NbCl5-Mg Reagent System in Regio- and Stereoselective Synthesis of (2Z)-Alkenylamines and (3Z)-Alkenylols from Substituted 2-Alkynylamines and 3-Alkynylols
Abstract
1. Introduction
2. Results
3. Materials and Methods
3.1. General Information
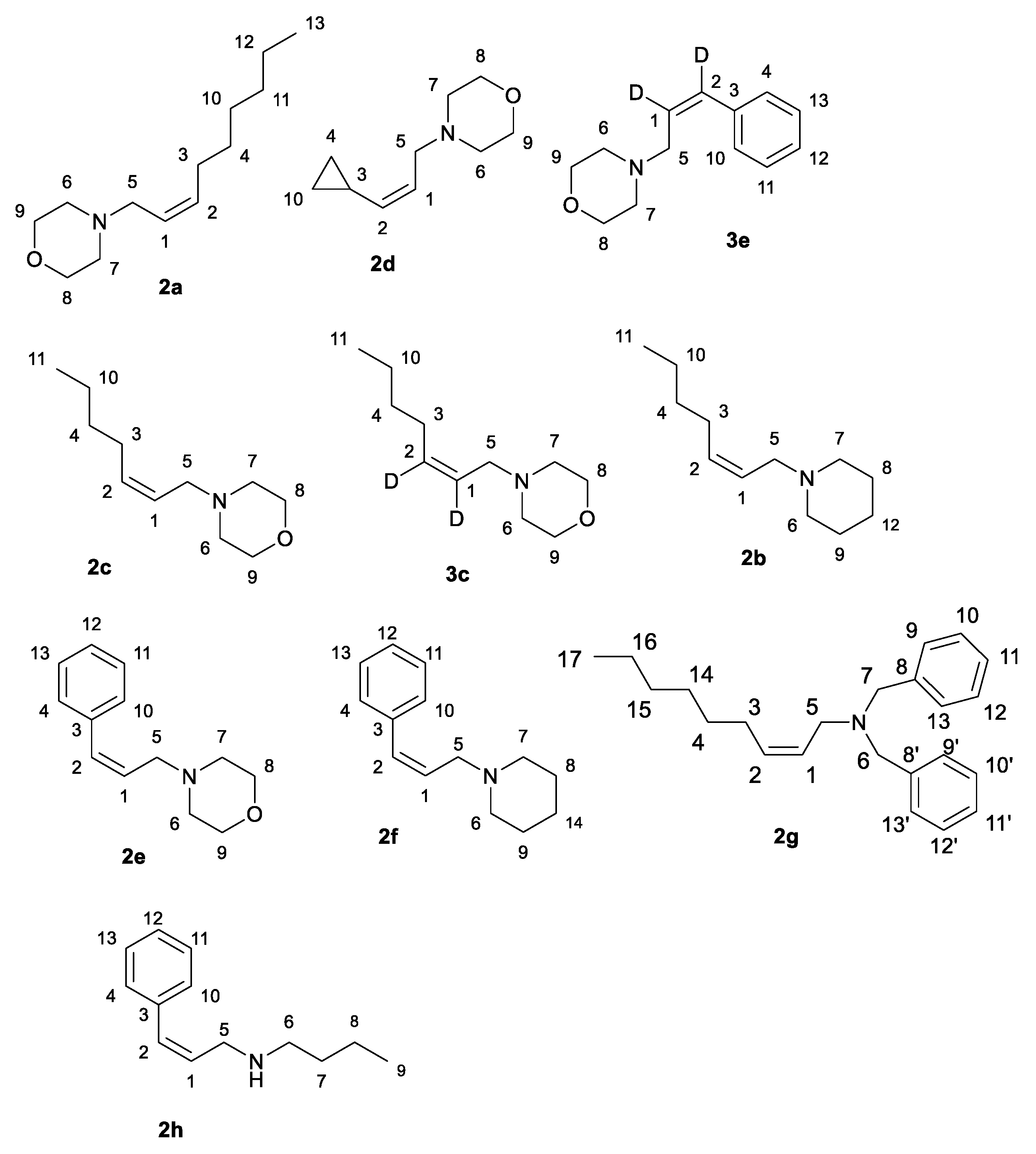
3.2. Preparation of (Z)-2-Alkenylamines 2a–h, 3c,e via Reduction of Substituted 2-Alkynylamines via Mg-NbCl5
- (Z)-4-(non-2-en-1-yl)morpholine; Typical Procedure
- (Z)-1-(hept-2-en-1-yl)piperidine (2b)
- (Z)-4-(hept-2-en-1-yl)morpholine (2c)
- (Z)-4-(3-cyclopropylallyl)morpholine (2d)
- (Z)-4-(3-phenylallyl)morpholine (2e)
- (Z)-1-(3-phenylallyl)piperidine (2f)
- (Z)-4-(hept-2-en-1-yl-2,3-d2)morpholine (3c)
- (Z)-4-(3-phenylallyl-2,3-d2)morpholine (3e)
- (Z)-N,N-dibenzylnon-2-en-1-amine (2g)
- (Z)-N-(3-phenylallyl)butan-1-amine (2h)
3.3. Preparation of (3Z)-Alkenylols 5a–e via Reduction of Substituted Alkynylols via Mg-NbCl5
- (Z)-dec-3-en-1-ol; Typical Procedure
- (Z)-non-3-en-1-ol (5b)
- (Z)-oct-3-en-1-ol (5c)
- (Z)-dodec-3-en-1-ol (5d)
- (Z)-4-phenylbut-3-en-1-ol (5e)
3.4. Preparation of (3Z)-Alkenylols 6a–d via Chlorothiolation of Alkynes with the NbCl5–Mg Reagent System
- (E)-4-(2-chloro-3-(methylthio)non-2-en-1-yl)morpholine; Typical Procedure
- (E)-1-(2-chloro-3-(methylthio)hept-2-en-1-yl)piperidine (6b)
- (E)-4-(2-chloro-3-(methylthio)hept-2-en-1-yl)morpholine (6c)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ohff, A.; Pulst, S.; Peulecke, N.; Arndt, P.; Burlakov, V.V.; Rosenthal, U. Unusual Reactions of Titanocene- and Zirconocene-Generating Complexes. Synlett 1996, 2, 111–118. [Google Scholar] [CrossRef]
- Rosenthal, U.; Burlakov, V.V.; Arndt, P.; Baumann, W.; Spannenberg, A. The Titanocene Complex of Bis(trimethylsilyl)acetylene: Synthesis, Structure, and Chemistry. Organometallics 2003, 22, 884–900. [Google Scholar] [CrossRef]
- Shur, V.B.; Bernadyuk, S.Z.; Burlakov, V.V.; Andrianov, V.G.; Yanovsky, A.I.; Struchkov, Y.T.; Vol’pin, M.E. Synthesis and x-ray structure determination of an organometallic titanoxane [Cp2TiC(Ph)CH(Ph)]2O. Evidence for the formation of a titanocene complex with tolane. J. Organomet. Chem. 1983, 243, 157–163. [Google Scholar]
- Shur, V.B.; Burlakov, V.V.; Vol’pin, M.E. Complex of titanocene with tolane. Isolation, spectral characteristics, reactivity. J. Organomet. Chem. 1988, 347, 77–83. [Google Scholar] [CrossRef]
- Broene, R.D.; Buchwald, S.L. Zirconocene complexes of unsaturated organic molecules: New vehicles for organic synthesis. Science 1993, 261, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Kawaji, T.; Shohji, N.; Miyashita, K.; Okamoto, S. Non-Cp titanium alkoxide-based homolytic ring-opening of epoxides by an intramolecular hydrogen abstraction in β-titanoxy radical intermediates. Chem. Commun. 2011, 47, 7857–7859. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, U.; Burlaakov, V.V.; Bach, M.A.; Beweries, T. Five-membered metallacycles of titanium and zirconium—Attractive compounds for organometallic chemistry and catalysis. Chem. Soc. Rev. 2007, 36, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, K.; Okamoto, S.; Sato, F. Asymmetric Synthesis of Allyl- and α-Allenylamines from Chiral Imines and Alkynes via (η2-Imine)Ti(O-i-Pr)2 Complexes. Org. Lett. 2003, 5, 2145–2148. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, I.L.; Hyung, K.K.; Lee, G.; Cha, J.K. Low-Valent Titanium-Mediated Stereoselective Alkylation of Allylic Alcohols. J. Am. Chem. Soc. 2008, 130, 15997–16002. [Google Scholar] [CrossRef] [PubMed]
- Tarselli, M.A.; Micalizio, G.C. Aliphatic Imines in Titanium-Mediated Reductive Cross-Coupling: Unique Reactivity of Ti(O-i-Pr)4/n-BuLi. Org. Lett. 2009, 11, 4596–4599. [Google Scholar] [CrossRef]
- Hanamoto, T.; Yamada, K. Generation and Reactions of Trifluoromethylethenyl Titanium(II) Species. J. Organomet. Chem. 2009, 74, 7559–7561. [Google Scholar] [CrossRef]
- Balaich, G.; Rothwell, I.P. Regio- and stereoselective formation and isomerization of 1,3-cyclohexadienes catalyzed by titanium aryloxide compounds. J. Am. Chem. Soc. 1993, 115, 1581–1583. [Google Scholar] [CrossRef]
- Johnson, E.S.; Balaich, G.J.; Rothwell, I.P. Regio- and Stereoselective Synthesis of the 1,3-Cyclohexadiene Nucleus by [2 + 2 + 2] Cycloaddition Reactions Catalyzed by Titanium Aryloxide Compounds. J. Am. Chem. Soc. 1997, 119, 7685–7693. [Google Scholar] [CrossRef]
- Harada, K.; Urabe, H.; Sato, F. Generation and reactions of low-valent titanium alkoxide-acetylene complexes. A practical preparation of allyl alcohols. Tetrahedron Lett. 1995, 36, 3203–3206. [Google Scholar] [CrossRef]
- Takahashi, T.; Tsai, F.-Y.; Li, Y.; Nakajima, K.; Kotora, M. Carbon–Carbon Bond Formation Reaction of Zirconacyclopentadienes with Alkynes in the Presence of Ni(II)-complexes. J. Am. Chem. Soc. 1999, 121, 11093–11100. [Google Scholar] [CrossRef]
- Bando, M.; Nakajima, K.; Song, Z.; Takahashi, T. Selective Linear Triene Formation from Different Alkynes using Zr/Cu system. J. Am. Chem. Soc. 2008, 130, 5624–5625. [Google Scholar]
- Dransfield, P.J.; Wang, S.; Dilley, A.; Romo, D. Highly Regioselective Diels–Alder Reactions toward Oroidin Alkaloids: Use of a Tosylvinyl Moiety as a Nitrogen Masking Group with Adjustable Electronics. J. Org. Chem. 2005, 7, 1679–1682. [Google Scholar]
- Nishihara, Y.; Miyasaka, M.; Okamoto, M.; Takahashi, H.; Inoue, E.; Tanemura, K.; Takagi, K. Zirconocene-Mediated Highly Regio- and Stereoselective Synthesis of Multisubstituted Olefins Starting from 1-Alkynylboronates. J. Am. Chem. Soc. 2007, 129, 12634–12635. [Google Scholar] [CrossRef] [PubMed]
- Cotton, F.A.; Hall, W.T. Reactions of niobium(III) and tantalum(III) compounds with acetylenes. 1. Preparation and structure of pyridinium tetrachloro(pyridine)(tolane)tantalate, [pyH][TaCl4(py)(PhC.tplbond.CPh)]. Inorg. Chem. 1981, 19, 2352–2354. [Google Scholar] [CrossRef]
- Cotton, F.A.; Roth, W.J. Reactions of niobium(III) and tantalum(III) compounds with acetylenes. 5. Preparation and structure of [NbCl2(SC4H8)(PhCCPh)]2(m̧-Cl)2 and its relationship to other alkyne complexes of niobium(III) and tantalum(III). Inorg. Chim. Acta 1984, 85, 17–21. [Google Scholar] [CrossRef]
- Roskamp, E.J.; Pedersen, S.F. The first practical niobium(III) reagent in organic synthesis. A convenient route to 2-amino alcohols via the coupling of imines with aldehydes or ketones promoted by NbCl3(DME). J. Am. Chem. Soc. 1987, 109, 6551–6553. [Google Scholar] [CrossRef]
- Hartung, J.B.; Pedersen, S.F. Synthesis and characterization of trihaloniobium alkyne complexes. Organometallics 1990, 9, 1414–1417. [Google Scholar] [CrossRef]
- Biasotto, F.; Etienne, M.; Dahan, F. Five-Membered Niobacycle Formation from an Allyl-Alkyne Coupling Reaction. Organometallics 1995, 14, 1870–1874. [Google Scholar] [CrossRef]
- Strickler, J.R.; Bruck, M.A.; Wexler, P.A.; Wigley, D.E. Synthesis and structural characterization of a Meerwein-Ponndorf-Verley/Oppenauer redox intermediate at a tantalum(V) center. Organometallics 1990, 9, 266–273. [Google Scholar] [CrossRef]
- Strickler, J.R.; Wigley, D.E. Intermolecular tautomerization of metallacyclic imines to enamines formed from tantalum alkyne complexes and nitriles. Organometallics 1990, 9, 1665–1669. [Google Scholar] [CrossRef]
- Roskamp, E.J.; Pedersen, S.F. Convenient routes to vicinal diamines. Coupling of nitriles or N-(trimethylsilyl) imines promoted by NbCl4(THF)2. J. Am. Chem. Soc. 1987, 109, 3152–3154. [Google Scholar] [CrossRef]
- Masuda, T.; Isobe, E.; Higashimura, T.; Takada, T. Poly[1-(trimethylsilyl)-1-propyne]: A new high polymer synthesized with transition-metal catalysts and characterized by extremely high gas permeability. J. Am. Chem. Soc. 1983, 105, 7473–7474. [Google Scholar] [CrossRef]
- Masuda, T.; Niki, A.; Isobe, E.; Higashimura, T. Effect of organometallic cocatalysts on the polymerization of 1-phenyl-1-propyne by tantalum pentachloride (TaCl5) and niobium pentachloride (NbCl5). Macromolecules 1985, 18, 2109–2113. [Google Scholar] [CrossRef]
- Obora, Y.; Takeshita, K.; Ishii, Y. NbCl3-catalyzed [2+2+2] intermolecular cycloaddition of alkynes and alkenes to 1,3-cyclohexadiene derivatives. Org. Biomol. Chem. 2009, 7, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Obora, Y.; Satoh, Y.; Ishii, Y. NbCl3-Catalyzed Intermolecular [2+2+2] Cycloaddition of Alkynes and α,ω-Dienes: Highly Chemo- and Regioselective Formation of 5-ω-Alkenyl-1,4-substituted-1,3-cyclohexadiene Derivatives. J. Org. Chem. 2010, 75, 6046–6049. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Obora, Y. NbCl3-Catalyzed Three-Component [2 + 2 + 2] Cycloaddition Reaction of Terminal Alkynes, Internal Alkynes, and Alkenes to 1,3,4,5-Tetrasubstituted 1,3-Cyclohexadienes. Org. Lett. 2011, 13, 2568–2571. [Google Scholar] [CrossRef]
- Satoh, Y.; Obora, Y. Low-Valent Niobium-Catalyzed Intermolecular [2 + 2 + 2] Cycloaddition of tert-Butylacetylene and Arylnitriles to Form 2,3,6-Trisubstituted Pyridine Derivatives. J. Org. Chem. 2013, 78, 7771–7776. [Google Scholar] [CrossRef]
- Satoh, Y.; Obora, Y. Niobium Complexes in Organic Transformations: From Stoichiometric Reactions to Catalytic [2+2+2] Cycloaddition Reactions. Eur. J. Org. Chem. 2015, 2015, 5041–5054. [Google Scholar] [CrossRef]
- Curtis, M.D.; Real, J. Monomeric and dimeric niobium(II) and niobium(III) cyclopentadienyl carbonyl and alkyne complexes. J. Organomet. 1985, 4, 940–942. [Google Scholar] [CrossRef]
- Curtis, M.D.; Real, J.; Kwon, D. Monocyclopentadienyl complexes of niobium(III) and tantalum(III). Structures of [Cp’NbCl(CO)2]2(.mu.-Cl)2 and Cp’NbCl2(ArCCAr) (Ar = p-tolyl). Organometallics 1989, 8, 1644–1651. [Google Scholar] [CrossRef]
- Galindo, A.; Gómez, M.; Gómez-Sal, P.; Martín, A.; del Río, D.; Sánchez, F. Alkyl Alkyne Mono((trimethylsilyl)cyclopentadienyl) Niobium Complexes. Synthesis and Chemical Behavior in Insertion Processes. X-ray Crystal Structures of [NbCp‘(CH2SiMe3)2(Me3SiCCSiMe3)] and [NbCp‘(NAr){η4-CH(SiMe3)C(SiMe3)C(CH2SiMe3)CH(SiMe3)}], (Cp‘ = η5-C5H4SiMe3, Ar = 2,6-Me2C6H3). DFT Studies of the Model Complexes [Nb(η5-C5H5)R2(HCCH)] (R = Cl, Me). Organometallics 2002, 21, 293–304. [Google Scholar]
- Green, M.L.H.; Jousseaume, B. Bis(η-isopropylcyclopentadienyl)tantalum chemistry: Some hydride, carbonyl, alkyl, alkyne and tertiaryphosphine derivatives, and an improved synthesis of dichlorobiscyclopentadienylniobium. J. Organomet. Chem. 1980, 193, 339–344. [Google Scholar] [CrossRef]
- Serrano, R.; Royo, P. Niobium(III) complexes containing acetylene, isocyanide, phosphine or phosphite ligands. J. Organomet. Chem. 1983, 247, 33–37. [Google Scholar] [CrossRef][Green Version]
- Antinõlo, A.; Martinez-Ripoll, M.; Mugnier, Y.; Otero, A.; Prashar, S.; Rodriguez, A.M. Ansa-Niobocene Complexes: Synthesis and Characterization of Novel Complexes [Me2Si(η5-C5H4)2]NbCl2 and [Me2Si(η5-C5H4)2]NbCl(RC⋮CR) (R = Me, Ph). X-ray Crystal Structure of [Me2Si(η5-C5H4)2]NbCl(MeC⋮CMe). Organometallics 1996, 15, 3241–3243. [Google Scholar] [CrossRef]
- Antiñolo, A.; dó Garcia-Lie, S.; de Ilarduya, J.M.; Otero, A. Synthesis and reactivity of bis(trimethylsilylcyclopentadienyl)- niobium compounds with cumulene ligands. J. Organomet. Chem. 1987, 335, 85–90. [Google Scholar] [CrossRef]
- García-Yebra, C.; Carrero, F.; López-Mardomingo, C.; Fajardo, M.; Rodríguez, A.; Antiñolo, A.; Otero, A.; Lucas, D.; Mugnier, Y. New Niobocene Alkyne Complexes: Synthesis and Characterization of Neutral and Cationic Niobium Complexes with Functionalized Alkynes. X-ray Crystal Structure of [Nb(η5-C5H4SiMe3)2(Cl)(η2(C,C)-R1C⋮CR2)] (R1 = C⋮CPh, R2 = Ph (2b); R1 = CH2CHC(CH3)2, R2 = Ph (3b)). Organometallics 1999, 18, 1287–1298. [Google Scholar]
- Kataoka, Y.; Takai, K.; Oshima, K.; Utimoto, K. Reduction of acetylenes to (Z)-olefins by means of low-valent niobium or tantalum. Tetrahedron Lett. 1990, 31, 365–368. [Google Scholar] [CrossRef]
- Gianetti, T.L.; Tomson, N.C.; Arnold, J.; Bergman, R.G. Z-Selective, Catalytic Internal Alkyne Semihydrogenation under H2/CO Mixtures by a Niobium(III) Imido Complex. J. Am. Chem. Soc. 2011, 133, 14904–14907. [Google Scholar] [CrossRef] [PubMed]
- Gianetti, T.L.; Bergman, R.G.; Arnold, J. Stoichiometric carbon–carbon bond formation mediated by well defined Nb(III) complexes. Polyhedron 2014, 84, 19–23. [Google Scholar] [CrossRef]
- Le Quere, J.-L.; Petillon, F.Y.; Guerchais, J.E.; Sala-Pala, J. Reactivity of transition metal hydrides and thiolates. Reaction of trihydridobis(π-cyclopentadienyl)niobium with dimethyldisulphide and reactions of niobium, molybdenum and tungsten thiolates with alkynes. Inorg. Chim. Acta 1980, 43, 5–10. [Google Scholar] [CrossRef]
- Mitsuyoshi, S.; Koichiro, O. Reduction of organic compounds with low-valent niobium (NbCl5/NaAlH4). Chem. Lett. 1982, 11, 157–160. [Google Scholar]
- Labinger, J.A.; Schwarz, J. Synthesis and reactivity of (dialkylacetylene)niobium(III) complexes and derivatives. Contrast between protonation and methylation pathways. J. Am. Chem. Soc. 1975, 97, 1596–1597. [Google Scholar] [CrossRef]
- Kataoka, Y. Generation and Synthetic Applications of Niobium- and Tantalum-Alkyne Complexes; Kyoto University: Kyoto, Japan, 1992. [Google Scholar]
- Kadikova, R.N.; Ramazanov, I.R.; Gabdullin, A.M.; Mozgovoi, O.S.; Dzhemilev, U.M. Niobium- and zirconium-catalyzed reactions of substituted 2 alkynylamines with Et2Zn. RSC Adv. 2021, 11, 4631–4638. [Google Scholar] [CrossRef]
- Sharma, S.; Vermani, O.P.; Narula, A.K. Synthesis and structural studies of complexes of niobium(III) chloride with ditertiary phosphines. Indian J. Chem. 1996, 35A, 60–62. [Google Scholar]
- Siemeling, U.; Gibson, V.C. Synthesis and characterization of the paramagnetic niobium(III) complex [Nb(η-C5Me5)Cl2(PMe3)2] and its reaction with carbon monoxide. J. Organomet. Chem. 1992, 424, 159–161. [Google Scholar] [CrossRef]
- Sultanov, R.M.; Ismagilov, R.R.; Popod’ko, N.R.; Tulyabaev, A.R.; Dzhemilev, U.M. TaCl5-catalyzed reaction of 1-alkenes with n-alkyl Grignard reagents. J. Organomet. Chem. 2013, 724, 51–56. [Google Scholar] [CrossRef]
- Sultanov, R.M.; Ismagilov, R.R.; Popod’ko, N.R.; Tulyabaev, A.R.; Sabirov, D.; Dzhemilev, U.M. TaCl5-catalyzed carbomagnesiation of some norbornenes with ethyl Grignard reagents. J. Organomet. Chem. 2013, 745–746, 120–125. [Google Scholar] [CrossRef]
- Sultanov, R.M.; Dzhemilev, U.M.; Samoilova, E.V.; Ismagilov, R.R.; Khalilov, L.M.; Popod’ko, N.R. Two routes of tantalum-catalyzed alkene carbomagnesiation with ethyl Grignard reagents. J. Organomet. Chem. 2012, 715, 5–8. [Google Scholar] [CrossRef]
- Parker, K.D.; Fryzuk, M.D. Synthesis, Structure, and Reactivity of Niobium and Tantalum Alkyne Complexes. Organometallics 2015, 34, 2037–2047. [Google Scholar] [CrossRef]
- Ramazanov, I.R.; Kadikova, R.N.; Dzhemilev, U.M. Cp2ZrCl2-Catalyzed cycloalumination of acetylenic alcohols and propargylamines by Et3A. Russ. Chem. Bull. 2011, 60, 99–106. [Google Scholar] [CrossRef]
- Ramazanov, I.R.; Kadikova, R.N.; Dzhemilev, U.M. Efficient halogenation of unsaturated organoaluminum compounds with sulfonyl halides. Russ. J. Org. Chem. 2013, 49, 335–340. [Google Scholar] [CrossRef]
- Kadikova, R.N.; Zosim, T.P.; Dzhemilev, U.M.; Ramazanov, I.R. The efficient method for the preparation of alkenylsilanes from organoaluminums. J. Organometal. Chem. 2014, 763, 14–19. [Google Scholar] [CrossRef]
- Kadikova, R.N.; Ramazanov, I.R.; Vyatkin, A.V.; Dzhemilev, U.M. Zirconocene Catalysis in Organoaluminum Synthesis of 1-Alkenyl Sulfones and Sulfides. Synthesis 2017, 49, 1889–1897. [Google Scholar] [CrossRef]
- Ramazanov, I.R.; Kadikova, R.N.; Zosim, T.P.; Dzhemilev, U.M. One-Pot Synthesis of 1-Alkenyl Sulfides from Alkynes and Organic Disulfides with the Use of Organoaluminums. Synthesis 2015, 47, 2670–2676. [Google Scholar] [CrossRef]
- Kadikova, R.N.; Ramazanov, I.R.; Vyatkin, A.V.; Dzhemilev, U.M. Zirconium-Catalyzed Alkyne Carbo-and Cycloalumination Reactions in Stereoselective Preparation of 1-Alkenyl Selenides. Synthesis 2017, 49, 4523–4534. [Google Scholar] [CrossRef]
- Manzer, L.E. Preparation of the paramagnetic alkyls bis(cyclopentadienyl)dimethylniobiumandbis(methylcyclopentadienyl)dimethyltantalum and some six- and eight-coordinate phosphine derivatives of niobium(IV). Inorg. Chem. 1977, 16, 525–528. [Google Scholar] [CrossRef]
- Amiel, Y. Addition of sulfonyl chlorides to acetylenes. Addition of sulfonyl chlorides to acetylenes. Tetrahedron Lett. 1971, 8, 661–663. [Google Scholar] [CrossRef]
- Amiel, Y. The thermal and the copper-catalyzed addition of sulfonyl bromides to phenylacetylene. J. Org. Chem. 1974, 39, 3867–3870. [Google Scholar] [CrossRef]
- Liang, S.; Jiang, L.; Yi, W.-b.; Wei, J. Copper-Catalyzed Vicinal Chloro-thiolation of Alkynes with Sulfonyl Chlorides. Org. Lett. 2018, 20, 7024–7028. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, H.; Che, J.; Yang, Y.; Zhu, G. Synthesis of cis-1,2-dichlorovinylsulfones via Fe-catalyzed regio- and stereoselective addition of sulfonyl chlorides to aromatic chloroalkynes. Tetrahedron Lett. 2014, 55, 1011–1013. [Google Scholar] [CrossRef]
- Cotton, F.A.; Lu, J. EPR and Crystallographic Studies of Some Reaction Products of VCl4, NbCl4, and TaCl4 with Trialkyl- and Triarylphosphines. Inorg. Chem. 1995, 34, 2639–2644. [Google Scholar] [CrossRef]
- Zeng, X.; Ilies, L.; Nakamura, E. Iron-Catalyzed Regio- and Stereoselective Chlorosulfonylation of Terminal Alkynes with Aromatic Sulfonyl Chlorides. Org. Lett. 2012, 14, 954–956. [Google Scholar] [CrossRef]
- Amiel, Y. Addition of sulfonyl chlorides to acetylenes. I. Stereoselective syntheses of .beta.-chlorovinyl sulfones. J. Org. Chem. 1971, 36, 3691–3696. [Google Scholar] [CrossRef]
- Calo, V.; Scorrano, G.; Modena, G. Additions of sulfenyl chlorides to acetylenes. XII. Addition to tert-butylacetylene. J. Org. Chem. 1969, 34, 2020–2022. [Google Scholar] [CrossRef]
- Benati, L.; Montevecchi, P.C.; Spagnolo, P. Synthetic utility of 4′-nitrobenzenesulfenanilide in the functionalization of carbon-carbon double and triple bonds: Its use in the bromosulfenylation of alkenes and alkynes. Tetrahedron 1993, 49, 5365–5376. [Google Scholar] [CrossRef]
- Iwasaki, M.; Fujii, T.; Yamamoto, A.; Nakajima, K.; Nishihara, Y. Palladium-Catalyzed Regio- and Stereoselective Chlorothiolation of Terminal Alkynes with Sulfenyl Chlorides. Chem. Asian J. 2014, 9, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Fujii, T.; Nakajima, K.; Nishihara, Y. Iron-Induced Regio- and Stereoselective Addition of Sulfenyl Chlorides to Alkynes by a Radical Pathway. Angew. Chem. Int. Ed. 2014, 53, 13880–13884. [Google Scholar] [CrossRef]
- Bieber, L.W.; da Silva, M.F. Mild and efficient synthesis of propargylamines by copper-catalyzed Mannich reaction. Tetrahedron Lett. 2004, 45, 8281–8283. [Google Scholar] [CrossRef]
- Knight, J.A.; Diamond, J.H. Synthesis of Some Octenoic Acids. J. Org. Chem. 1959, 24, 400–403. [Google Scholar] [CrossRef]
- Brenna, E.; Crotti, M.; Gatti, F.G.; Marinoni, L.; Monti, D.; Quaiato, S. Exploitation of a Multienzymatic Stereoselective Cascade Process in the Synthesis of 2-Methyl-3-Substituted Tetrahydrofuran Precursors. J. Org. Chem. 2017, 82, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.J.; Guoa, B.; Njardarson, J.T. Synthesis of allylic and homoallylic alcohols from unsaturated cyclic ethers using a mild and selective C–O reduction approach. Chem. Commun. 2012, 48, 7844–7846. [Google Scholar] [CrossRef]
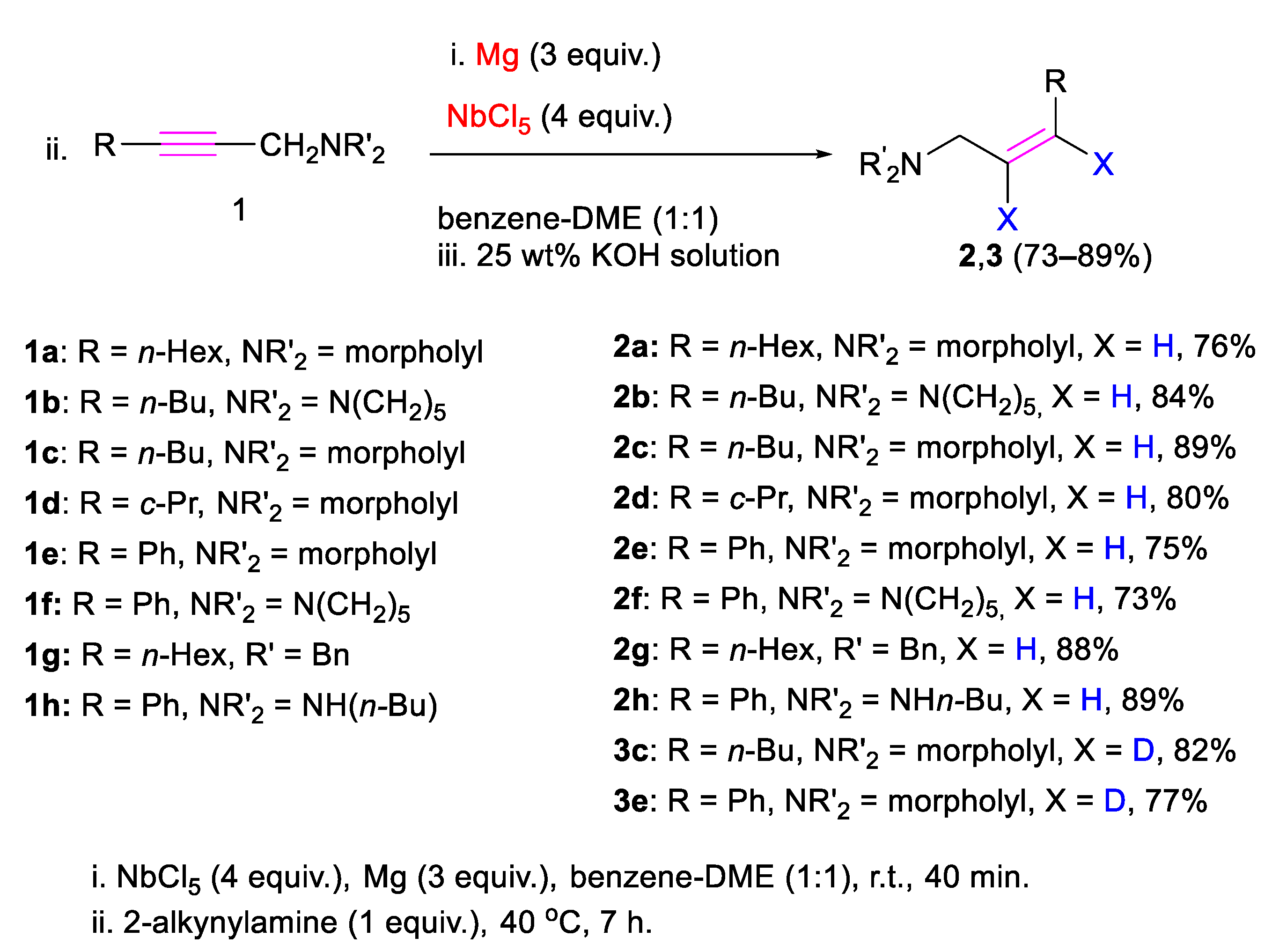
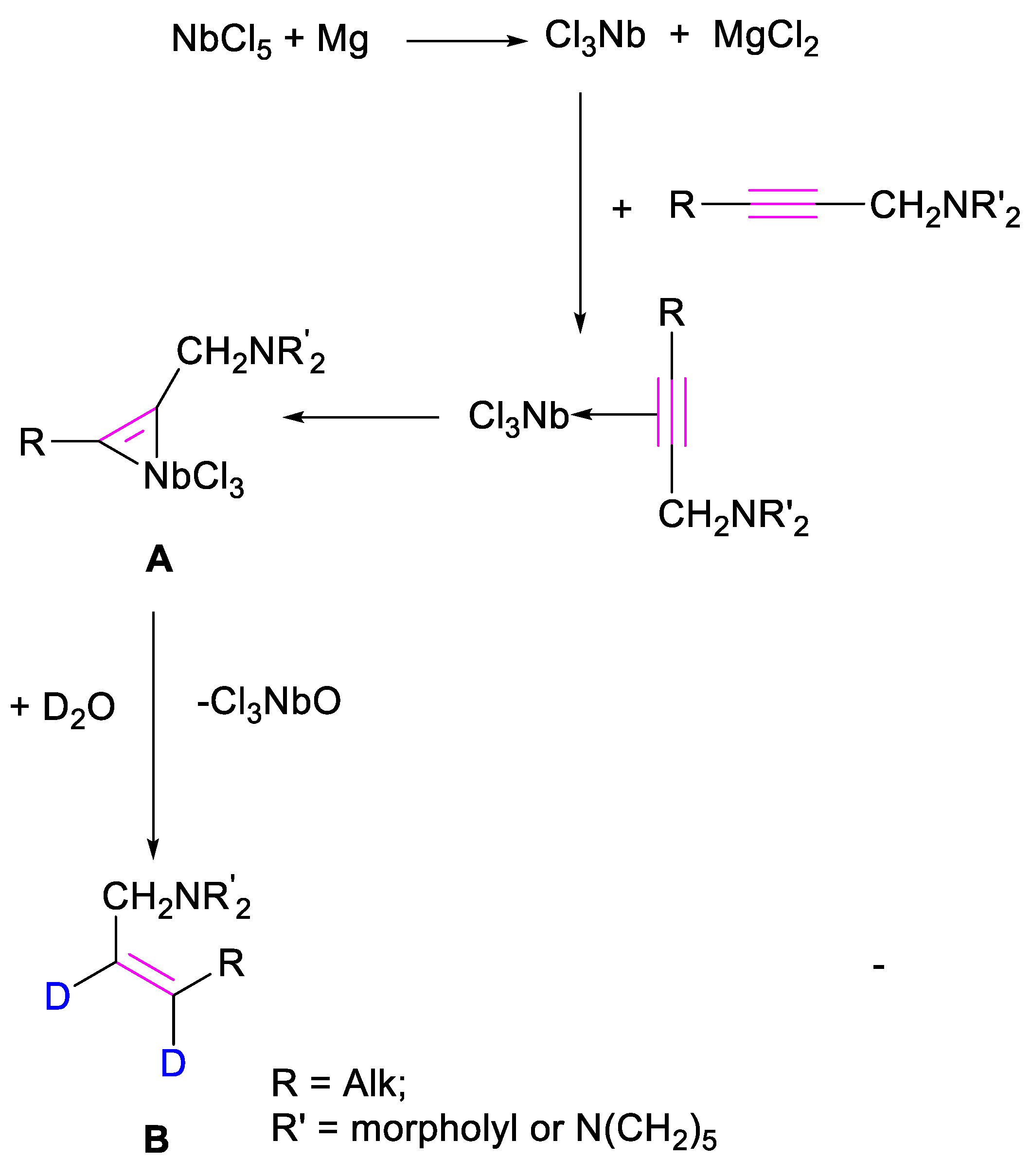





| Entry | Solvent | Conversion of 2-Alkynylamine (%) | Yield of 2a (%) |
|---|---|---|---|
| 1 | DME b: Benzene b | >99 | 89 |
| 2 | Et2O b: Benzene b | >80 | 81 |
| 3 | CH2Cl2 b: Benzene b | >99 | 72 |
| 4 | Toluene | <5 | 4 (GC) |
| 5 | Benzene | <5 | 3 (GC) |
| 6 | DME | <5 | <1 (GC) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadikova, R.N.; Gabdullin, A.M.; Mozgovoj, O.S.; Ramazanov, I.R.; Dzhemilev, U.M. NbCl5-Mg Reagent System in Regio- and Stereoselective Synthesis of (2Z)-Alkenylamines and (3Z)-Alkenylols from Substituted 2-Alkynylamines and 3-Alkynylols. Molecules 2021, 26, 3722. https://doi.org/10.3390/molecules26123722
Kadikova RN, Gabdullin AM, Mozgovoj OS, Ramazanov IR, Dzhemilev UM. NbCl5-Mg Reagent System in Regio- and Stereoselective Synthesis of (2Z)-Alkenylamines and (3Z)-Alkenylols from Substituted 2-Alkynylamines and 3-Alkynylols. Molecules. 2021; 26(12):3722. https://doi.org/10.3390/molecules26123722
Chicago/Turabian StyleKadikova, Rita N., Azat M. Gabdullin, Oleg S. Mozgovoj, Ilfir R. Ramazanov, and Usein M. Dzhemilev. 2021. "NbCl5-Mg Reagent System in Regio- and Stereoselective Synthesis of (2Z)-Alkenylamines and (3Z)-Alkenylols from Substituted 2-Alkynylamines and 3-Alkynylols" Molecules 26, no. 12: 3722. https://doi.org/10.3390/molecules26123722
APA StyleKadikova, R. N., Gabdullin, A. M., Mozgovoj, O. S., Ramazanov, I. R., & Dzhemilev, U. M. (2021). NbCl5-Mg Reagent System in Regio- and Stereoselective Synthesis of (2Z)-Alkenylamines and (3Z)-Alkenylols from Substituted 2-Alkynylamines and 3-Alkynylols. Molecules, 26(12), 3722. https://doi.org/10.3390/molecules26123722






