Accelerated Solvent Extractions (ASE) of Mitragyna speciosa Korth. (Kratom) Leaves: Evaluation of Its Cytotoxicity and Antinociceptive Activity
Abstract
1. Introduction
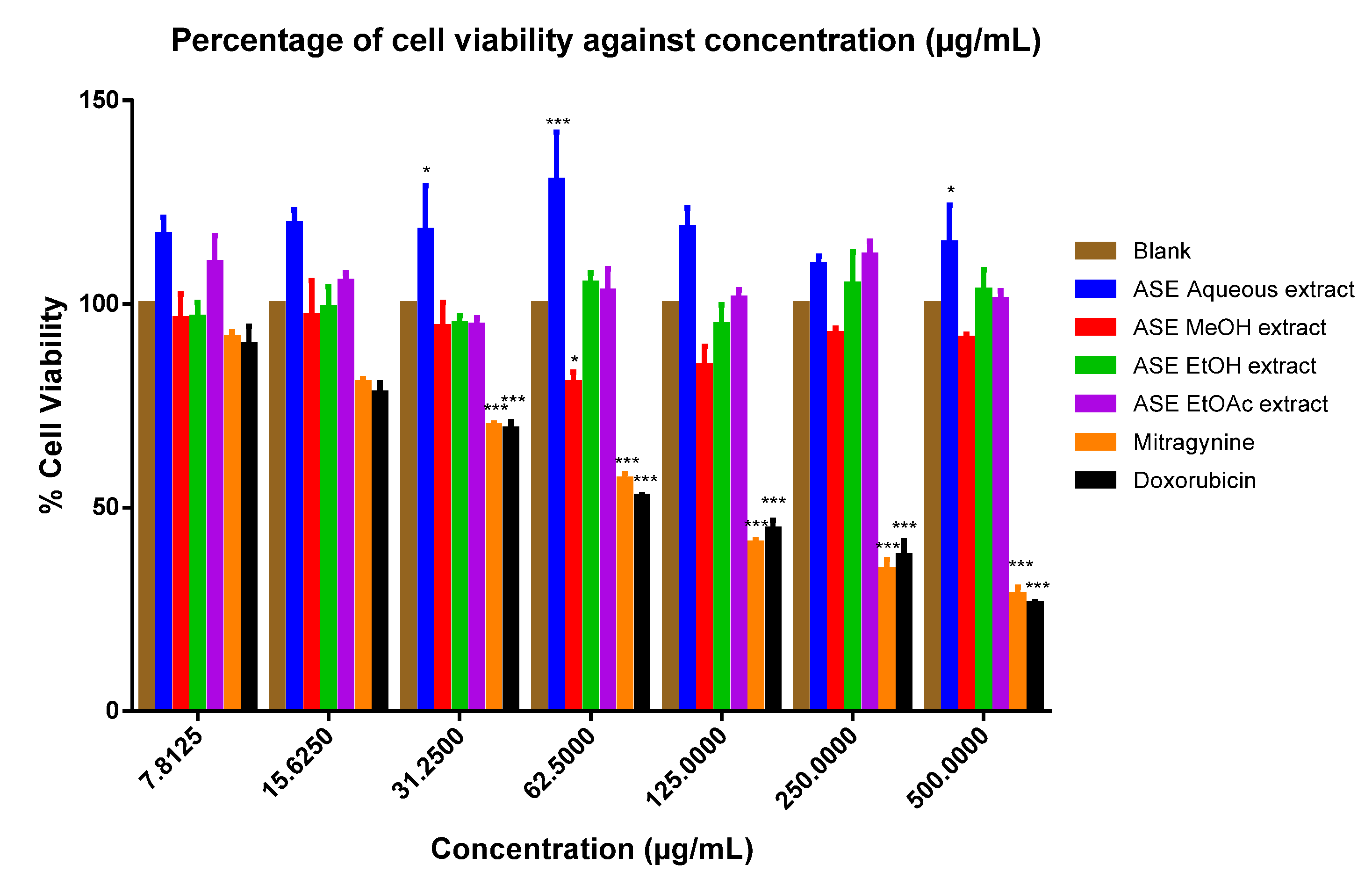
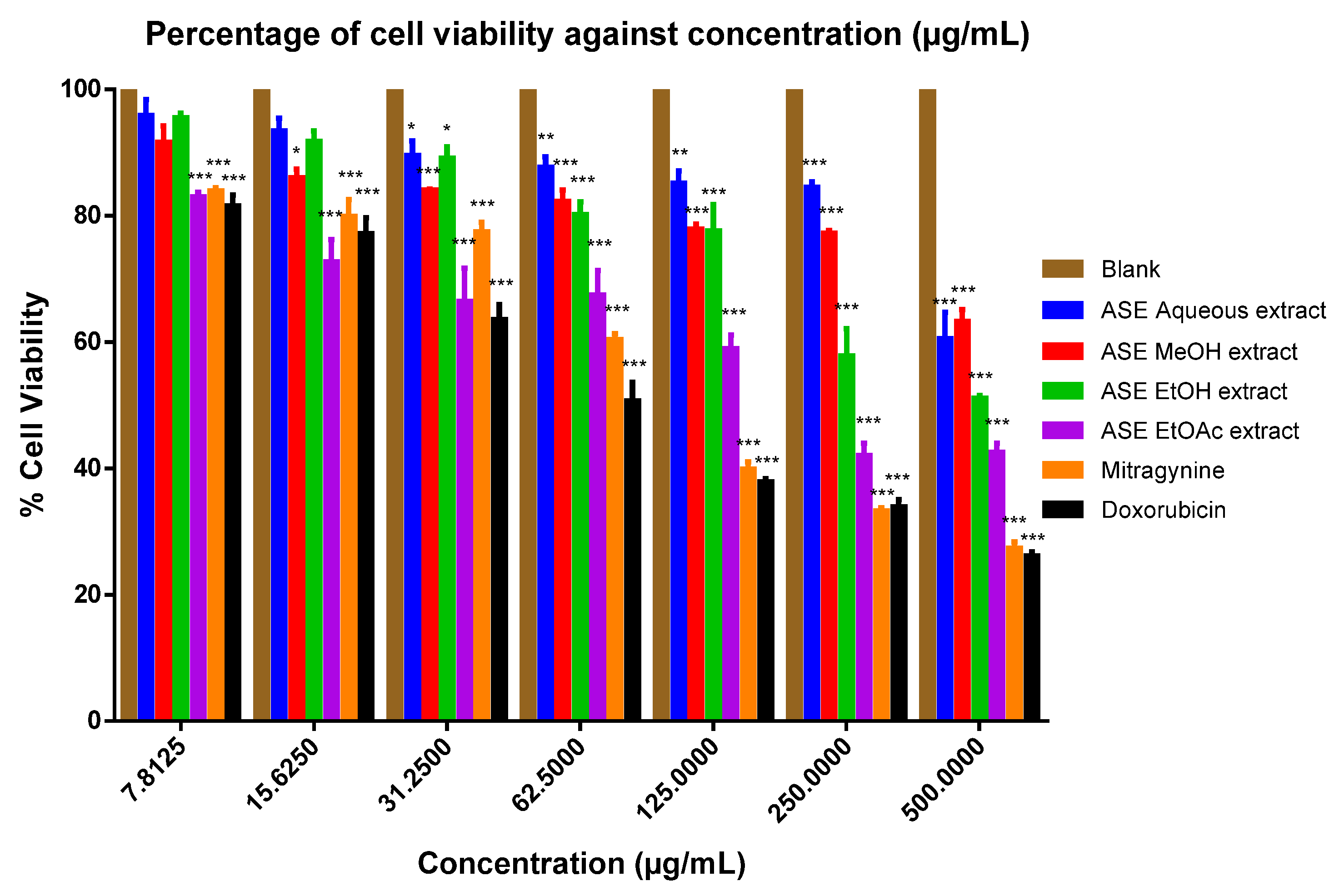
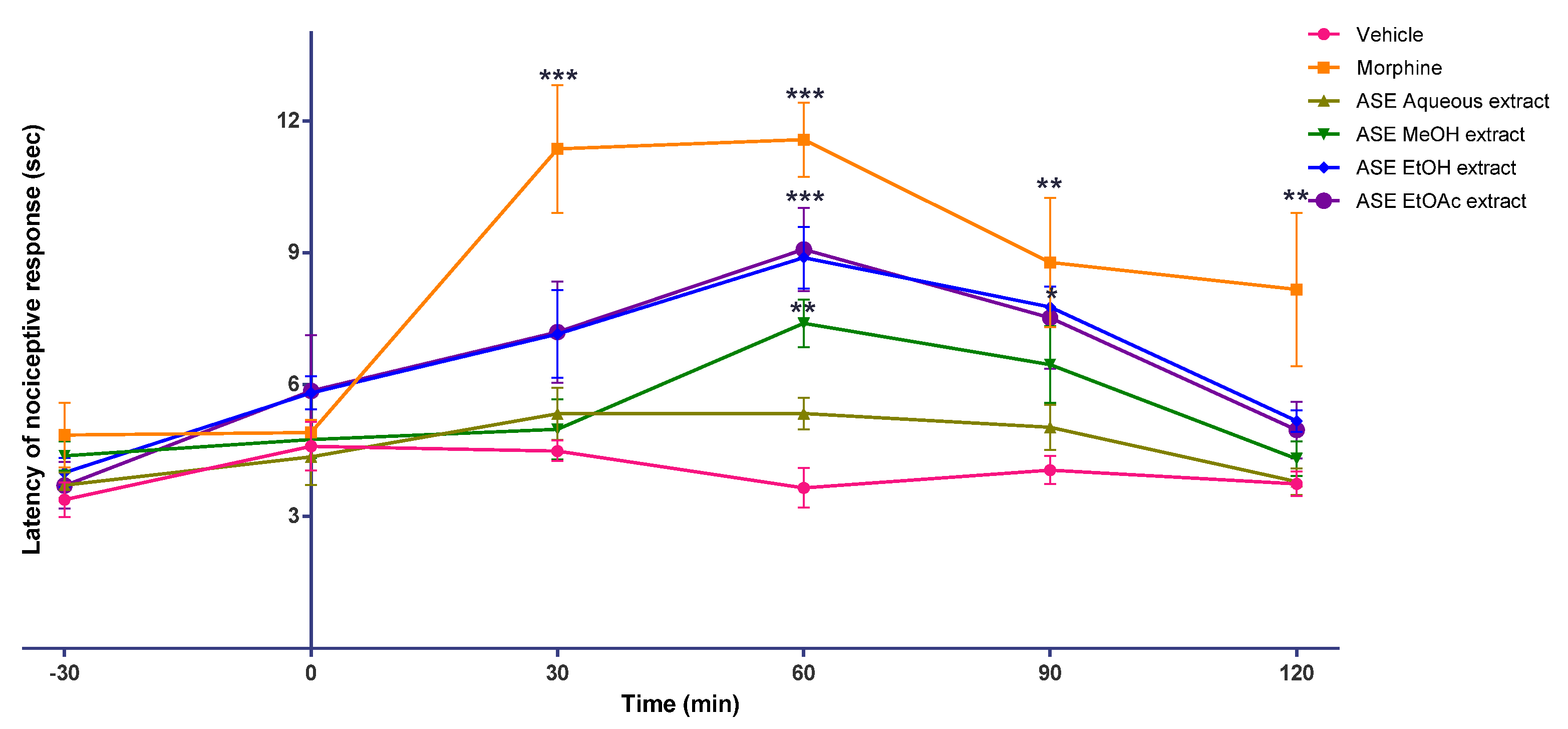
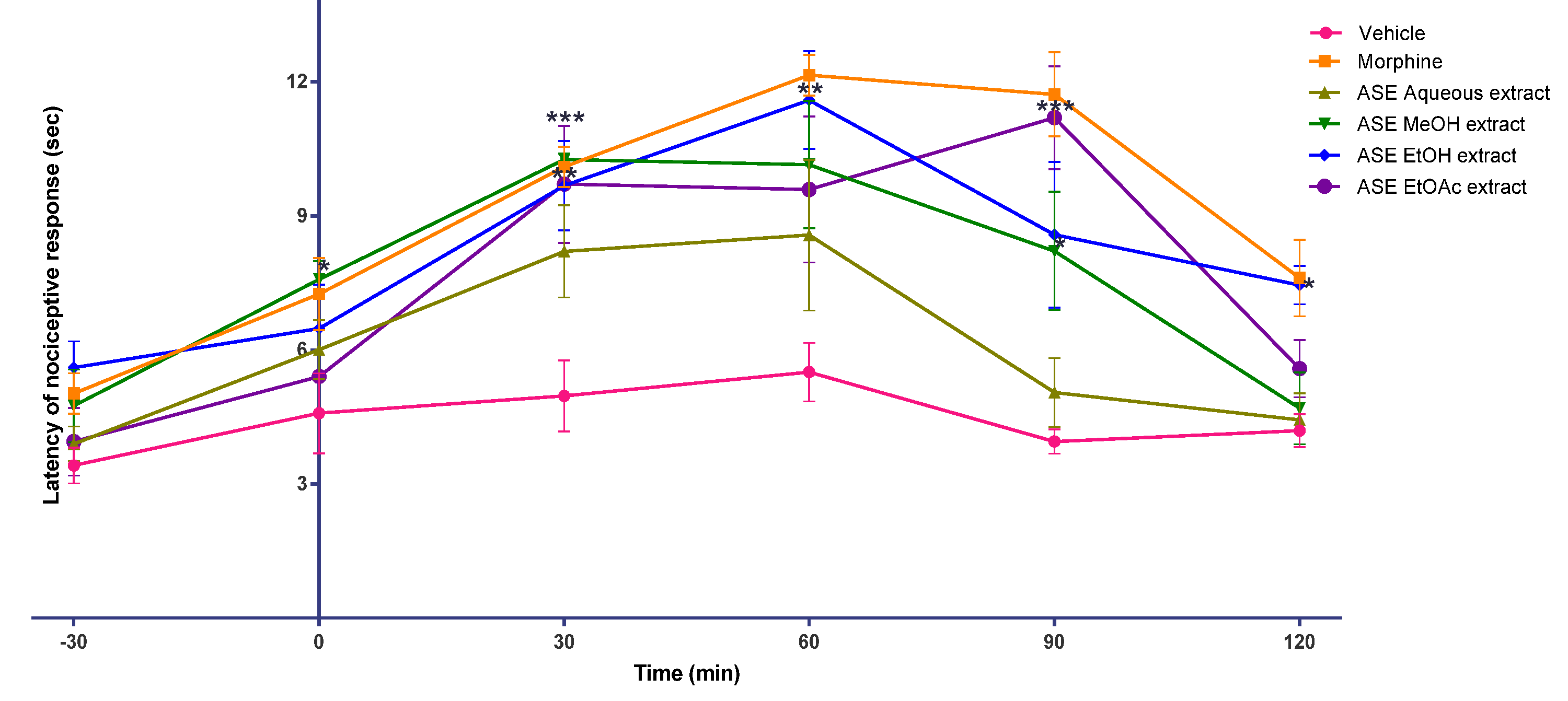
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals
4.3. General Instrumentations
4.4. Preparation of Extracts
4.5. Isolation of the Mitragynine
4.6. Determination of Phenolic Contents and Total Flavonoid Contents
4.6.1. Total Phenolic Contents (TPC)
4.6.2. Total Flavonoid Contents (TFC)
4.7. Preparation of Extracts and Cell Treatment
4.7.1. Cell Cultures and Conditions
4.7.2. MTT Cell Viability Assay
4.8. Animal Behaviour Study
4.8.1. Preparation of Extracts for Animal Behaviour Study
4.8.2. Animals
4.8.3. Hot Plate Test
4.8.4. Tail-flick Test
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singh, D.; Chear, N.J.Y.; Narayanan, S.; Leon, F.; Sharma, A.; McCurdy, C.R.; Avery, B.A.; Balasingam, V. Patterns and reasons for kratom (Mitragyna speciosa) use among current and former opioid poly-drug users. J. Ethnopharmacol. 2020, 249. [Google Scholar] [CrossRef] [PubMed]
- Vicknasingam, B.; Narayanan, S.; Beng, G.T.; Mansor, S.M. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int. J. Drug Policy 2010, 21, 283–288. [Google Scholar] [CrossRef]
- Ilmie, M.U.; Mansor, S.M.; Abdullah, J.M. Behavioural and Electrophysiological Evidence of Impaired Learning and Memory in Male Sprague Dawley Rats following Subchronic Exposure to Standardised Methanolic Extract of Mitragyna speciosa Korth. Malays. J. Med. Sci. 2015, 22, 45. [Google Scholar]
- Raffa, R.B. (Ed.) Kratom and Other Mitragynines: The Chemistry and Pharmacology of Opioids from a Non-opium Source; CRC Press: New York, NY, USA, 2015. [Google Scholar]
- Parthasarathy, S.; Ramanathan, S.; Murugaiyah, V.; Hamdan, M.R.; Said, M.I.M.; Lai, C.S.; Mansor, S.M. A simple HPLC–DAD method for the detection and quantification of psychotropic mitragynine in Mitragyna speciosa (ketum) and its products for the application in forensic investigation. Forensic Sci. Int. 2013, 226, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.; Muzaimi, M.; Navaratnam, V.; Yusoff, N.H.; Suhaimi, F.W.; Vadivelu, R.; Vicknasingam, B.K.; Amato, D.; von Hörsten, S.; Ismail, N.I.; et al. From Kratom to mitragynine and its derivatives: Physiological and behavioural effects related to use, abuse, and addiction. Neurosci. Biobehav. Rev. 2013, 37, 138–151. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Alexovic, M.; Balogh, I.S.; Sandrejov, J.; Andruch, V. A dispersive liquid–liquid microextraction procedure for UV-Vis spectrophotometric determination of chromium(vi) in water samples. Anal. Methods 2012, 4, 1410–1414. [Google Scholar] [CrossRef]
- Alexovic, M.; Andruch, V.; Balogh, I.S.; Sandrejov, J. A single-valve sequential injection manifold (SV-SIA) for automation of air-assisted liquid-phase microextraction: Stopped flow spectrophotometric determination of chromium(vi). Anal. Methods 2013, 5, 2497–2502. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. An introduction to natural products isolation. In Natural Products Isolation (1–25); Humana Press: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- De Silva, G.O.; Abeysundara, A.T.; Aponso, M.M.W. Extraction methods, qualitative and quantitative techniques for screening of phytochemicals from plants. Am. J. Essent. Oils 2017, 5, 29–32. [Google Scholar]
- Ahmad, R.; Ahmad, N.; Al-Anaki, W.S.; Ismail, F.A.; Al-Jishi, F. Solvent and temperature effect of accelerated solvent extraction (ASE) coupled with ultra-high-pressure liquid chromatography (UHPLC-PDA) for the determination of methyl xanthines in commercial tea and coffee. Food Chem. 2020, 311, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Trevino, L.M.; Turner, C. Pressurised hot ethanol extraction of carotenoids from carrot by-products. Molecules 2012, 17, 1809–1818. [Google Scholar] [CrossRef]
- Mohamed, H.M. Green, environment-friendly, analytical tools give insights in pharmaceuticals and cosmetics analysis. TrAC Trend Anal. Chem. 2015, 66, 176–192. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, D.; Chopra, K. Participation of α 2 receptors in the antinociceptive activity of Quercetin. J. Med. Food 2005, 8, 529–532. [Google Scholar] [CrossRef]
- Lin, H.; Zhu, H.; Tan, J.; Wang, H.; Wang, Z.; Li, P.; Zhao, C.; Liu, J. Comparative analysis of chemical constituents of Moringa oleifera leaves from China and India by Ultra-Performance Liquid Chromatography coupled with Quadrupole-time-of-flight mass spectrometry. Molecules 2019, 24, 942. [Google Scholar] [CrossRef]
- Wang, F.; Huang, S.; Chen, Q.; Hu, Z.; Li, Z.; Zheng, P.; Liu, X.; Li, S.; Zhang, S.; Chen, J. Chemical characterisation and quantification of the major constituents in the Chinese herbal formula Jian-Pi-Yi-Shen pill by UPLC-Q- TOF-MS/MS and HPLC-QQQ-MS/MS. Phytochem. Anal. 2020, 31, 915–929. [Google Scholar] [CrossRef]
- Pandey, B.P.; Pradhan, S.P.; Adhikari, K. LC-ESI-QTOF-MS for the Profiling of the Metabolites and in vitro Enzymes Inhibition Activity of Bryophyllum pinnatum and Oxalis corniculata Collected from Ramechhap District of Nepal. Chem. Biodivers. 2020, 17, 1–28. [Google Scholar]
- Wang, X.; Sun, W.; Sun, H.; Lv, H.; Wu, Z.; Wang, P.; Liu, L.; Cao, H. Analysis of the constituents in the rat plasma after oral administration of Yin Chen Hao Tang by UPLC/Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2008, 46, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Akhgari, A.; Laakso, I.; Seppänen-Laakso, T.; Yrjönen, T.; Vuorela, H.; OksmanCaldentey, K.M.; Rischer, H. Analysis of Indole Alkaloids from Rhazya stricta Hairy Roots by Ultra-Performance Liquid Chromatography Mass Spectrometry. Molecules 2015, 20, 22621–22634. [Google Scholar] [CrossRef]
- Gai, Y.; Chen, H.; Wu, C.; Feng, F.; Wang, Y.; Liu, W.; Wang, S. Analysis of the traditional medicine Yi Gan San by the fragmentation patterns of cadambine indole alkaloids using HPLC coupled with high-resolution MS. J. Sep. Sci. 2013, 36, 3723–3732. [Google Scholar] [CrossRef]
- Avula, B.; Sagi, S.; Wang, Y.H.; Wang, M.; Ali, Z.; Smillie, T.J.; Zweigenbaum, J.; Khan, I.A. Identification and Characterisation of Indole and Oxindole Alkaloids from Leaves of Mitragyna speciosa Korth using Liquid Chromatography—Accurate QTOF Mass Spectrometry. J. AOAC Int. 2015, 98, 13–21. [Google Scholar] [CrossRef]
- Orio, L.; Alexandru, L.; Cravotto, G.; Mantegna, S.; Barge, A. UAE, MAE, SFE-CO2 and classical methods for the extraction of Mitragyna speciosa leaves. Ultrason. Sonochem. 2012, 19, 591–595. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Metabolomics: What you see is what you extract. Phytochem. Anal. 2014, 25, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.S.; Khanuja, S.P.S.; Longa, G.; Rakesh, D.D. Extraction Technologies for Medicinal and Aromatic Plants. Available online: http://www.filedump.net/dumped/extractiontechnologiesformedicinalandaromaticplants1348238050.pdf#page=25 (accessed on 1 October 2020).
- Kislik, V.S. Solvent Extraction: Classical and Novel Approaches; Elsevier: Oxford, UK, 2012. [Google Scholar]
- Nafiu, M.O.; Hamid, A.A.; Muritala, H.F.; Adeyemi, S.B. Preparation, standardisation, and quality control of medicinal plants in Africa. In Medicinal Spices and Vegetables from Africa; Academic Press: Cambridge, MA, USA, 2017; pp. 171–204. [Google Scholar] [CrossRef]
- Truong, D.H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J. Food Qual. 2019, 1–9. [Google Scholar] [CrossRef]
- Nawaz, H.; Aslam, M.; Muntaha, S.T. Effect of Solvent Polarity and Extraction Method on Phytochemical Composition and Antioxidant Potential of Corn Silk. Free Radic. Antioxidants 2019, 9. [Google Scholar] [CrossRef]
- Sulaiman, C.T.; Balachandran, I. LC/MS Characterisation of Phenolic Antioxidants of Brindle Berry (Garcinia gummi-gutta (L.) Robson). Nat. Prod. Res. 2017, 31, 1191–1194. [Google Scholar] [CrossRef]
- Ngo, T.V.; Scarlett, C.J.; Bowyer, M.C.; Ngo, P.D.; Vuong, Q.V. Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. J. Food Qual. 2017, 8. [Google Scholar] [CrossRef]
- Kikura-Hanajiri, R.; Kawamura, M.; Maruyama, T.; Kitajima, M.; Takayama, H.; Goda, Y. Simultaneous analysis of mitragynine, 7-hydroxymitragynine, and other alkaloids in the psychotropic plant “kratom” (Mitragyna speciosa) by LC-ESI-MS. Forensic Toxicol. 2009, 27, 67–74. [Google Scholar] [CrossRef]
- Sharma, A.; Kamble, S.H.; León, F.; Chear, N.J.Y.; King, T.I.; Berthold, E.C.; Ramanathan, S.; McCurdy, C.R.; Avery, B.A. Simultaneous quantification of ten key Kratom alkaloids in Mitragyna speciosa leaf extracts and commercial products by ultra-performance liquid chromatography− tandem mass spectrometry. Drug Test. Anal. 2019, 11, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Sabetghadam, A.; Ramanathan, S.; Mansor, S.M. The evaluation of antinociceptive activity of alkaloid, methanolic, and aqueous extracts of Malaysian Mitragyna speciosa Korth leaves in rats. Pharmacogn. Res. 2010, 2, 181. [Google Scholar] [CrossRef]
- Wiji, P.P.; Bahtiar, A.; Hayun, H. Synthesis and cytotoxicity evaluation of novel asymmetrical mono-carbonyl analogs of curcumin (AMACs) against Vero, HeLa, and MCF7 Cell Lines. Sci. Pharm. 2018, 86, 25. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, T.; Firouz, N.S.; Santhanam, R.; Jong, V.Y.M. Phytochemicals from Calophyllum macrocarpum Hook. f. and its cytotoxic activities. Nat. Prod. Res. 2020, 1–6. [Google Scholar] [CrossRef]
- Souid, G.; Sfar, M.; Timoumi, R.; Romdhane, M.H.; Essefi, S.A.; Majdoub, H. Protective Effect Assessment of Moringa oleifera against Cadmium-induced Toxicity in HCT116 and HEK-293 Cell Lines. Environ. Sci. Pollut. Res. Int. 2020, 1–10. [Google Scholar] [CrossRef]
- Xu, J.; Brennan, T.J. The pathophysiology of acute pain: Animal models. Curr. Opin. Anaesthesiol. 2011, 24, 508. [Google Scholar] [CrossRef]
- Yaksh, T.L.; Rudy, T.A. Studies on the direct spinal action of narcotics in the production of analgesia in the rat. J. Pharmacol. Exp. Ther. 1977, 202, 411–428. [Google Scholar]
- Smith, T.W.; Buchan, P.; Parsons, D.N.; Wilkinson, S. Peripheral antinociceptive effects of N-methyl morphine. Life Sci. 1982, 31, 1205–1208. [Google Scholar] [CrossRef]
- Smith, T.W.; Follenfant, R.L.; Ferreira, S.H. Antinociceptive models displaying peripheral opioid activity. Int. J. Tissue React. 1985, 7, 61–67. [Google Scholar]
- Takayama, H.; Ishikawa, H.; Kurihara, M.; Kitajima, M.; Aimi, N.; Ponglux, D.; Koyama, F.; Matsumoto, K.; Moriyama, T.; Yamamoto, L.T.; et al. Studies on the Synthesis and Opioid Agonistic Activities of Mitragynine-related Indole alkaloids: Discovery of opioid agonists structurally different from other opioid ligands. J. Med. Chem. 2002, 45, 1949–1956. [Google Scholar] [CrossRef]
- Kruegel, A.C.; Gassaway, M.M.; Kapoor, A.; Váradi, A.; Majumdar, S.; Filizola, M.; Javitch, J.A.; Sames, D. Synthetic and receptor signaling explorations of the Mitragyna alkaloids: Mitragynine as an atypical molecular framework for opioid receptor modulators. J. Am. Chem. Soc. 2016, 138, 6754–6764. [Google Scholar] [CrossRef]
- Obeng, S.; Kamble, S.H.; Reeves, M.E.; Restrepo, L.F.; Patel, A.; Behnke, M.; Chear, N.J.Y.; Ramanathan, S.; Sharma, A.; Leon, F. Investigation of the adrenergic and opioid binding affinities, metabolic stability, plasma protein binding properties, and functional effects of selected indole-based kratom alkaloids. J. Med. Chem. 2020, 63, 433–439. [Google Scholar] [CrossRef]
- Devaraj, S.; Esfahani, A.S.; Ismail, S.; Ramanathan, S.; Yam, M.F. Evaluation of the antinociceptive activity and acute oral toxicity of standardised ethanolic extract of the rhizome of Curcuma xanthorrhiza Roxb. Molecules 2010, 15, 2925–2934. [Google Scholar] [CrossRef]
- Ullah, H.A.; Zaman, S.; Juhara, F.; Akter, L.; Tareq, S.M.; Masum, E.H.; Bhattacharjee, R. Evaluation of antinociceptive, in-vivo & in-vitro anti-inflammatory activity of ethanolic extract of Curcuma zedoaria rhizome. BMC Complement. Altern. Med. 2014, 14, 346. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A. Therapeutic potential of flavonoids in pain and inflammation: Mechanisms of action, pre-clinical and clinical data, and pharmaceutical development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef]
- Hazlina, A.H.; Azfar, H.A.A.; Rosniza, R. Accelerated Solvent Extraction: An Innovative Sample Extraction Technique for Natural Products. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/47/122/47122414.pdf?r=1 (accessed on 1 October 2020).
- Goh, T.B.; Hamdan, M.R.; Siddiqui, M.J.; Mordi, M.N.; Mansor, S.M. A simple and cost effective isolation and purification protocol of mitragynine from Mitragyna speciosa Korth (Ketum) Leaves. Malays. J. Anal. Sci. 2011, 15, 54–60. [Google Scholar]
- Jamil, M.F.A.; Subki, M.F.M.; Lan, T.M.; Majid, M.I.A.; Adenan, M.I. The effect of mitragynine on cAMP formation and mRNA expression of mu-opioid receptors mediated by chronic morphine treatment in SK–N–SH neuroblastoma cell. J. Ethnopharmacol. 2013, 148, 135–143. [Google Scholar] [CrossRef]
- Farooq, M.U.; Mumtaz, M.W.; Mukhtar, H.; Rashid, U.; Akhtar, M.T.; Raza, S.A.; Nadeem, M. UHPLC-QTOF-MS/MS based phytochemical characterisation and anti-hyperglycemic prospective of hydro-ethanolic leaf extract of Butea monosperma. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Tohar, N.; Shilpi, J.A.; Sivasothy, Y.; Ahmad, S.; Awang, K. Chemical constituents and nitric oxide inhibitory activity of supercritical carbon dioxide extracts from Mitragyna speciosa leaves. Arab. J. Chem. 2019, 12, 350–359. [Google Scholar] [CrossRef]
- Woolfe, G.; MacDonald, A.D. The evaluation of the analgesic action of pethidine hydrochloride (Demerol). J. Pharmacol. Exp. Ther. 1944, 80, 300–307. [Google Scholar]
- Sabetghadam, A.; Navaratnam, V.; Mansor, S.M. Dose–Response Relationship, Acute Toxicity, and Therapeutic Index between the Alkaloid Extract of Mitragyna speciosa and Its Main Active Compound Mitragynine in Mice. Drug Dev. Res. 2013, 74, 23–30. [Google Scholar] [CrossRef]
- D’amour, F.E.; Smith, D.L. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941, 72, 74–79. [Google Scholar]
| Sample | Time (min) | Mean of Dry Yield (g) | Mean Percentage of Mitragynine (%) Mean ± SEM |
|---|---|---|---|
| ASE aqueous | 5 | 2.14 ± 0.15 | 1.83 ± 0.08 |
| 10 | 2.01 ± 0.04 | 1.57 ± 0.29 | |
| 20 | 2.00 ± 0.29 | 1.54 ± 0.17 |
| Sample | Time (min) | Mean of Dry Yield (g) | Mean Percentage of Mitragynine (%) Mean ± SEM |
|---|---|---|---|
| ASE aqueous | 5 | 2.14 ± 0.15 b | 1.83 ± 0.08 a |
| ASE MeOH | 5 | 2.91 ± 0.21c | 7.19 ± 0.30 b |
| ASE EtOH | 5 | 2.26 ± 0.09 bc | 6.53 ± 0.20 b |
| ASE EtOAc | 5 | 0.53 ± 0.11 a | 6.79 ± 0.59 b |
| Extract | Mean of Absorbance | Total Phenolic Content in Dry Extract (GAE mg/g), Mean ± SEM |
|---|---|---|
| ASE aqueous | 0.430 | 367.42 ± 3.06 a |
| ASE MeOH | 0.527 | 448.67 ± 8.33 c |
| ASE EtOH | 0.478 | 407.83 ± 2.50 b |
| ASE EtOAc | 0.540 | 459.78 ± 5.47 c |
| Extract | Mean of Absorbance | Total Flavonoids Content in Dry Extract (QE mg/g), Mean ± SEM |
|---|---|---|
| ASE aqueous | 0.055 | 115.25 ± 6.25 a |
| ASE MeOH | 0.059 | 125.25 ± 1.25 a |
| ASE EtOH | 0.086 | 194.00 ± 5.00 b |
| ASE EtOAc | 0.065 | 141.50 ± 10.00 a |
| Sample | IC50 Value | |
|---|---|---|
| HEK-293 Kidney Cells | HeLa Chang Liver Cells | |
| ASE aqueous extract | >500 µg/mL | >500 µg/mL |
| ASE MeOH extract | >500 µg/mL | >500 µg/mL |
| ASE EtOH extract | >500 µg/mL | >500 µg/mL |
| ASE EtOAc extract | >500 µg/mL | 153.75 ± 31.75 µg/mL |
| Mitragynine | 112.30 ± 17.59 µM | 210.04 ± 0.80 µM |
| Doxorubicin a | 80.82 ± 12.05 µM | 86.23 ± 27.49 µM |
| Identification | Calculated m/z [M+H]+ | Precursor ion Experimental m/z [M+H]+ | Elemental Composition | Major Ions in MS/MS Spectra (Key Fragment Ions) | ASE Aqueous RT, min | ASEMeOH RT, min | ASE EtOH RT, min | ASE EtOAc RT, min | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Chlorogenic acid | 355.1014 | 355.1023 | C16H18O9 | 195.0649, 163.0393, 135.0445 | 2.99 | - | 2.49 | 2.39 | [16] |
| Umbelliferone | 163.0390 | 163.0394 | C9H6O3 | 145.0280, 135.0445, 117.0340 | 3.00 | 3.25 | 3.06 | 3.09 | [17] |
| O-coumaric acid | 163.0427 | 165.0553 | C9H8O3 | 147.0439, 109.0644, 165.0550, 121.0652 | - | 3.93 | 4.17 | 4.20 | [16] |
| Quercetin 3-galactoside 7-rhamnoside | 611.1607 | 611.1619 | C27H30O16 | 465.1024, 449.1070, 303.0500 | 4.85 | 4.84 | 4.86 | 4.86 | [16] |
| Rutin | 611.1602 | 611.1621 | C27H30O16 | 465.1022, 449.1072, 303.0496 | 5.01 | 5.03 | 5.03 | 5.03 | [16] |
| Quercetin | 303.0508 | 303.0509 | C15H10O7 | 285.0395, 229.0496, 153.0183 | - | 5.26 | 5.30 | 5.25 | [18] |
| Isoquercitrin | 465.1028 | 465.1036 | C21H20O12 | 303.0499, 153.0179 | - | - | - | 5.29 | [19] |
| Vincamine | 355.2016 | 355.2023 | C21H26N2O3 | 338.1942, 224.1290, 144.0809 | 5.41 | 5.48 | 5.47 | 5.44 | [20] |
| Rhynchophylline | 385.2122 | 385.2122 | C22H28N2O4 | 160.0759, 110.0965, 129.0544 | - | 5.87 | 5.93 | 5.89 | [21] |
| Corynoxine B | 385.2122 | 385.2132 | C22H28N2O4 | 241.1338, 160.0758, 110.0964 | 6.55 | 6.44 | 6.44 | 6.44 | [22] |
| Corynoxine | 385.2122 | 385.2132 | C22H28N2O4 | 353.1851, 160.0758, 110.0966 | - | 6.65 | 6.65 | 6.65 | [22] |
| 7-hydroxymitragynine | 415.2227 | 415.2239 | C23H30N2O5 | 400.1984, 190.0863, 110.0965 | 6.83 | 6.74 | 6.79 | 6.79 | [22] |
| Mitragynine | 399.2278 | 399.2278 | C23H30N2O4 | 238.1440, 226.1441, 174.0913, 110.0967 | 7.44 | 7.34 | 7.34 | 7.49 | [22] |
| Corynantheidine | 369.2170 | 369.2173 | C22H28 N2O3 | 238.1441, 226.1441, 110.0963 | - | - | - | 7.73 | [22] |
| Speciogynine | 399.2278 | 399.2302 | C23H30N2O4 | 238.1443, 226.1445, 174.0916, 110.0965 | - | - | 7.74 | 7.79 | [22] |
| Paynantheine | 397.2122 | 397.2133 | C23H28N2O4 | 236.1287, 224.1288, 174.0916, 159.0682 | 8.54 | - | 8.23 | 8.29 | [22] |
| 3-isopaynantheine | 397.2122 | 397.2131 | C23H28N2O4 | 200.1071, 174.0914, 159.0681 | 9.20 | - | 9.18 | 9.24 | [22] |
| Speciociliatine | 399.2278 | 399.2283 | C23H30N2O4 | 238.1437, 226.1443, 174.0911, 110.0962 | - | 11.49 | 11.43 | 11.43 | [22] |
| α-linolenic acid | 279.2319 | 279.2325 | C18H30O2 | 277.2318, 223.1688, 137.1329 | - | - | 13.33 | 13.48 | [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, Y.S.; Karunakaran, T.; Murugaiyah, V.; Santhanam, R.; Abu Bakar, M.H.; Ramanathan, S. Accelerated Solvent Extractions (ASE) of Mitragyna speciosa Korth. (Kratom) Leaves: Evaluation of Its Cytotoxicity and Antinociceptive Activity. Molecules 2021, 26, 3704. https://doi.org/10.3390/molecules26123704
Goh YS, Karunakaran T, Murugaiyah V, Santhanam R, Abu Bakar MH, Ramanathan S. Accelerated Solvent Extractions (ASE) of Mitragyna speciosa Korth. (Kratom) Leaves: Evaluation of Its Cytotoxicity and Antinociceptive Activity. Molecules. 2021; 26(12):3704. https://doi.org/10.3390/molecules26123704
Chicago/Turabian StyleGoh, Yong Sean, Thiruventhan Karunakaran, Vikneswaran Murugaiyah, Rameshkumar Santhanam, Mohamad Hafizi Abu Bakar, and Surash Ramanathan. 2021. "Accelerated Solvent Extractions (ASE) of Mitragyna speciosa Korth. (Kratom) Leaves: Evaluation of Its Cytotoxicity and Antinociceptive Activity" Molecules 26, no. 12: 3704. https://doi.org/10.3390/molecules26123704
APA StyleGoh, Y. S., Karunakaran, T., Murugaiyah, V., Santhanam, R., Abu Bakar, M. H., & Ramanathan, S. (2021). Accelerated Solvent Extractions (ASE) of Mitragyna speciosa Korth. (Kratom) Leaves: Evaluation of Its Cytotoxicity and Antinociceptive Activity. Molecules, 26(12), 3704. https://doi.org/10.3390/molecules26123704








