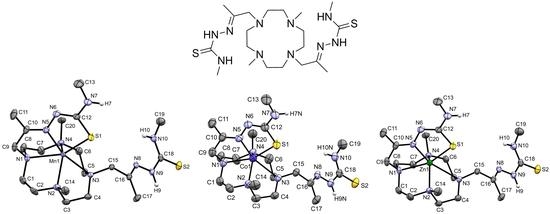
The Synthesis of a Bis(thiosemicarbazone) Macrocyclic Ligand and the Mn(II), Co(II), Zn(II) and 68Ga(III) Complexes
Abstract
1. Introduction
2. Results and Discussion
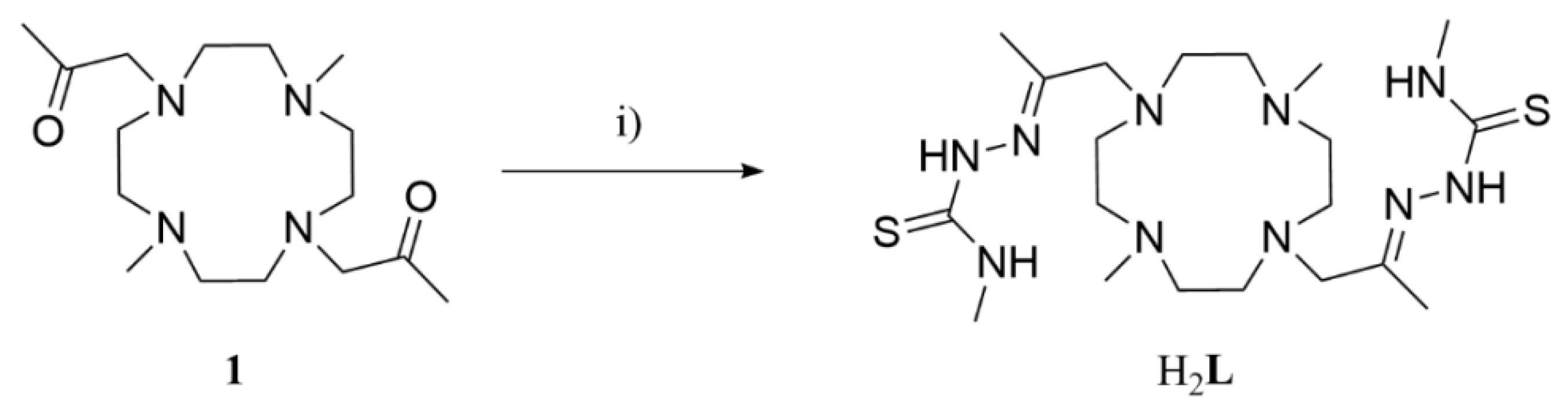
2.1. Synthesis of H2L and the Manganese(II), Cobalt(II) and Zinc(II) Complexes
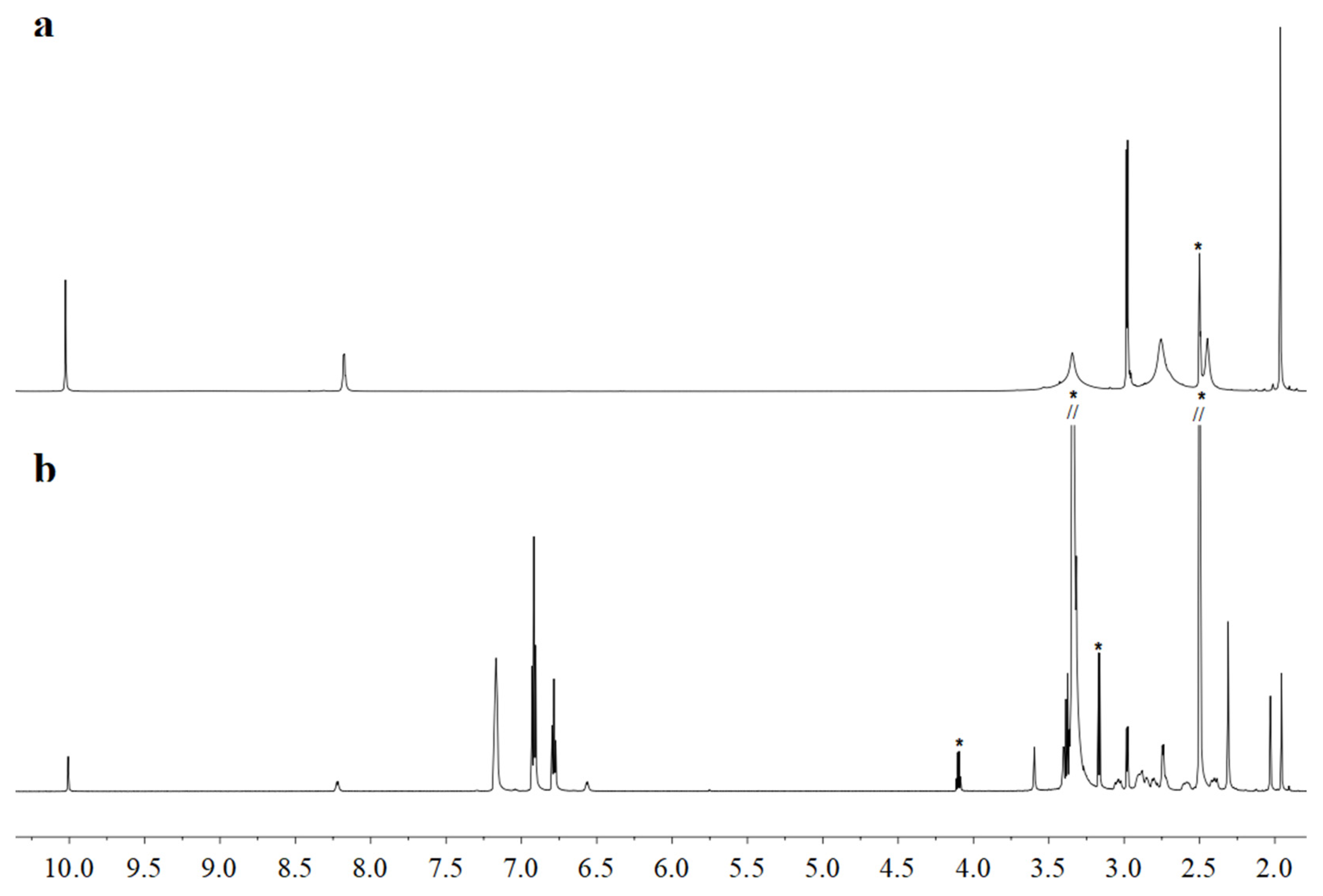
2.2. NMR Studies
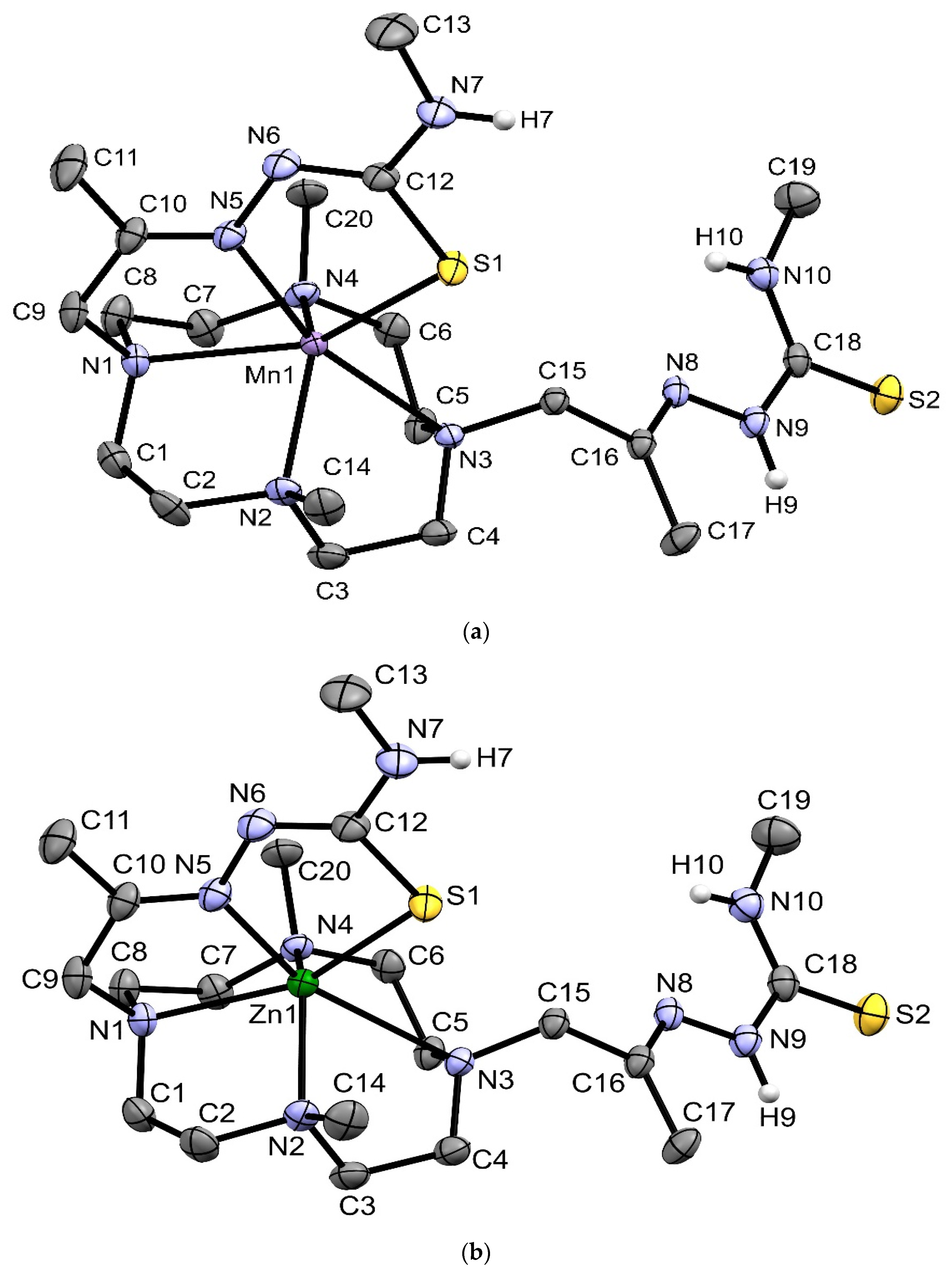
2.3. X-ray Crystal Structures of [MnHL](BPh4), [CoHL](BPh4) and [ZnHL](BPh4)
2.4. Magnetic Susceptibility
2.5. Density Functional Theory Calculations
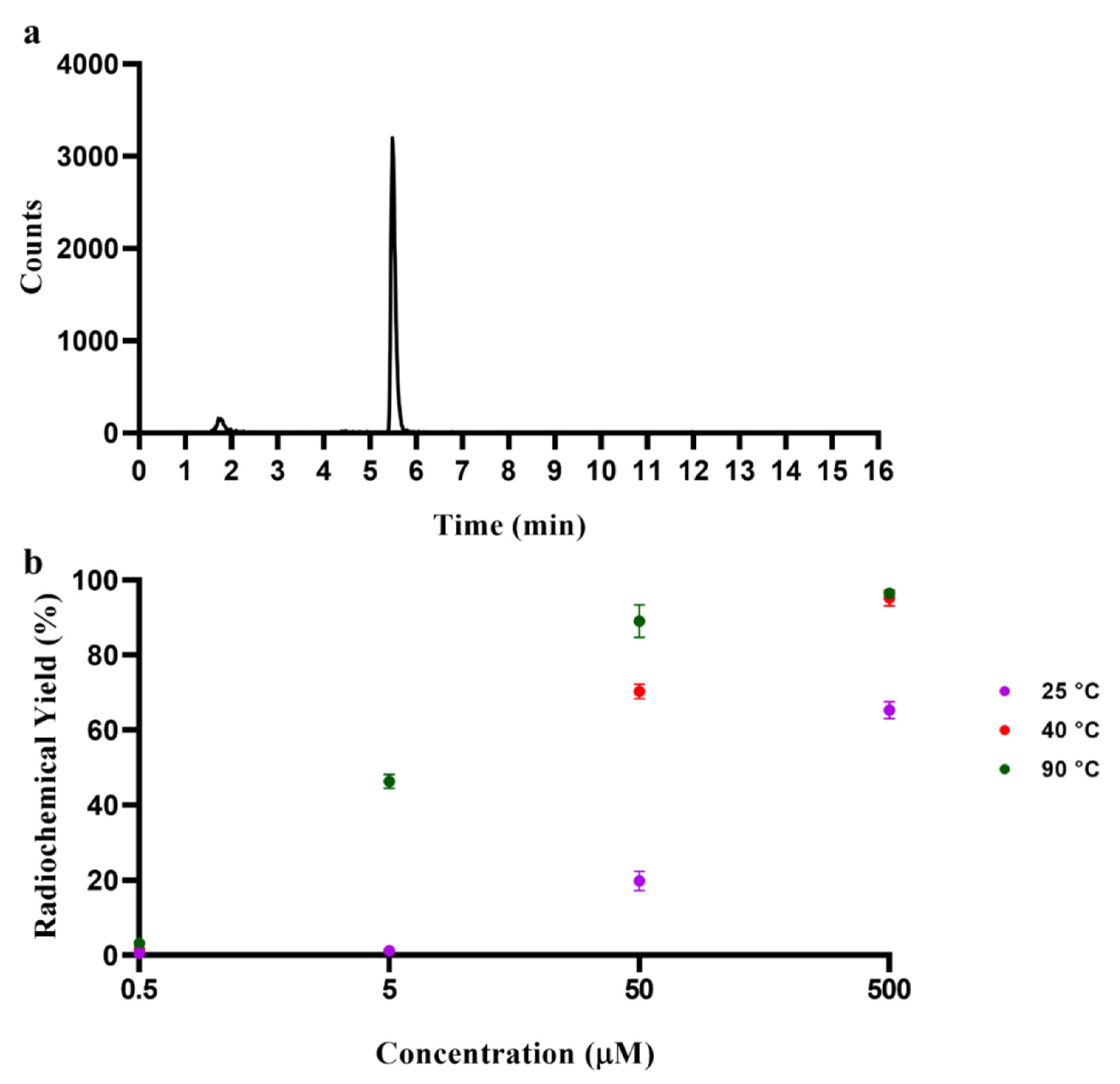
2.6. Radiolabelling with 68Ga3+
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis
General Procedure for the Synthesis of the Complexes
3.3. Radiolabelling with 68Ga
3.4. Single-Crystal X-ray Diffraction Procedure
3.5. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Richardson, D.; Kalinowski, D.S.; Richardson, V.; Sharpe, P.; Lovejoy, D.B.; Islam, M.; Bernhardt, P.V. 2-Acetylpyridine Thiosemicarbazones are Potent Iron Chelators and Antiproliferative Agents: Redox Activity, Iron Complexation and Characterization of their Antitumor Activity. J. Med. Chem. 2009, 52, 1459–1470. [Google Scholar] [CrossRef]
- Pedrido, R.; González-Noya, A.M.; Romero, M.J.; Martínez-Calvo, M.; López, M.V.; Gómez-Fórneas, E.; Zaragoza, G.; Bermejo, M.R.; Verez, G.Z. Pentadentate thiosemicarbazones as versatile chelating systems. A comparative structural study of their metallic complexes. Dalton Trans. 2008, 6776–6787. [Google Scholar] [CrossRef] [PubMed]
- Nomiya, K.; Sekino, K.; Ishikawa, M.; Honda, A.; Yokoyama, M.; Kasuga, N.C.; Yokoyama, H.; Nakano, S.; Onodera, K. Syntheses, crystal structures and antimicrobial activities of monomeric 8-coordinate, and dimeric and monomeric 7-coordinate bismuth(III) complexes with tridentate and pentadentate thiosemicarbazones and pentadentate semicarbazone ligands. J. Inorg. Biochem. 2004, 98, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Paterson, B.M.; White, J.M.; Donnelly, P.S. A hexadentate bis(thiosemicarbazonato) ligand: Rhenium(V), iron(III) and cobalt(III) complexes. Dalton Trans. 2010, 39, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Pavlishchuk, V.V.; Kolotilov, S.V.; Addison, A.W.; Butcher, R.J.; Sinn, E. Nickel(II) complexes with dithiadiiminoamine and dithiabis(thiosemicarbazone) ligands. J. Chem. Soc. Dalton Trans. 2000, 335–341. [Google Scholar] [CrossRef]
- Zaltariov, M.F.; Hammerstad, M.; Arabshahi, H.J.; Jovanović, K.; Richter, K.W.; Cazacu, M.; Shova, S.; Balan, M.; Andersen, N.H.; Radulović, S.; et al. New Iminodiacetate–Thiosemicarbazone Hybrids and Their Copper(II) Complexes Are Potential Ribonucleotide Reductase R2 Inhibitors with High Antiproliferative Activity. Inorg. Chem. 2017, 56, 3532–3549. [Google Scholar] [CrossRef]
- Paterson, B.M.; White, K.F.; White, J.M.; Abrahams, B.F.; Donnelly, P.S. Guest-induced Assembly of Bis(thiosemicarbazonato) Zinc(II) Coordination Nanotubes. Angew. Chem. Int. Ed. 2017, 56, 8370–8374. [Google Scholar] [CrossRef]
- Dayal, D.; Palanimuthu, D.; Shinde, S.V.; Somasundaram, K.; Samuelson, A.G. A novel zinc bis(thiosemicarbazone) complex for live cell imaging. JBIC J. Biol. Inorg. Chem. 2011, 16, 621–632. [Google Scholar] [CrossRef]
- Huseynova, M.; Medjidov, A.; Taslimi, P.; Aliyeva, M. Synthesis, characterization, crystal structure of the coordination polymer Zn(II) with thiosemicarbazone of glyoxalic acid and their inhibitory properties against some metabolic enzymes. Bioorg. Chem. 2019, 83, 55–62. [Google Scholar] [CrossRef]
- Crouch, P.J.; Hung, L.W.; Adlard, P.A.; Cortes, M.; Lal, V.; Filiz, G.; Perez, K.A.; Nurjono, M.; Caragounis, A.; Du, T.; et al. Increasing Cu bioavailability inhibits A oligomers and tau phosphorylation. Proc. Natl. Acad. Sci. USA 2009, 106, 381–386. [Google Scholar] [CrossRef]
- Oliveira, C.; Maia, P.I.D.S.; Souza, P.C.; Pavan, F.R.; Leite, C.Q.F.; Viana, R.B.; Batista, A.A.; Nascimento, O.R.; Deflon, V.M. Manganese(II) complexes with thiosemicarbazones as potential anti-Mycobacterium tuberculosis agents. J. Inorg. Biochem. 2014, 132, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Beraldo, H.; Gambino, D. The Wide Pharmacological Versatility of Semicarbazones, Thiosemicarbazones and Their Metal Complexes. Mini-Rev. Med. Chem. 2004, 4, 31–39. [Google Scholar] [CrossRef]
- Paterson, B.; Donnelly, P.S. Copper complexes of bis(thiosemicarbazones): From chemotherapeutics to diagnostic and therapeutic radiopharmaceuticals. Chem. Soc. Rev. 2011, 40, 3005–3018. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, J.R.; Hueting, R. Metal complexes of thiosemicarbazones for imaging and therapy. Inorg. Chim. Acta 2012, 389, 3–15. [Google Scholar] [CrossRef]
- Mendes, I.C.; Soares, M.A.; Dos Santos, R.G.; Pinheiro, C.; Beraldo, H. Gallium(III) complexes of 2-pyridineformamide thiosemicarbazones: Cytotoxic activity against malignant glioblastoma. Eur. J. Med. Chem. 2009, 44, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; Chen, C.L.; Zhang, D.; Niu, J.Y.; Ji, B.S. Mn(II), Co(II) and Zn(II) complexes with heterocyclic substituted thiosemicarbazones: Synthesis, characterization, X-ray crystal structures and antitumor comparison. Eur. J. Med. Chem. 2010, 45, 3169–3177. [Google Scholar] [CrossRef]
- Yu, Y.; Kalinowski, D.S.; Kovacevic, Z.; Siafakas, A.R.; Jansson, P.J.; Stefani, C.; Lovejoy, D.B.; Sharpe, P.; Bernhardt, P.V.; Richardson, D.R. Thiosemicarbazones from the Old to New: Iron Chelators That Are More Than Just Ribonucleotide Reductase Inhibitors. J. Med. Chem. 2009, 52, 5271–5294. [Google Scholar] [CrossRef]
- Li, M.-X.; Zhang, D.; Zhang, L.-Z.; Niu, J.-Y.; Ji, B.-S. Synthesis, crystal structures and biological activities of 2-acetylpyridine N(4)-cyclohexylthiosemicarbazone and its manganese(II) and nickel(II) complexes. Inorg. Chem. Commun. 2010, 13, 1572–1575. [Google Scholar] [CrossRef]
- Bernhardt, P.V.; Sharpe, P.C.; Islam, M.; Lovejoy, D.B.; Kalinowski, D.S.; Richardson, D.R. Iron Chelators of the Dipyridylketone Thiosemicarbazone Class: Precomplexation and Transmetalation Effects on Anticancer Activity. J. Med. Chem. 2009, 52, 407–415. [Google Scholar] [CrossRef]
- Stacy, A.E.; Palanimuthu, D.; Bernhardt, P.V.; Kalinowski, D.S.; Jansson, P.J.; Richardson, D.R. Zinc(II)–Thiosemicarbazone Complexes Are Localized to the Lysosomal Compartment Where They Transmetallate with Copper Ions to Induce Cytotoxicity. J. Med. Chem. 2016, 59, 4965–4984. [Google Scholar] [CrossRef]
- Arion, V.B.; Jakupec, M.; Galanski, M.; Unfried, P.; Keppler, B.K. Synthesis, structure, spectroscopic and in vitro antitumour studies of a novel gallium(III) complex with 2-acetylpyridine 4N-dimethylthiosemicarbazone. J. Inorg. Biochem. 2002, 91, 298–305. [Google Scholar] [CrossRef]
- Kowol, C.R.; Berger, R.; Eichinger, R.; Roller, A.; Jakupec, M.; Schmidt, P.P.; Arion, V.B.; Keppler, B.K. Gallium(III) and Iron(III) Complexes of α-N-Heterocyclic Thiosemicarbazones: Synthesis, Characterization, Cytotoxicity, and Interaction with Ribonucleotide Reductase. J. Med. Chem. 2007, 50, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Deng, J.; Qian, K.; Tian, L.; Li, J.; He, K.; Huang, X.; Cheng, Z.; Zheng, Y.; Wang, Y. Novel 2-pyridinecarboxaldehyde thiosemicarbazones Ga(III) complexes with a high antiproliferative activity by promoting apoptosis and inhibiting cell cycle. Eur. J. Med. Chem. 2017, 134, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Qian, K.; Tian, L.; Cheng, Z.; Wang, Y. Gallium(III)–2-benzoylpyridine-thiosemicarbazone complexes promote apoptosis through Ca2+ signaling and ROS-mediated mitochondrial pathways. New J. Chem. 2018, 42, 10226–10233. [Google Scholar] [CrossRef]
- King, A.P.; Gellineau, H.A.; Ahn, J.-E.; MacMillan, S.N.; Wilson, J.J. Bis(thiosemicarbazone) Complexes of Cobalt(III). Synthesis, Characterization, and Anticancer Potential. Inorg. Chem. 2017, 56, 6609–6623. [Google Scholar] [CrossRef]
- West, D.X.; Nassar, A.; El-Saied, F.A.; Ayad, M.I. Cobalt(II) complexes with 2-aminoacetophenone N(4)-substituted thiosemicarbazones. Transit. Met. Chem. 1999, 24, 617–621. [Google Scholar] [CrossRef]
- Deng, J.; Li, T.; Su, G.; Qin, Q.-P.; Liu, Y.; Gou, Y. Co(III) complexes based on α-N-heterocyclic thiosemicarbazone ligands: DNA binding, DNA cleavage, and topoisomerase I/II inhibitory activity studies. J. Mol. Struct. 2018, 1167, 33–43. [Google Scholar] [CrossRef]
- Di Vaira, M.; Bazzicalupi, C.; Orioli, P.; Messori, L.; Bruni, A.B.; Zatta, P. Clioquinol, a Drug for Alzheimer’s Disease Specifically Interfering with Brain Metal Metabolism: Structural Characterization of Its Zinc(II) and Copper(II) Complexes. Inorg. Chem. 2004, 43, 3795–3797. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, Y.; Yasui, H. Zinc complexes developed as metallopharmaceutics for treating diabetes mellitus based on the bio-medicinal inorganic chemistry. Curr. Top. Med. Chem. 2012, 12, 210–218. [Google Scholar] [CrossRef]
- Chukwuma, C.I.; Mashele, S.S.; Eze, K.C.; Matowane, G.R.; Islam, S.M.; Bonnet, S.L.; Noreljaleel, A.E.; Ramorobi, L.M. A comprehensive review on zinc(II) complexes as anti-diabetic agents: The advances, scientific gaps and prospects. Pharmacol. Res. 2020, 155, 104744. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Wei, J.-H.; Chen, Z.-F.; Liu, M.; Gu, Y.-Q.; Huang, K.-B.; Li, Z.-Q.; Liang, H. The antitumor activity of zinc(II) and copper(II) complexes with 5,7-dihalo-substituted-8-quinolinoline. Eur. J. Med. Chem. 2013, 69, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Li, J.; Khan, Z.H.; Ma, F.; Liu, X. A novel zinc complex with antibacterial and antioxidant activity. BMC Chem. 2021, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, N.C.; Sekino, K.; Ishikawa, M.; Honda, A.; Yokoyama, M.; Nakano, S.; Shimada, N.; Koumo, C.; Nomiya, K. Synthesis, structural characterization and antimicrobial activities of 12 zinc(II) complexes with four thiosemicarbazone and two semicarbazone ligands. J. Inorg. Biochem. 2003, 96, 298–310. [Google Scholar] [CrossRef]
- Tarushi, A.; Totta, X.; Raptopoulou, C.; Psycharis, V.; Psomas, G.; Kessissoglou, D.P. Structural features of mono- and tri-nuclear Zn(II) complexes with a non-steroidal anti-inflammatory drug as ligand. Dalton Trans. 2012, 41, 7082. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, Y.; Taniuchi, H.; Yonekura, Y.; Ohtani, H.; Konishi, J.; Yokoyama, A. Copper-62-ATSM: A new hypoxia imaging agent with high membrane permeability and low redox potential. J. Nucl. Med. 1997, 38, 1155–1160. [Google Scholar]
- Torres, J.B.; Andreozzi, E.M.; Dunn, J.T.; Siddique, M.; Szanda, I.; Howlett, D.R.; Sunassee, K.; Blowera, P.J.; Torres, J.B. PET Imaging of Copper Trafficking in a Mouse Model of Alzheimer Disease. J. Nucl. Med. 2015, 57, 109–114. [Google Scholar] [CrossRef]
- Andreozzi, E.M.; Torres, J.B.; Sunassee, K.; Dunn, J.; Walker-Samuel, S.; Szanda, I.; Blower, P.J. Studies of copper trafficking in a mouse model of Alzheimer’s disease by positron emission tomography: Comparison of 64Cu acetate and 64CuGTSM. Metallomics 2017, 9, 1622–1633. [Google Scholar] [CrossRef]
- Paterson, B.M.; Cullinane, C.; Crouch, P.J.; White, A.R.; Barnham, K.J.; Roselt, P.D.; Noonan, W.; Binns, D.; Hicks, R.J.; Donnelly, P.S. Modification of Biodistribution and Brain Uptake of Copper Bis(thiosemicarbazonato) Complexes by the Incorporation of Amine and Polyamine Functional Groups. Inorg. Chem. 2019, 58, 4540–4552. [Google Scholar] [CrossRef]
- Hickey, J.L.; Lim, S.; Hayne, D.; Paterson, B.; White, J.M.; Villemagne, V.L.; Roselt, P.; Binns, D.; Cullinane, C.; Jeffery, C.M.; et al. Diagnostic Imaging Agents for Alzheimer’s Disease: Copper Radiopharmaceuticals that Target Aβ Plaques. J. Am. Chem. Soc. 2013, 135, 16120–16132. [Google Scholar] [CrossRef]
- McInnes, L.E.; Noor, A.; Kysenius, K.; Cullinane, C.; Roselt, P.; McLean, C.A.; Chiu, F.C.K.; Powell, A.K.; Crouch, P.J.; White, J.M.; et al. Potential Diagnostic Imaging of Alzheimer’s Disease with Copper-64 Complexes That Bind to Amyloid-β Plaques. Inorg. Chem. 2019, 58, 3382–3395. [Google Scholar] [CrossRef]
- Cowley, A.R.; Dilworth, J.R.; Donnelly, P.S.; Heslop, J.M.; Ratcliffe, S.J. Bifunctional chelators for copper radiopharmaceuticals: The synthesis of [Cu(ATSM)–amino acid] and [Cu(ATSM)–octreotide] conjugates. Dalton Trans. 2007, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Paterson, B.; Karas, J.A.; Scanlon, D.B.; White, J.M.; Donnelly, P.S. Versatile New Bis(thiosemicarbazone) Bifunctional Chelators: Synthesis, Conjugation to Bombesin(7−14)-NH2, and Copper-64 Radiolabeling. Inorg. Chem. 2010, 49, 1884–1893. [Google Scholar] [CrossRef]
- Hueting, R.; Christlieb, M.; Dilworth, J.R.; Garayoa, E.G.; Gouverneur, V.; Jones, M.W.; Maes, V.; Schibli, R.; Sun, X.; Tourwé, D.A. Bis(thiosemicarbazones) as bifunctional chelators for the room temperature 64-copper labeling of peptides. Dalton Trans. 2010, 39, 3620–3632. [Google Scholar] [CrossRef] [PubMed]
- Rösch, F. Past, present and future of 68Ge/68Ga generators. Appl. Radiat. Isot. 2013, 76, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Ramogida, C.F.; Orvig, C. Tumour targeting with radiometals for diagnosis and therapy. Chem. Commun. 2013, 49, 4720–4739. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Benešová, M. [68Ga]Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals 2020, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Neels, O.; Müller, M.; Bauder-Wüst, U.; Remde, Y.; Schafer, M.A.; Hennrich, U.; Eisenhut, M.; Afshar-Oromieh, A.; Haberkorn, U.; et al. Novel Preclinical and Radiopharmaceutical Aspects of [68Ga]Ga-PSMA-HBED-CC: A New PET Tracer for Imaging of Prostate Cancer. Pharmaceuticals 2014, 7, 779–796. [Google Scholar] [CrossRef]
- Tsionou, M.I.; Knapp, C.E.; Foley, C.A.; Munteanu, C.R.; Cakebread, A.; Imberti, C.; Eykyn, T.R.; Young, J.D.; Paterson, B.M.; Blower, P.J.; et al. Comparison of macrocyclic and acyclic chelators for gallium-68 radiolabelling. RSC Adv. 2017, 7, 49586–49599. [Google Scholar] [CrossRef] [PubMed]
- Sinnes, J.-P.; Nagel, J.; Waldron, B.P.; Maina, T.; Nock, B.A.; Bergmann, R.K.; Ullrich, M.; Pietzsch, J.; Bachmann, M.; Baum, R.P.; et al. Instant kit preparation of 68Ga-radiopharmaceuticals via the hybrid chelator DATA: Clinical translation of [68Ga]Ga-DATA-TOC. EJNMMI Res. 2019, 9, 48. [Google Scholar] [CrossRef]
- Joshi, T.; Graham, B.; Spiccia, L. Macrocyclic Metal Complexes for Metalloenzyme Mimicry and Sensor Development. Accounts Chem. Res. 2015, 48, 2366–2379. [Google Scholar] [CrossRef]
- Lejault, P.; Duskova, K.; Bernhard, C.; Valverde, I.E.; Romieu, A.; Monchaud, D. The Scope of Application of Macrocyclic Polyamines Beyond Metal Chelation. Eur. J. Org. Chem. 2019, 2019, 6146–6157. [Google Scholar] [CrossRef]
- Shinoda, S. Dynamic cyclen–metal complexes for molecular sensing and chirality signaling. Chem. Soc. Rev. 2013, 42, 1825–1835. [Google Scholar] [CrossRef]
- Rashid, H.U.; Martines, M.A.U.; Jorge, J.; De Moraes, P.M.; Umar, M.N.; Khan, K.; Rehman, H.U. Cyclen-based Gd3+ complexes as MRI contrast agents: Relaxivity enhancement and ligand design. Bioorg. Med. Chem. 2016, 24, 5663–5684. [Google Scholar] [CrossRef] [PubMed]
- Cabbiness, D.K.; Margerum, D.W. Macrocyclic effect on the stability of copper(II) tetramine complexes. J. Am. Chem. Soc. 1969, 91, 6540–6541. [Google Scholar] [CrossRef]
- Hancock, R.D.; Martell, A.E. The Chelate, Cryptate and Macrocyclic Effects. Comments Inorg. Chem. 1988, 6, 237–284. [Google Scholar] [CrossRef]
- Baranyai, Z.; Tircsó, G.; Rösch, F. The Use of the Macrocyclic Chelator DOTA in Radiochemical Separations. Eur. J. Inorg. Chem. 2020, 2020, 36–56. [Google Scholar] [CrossRef]
- Stasiuk, G.; Long, N.J. The ubiquitous DOTA and its derivatives: The impact of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid on biomedical imaging. Chem. Commun. 2013, 49, 2732–2746. [Google Scholar] [CrossRef] [PubMed]
- Burke, B.P.; Clemente, G.S.; Archibald, S.J. Recent advances in chelator design and labelling methodology for 68Ga radiopharmaceuticals. J. Label. Compd. Radiopharm. 2014, 57, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Molnár, E.; Camus, N.; Patinec, V.; Rolla, G.A.; Botta, M.; Tircsó, G.; Kálmán, F.K.; Fodor, T.; Tripier, R.; Platas-Iglesias, C. Picolinate-Containing Macrocyclic Mn2+ Complexes as Potential MRI Contrast Agents. Inorg. Chem. 2014, 53, 5136–5149. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, A.; Knigge, U.; Mortensen, J.; Oturai, P.; Berthelsen, A.K.; Loft, A.; Binderup, T.; Rasmussen, P.; Elema, D.; Klausen, T.L.; et al. Clinical PET of Neuroendocrine Tumors Using 64Cu-DOTATATE: First-in-Humans Study. J. Nucl. Med. 2012, 53, 1207–1215. [Google Scholar] [CrossRef]
- Demirci, E.; Kabasakal, L.; Toklu, T.; Ocak, M.; Şahin, O.E.; Alan-Selcuk, N.; Araman, A. 177Lu-DOTATATE therapy in patients with neuroendocrine tumours including high-grade (WHO G3) neuroendocrine tumours: Response to treatment and long-term survival update. Nucl. Med. Commun. 2018, 39, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.L.; Davey, P.R.W.J.; Ma, M.T.; White, J.M.; Morgenstern, A.; Bruchertseifer, F.; Blower, P.J.; Paterson, B.M. An octadentate bis(semicarbazone) macrocycle: A potential chelator for lead and bismuth radiopharmaceuticals. Dalton Trans. 2020, 49, 14962–14974. [Google Scholar] [CrossRef] [PubMed]
- Alam, I.S.; Arrowsmith, R.L.; Cortezon-Tamarit, F.; Twyman, F.; Kociok-Köhn, G.; Botchway, S.W.; Dilworth, J.R.; Carroll, L.; Aboagye, E.O.; Pascu, S.I. Microwave gallium-68 radiochemistry for kinetically stable bis(thiosemicarbazone) complexes: Structural investigations and cellular uptake under hypoxia. Dalton Trans. 2015, 45, 144–155. [Google Scholar] [CrossRef]
- Arrowsmith, R.L.; Waghorn, P.A.; Jones, M.W.; Bauman, A.; Brayshaw, S.K.; Hu, Z.; Kociok-Kohn, G.D.; Mindt, T.L.; Tyrrell, R.M.; Botchway, S.W.; et al. Fluorescent gallium and indium bis(thiosemicarbazonates) and their radiolabelled analogues: Synthesis, structures and cellular confocal fluorescence imaging investigations. Dalton Trans. 2011, 40, 6238–6252. [Google Scholar] [CrossRef]
- Jalilian, A.R.; Mehdipour, P.; Akhlaghi, M.; Yousefnia, H.; Shafaii, K. Evaluation of a [67Ga]-Thiosemicarbazone Complex as Tumor Imaging Agent. Sci. Pharm. 2009, 77, 343–354. [Google Scholar] [CrossRef][Green Version]
- Moghadam, F.H.; Jalilian, A.R.; Nemati, A.; Abedini, M. Preparation and biodistribution studies of [67Ga]2-acetylpyridine 4,4-dimethyl thiosemicarbazone complex as a possible SPECT tracer for detection of malignancies. J. Radioanal. Nucl. Chem. 2007, 272, 115–121. [Google Scholar] [CrossRef]
- Lima, L.; Beyler, M.; Delgado, R.; Platas-Iglesias, C.; Tripier, R. Investigating the Complexation of the Pb2+/Bi3+ Pair with Dipicolinate Cyclen Ligands. Inorg. Chem. 2015, 54, 7045–7057. [Google Scholar] [CrossRef]
- Corey, E.J.; Bailar, J.C., Jr. The Stereochemistry of Complex Inorganic Compounds. XXII. Stereospecific Effects in Complex Ions. J. Am. Chem. Soc. 1959, 81, 2620–2629. [Google Scholar] [CrossRef]
- Eisenberg, R.; Brennessel, W.W. Redetermination of the trigonal prismatic complex tris(cis-1,2-diphenylethylene-1,2-dithiolato)rhenium. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2006, 62, 464–466. [Google Scholar] [CrossRef]
- Tsuboyama, S.; Matsudo, M.; Tsuboyama, K.; Sakurai, T. Structures of [(R)- and (S)-prolinato](optically active cyclen)cobalt(III) complexes. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1989, 45, 872–876. [Google Scholar] [CrossRef]
- Danker, F.; Näther, C.; Bensch, W. Synthesis and crystal structure of (1, 4, 7, 10-tetraazacyclododecane-κ4N)(tetrasulfido-κ2S1, S4) manganese(II). Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Vargová, Z.; Kotek, J.; Rudovsky, J.; Plutnar, J.; Gyepes, R.; Hermann, P.; Györyová, K.; Lukeš, I. Ternary Complexes of Zinc(II), Cyclen and Pyridinecarboxylic Acids. Eur. J. Inorg. Chem. 2007, 2007, 3974–3987. [Google Scholar] [CrossRef]
- Martinelli, J.; Balali-Mood, B.; Panizzo, R.; Lythgoe, M.F.; White, A.J.P.; Ferretti, P.; Steinke, J.H.G.; Vilar, R. Coordination chemistry of amide-functionalised tetraazamacrocycles: Structural, relaxometric and cytotoxicity studies. Dalton Trans. 2010, 39, 10056–10067. [Google Scholar] [CrossRef]
- El Safadi, M.; Bhadbhade, M.; Shimmon, R.; Baker, A.T.; McDonagh, A.M. Cyclen-based chelators for the inhibition of Aβ aggregation: Synthesis, anti-oxidant and aggregation evaluation. Inorg. Chim. Acta 2017, 467, 343–350. [Google Scholar] [CrossRef]
- Bernier, N.; Costa, J.; Delgado, R.; Félix, V.; Royal, G.; Tripier, R. trans-Methylpyridine cyclen versus cross-bridged trans-methylpyridine cyclen. Synthesis, acid–base and metal complexation studies (metal = Co2+, Cu2+, and Zn2+). Dalton Trans. 2011, 40, 4514–4526. [Google Scholar] [CrossRef]
- Tsitovich, P.B.; Tittiris, T.Y.; Cox, J.M.; Benedict, J.B.; Morrow, J.R. Fe(II) and Co(II) N-methylated CYCLEN complexes as paraSHIFT agents with large temperature dependent shifts. Dalton Trans. 2017, 47, 916–924. [Google Scholar] [CrossRef]
- Knight, J.C.; Alvarez, S.; Angelo, J.A.; Edwards, P.G.; Singh, N. A novel bipyridine-based hexadentate tripodal framework with a strong preference for trigonal prismatic co-ordination geometries. Dalton Trans. 2010, 39, 3870–3883. [Google Scholar] [CrossRef]
- Aoki, S.; Zulkefeli, M.; Shiro, M.; Kimura, E. New supramolecular trigonal prisms from zinc(II)-1,4,7,10-tetraazacyclododecane (cyclen) complexes and trithiocyanurate in aqueous solution. Proc. Natl. Acad. Sci. USA 2002, 99, 4894–4899. [Google Scholar] [CrossRef]
- Kojima, M.; Nakabayashi, K.; Ohba, S.; Okumoto, S.; Saito, Y.; Fujita, J. Green and Brown Isomers of the ((S)-1-Amino-2-propanethiolato-N,S)(1,4,7,10-tetraazacyclododecane)cobalt(III) Ion and Crystal Structure of the Green Isomer. Bull. Chem. Soc. Jpn. 1986, 59, 277–283. [Google Scholar] [CrossRef]
- Chandra, S. and Sangeetika. Synthesis and spectral studies on copper(II) and cobalt(II) complexes of macrocyclic ligand containing thiosemicarbazone moiety. Ind. J. Chem. A 2002, 41, 1629. [Google Scholar]
- Chandra, S.; Pundir, M. Spectral studies of cobalt(II) complexes of 12-membered macrocyclic ligands having thiosemicarbazone moieties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Tsitovich, P.B.; Morrow, J.R. Macrocyclic ligands for Fe(II) paraCEST and chemical shift MRI contrast agents. Inorg. Chim. Acta 2012, 393, 3–11. [Google Scholar] [CrossRef]
- Howard, J.A.K.; Kenwright, A.; Moloney, J.M.; Parker, D.; Woods, M.; Port, M.; Navet, M.; Rousseau, O. Structure and dynamics of all of the stereoisomers of europium complexes of tetra(carboxyethyl) derivatives of dota: Ring inversion is decoupled from cooperative arm rotation in the RRRR and RRRS isomers. Chem. Commun. 1998, 1381–1382. [Google Scholar] [CrossRef]
- Enamullah, M.; Vasylyeva, V.; Janiak, C. Chirality and diastereoselection of Δ/Λ-configured tetrahedral zinc(II) complexes with enantiopure or racemic Schiff base ligands. Inorg. Chim. Acta 2013, 408, 109–119. [Google Scholar] [CrossRef]
- Chamayou, A.-C.; Lüdeke, S.; Brecht, V.; Freedman, T.B.; Nafie, L.A.; Janiak, C. Chirality and Diastereoselection of Δ/Λ-Configured Tetrahedral Zinc Complexes through Enantiopure Schiff Base Complexes: Combined Vibrational Circular Dichroism, Density Functional Theory, 1H NMR, and X-ray Structural Studies. Inorg. Chem. 2011, 50, 11363–11374. [Google Scholar] [CrossRef] [PubMed]
- Pescitelli, G.; Lüdeke, S.; Chamayou, A.-C.; Marolt, M.; Justus, V.; Górecki, M.; Arrico, L.; Di Bari, L.; Islam, M.A.; Gruber, I.; et al. Broad-Range Spectral Analysis for Chiral Metal Coordination Compounds: (Chiro)optical Superspectrum of Cobalt(II) Complexes. Inorg. Chem. 2018, 57, 13397–13408. [Google Scholar] [CrossRef]
- Vargas, A.; Zerara, M.; Krausz, E.; Hauser, A.; Daku, L.M.L. Density-Functional Theory Investigation of the Geometric, Energetic, and Optical Properties of the Cobalt(II)tris(2,2′-bipyridine) Complex in the High-Spin and the Jahn−Teller Active Low-Spin States. J. Chem. Theory Comput. 2006, 2, 1342–1359. [Google Scholar] [CrossRef]
- Wadas, T.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef]
- Prado, V.D.S.; Leitao, R.C.F.; Silva, F.; Gano, L.; Santos, I.C.; Marques, F.L.N.; Paulo, A.R.; Deflon, V.M. Gallium and indium complexes with new hexadentate bis(semicarbazone) and bis(thiosemicarbazone) chelators. Dalton Trans. 2021, 50, 1631–1640. [Google Scholar] [CrossRef]
- Earnshaw, A. Introduction to Magnetochemistry; Elsevier: London, UK, 1968. [Google Scholar]
- Kahn, O. Molecular Magnetism; Wiley-VCH: New York, NY, USA, 1993. [Google Scholar]
- CrysAlisPro; Rigaku Oxford Diffraction: Yarnton, UK, 2018.
- Sheldrick, G. Crystal Structure refinement with SHELXL. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.A. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian-type basis sets for local spin density functional calculations. Part I. Boron through neon, optimization technique and validation. Can. J. Chem. 1992, 70, 560–571. [Google Scholar] [CrossRef]
- Sosa, C.; Andzelm, J.; Elkin, B.C.; Wimmer, E.; Dobbs, K.D.; Dixon, D.A. A local density functional study of the structure and vibrational frequencies of molecular transition-metal compounds. J. Phys. Chem. 1992, 96, 6630–6636. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Dennington, T.A.K.R.; Millam, J.M. GaussView; Version 6.1.1; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]


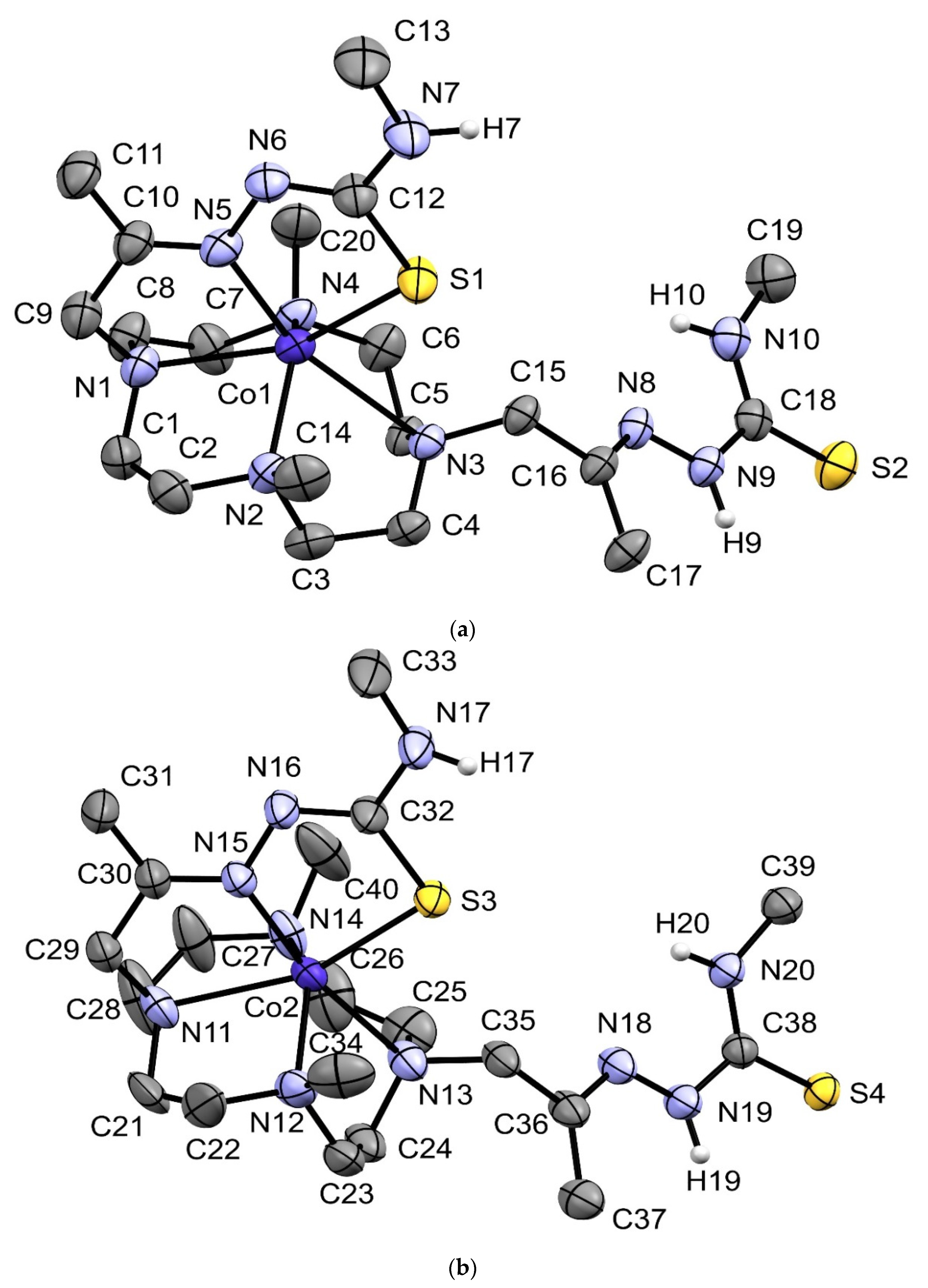
| [MnHL](BPh4) | [CoHL](BPh4)·(C3H6O) | [ZnHL](BPh4)·1.67(C3H6O) | |
|---|---|---|---|
| Empirical formula | C44H61BMnN10S2 | C47H67BCoN10OS2 | C49H70.75BN10O1.67S2Zn |
| Formula weight | 859.89 | 921.96 | 966.86 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c | P21/c |
| Temperature (K) | 123(2) | 173(2) | 123(1) |
| a (Å) | 17.4842(3) | 17.2017(2) | 17.32220(10) |
| b (Å) | 35.9904(5) | 35.8919(4) | 36.0585(2) |
| c (Å) | 17.4662(3) | 17.3716(2) | 17.36950(10) |
| α (°) | 90 | 90 | 90 |
| β (°) | 100.8860(10) | 102.2170(10) | 101.7190(10) |
| γ (°) | 90 | 90 | 90 |
| V (Å3) | 10,793.1(3) | 10,482.4 | 10,623.06(11) |
| Z | 8 | 8 | 8 |
| Dc (g cm−3) | 1.058 | 1.168 | 1.209 |
| Absorption coefficient (mm−1) | 0.358 | 3.639 | 1.729 |
| F(000) | 3656 | 3928 | 4121 |
| Angle range 2θ, ° | MoKα 6.630 to 55.754 | CuKα 7.168 to 154.048 | CuKα 7.012 to 154.846 |
| Reflections collected | 143,110 | 102,026 | 115,251 |
| Independent reflections | 25,679 [R(int) = 0.0469] | 21,504 [R(int) = 0.0832] | 22,186 [R(int) = 0.0456] |
| Final R1 values (I > 2σ(I)) | 0.0440 | 0.0889 | 0.0572 |
| Final wR1(F2) values (I > 2σ(I)) | 0.1111 | 0.2389 | 0.1622 |
| Final R1 values (all data) | 0.0606 | 0.1252 | 0.0617 |
| Final wR1(F2) values (all data) | 0.1180 | 0.2764 | 0.1667 |
| GoF on F2 | 1.076 | 1.036 | 1.023 |
| CSD no. | 2,072,659 | 2,072,660 | 2,072,661 |
| Mn1 (Å) | Mn2 (Å) | Co1 (Å) | Co2 (Å) | Zn1 (Å) | Zn2 (Å) | |
|---|---|---|---|---|---|---|
| M-N1/11 | 2.362(2) | 2.397(1) | 2.331(4) | 2.325(4) | 2.340(2) | 2.393(2) |
| M-N2/12 | 2.264(2) | 2.254(2) | 2.156(4) | 2.183(5) | 2.152(2) | 2.140(2) |
| M-N3/13 | 2.501(2) | 2.471(2) | 2.620(4) | 2.317(4) | 2.737(2) | 2.630(2) |
| M-N4/14 | 2.244(2) | 2.250(2) | 2.113(4) | 2.159(4) | 2.121(2) | 2.125(2) |
| M-N5/15 | 2.257(2) | 2.218(2) | 2.120(4) | 2.087(4) | 2.147(2) | 2.130(2) |
| M-S1/3 | 2.462(1) | 2.455(1) | 2.323(2) | 2.365(1) | 2.346(7) | 2.344(7) |
| θ1 (°) | θ2 (°) | θ3 (°) | Av (°) | |
|---|---|---|---|---|
| Mn1 | 35.7 | 20.2 | 8.1 | 21.4 |
| Mn2 | 32.0 | 18.6 | 7.0 | 19.2 |
| Co1 | 37.5 | 21.4 | 12.3 | 23.7 |
| Co2 | 40.7 | 31.2 | 20.9 | 30.9 |
| Zn1 | 37.4 | 21.7 | 13.1 | 24.1 |
| Zn2 | 33.7 | 21.5 | 12.6 | 22.6 |
| Zn1 XRD | Zn1 DFT | Zn2 XRD | Zn2 DFT | Co1 XRD | Co1 DFT | Co2 XRD | Co2 DFT | |
|---|---|---|---|---|---|---|---|---|
| M-N1/N11 | 2.340(2) | 2.390 | 2.393(2) | 2.411 | 2.331(4) | 2.351 | 2.325(4) | 2.402 |
| M-N2/N12 | 2.151(2) | 2.240 | 2.140(2) | 2.260 | 2.156(4) | 2.248 | 2.183(5) | 2.259 |
| M-N3/N13 | 2.737(2) | 2.727 | 2.630(2) | 2.701 | 2.620(4) | 2.680 | 2.317(4) | 2.400 |
| M-N4/N14 | 2.120(2) | 2.228 | 2.125(2) | 2.238 | 2.113(4) | 2.217 | 2.159(4) | 2.239 |
| M-N5N/15 | 2.147(2) | 2.211 | 2.130(2) | 2.212 | 2.120(4) | 2.164 | 2.087(4) | 2.128 |
| M-S1/S3 | 2.346(7) | 2.420 | 2.344(7) | 2.419 | 2.323(2) | 2.401 | 2.365(1) | 2.412 |
| H2L | DOTA | |||
|---|---|---|---|---|
| pH 3.5 | pH 6 | pH 3.5 | pH 6 | |
| 25 °C | 1.8 ± 1.7 | 19.8 ± 2.6 | 86.7 ± 5.0 | 73.2 ± 6.4 |
| 40 °C | 60.4 ± 3.1 | 70.3 ± 1.9 | - | - |
| 90 °C | 95.1 ± 2.3 | 89.0 ± 4.3 | 95.3 ± 0.9 | 97.2 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grieve, M.L.; Davey, P.R.W.J.; Forsyth, C.M.; Paterson, B.M. The Synthesis of a Bis(thiosemicarbazone) Macrocyclic Ligand and the Mn(II), Co(II), Zn(II) and 68Ga(III) Complexes. Molecules 2021, 26, 3646. https://doi.org/10.3390/molecules26123646
Grieve ML, Davey PRWJ, Forsyth CM, Paterson BM. The Synthesis of a Bis(thiosemicarbazone) Macrocyclic Ligand and the Mn(II), Co(II), Zn(II) and 68Ga(III) Complexes. Molecules. 2021; 26(12):3646. https://doi.org/10.3390/molecules26123646
Chicago/Turabian StyleGrieve, Melyssa L., Patrick R. W. J. Davey, Craig M. Forsyth, and Brett M. Paterson. 2021. "The Synthesis of a Bis(thiosemicarbazone) Macrocyclic Ligand and the Mn(II), Co(II), Zn(II) and 68Ga(III) Complexes" Molecules 26, no. 12: 3646. https://doi.org/10.3390/molecules26123646
APA StyleGrieve, M. L., Davey, P. R. W. J., Forsyth, C. M., & Paterson, B. M. (2021). The Synthesis of a Bis(thiosemicarbazone) Macrocyclic Ligand and the Mn(II), Co(II), Zn(II) and 68Ga(III) Complexes. Molecules, 26(12), 3646. https://doi.org/10.3390/molecules26123646