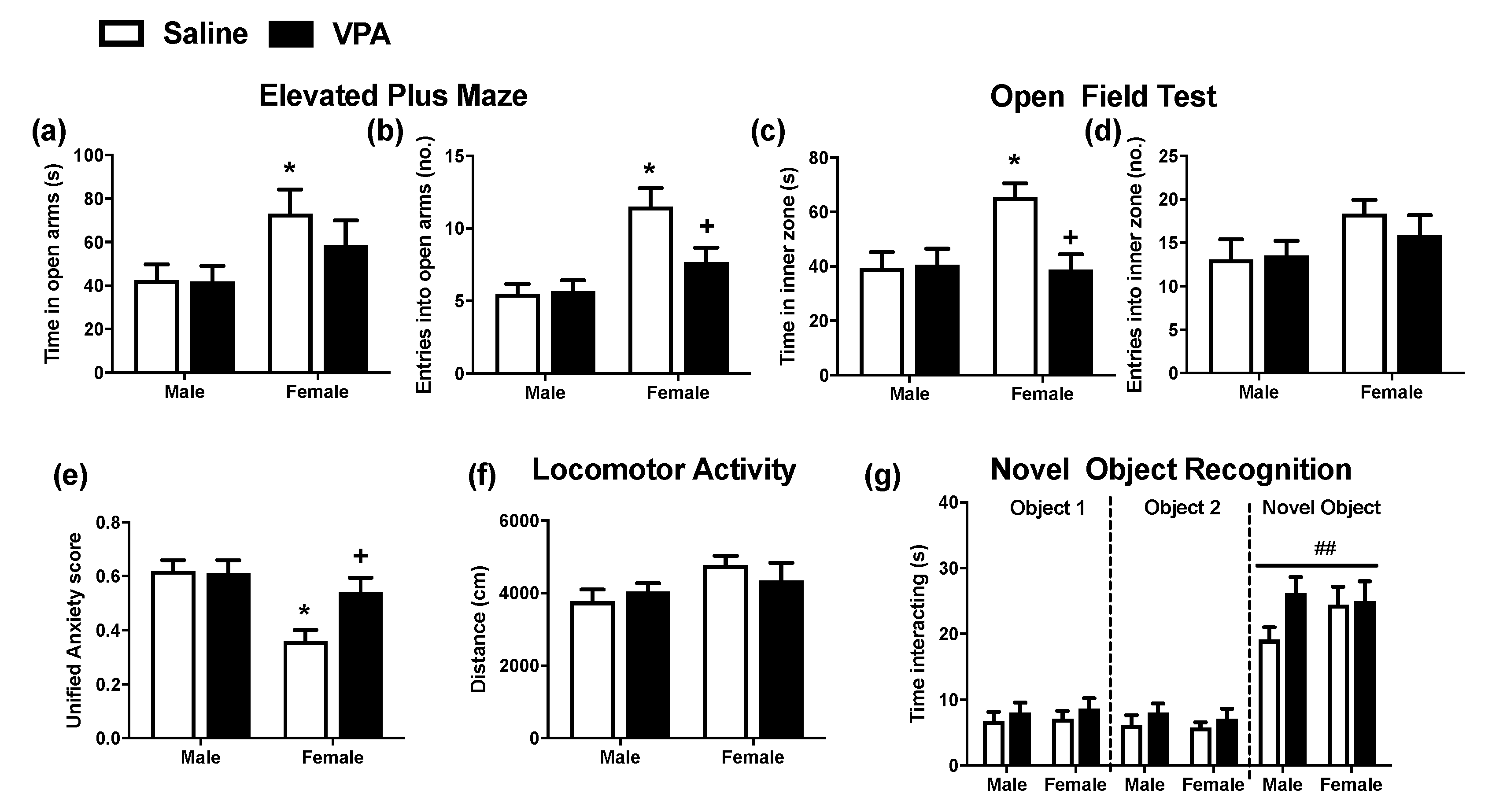
2.2. Prenatal VPA Exposure Induces Anxiety-Like Behaviour in Female, But Not Male, Adolescent Rats
2.3. Prenatal VPA Exposure Does Not Alter Locomotor Activity or Novel Object Recognition in Female or Male Adolescent Rats
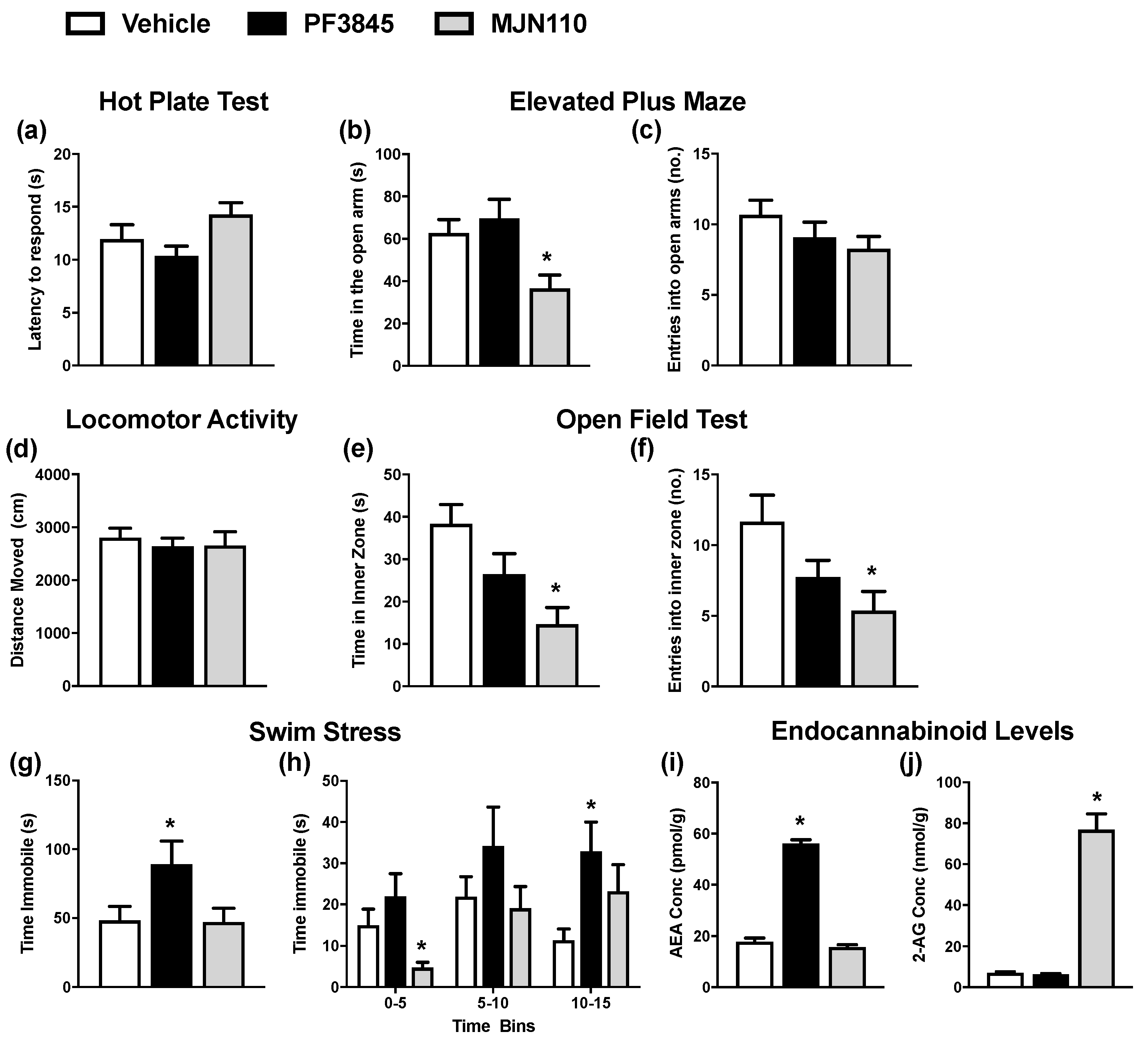
2.4. Enhancing 2-AG and AEA Levels Alters Anxiety-Like and Stress Coping Behaviour in VPA-Exposed Female Rats, Respectively
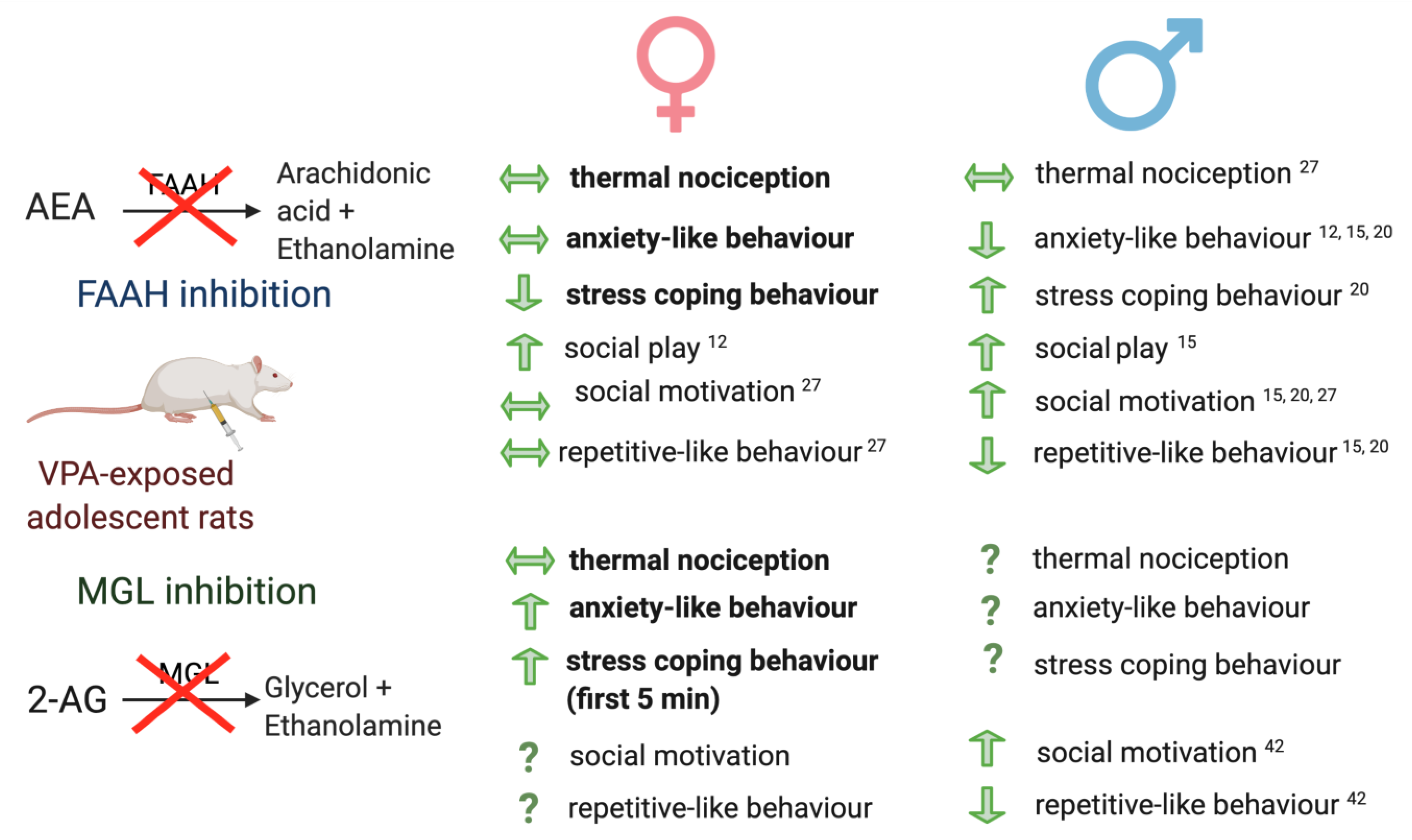
3. Discussion
4. Materials and Methods
4.1. Animals and Prenatal Administration of Valproic Acid
4.2. Experimental Design
4.3. Behaviour
4.3.1. Nest Seeking
4.3.2. Three-Chamber Test
4.3.3. Olfactory Habituation/Dishabituation Test
4.3.4. Direct Social Interaction
4.3.5. Elevated Plus Maze
4.3.6. Open Field Test
4.3.7. Novel Object Recognition
4.3.8. Hot Plate Test
4.3.9. Forced Swim Stress
4.4. Unified Behavioural Scoring
4.5. Quantification of Endocannabinoid Concentrations Using Liquid Chromatography–Tandem Mass Spectrometry
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- American Psychiatric Assocation. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Failla, M.D.; Schwartz, K.L.; Chaganti, S.; Cutting, L.E.; Landman, B.A.; Cascio, C.J. Using phecode analysis to characterize co-occurring medical conditions in autism spectrum disorder. Autism 2021, 25, 800–811. [Google Scholar] [CrossRef]
- Simonoff, E.; Pickles, A.; Charman, T.; Chandler, S.; Loucas, T.; Baird, G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Daniels, J.; Warren, Z.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years-Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef]
- Head, A.M.; McGillivray, J.A.; Stokes, M.A. Gender differences in emotionality and sociability in children with autism spectrum disorders. Mol. Autism 2014, 5, 19. [Google Scholar] [CrossRef]
- Supekar, K.; Menon, V. Sex differences in structural organization of motor systems and their dissociable links with repetitive/restricted behaviors in children with autism. Mol. Autism 2015, 6, 50. [Google Scholar] [CrossRef]
- Roullet, F.I.; Lai, J.K.; Foster, J.A. In utero exposure to valproic acid and autism--a current review of clinical and animal studies. Neurotoxicol. Teratol. 2013, 36, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, A.M.; Schiavi, S.; Calamandrei, G.; Trezza, V. Prenatal valproate in rodents as a tool to understand the neural underpinnings of social dysfunctions in autism spectrum disorder. Neuropharmacology 2019, 159, 107477. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Gronborg, T.K.; Sorensen, M.J.; Schendel, D.; Parner, E.T.; Pedersen, L.H.; Vestergaard, M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013, 309, 1696–1703. [Google Scholar] [CrossRef]
- Nicolini, C.; Fahnestock, M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Melancia, F.; Schiavi, S.; Servadio, M.; Cartocci, V.; Campolongo, P.; Palmery, M.; Pallottini, V.; Trezza, V. Sex-specific autistic endophenotypes induced by prenatal exposure to valproic acid involve anandamide signalling. Br. J. Pharmacol. 2018, 175, 3699–3712. [Google Scholar] [CrossRef]
- Schneider, T.; Przewlocki, R. Behavioral alterations in rats prenatally exposed to valproic acid: Animal model of autism. Neuropsychopharmacology 2005, 30, 80–89. [Google Scholar] [CrossRef]
- Servadio, M.; Manduca, A.; Melancia, F.; Leboffe, L.; Schiavi, S.; Campolongo, P.; Palmery, M.; Ascenzi, P.; di Masi, A.; Trezza, V. Impaired repair of DNA damage is associated with autistic-like traits in rats prenatally exposed to valproic acid. Eur. Neuropsychopharmacol. 2018, 28, 85–96. [Google Scholar] [CrossRef]
- Servadio, M.; Melancia, F.; Manduca, A.; di Masi, A.; Schiavi, S.; Cartocci, V.; Pallottini, V.; Campolongo, P.; Ascenzi, P.; Trezza, V. Targeting anandamide metabolism rescues core and associated autistic-like symptoms in rats prenatally exposed to valproic acid. Transl. Psychiatry 2016, 6, e902. [Google Scholar] [CrossRef]
- Scheggi, S.; Guzzi, F.; Braccagni, G.; De Montis, M.G.; Parenti, M.; Gambarana, C. Targeting PPARalpha in the rat valproic acid model of autism: Focus on social motivational impairment and sex-related differences. Mol. Autism 2020, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Favre, M.R.; Barkat, T.R.; Lamendola, D.; Khazen, G.; Markram, H.; Markram, K. General developmental health in the VPA-rat model of autism. Front. Behav. Neurosci. 2013, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, S.; Iezzi, D.; Manduca, A.; Leone, S.; Melancia, F.; Carbone, C.; Petrella, M.; Mannaioni, G.; Masi, A.; Trezza, V. Reward-Related Behavioral, Neurochemical and Electrophysiological Changes in a Rat Model of Autism Based on Prenatal Exposure to Valproic Acid. Front. Cell. Neurosci. 2019, 13, 479. [Google Scholar] [CrossRef]
- Hughes, E.M.; Calcagno, P.; Clarke, M.; Sanchez, C.; Smith, K.; Kelly, J.P.; Finn, D.P.; Roche, M. Prenatal exposure to valproic acid reduces social responses and alters mRNA levels of opioid receptor and pre-pro-peptide in discrete brain regions of adolescent and adult male rats. Brain Res. 2020, 1732, 146675. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.F.; Lu, T.Y.; Chu, M.C.; Chen, P.S.; Lee, C.W.; Lin, H.C. Targeting the inhibition of fatty acid amide hydrolase ameliorate the endocannabinoid-mediated synaptic dysfunction in a valproic acid-induced rat model of Autism. Neuropharmacology 2019, 162, 107736. [Google Scholar] [CrossRef] [PubMed]
- Zamberletti, E.; Gabaglio, M.; Woolley-Roberts, M.; Bingham, S.; Rubino, T.; Parolaro, D. Cannabidivarin Treatment Ameliorates Autism-Like Behaviors and Restores Hippocampal Endocannabinoid System and Glia Alterations Induced by Prenatal Valproic Acid Exposure in Rats. Front. Cell. Neurosci. 2019, 13, 367. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Sharma, B. Minocycline ameliorates prenatal valproic acid induced autistic behaviour, biochemistry and blood brain barrier impairments in rats. Brain Res. 2016, 1630, 83–97. [Google Scholar] [CrossRef]
- Luhach, K.; Kulkarni, G.T.; Singh, V.P.; Sharma, B. Attenuation of neurobehavioural abnormalities by papaverine in prenatal valproic acid rat model of ASD. Eur. J. Pharmacol. 2021, 890, 173663. [Google Scholar] [CrossRef]
- Edalatmanesh, M.A.; Nikfarjam, H.; Vafaee, F.; Moghadas, M. Increased hippocampal cell density and enhanced spatial memory in the valproic acid rat model of autism. Brain Res. 2013, 1526, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Gzielo, K.; Potasiewicz, A.; Holuj, M.; Litwa, E.; Popik, P.; Nikiforuk, A. Valproic acid exposure impairs ultrasonic communication in infant, adolescent and adult rats. Eur. Neuropsychopharmacol. 2020, 41, 52–62. [Google Scholar] [CrossRef]
- Kazlauskas, N.; Seiffe, A.; Campolongo, M.; Zappala, C.; Depino, A.M. Sex-specific effects of prenatal valproic acid exposure on sociability and neuroinflammation: Relevance for susceptibility and resilience in autism. Psychoneuroendocrinology 2019, 110, 104441. [Google Scholar] [CrossRef]
- Kerr, D.M.; Gilmartin, A.; Roche, M. Pharmacological inhibition of fatty acid amide hydrolase attenuates social behavioural deficits in male rats prenatally exposed to valproic acid. Pharmacol. Res. 2016, 113 Pt A, 228–235. [Google Scholar] [CrossRef]
- Kim, K.C.; Kim, P.; Go, H.S.; Choi, C.S.; Park, J.H.; Kim, H.J.; Jeon, S.J.; Dela Pena, I.C.; Han, S.H.; Cheong, J.H.; et al. Male-specific alteration in excitatory post-synaptic development and social interaction in pre-natal valproic acid exposure model of autism spectrum disorder. J. Neurochem. 2013, 124, 832–843. [Google Scholar] [CrossRef]
- Dai, Y.C.; Zhang, H.F.; Schon, M.; Bockers, T.M.; Han, S.P.; Han, J.S.; Zhang, R. Neonatal Oxytocin Treatment Ameliorates Autistic-Like Behaviors and Oxytocin Deficiency in Valproic Acid-Induced Rat Model of Autism. Front. Cell. Neurosci. 2018, 12, 355. [Google Scholar] [CrossRef] [PubMed]
- Win-Shwe, T.T.; Nway, N.C.; Imai, M.; Lwin, T.T.; Mar, O.; Watanabe, H. Social behavior, neuroimmune markers and glutamic acid decarboxylase levels in a rat model of valproic acid-induced autism. J. Toxicol. Sci. 2018, 43, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef]
- Vasa, R.A.; Mazurek, M.O. An update on anxiety in youth with autism spectrum disorders. Curr. Opin. Psychiatry 2015, 28, 83–90. [Google Scholar] [CrossRef]
- Mirza, R.; Sharma, B. Benefits of Fenofibrate in prenatal valproic acid-induced autism spectrum disorder related phenotype in rats. Brain Res. Bull. 2019, 147, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, S.; Takuma, K.; Hara, Y.; Maeda, Y.; Ago, Y.; Matsuda, T. Autism-like behaviours with transient histone hyperacetylation in mice treated prenatally with valproic acid. Int. J. Neuropsychopharmacol. 2013, 16, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Morena, M.; Nastase, A.S.; Santori, A.; Cravatt, B.F.; Shansky, R.M.; Hill, M.N. Sex-dependent effects of endocannabinoid modulation of conditioned fear extinction in rats. Br. J. Pharmacol. 2021, 178, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Simone, J.J.; Baumbach, J.L.; McCormick, C.M. Sex-specific effects of CB1 receptor antagonism and stress in adolescence on anxiety, corticosterone concentrations, and contextual fear in adulthood in rats. Int. J. Dev. Neurosci. 2018, 69, 119–131. [Google Scholar] [CrossRef]
- Craft, R.M.; Wakley, A.A.; Tsutsui, K.T.; Laggart, J.D. Sex differences in cannabinoid 1 vs. cannabinoid 2 receptor-selective antagonism of antinociception produced by delta9-tetrahydrocannabinol and CP55,940 in the rat. J. Pharmacol. Exp. Ther. 2012, 340, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, L.; Cavone, V.; Tinacci, S.; Concas, I.; Petralia, A.; Signorelli, M.S.; Diaz-Caneja, C.M.; Aguglia, E. Cannabinoids for People with ASD: A Systematic Review of Published and Ongoing Studies. Brain Sci. 2020, 10, 572. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Bellocchio, L.; Bouzon-Arnaiz, I.; Yee, B.K. The role of the endocannabinoid system in autism spectrum disorders: Evidence from mouse studies. Prog. Mol. Biol. Transl. Sci. 2020, 173, 183–208. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.; Manduca, A.; Cacchione, C.; Vicari, S.; Trezza, V. Healing autism spectrum disorder with cannabinoids: A neuroinflammatory story. Neurosci. Biobehav. Rev. 2021, 121, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.M.; Downey, L.; Conboy, M.; Finn, D.P.; Roche, M. Alterations in the endocannabinoid system in the rat valproic acid model of autism. Behav. Brain Res. 2013, 249, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Liu, Y.; Xie, S.; Wang, L.; Li, D.; Li, L.; Wang, F.; Zhang, Y.; Xia, W.; Sun, C.; et al. Alterations of the endocannabinoid system and its therapeutic potential in autism spectrum disorder. Open Biol. 2021, 11, 200306. [Google Scholar] [CrossRef] [PubMed]
- Moy, S.S.; Nadler, J.J.; Perez, A.; Barbaro, R.P.; Johns, J.M.; Magnuson, T.R.; Piven, J.; Crawley, J.N. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004, 3, 287–302. [Google Scholar] [CrossRef]
- Yang, M.; Crawley, J.N. Simple behavioral assessment of mouse olfaction. Curr. Protoc. Neurosci. 2009, 48, 8–24. [Google Scholar] [CrossRef]
- Vanderschuren, L.J.; Achterberg, E.J.; Trezza, V. The neurobiology of social play and its rewarding value in rats. Neurosci. Biobehav. Rev. 2016, 70, 86–105. [Google Scholar] [CrossRef]
- Du, L.; Zhao, G.; Duan, Z.; Li, F. Behavioral improvements in a valproic acid rat model of autism following vitamin D supplementation. Psychiatry Res. 2017, 253, 28–32. [Google Scholar] [CrossRef]
- Lai, M.C.; Lombardo, M.V.; Pasco, G.; Ruigrok, A.N.; Wheelwright, S.J.; Sadek, S.A.; Chakrabarti, B.; Consortium, M.A.; Baron-Cohen, S. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS ONE 2011, 6, e20835. [Google Scholar] [CrossRef]
- Wu, J.; Dai, Y.C.; Lan, X.Y.; Zhang, H.F.; Bai, S.Z.; Hu, Y.; Han, S.P.; Han, J.S.; Zhang, R. Postnatal AVP treatments prevent social deficit in adolescence of valproic acid-induced rat autism model. Peptides 2021, 137, 170493. [Google Scholar] [CrossRef]
- Mirza, R.; Sharma, B. Beneficial effects of pioglitazone, a selective peroxisome proliferator-activated receptor-gamma agonist in prenatal valproic acid-induced behavioral and biochemical autistic like features in Wistar rats. Int. J. Dev. Neurosci. 2019, 76, 6–16. [Google Scholar] [CrossRef]
- Campolongo, M.; Kazlauskas, N.; Falasco, G.; Urrutia, L.; Salgueiro, N.; Hocht, C.; Depino, A.M. Sociability deficits after prenatal exposure to valproic acid are rescued by early social enrichment. Mol. Autism 2018, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, B.; Zhou, D.; Xing, J.; Li, H.; Li, J.; Zhang, Z.; Zhang, B.; Li, P. Supplementation of Diet With Different n-3/n-6 PUFA Ratios Ameliorates Autistic Behavior, Reduces Serotonin, and Improves Intestinal Barrier Impairments in a Valproic Acid Rat Model of Autism. Front. Psychiatry 2020, 11, 552345. [Google Scholar] [CrossRef] [PubMed]
- Niesink, R.J.; Van Ree, J.M. Involvement of opioid and dopaminergic systems in isolation-induced pinning and social grooming of young rats. Neuropharmacology 1989, 28, 411–418. [Google Scholar] [CrossRef]
- Banerjee, A.; Engineer, C.T.; Sauls, B.L.; Morales, A.A.; Kilgard, M.P.; Ploski, J.E. Abnormal emotional learning in a rat model of autism exposed to valproic acid in utero. Front. Behav. Neurosci. 2014, 8, 387. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Roman, A.; Basta-Kaim, A.; Kubera, M.; Budziszewska, B.; Schneider, K.; Przewlocki, R. Gender-specific behavioral and immunological alterations in an animal model of autism induced by prenatal exposure to valproic acid. Psychoneuroendocrinology 2008, 33, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Olexova, L.; Stefanik, P.; Krskova, L. Increased anxiety-like behaviour and altered GABAergic system in the amygdala and cerebellum of VPA rats—An animal model of autism. Neurosci. Lett. 2016, 629, 9–14. [Google Scholar] [CrossRef]
- Lin, H.C.; Gean, P.W.; Wang, C.C.; Chan, Y.H.; Chen, P.S. The amygdala excitatory/inhibitory balance in a valproate-induced rat autism model. PLoS ONE 2013, 8, e55248. [Google Scholar] [CrossRef]
- Rubino, T.; Realini, N.; Castiglioni, C.; Guidali, C.; Vigano, D.; Marras, E.; Petrosino, S.; Perletti, G.; Maccarrone, M.; Di Marzo, V.; et al. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb. Cortex 2008, 18, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Bedse, G.; Bluett, R.J.; Patrick, T.A.; Romness, N.K.; Gaulden, A.D.; Kingsley, P.J.; Plath, N.; Marnett, L.J.; Patel, S. Therapeutic endocannabinoid augmentation for mood and anxiety disorders: Comparative profiling of FAAH, MAGL and dual inhibitors. Transl. Psychiatry 2018, 8, 92. [Google Scholar] [CrossRef]
- Sciolino, N.R.; Zhou, W.; Hohmann, A.G. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol. Res. 2011, 64, 226–234. [Google Scholar] [CrossRef]
- Henry, R.J.; Kerr, D.M.; Flannery, L.E.; Killilea, M.; Hughes, E.M.; Corcoran, L.; Finn, D.P.; Roche, M. Pharmacological inhibition of FAAH modulates TLR-induced neuroinflammation, but not sickness behaviour: An effect partially mediated by central TRPV1. Brain Behav. Immun. 2017, 62, 318–331. [Google Scholar] [CrossRef]
- Flannery, L.E.; Henry, R.J.; Kerr, D.M.; Finn, D.P.; Roche, M. FAAH, but not MAGL, inhibition modulates acute TLR3-induced neuroimmune signaling in the rat, independent of sex. J. Neurosci. Res. 2017, 96, 989–1001. [Google Scholar] [CrossRef]
- Ignatowska-Jankowska, B.; Wilkerson, J.L.; Mustafa, M.; Abdullah, R.; Niphakis, M.; Wiley, J.L.; Cravatt, B.F.; Lichtman, A.H. Selective monoacylglycerol lipase inhibitors: Antinociceptive versus cannabimimetic effects in mice. J. Pharmacol. Exp. Ther. 2015, 353, 424–432. [Google Scholar] [CrossRef]
- Hughes, E.M.; Thornton, A.M.; Kerr, D.M.; Smith, K.; Sanchez, C.; Kelly, J.P.; Finn, D.P.; Roche, M. Kappa Opioid Receptor-mediated Modulation of Social Responding in Adolescent Rats and in Rats Prenatally Exposed to Valproic Acid. Neuroscience 2020, 444, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Flannery, L.E.; Kerr, D.M.; Finn, D.P.; Roche, M. FAAH inhibition attenuates TLR3-mediated hyperthermia, nociceptive- and anxiety-like behaviour in female rats. Behav. Brain Res. 2018, 353, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.J.; Creeth, H.D.J.; Tyson, H.R.; Boque-Sastre, R.; Isles, A.R.; Palme, R.; Touma, C.; John, R.M. Unified Behavioral Scoring for Preclinical Models. Front. Neurosci. 2020, 14, 313. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thornton, A.M.; Humphrey, R.M.; Kerr, D.M.; Finn, D.P.; Roche, M. Increasing Endocannabinoid Tone Alters Anxiety-Like and Stress Coping Behaviour in Female Rats Prenatally Exposed to Valproic Acid. Molecules 2021, 26, 3720. https://doi.org/10.3390/molecules26123720
Thornton AM, Humphrey RM, Kerr DM, Finn DP, Roche M. Increasing Endocannabinoid Tone Alters Anxiety-Like and Stress Coping Behaviour in Female Rats Prenatally Exposed to Valproic Acid. Molecules. 2021; 26(12):3720. https://doi.org/10.3390/molecules26123720
Chicago/Turabian StyleThornton, Aoife M., Rachel M. Humphrey, Daniel M. Kerr, David P. Finn, and Michelle Roche. 2021. "Increasing Endocannabinoid Tone Alters Anxiety-Like and Stress Coping Behaviour in Female Rats Prenatally Exposed to Valproic Acid" Molecules 26, no. 12: 3720. https://doi.org/10.3390/molecules26123720
APA StyleThornton, A. M., Humphrey, R. M., Kerr, D. M., Finn, D. P., & Roche, M. (2021). Increasing Endocannabinoid Tone Alters Anxiety-Like and Stress Coping Behaviour in Female Rats Prenatally Exposed to Valproic Acid. Molecules, 26(12), 3720. https://doi.org/10.3390/molecules26123720







