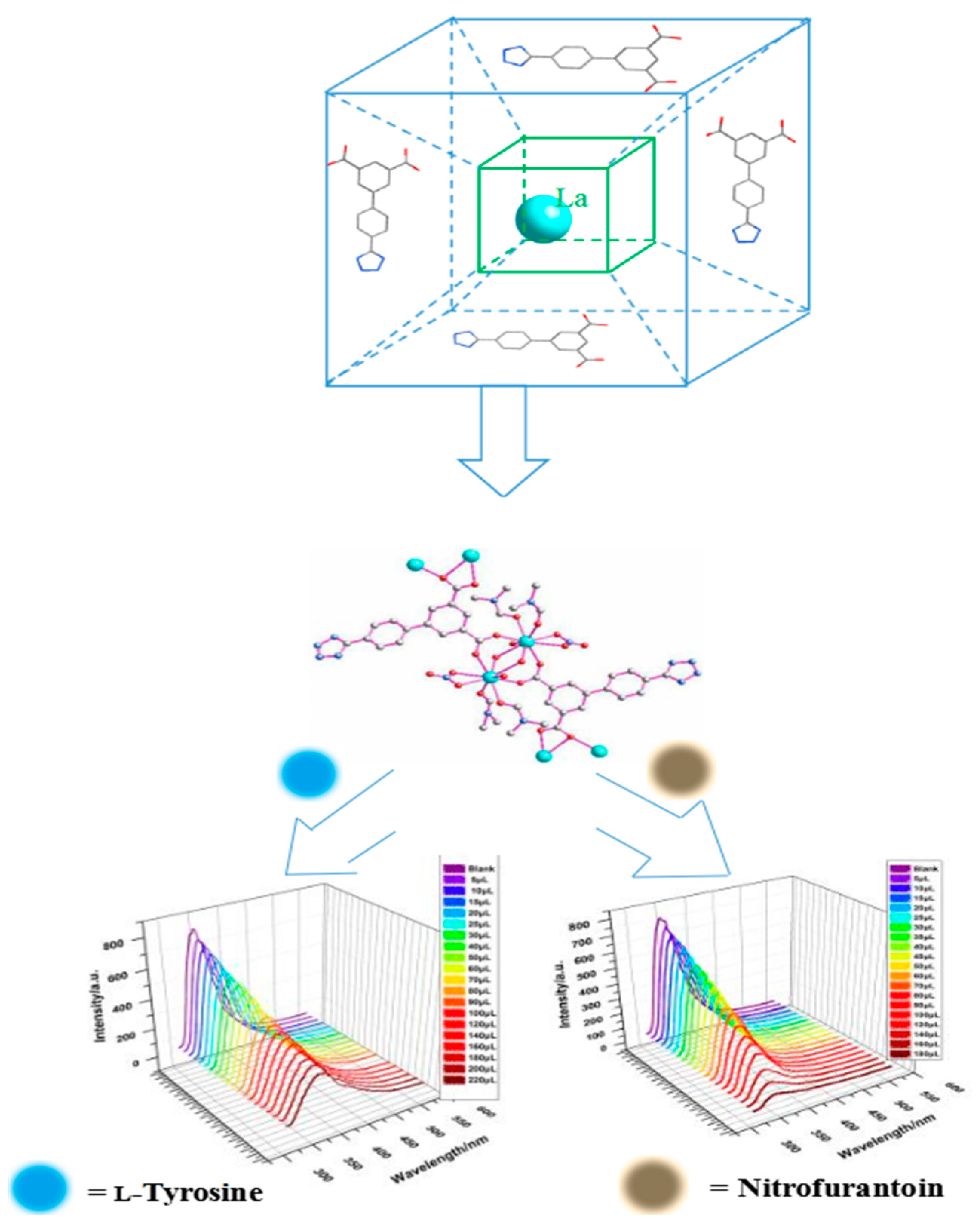
Solvothermal Preparation of a Lanthanide Metal-Organic Framework for Highly Sensitive Discrimination of Nitrofurantoin and l-Tyrosine
Abstract
1. Introduction
2. Results and Discussion
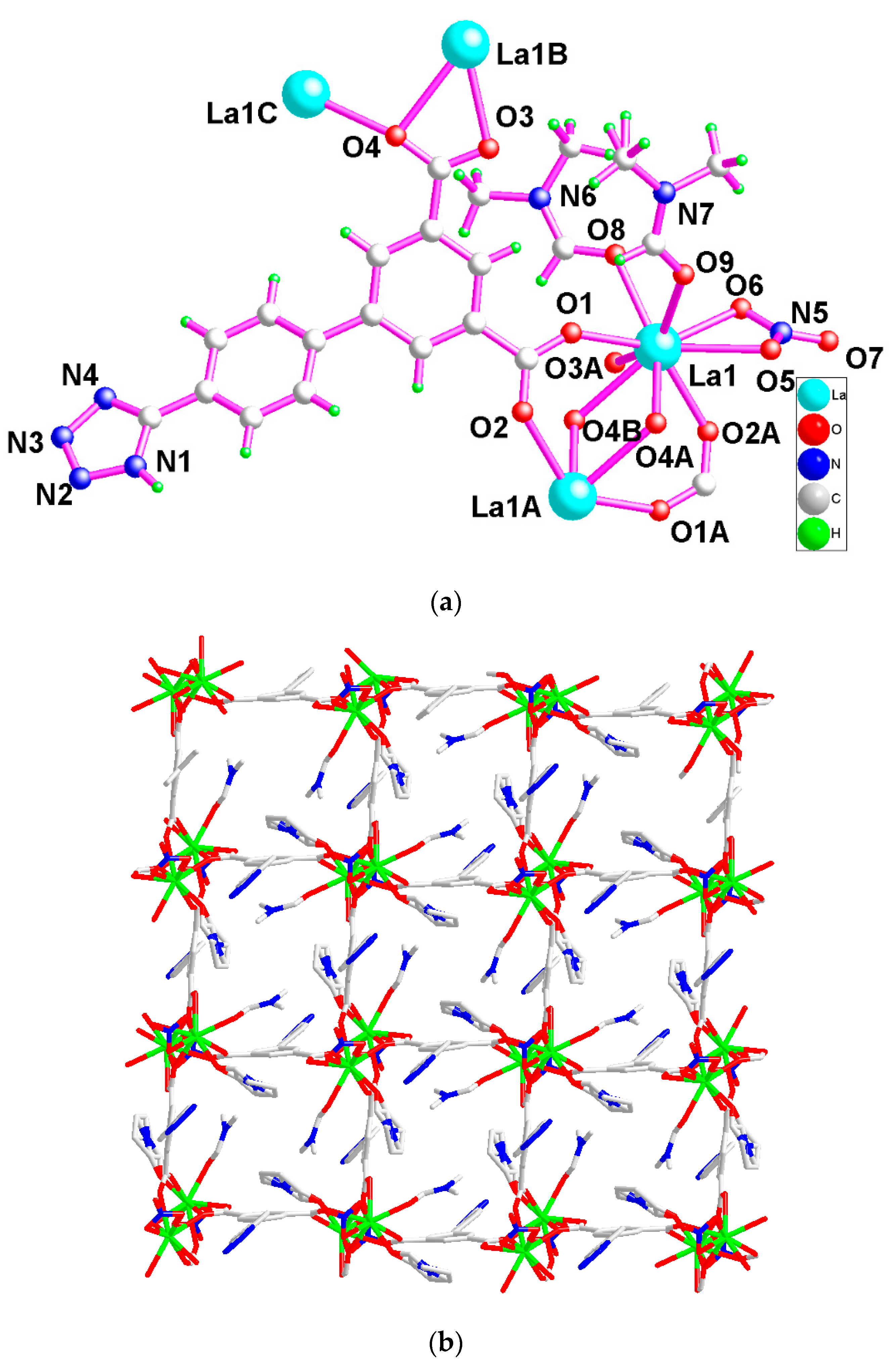
2.1. Structural Description of 1
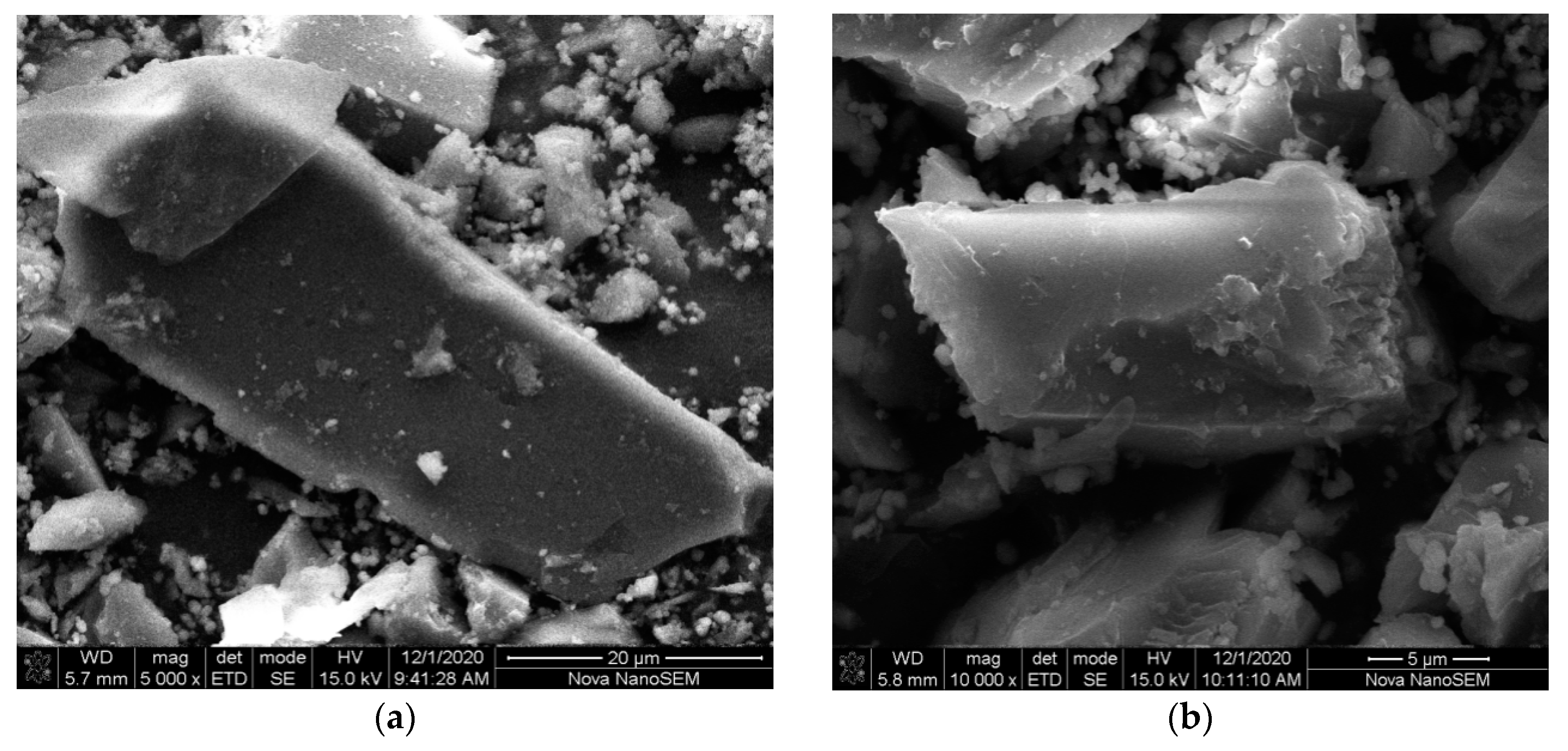
2.2. PXRD, FT-IR and SEM Characterizations of 1
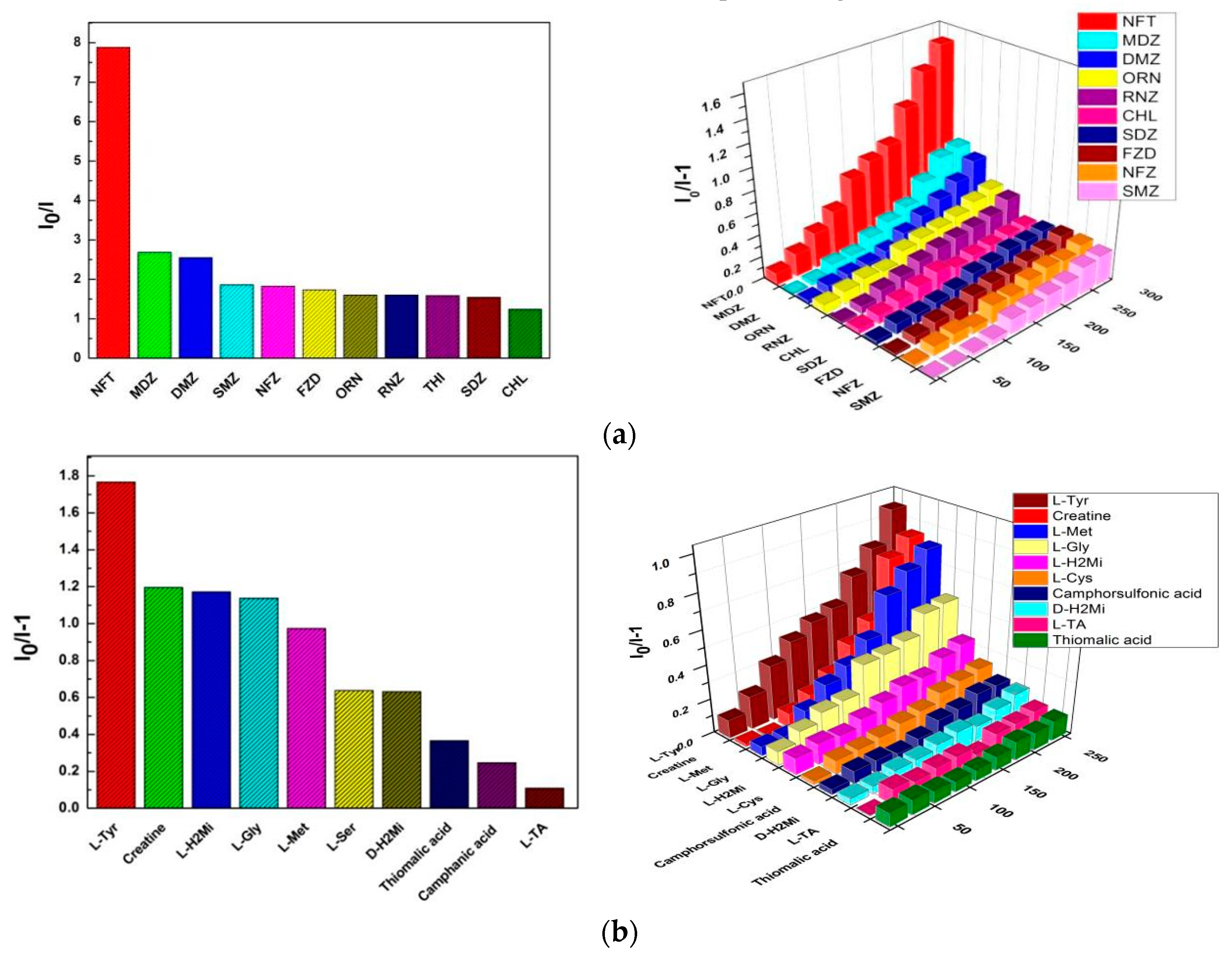
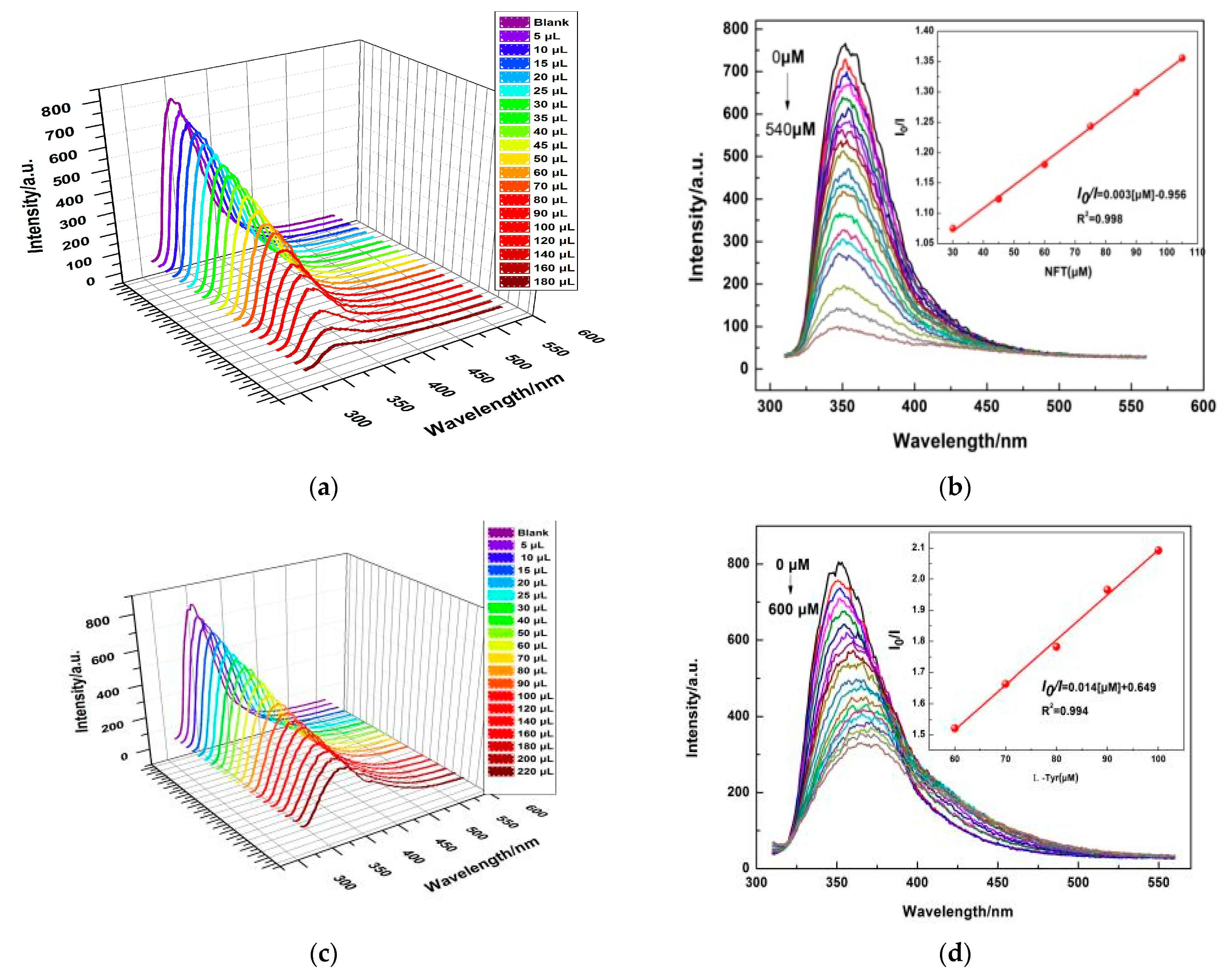
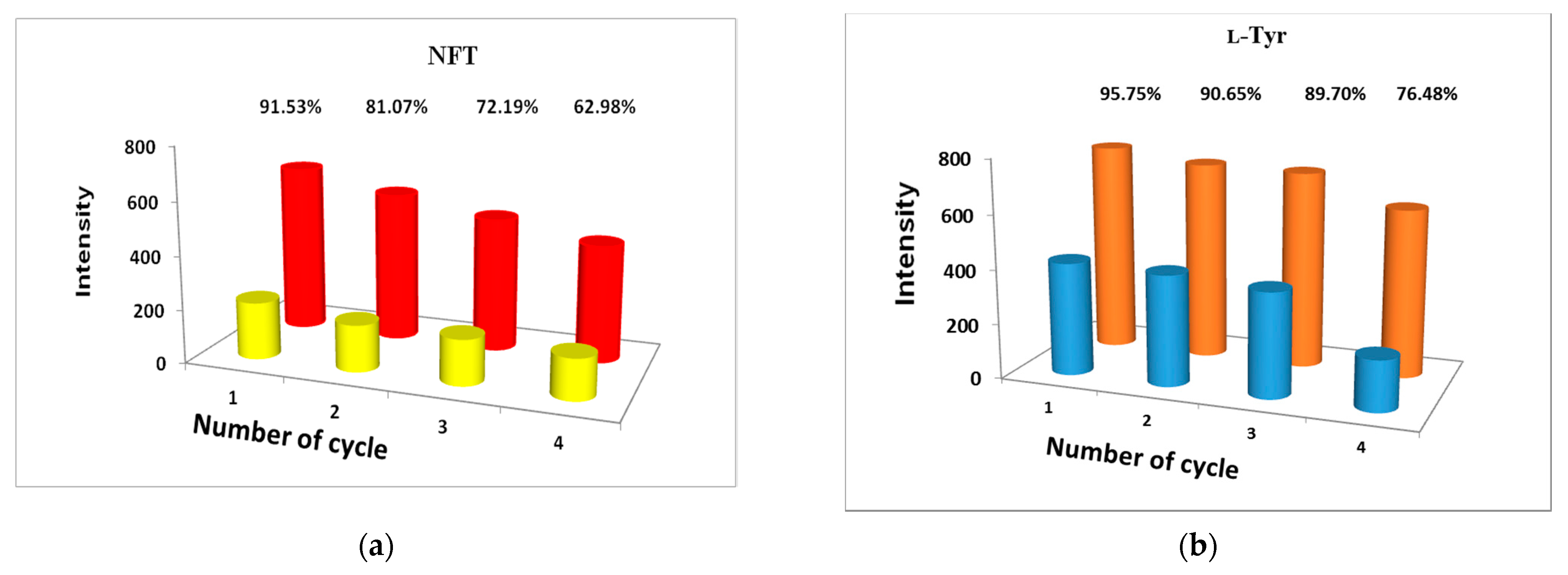
2.3. Photo-Luminescent Properties of 1
3. Materials and Methods
3.1. General Remarks
3.2. Preparation of [La(HL)(DMF)2(NO3)] (1)
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Calahorro, A.J.; Briones, D.; Seco, J.M.; Salinas-Castillo, A.; Rodriguez-Dieguez, A.; Mendicute-Fierro, C. Bidimensional cadmium metal-organic frameworks based on 1,3-bis(4-pyridyl)propane displaying long lifetime photoluminescence emission. Polyhedron 2015, 91, 47–51. [Google Scholar] [CrossRef]
- Al-Janabi, N.; Deng, H.R.; Borges, J.; Liu, X.F.; Garforth, A.; Siperstein, F.R.; Fan, X.L. A facile post-synthetic modification method to improve hydrothermal stability and CO2 selectivity of CuBTC metal−organic framework. Ind. Eng. Chem. 2016, 55, 7941–7949. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A.; Dhakshinamoorthy, A.; Garcia, H. Mixed-metal MOFs: Unique opportunities in metal-organic framework (MOF) functionality and design. Angew. Chem. Int. Ed. 2019, 58, 15188–15205. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.-J.; Zhou, H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.P.; Young, A.E.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Simagina, A.A.; Polynski, M.V.; Vinogradov, A.V.; Pidko, E.A. Towards rational design of metal-organic framework-based drug delivery systems. Russ. Chem. Rev. 2018, 87, 831–858. [Google Scholar] [CrossRef]
- Lysova, A.A.; Samsonenko, D.G.; Kovalenko, K.A.; Nizovtsev, A.S.; Dybtsev, D.N.; Fedin, V.P. A Series of Mesoporous Metal-Organic Frameworks with Tunable Windows Sizes and Exceptionally High Ethane over Ethylene Adsorption Selectivity. Angew. Chem. Int. Ed. 2020, 59, 20561–20567. [Google Scholar] [CrossRef] [PubMed]
- Sapianik, A.A.; Kovalenko, K.A.; Samsonenko, D.G.; Barsukova, M.O.; Dybtsev, D.N.; Fedin, V.P. Exceptionally effective benzene/cyclohexane separation using a nitro-decorated metal-organic framework. Chem. Commun. 2020, 56, 8241–8244. [Google Scholar] [CrossRef] [PubMed]
- Sapianik, A.A.; Fedin, V.P. Main Approaches to the Synthesis of Heterometallic Metal-Organic Frameworks. Russ. J. Coord. Chem. 2020, 46, 443–457. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Meng, J.-S.; Liu, X.; Niu, C.-J.; Pang, Q.; Li, J.-T.; Liu, F.; Liu, Z.; Mai, L.-Q. Advances in metal-organic framework coatings: Versatile synthesis and broad applications. Chem. Soc. Rev. 2020, 49, 3142–3186. [Google Scholar] [CrossRef]
- Chen, K.; Wu, C.-D. Development of photoluminescence metal-organic framework sensors consisting of dual-emission centers. Chin. Chem. Lett. 2019, 29, 823–826. [Google Scholar] [CrossRef]
- Lysova, A.A.; Samsonenko, D.G.; Dorovatovskii, P.V.; Lazarenko, V.A.; Khrustalev, V.N.; Kovalenko, K.A.; Dybtsev, D.N.; Fedin, V.P. Tuning the Molecular and Cationic Affinity in a Series of Multifunctional Metal-Organic Frameworks Based on Dodecanuclear Zn(II) Carboxylate Wheels. J. Am. Chem. Soc. 2019, 141, 17260–17269. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.; Min, H.; Shi, W.; Cheng, P. Multicenter Metal-Organic Framework-Based Ratiometric Fluorescent Sensors. Adv. Mater. 2020, 32, 1805871. [Google Scholar] [CrossRef]
- Han, Z.-S.; Wang, K.-Y.; Guo, Y.-F.; Chen, W.-J.; Zhang, J.-L.; Zhang, X.-R.; Siligard, G.; Yang, S.-H.; Zhou, Z.; Sun, P.-C.; et al. Cation-induced chirality in a bifunctional metal-organic framework for quantitative enantioselective recognition. Nat. Commun. 2019, 10, 5117. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Wang, Z.-Y.; Gao, J.; Wang, K.-Y.; Gianolio, E.; Aime, S.; Shi, W.; Zhou, Z.; Cheng, P.; Zaworotko, M.J. A Gadolinium(III) Zeolite-like Metal-Organic-Framework-Based Magnetic Resonance Thermometer. Chem 2019, 5, 1609–1618. [Google Scholar] [CrossRef]
- Wang, D.-B.; Tan, Q.-H.; Liu, J.-J.; Liu, Z.-L. A stable europium metal-organic framework as a dual-functional luminescent sensor for quantitatively detecting temperature and humidity. Dalton Trans. 2016, 45, 18450–18454. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-H.; Han, Z.-S.; Shi, W.; Cheng, P. Design, Synthesis and Applications of Chiral Metal-Organic Frameworks. Acta Chim. Sin. 2020, 78, 1336–1348. [Google Scholar] [CrossRef]
- Hou, Y.-L.; Xu, H.; Cheng, Q.-Q.; Zhao, B. Controlled lanthanide-organic framework nanospheres as reversible and sensitive luminescent sensors for practical applications. Chem. Commun. 2015, 51, 6769–6772. [Google Scholar] [CrossRef]
- Dong, M.-J.; Zhao, M.; Ou, S.; Zou, C.; Wu, C.-D. A luminescent Dye@MOF platform: Emission fingerprint relationships of volatile organic molecules. Angew. Chem. Int. Ed. 2014, 53, 1575–1579. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, L.; Gao, L.-X.; Zhu, P.-P.; Chen, Q.; Tan, K.-J. A highly fluorescent lanthanide metal-organic framework as dual-mode visual sensor for berberine hydrochloride and tetracycline. Anal. Bioanal. Chem. 2019, 411, 5963–5973. [Google Scholar] [CrossRef]
- Lian, X.; Yan, B. A lanthanide metal-organic framework (MOF-76) for adsorbing dyes and fluorescence detecting aromatic pollutants. RSC Adv. 2016, 6, 11570–11576. [Google Scholar] [CrossRef]
- Wencewicz, T.A. Crossroads of antibiotic resistance and biosynthesis. J. Mol. Biol. 2016, 431, 3370–3399. [Google Scholar] [CrossRef] [PubMed]
- Ruppe, E.; Burdet, C.; Grall, N.; Lastours, V.; Lescure, F.X.; Andremont, A.; Armand-Lefevre, L. Impact of antibiotics on the intestinal microbiota needs to be re-defined to optimize antibiotic usage. Clin. Microbiol. Infect. 2018, 24, 783–784. [Google Scholar] [CrossRef]
- Pruden, A.; Pei, R.-T.; Storteboom, H. Antibiotic resistance genes as emerging contaminants: Studies in Northern Colorado. Environ. Sci. Technol. 2006, 40, 7445–7450. [Google Scholar] [CrossRef] [PubMed]
- Jager, L.P.; Graaf, G.J.; Widjaja-Greefkes, H.C.A. Effects of alpha(1)-antagonists on production and release of aldosterone and other steroid hormones by porcine adrenocortical cells in vitro. Can. J. Physiol. Pharm. 1998, 76, 676–683. [Google Scholar] [CrossRef]
- McEvoy, J.D.G. Contamination of animal feedingstuffs as a cause of residues in food: A review of regulatory aspects, incidence and control. Anal. Chim. Acta 2002, 473, 3–26. [Google Scholar] [CrossRef]
- Available online: http://www.moa.gov.cn/nybgb/2008/djiuq/201806/t20180611_6151661.htm (accessed on 14 May 2021).
- Kalser, L.R.; Robr, F.J.; Koron, M.S.; Levy, H.L. Tyrosine supplementation in phenylketonuria: Diurnal blood tyrosine levels and presumptive brain influx of tyrosine and other large neutral amino acids. J. Pediatr. 2001, 139, 421–427. [Google Scholar] [CrossRef]
- Rohr, F.J.; Lobbregt, D.; Levy, H.L. Tyrosine supplementation in the treatment of maternal phenylketonuria. Am. J. Clin. Nutr. 1998, 67, 473–476. [Google Scholar] [CrossRef][Green Version]
- Smith, M.L.; Hanley, W.B.; Clarke, J.T.R.; Klim, P.; Schoonheyt, W.; Austin, V.; Lehotay, D.C. Randomised controlled trial of tyrosine supplementation on neuropsychological performance in phenylketonuria. Arch. Dis. Child. 1998, 78, 116–121. [Google Scholar] [CrossRef]
- Levy, P.A.; Miller, J.B.; Shapira, E. The advantage of phenylalanine to tyrosine ratio for the early detection of phenylketonuria. Clin. Chim. Acta 1998, 270, 177–181. [Google Scholar] [CrossRef]
- Mcardle, L.; Rafferty, M.; Maelandsmo, G.M.; Bergin, O.; Farr, C.J.; Dervan, P.A.; O’Loughlin, S.; Herlyn, M.; Easty, D.J. Protein tyrosine phosphatase genes downregulated in melanoma. J. Investig. Dermatol. 2001, 117, 1255–1260. [Google Scholar] [CrossRef]
- Buron, G.; Hacquemand, R.; Pourie, G.; Brand, G. Inhalation exposure to acetone induces selective damage on olfactory neuroepithelium in mice. Neurotoxicology 2009, 30, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Kumarvel, V.; Da Fonseca, J. Acetone poisoning-a diagnostic dilemma. Eur. J. Anaesthesiol. 2007, 24, 805–806. [Google Scholar] [CrossRef]
- Pak, Y.L.; Swamy, K.M.K.; Yoon, J. Recent Progress in Fluorescent Imaging Probes. Sensors 2015, 15, 24374–24396. [Google Scholar] [CrossRef]
- Clementino, R.F.P.; Santos, A.B.D.; Marques, O.J.B.J.; Ratkovski, D.R.; Gatto, C.C.; Malvestiti, I.; Machado, F.L.D.; Falcao, E.H.L. Structural description, luminescent and magnetic properties of novel 2-D coordination polymers containing thiazolo[5,4-d]thiazole rings and trivalent lanthanide ions. J. Solid State Chem. 2018, 268, 94–101. [Google Scholar] [CrossRef]
- Kostakis, G.E.; Ako, A.M.; Powell, A.K. Structural motifs and topological representation of Mn coordination clusters. Chem. Soc. Rev. 2010, 39, 2238–2271. [Google Scholar] [CrossRef] [PubMed]
- Tabacaru, A.; Pettinari, C.; Galli, S. Coordination polymers and metal-organic frameworks built up with poly(tetrazolate) ligands. Coord. Chem. Rev. 2018, 372, 1–30. [Google Scholar] [CrossRef]
- Putnam, C.D.; Hammel, M.; Hura, G.L.; Tainer, J.A. X-ray solution scattering (SAXS) combined with crystallography and computation: Defining accurate macromolecular structures, conformations and assemblies in solution. Q. Rev. Biophys. 2007, 40, 191–285. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-Y.; Zhu, H.-X.; Wang, P.; Wu, C.-Y.; Gao, W.-C.; Mu, J.-Y.; Wei, S.-Y. Performance optimization of UV curable waterborne polyurethane acrylate wood coatings modified by castor oil. J. For. Eng. 2020, 5, 89–95. [Google Scholar]
- Zheng, C.-X.; Zhu, S.-L.; Lu, Y.; Mei, C.-T.; Xu, X.-W.; Yue, Y.-Y.; Han, J.-Q. Synthesis and characterization of cellulose nanofibers/polyacrylic acid-polyacrylamide double network electroconductive hydrogel. J. For. Eng. 2020, 5, 93–100. [Google Scholar]
- Ibitoye, S.E.; Adegun, I.K.; Omoniyi, P.O.; Ogedengbe, T.S.; Alabi, O.O. Numerical investigation of thermo-physical properties of non-newtonian fliud in a modelled intestine. J. Bioresour. Bioprod. 2020, 5, 211–221. [Google Scholar] [CrossRef]
- Tan, W.; Hao, X.-L.; Wang, Q.-W.; Ou, R.-X. Mechanical and thermal properties of bamboo plastic composites reinforced by thermotropic liquid crystal copolyesters. J. For. Eng. 2020, 5, 97–103. [Google Scholar]
- Zhu, L.-T.; Gu, Y.-F.; Wu, G.-S. Biomass thermal conductivity measurement system design. J. For. Eng. 2020, 5, 97–102. [Google Scholar]
- Krishnakumar, V.; Xavier, R.J. FT Raman and FT-IR spectral studies of 3-mercapto-1,2,4-triazole. Spectrochim. Acta A 2004, 60, 709–714. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.-Z.; Li, Y.-J.; Shen, D.-H.; Dai, Y.-P.; Zhang, S.-Z.; Zhang, W.-G. Effect of high temperature oil heat treatment on the starch content and mold-resistant property of bamboo. J. For. Eng. 2020, 5, 109–115. [Google Scholar]
- Zhang, M.-J.; Ma, W.-J.; Cui, J.-X.; Wu, S.-T.; Han, J.-Q.; Zou, Y.; Huang, C.-B. Hydrothermal synthesized UV-resistance and transparent coating composited superoloephilic electrospun membrane for high efficiency oily wastewater treatment. J. Hazard. Mater. 2020, 383, 121152. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Wang, R.-X.; Tang, G.-S.; Mou, Z.-P.; Lei, J.-D.; Han, J.-Q.; Smedt, S.D.; Xiong, R.-H.; Huang, C.-B. Ecofriendly electrospun membranes loaded with visible-lightresponding nanoparticles for multifunctional usages: Highly efficient air filtration, dye scavenging, and bactericidal activity. ACS Appl. Mater. Interfaces 2019, 11, 12880–12889. [Google Scholar] [CrossRef]
- Zhu, M.-M.; Xiong, R.-H.; Huang, C.-B. Bio-Based and Photocrosslinked Electrospun Antibacterial Nanofibrous Membranes for Air Filtration. Carbohydr. Polym. 2019, 205, 55–62. [Google Scholar] [CrossRef]
- Tang, G.-S.; Chen, L.; Wang, Z.-X.; Gao, S.-T.; Qu, Q.-L.; Xiong, R.-H.; Braeckmans, K.; Smedt, S.C.D.; Zhang, Y.-S.; Huang, C.-B. Faithful Fabrication of Biocompatible Multicompartmental Memomicrospheres for Digitally Color-Tunable Barcoding. Small 2020, 16, 1907586. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Hua, D.-W.; Huang, C.-B.; Samal, S.K.; Xiong, R.-H.; Sauvage, F.; Braeckmans, K.; Remaut, K.; Smedt, S.C.D. Materials and Technologies to Combat Counterfeiting of Pharmaceuticals: Current and Future Problem Tackling. Adv. Mater. 2020, 32, 1905486. [Google Scholar] [CrossRef]
- Tang, G.-S.; Chen, L.; Lian, L.-M.; Li, F.-H.; Ravanbakhsh, H.; Wang, M.; Zhang, Y.-S.; Huang, C.-B. Designable dual-power micromotors fabricated from a biocompatible gas-shearing strategy. Chem. Eng. J. 2021, 407, 127187. [Google Scholar] [CrossRef]
- Hua, D.-W.; Gao, S.-T.; Zhang, M.-J.; Ma, W.-J.; Huang, C.-B. A Novel Xanthan Gum-Based Conductive Hydrogel with Excellent Mechanical, Biocompatible, and Self-Healing Performances. Carbohydr. Polym. 2020, 247, 116743. [Google Scholar] [CrossRef]
- Xiong, R.-H.; Xu, R.-X.; Huang, C.-B.; Smedt, S.D.; Braeckmans, K. Stimuli-responsive nanobubbles for biomedical applications. Chem. Soc. Rev. 2021, 50, 5746–5776. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.-S.; Xiong, R.-H.; Lv, D.; Xu, R.-X.; Braeckmans, K.; Huang, C.-B.; Smedt, S.C.D. Gas-Shearing Fabrication of Multicompartmental Microspheres: A One-Step and Oil-FreeApproach. Adv. Sci. 2019, 6, 1802342–1802351. [Google Scholar] [CrossRef]
- An, J.; Kim, S.; Shrinidhi, A.; Kim, J.; Banna, H.; Sung, G.H.; Park, K.M.; Kim, K. Purification of protein therapeutics via high-affinity supramolecular host-guest interactions. Nat. Biomed. Eng. 2020, 4, 1044. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-T.; Tang, G.-S.; Hua, D.-W.; Xiong, R.-H.; Han, J.-Q.; Jiang, S.-H.; Zhang, Q.-L.; Huang, C.-B. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B. 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Hwang, W.; Yoo, J.; Hwang, I.; Lee, J.; Ko, Y.H.; Kim, H.W.; Kim, Y.; Lee, Y.; Hur, M.Y.; Park, K.M.; et al. Hierarchical Self-assembly of Polypseudorotaxanes into Artificial Microtubules. Angew. Chem. Int. Ed. 2020, 59, 3460–3464. [Google Scholar] [CrossRef]
- Hua, D.-W.; Xiong, R.-H.; Braeckmans, K.; Scheid, B.; Huang, C.-B.; Sauvage, F.; Smedt, S.C.D. Concentration Gradients in Material Sciences: Methods to Design and Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2009005. [Google Scholar] [CrossRef]
- Zhang, M.-M.; Ma, W.-J.; Wu, S.-T.; Tang, G.-S.; Cui, J.-X.; Zhang, Q.-L.; Chen, F.; Xiong, R.-H.; Huang, C.-B. Electrospun frogspawn structured membrane for gravity-driven oil-water separation. J. Colloid Interface Sci. 2019, 547, 136–144. [Google Scholar] [CrossRef]
- Cui, J.-X.; Lu, T.; Li, F.-H.; Wang, Y.-L.; Lei, J.-D.; Ma, W.-J.; Zou, Y.; Huang, C.-B. Flexible and transparent composite nanofibre membrane that was fabricated via a “green” electrospinning method for efficient particulate matter 2.5 capture. J. Colloid Interface Sci. 2021, 582, 506–514. [Google Scholar] [CrossRef]
- Lv, D.; Tang, G.-S.; Chen, L.; Zhang, M.-J.; Cui, J.-X.; Xiong, R.-H.; Huang, C.-B. Multifunctional Gas-Spinning Hierarchical Architecture: A Robust and Efficient Nanofiber Membrane for Simultaneous Air and Water Contaminant Remediation. ACS Appl. Polym. Mater. 2020, 2, 5686–5697. [Google Scholar] [CrossRef]
- Yorseng, K.; Siengchin, S.; Ashok, B.; Rajulu, A.V. Nanocomposite egg shell powder with in situ generated silver nanoparticles using inherent collagen as reducing agent. J. Bioresour. Bioprod. 2020, 5, 101–107. [Google Scholar] [CrossRef]
- Ashok, B.; Hariram, N.; Siengchin, S.; Rajulu, A.V. Modification of tamarind fruit shell powder with in situ generated copper nanoparticles by single step hydrothermal method. J. Bioresour. Bioprod. 2020, 5, 180–185. [Google Scholar] [CrossRef]
- Shuitz, A.M.; Sarjeant, A.A.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. Post-Synthesis Modification of a Metal-Organic Framework to Form Metallosalen-Containing MOF Materials. J. Am. Chem. Soc. 2011, 133, 13252–13255. [Google Scholar] [CrossRef]
- Qu, Q.-L.; Zhang, J.; Chen, X.-Q.; Ravanbakhsh, H.; Tang, G.-S.; Xiong, R.-H.; Manshian, B.-B.; Soenen, S.-J.; Sauvage, F.; Braeckmans, K.; et al. Triggered Release from Cellulose Microparticles Inspired by Wood Degradation by Fungi. ACS Sustain. Chem. Eng. 2021, 9, 387–397. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Zhao, Y.-L.; Song, B.; Huang, C.-B. Spectroscopic behavior and intracellular application of a highly sensitive UV-fluorescence double ratio probe based on water-soluble indole for detection acid pH. Dyes Pigments 2021, 188, 109205. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, G.X.; Song, Z.G.; Zhou, Y.P.; Chao, H.Y.; Yuan, D.Q.; Tan, T.T.Y.; Guo, Z.G.; Hu, Z.G.; Tang, B.Z.; et al. Two-Dimensional Metal-Organic Framework with Wide Channels and Responsive Turn-On Fluorescence for the Chemical Sensing of Volatile Organic Compounds. J. Am. Chem. Soc. 2014, 136, 7241–7244. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Song, Y.-H.; Wang, L. Multi-emission metal-organic framework composites for multicomponent ratiometric fluorescence sensing: Recent developments and future challenges. J. Mater. Chem. B 2020, 8, 3292–3315. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-Y.; Chen, D.-X.; Wu, M.-K.; Han, L.; Jiang, H.-L. Chemical Sensors Based on Metal-Organic Frameworks. Chem. Plus. Chem. 2016, 81, 675–690. [Google Scholar]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Calvez, G.; Le Natur, F.; Daiguebonne, C.; Bernot, K.; Suffren, Y.; Guilliou, O. Lanthanide-based hexa-nuclear complexes and their use as molecular precursors. Coord. Chem. Rev. 2017, 340, 134–153. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Zhao, Y.-L.; Wu, Y.-N.; Zhao, B.; Wang, L.-Y.; Song, B.; Huang, C.-B. Benzoindole-based bifunctional ratiometric turn-on sensor with an ICT effect for trapping of H+ and Al3+ in dual-channel cell imaging and samples. Spectrochim. Acta A 2021, 247, 119123. [Google Scholar] [CrossRef]
- Cisse, L.; Djande, A.; Capo-Chichi, M.; Delattre, F.; Saba, A.; Brochon, J.C.; Sanouski, S.; Tine, A.; Aaron, J.J. Fluorescence Quenching of Two Coumarin-3-carboxylic Acids by Trivalent Lanthanide Ions. J. Fluoresc. 2017, 27, 619–628. [Google Scholar] [CrossRef]
- Tarakanova, E.N.; Hamdoush, M.; Eroshin, A.V.; Ryzhov, I.V.; Zhabanov, Y.A.; Stuzhin, P.A. Preparation and Fluorescence Property of Metal-organic Framework Composite RhB/MOF-5. Chem. J. Chin. Univ. 2009, 30, 11–13. [Google Scholar]
- Ying, Y.-M.; Tao, C.-L.; Yu, M.-X.; Xiong, Y.; Guo, C.-R.; Liu, X.-G.; Zhao, Z.-J. In situ encapsulation of pyridine-substituted tetraphenylethene cations in metal-organic framework for the detection of antibiotics in aqueous medium. J. Mater. Chem. C 2019, 7, 8383–8388. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Huang, Y.H.; Lin, X.H.; Lu, F.F.; Zhang, Z.S.; Hu, Z.B. Lanthanum loaded graphitic carbon nitride nanosheets for highly sensitive and selective fluorescent detection of iron ions. Sens. Actuators B Chem. 2018, 255, 2218–2222. [Google Scholar] [CrossRef]
- Qin, C.-J.; Wang, W.-Y.; Wang, L.-X. A water-soluble organometallic conjugated polyelectrolyte for the direct colorimetric detection of silver ion in aqueous media with high selectivity and sensitivity. Macromolecules 2011, 44, 483–489. [Google Scholar] [CrossRef]
- Li, P.; Guo, M.-Y.; Gao, L.-L.; Yin, X.-M.; Yang, S.-L.; Bu, R.; Gao, E.-Q. Photoresponsivity and antibiotic sensing properties of an entangled tris(pyridinium)-based metal-organic framework. Dalton Trans. 2020, 49, 7488–7495. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wang, S.D.; Cao, K.Z.; Zou, G.D. A dual-functional fluorescent Co(II) coordination polymer sensor for the selective sensing of ascorbic acid and acetylacetone. J. Photochem. Photobiol. A 2021, 411, 113204. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Q.; Kapustin, E.A.; Yin, S.X.; Liang, L.; Zhou, Z.Y.; Niu, J.; Li, L.H.; Wang, Y.Y.; Su, J.; Li, J.; et al. Single-crystal x-ray diffraction structures of covalent organic frameworks. Science 2018, 361, 48–52. [Google Scholar] [CrossRef] [PubMed]

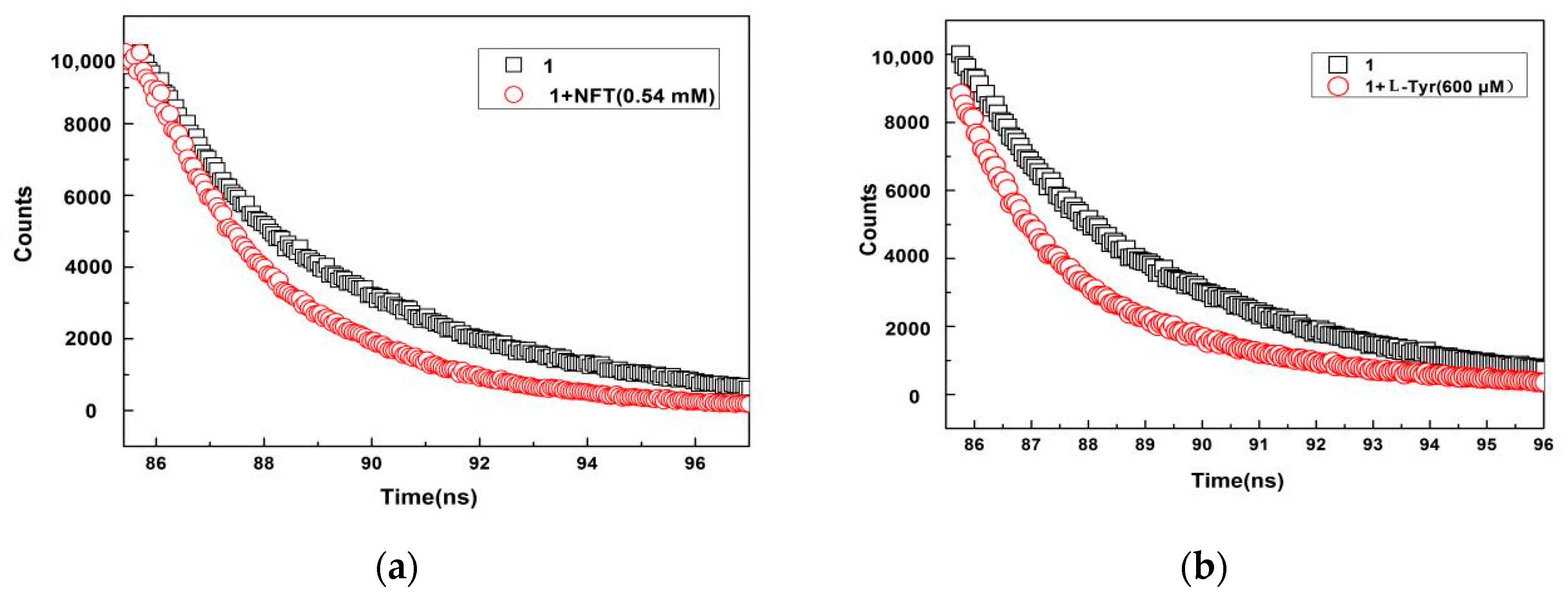

| Caption | T1/ns | T2/ns | B1/% | B2/% | Combined Life Rating/ns |
| 1 | 3.487773 | 6.584965 | 69.38 | 30.62 | 4.436 |
| 1 + NFT | 1.997302 | 3.409927 | 41.78 | 58.22 | 2.820 |
| 1 + l-Tyr | 2.528950 | 10.37314 | 71.39 | 28.61 | 4.773 |
| 1 | |
|---|---|
| Formula | C21H22LaN7O9 |
| M (g·mol−1) | 655.36 |
| Crystal system | Monoclinic |
| Space group | C2/c |
| Temperature | 133.32(16) |
| a (Å) | 28.2179(10) |
| b (Å) | 14.0169(5) |
| c (Å) | 14.3437(5) |
| α (°) | 90 |
| β (°) | 99.720(4) |
| γ (°) | 90 |
| V (Å3) | 5591.9(3) |
| Z | 8 |
| F (000) | 2608 |
| ρcalc (Mg·m−3) | 1.557 |
| μ (mm−1) | 1.586 |
| Data/restraints/parameters | 4916/36/362 |
| GOF on F2 | 1.069 |
| R1a (I ≥ 2σ(I)) | 0.0273 |
| wR2 b (all data) | 0.0643 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-T.; Liu, J.-Y.; Guo, R.; An, J.-D.; Huo, J.-Z.; Liu, Y.-Y.; Shi, W.; Ding, B. Solvothermal Preparation of a Lanthanide Metal-Organic Framework for Highly Sensitive Discrimination of Nitrofurantoin and l-Tyrosine. Molecules 2021, 26, 3673. https://doi.org/10.3390/molecules26123673
Wang T-T, Liu J-Y, Guo R, An J-D, Huo J-Z, Liu Y-Y, Shi W, Ding B. Solvothermal Preparation of a Lanthanide Metal-Organic Framework for Highly Sensitive Discrimination of Nitrofurantoin and l-Tyrosine. Molecules. 2021; 26(12):3673. https://doi.org/10.3390/molecules26123673
Chicago/Turabian StyleWang, Tian-Tian, Jing-Yi Liu, Rui Guo, Jun-Dan An, Jian-Zhong Huo, Yuan-Yuan Liu, Wei Shi, and Bin Ding. 2021. "Solvothermal Preparation of a Lanthanide Metal-Organic Framework for Highly Sensitive Discrimination of Nitrofurantoin and l-Tyrosine" Molecules 26, no. 12: 3673. https://doi.org/10.3390/molecules26123673
APA StyleWang, T.-T., Liu, J.-Y., Guo, R., An, J.-D., Huo, J.-Z., Liu, Y.-Y., Shi, W., & Ding, B. (2021). Solvothermal Preparation of a Lanthanide Metal-Organic Framework for Highly Sensitive Discrimination of Nitrofurantoin and l-Tyrosine. Molecules, 26(12), 3673. https://doi.org/10.3390/molecules26123673






