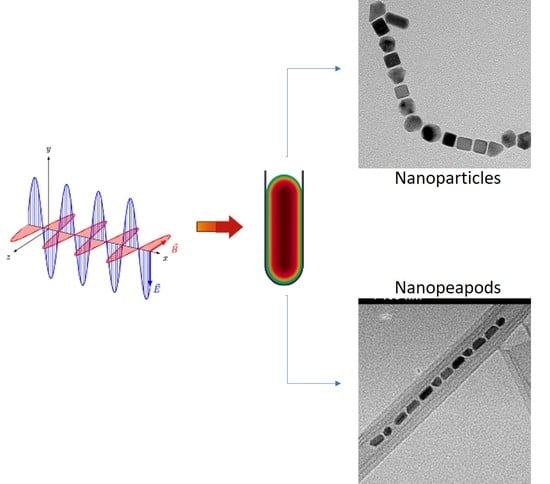
Microwave Synthetic Routes for Shape-Controlled Catalyst Nanoparticles and Nanocomposites
Abstract
1. Introduction
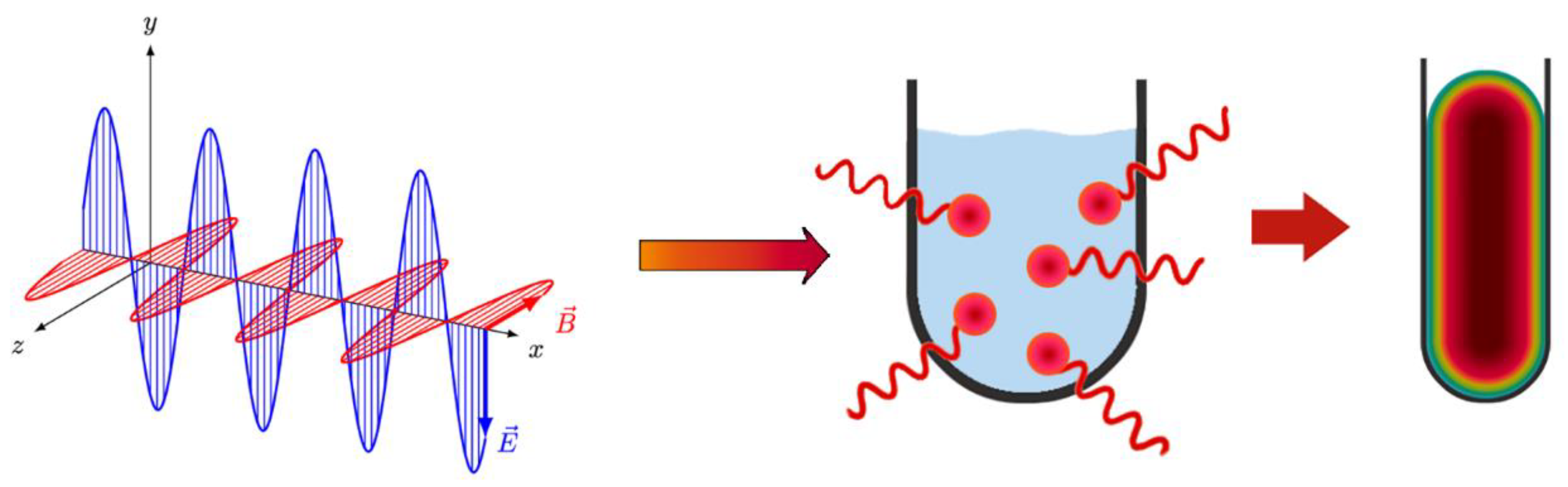
2. Fundamentals of MW Heating
3. Advances in Commercial MW Design for Research Applications
3.1. Single-Mode Cavity Design for Improved MW Energy Distribution
3.2. Expanded MW Capabilities for Chemical Synthesis and Processing
3.3. Advanced Hardware for MW Process Elucidation and Enhanced Reaction Control
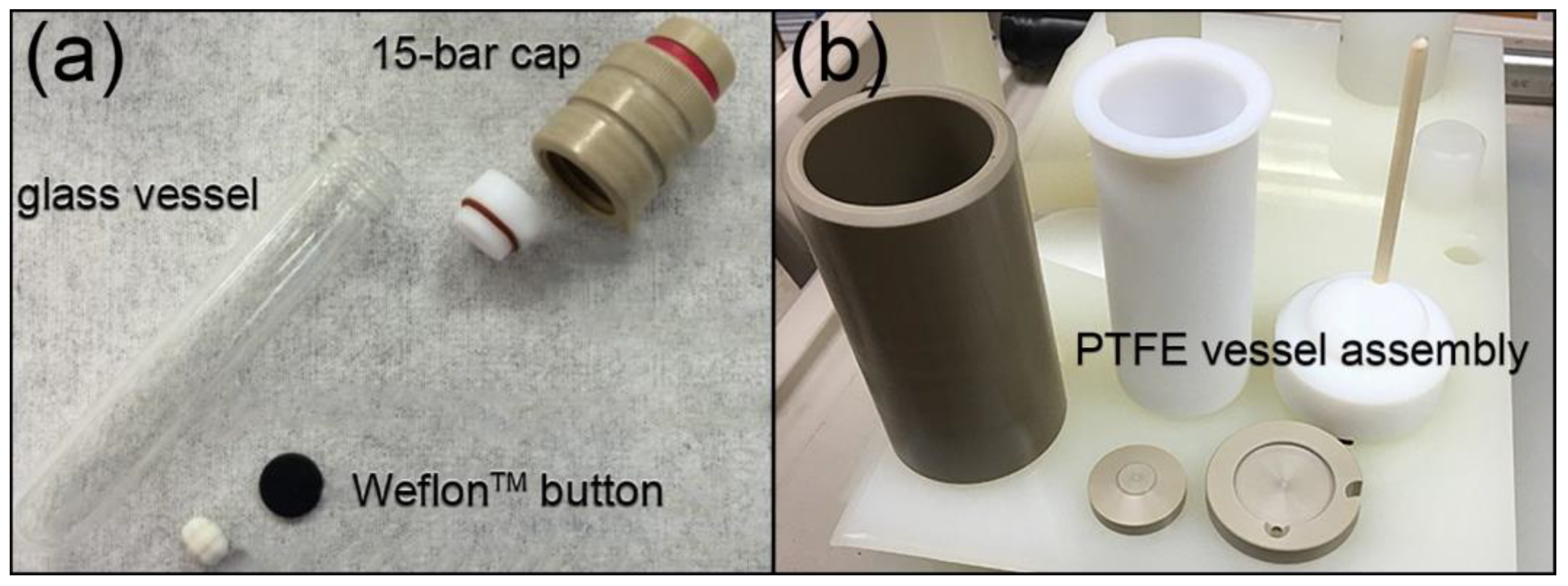
3.3.1. Reaction Vessel and Susceptor Materials
3.3.2. Fiber Optic Temperature and Pressure Controls
4. Future Directions: Facilitating Scale-Up
4.1. Quantitative Computational Modeling
4.2. Continuous-Flow MW Reactors
5. Microwave Synthetic Routes to Catalyst Nanomaterials
5.1. Synthesis of Colloidal Catalyst Nanocrystals via Conventional Heating Methods
5.2. MW Heating as an Alternative Route to Shape-Controlled Colloidal Nanocrystals
5.3. Nanoscale Platinum Catalysts
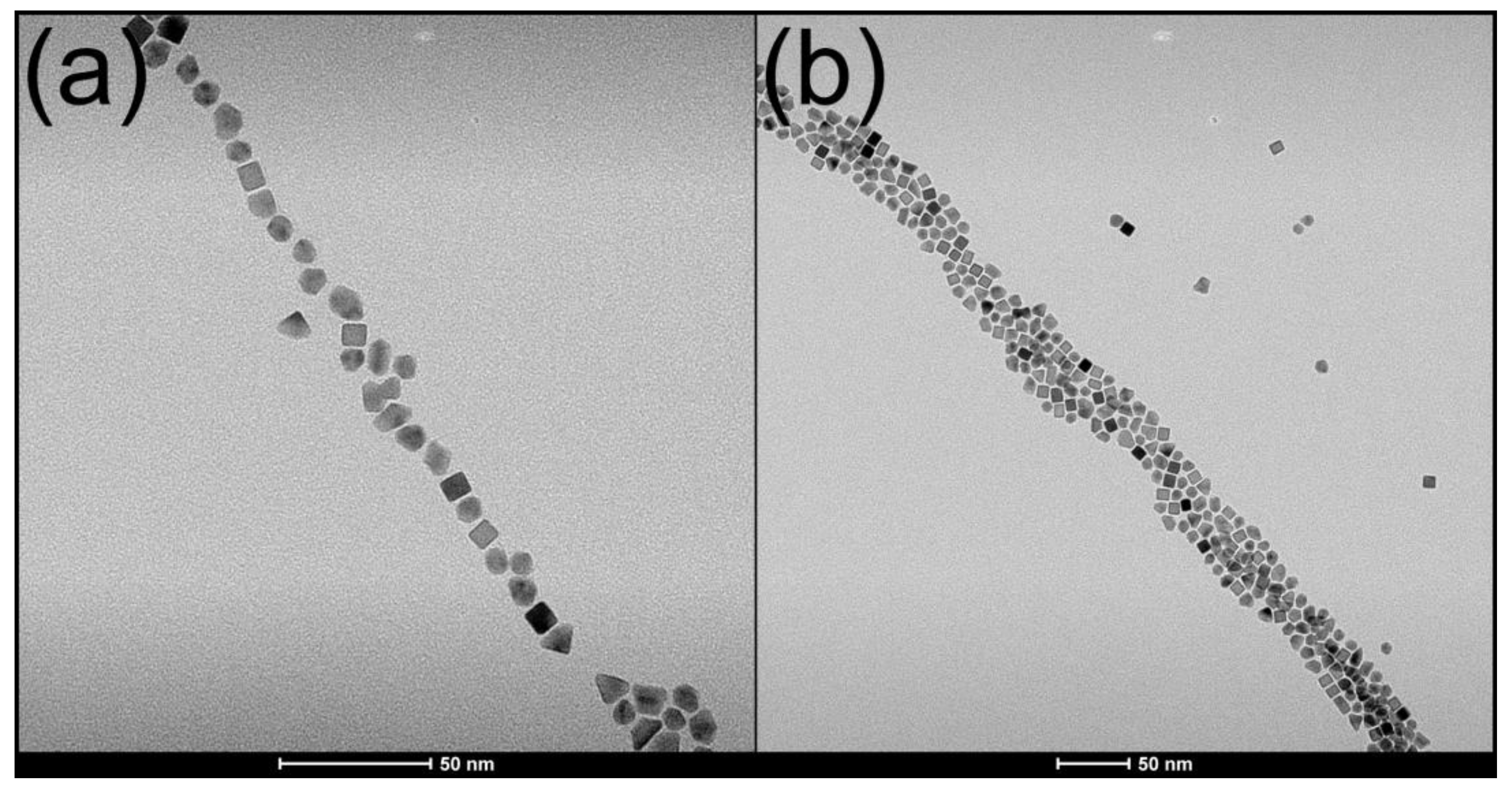
5.4. MW Synthesis of Catalyst Nanoparticles
6. Nanopeapods
6.1. Hexaniobate Nanopeapods
6.2. MW Synthesis of Platinum@Hexaniobate Nanopeapods
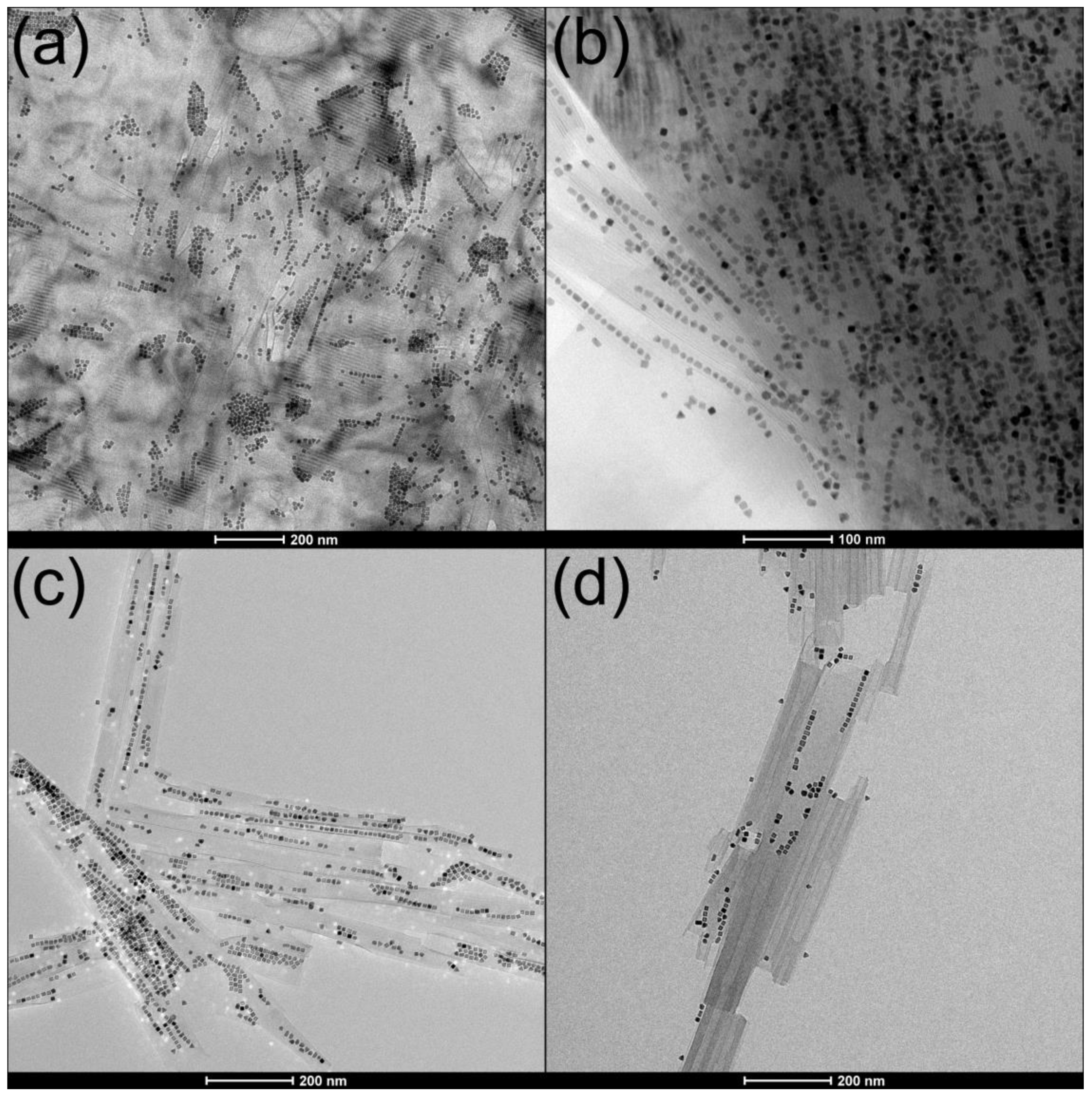
6.3. Formation and Morphology of Platinum@Hexaniobate Nanopeapods
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kappe, C.O.; Dallinger, D. Controlled microwave heating in modern organic synthesis: Highlights from the 2004–2008 literature. Mol. Divers. 2009, 13, 71–193. [Google Scholar] [CrossRef]
- Hayes, B.L. Microwave Synthesis-Chemistry at the Speed of Light; CEM Publishing: Matthews, NC, USA, 2002; ISBN 0-9722229-0-1. [Google Scholar]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O. High-speed combinatorial synthesis utilizing microwave irradiation. Curr. Opin. Chem. Biol. 2002, 6, 314–320. [Google Scholar] [CrossRef]
- Kappe, C.O. Microwave dielectric heating in synthetic organic chemistry. Chem. Soc. Rev. 2008, 37, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Davis-Wheeler Chin, C. Platinum@hexaniobate Nanopeapods: Sensitized Composite Architectures for Photocatalytic Hydrogen Evolution under Visible Light Irradiation; University of New Orleans: New Orleans, LA, USA, 2018. [Google Scholar]
- Davis-Wheeler Chin, C.; Akbarian-Tefaghi, S.; Reconco-Ramirez, J.; Wiley, J.B. Rapid microwave synthesis and optical activity of highly crystalline platinum nanocubes. MRS Commun. 2018, 8, 71–78. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-Assisted Synthesis of Colloidal Inorganic Nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 11312–11359. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef]
- Goyal, H.; Mehdad, A.; Lobo, R.F.; Stefanidis, G.; Vlachos, D.G. Scaleup of a Single-Mode Microwave Reactor. Ind. Eng. Chem. Res. 2019, 59, 2516–2523. [Google Scholar] [CrossRef]
- Kappe, C.O. ChemInform Abstract: How to Measure Reaction Temperature in Microwave-Heated Transformations. Chem. Soc. Rev. 2013, 44, 4977–4990. [Google Scholar] [CrossRef]
- Hayden, S.; Damm, M.; Kappe, C.O. On the Importance of Accurate Internal Temperature Measurements in the Microwave Dielectric Heating of Viscous Systems and Polymer Synthesis. Macromol. Chem. Phys. 2012, 214, 423–434. [Google Scholar] [CrossRef]
- Carbone, L. Wet-Chemical Synthesis Techniques for Colloidal Plasmonic Nanostructures Assisted by Convective or Microwave Dielectric Heating. In Handbook of Molecular Plasmonics; CRC Press: Boca Raton, FL, USA, 2013; pp. 413–482. [Google Scholar]
- Carbone, L.; Cozzoli, P.D. Colloidal heterostructured nanocrystals: Synthesis and growth mechanisms. Nano Today 2010, 5, 449–493. [Google Scholar] [CrossRef]
- Davis-Wheeler Chin, C.; Fontenot, P.R.; Rostamzadeh, T.; Treadwell, L.J.; Schmehl, R.H.; Wiley, J.B. Platinum@Hexaniobate Nanopeapods: A Directed Catalytic Nanocomposite Architecture for Sensitized H2 Production Under Visible Light Irradiation. 2021. Submitted. [Google Scholar]
- Horikoshi, S.; Serpone, N. Nanoparticle Synthesis through Microwave Heating. In Microwaves in Nanoparticle Synthesis; Wiley: Hoboken, NJ, USA, 2013; pp. 75–105. [Google Scholar]
- Zhu, Y.-J.; Chen, F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef]
- Deshayes, S.; Liagre, M.; Loupy, A.; Luche, J.-L.; Petit, A. Microwave activation in phase transfer catalysis. Tetrahedron 1999, 55, 10851–10870. [Google Scholar] [CrossRef]
- Kitchen, H.J.; Vallance, S.R.; Kennedy, J.L.; Ruiz, N.T.; Carassiti, L.; Harrison, A.; Whittaker, A.G.; Drysdale, T.D.; Kingman, S.; Gregory, D.H. Modern Microwave Methods in Solid-State Inorganic Materials Chemistry: From Fundamentals to Manufacturing. Chem. Rev. 2014, 114, 1170–1206. [Google Scholar] [CrossRef]
- Nishioka, M.; Miyakawa, M.; Daino, Y.; Kataoka, H.; Koda, H.; Sato, K.; Suzuki, T.M. Single-Mode Microwave Reactor Used for Continuous Flow Reactions under Elevated Pressure. Ind. Eng. Chem. Res. 2013, 52, 4683–4687. [Google Scholar] [CrossRef]
- Sauks, J.M.; Mallik, D.; Lawryshyn, Y.; Bender, T.; Organ, M. A Continuous-Flow Microwave Reactor for Conducting High-Temperature and High-Pressure Chemical Reactions. Org. Process. Res. Dev. 2013, 18, 1310–1314. [Google Scholar] [CrossRef]
- CEM Discover 2.0. Available online: https://cem.com/en/discover-2 (accessed on 3 June 2021).
- Biotage Biotage® Initiator + SP Wave. Available online: https://www.biotage.com/spwave-peptide-synthesis-0 (accessed on 3 June 2021).
- Anton-Paar MonowaveTM 400/200. Available online: https://www.anton-paar.com/corp-en/products/details/microwave-synthesis-monowave-400200/ (accessed on 3 June 2021).
- Sturm, G.S.; Verweij, M.D.; Stankiewicz, A.I.; Stefanidis, G. Microwaves and microreactors: Design challenges and remedies. Chem. Eng. J. 2014, 243, 147–158. [Google Scholar] [CrossRef]
- Microwave Research & Applications BP-210. Available online: https://www.microwaveresearch.com/BP-210.php (accessed on 3 June 2021).
- CEM Liberty Blue. Available online: https://cem.com/liberty-blue/ (accessed on 3 June 2021).
- CEM MARS6. Available online: https://cem.com/en/mars-6-synthesis (accessed on 3 June 2021).
- Milestone Segmented Rotor and Fiber Optic Probes; Milestone Srl: Sorisole, Italy, 2007.
- Robinson, J.; Kingman, S.; Irvine, D.; Licence, P.; Smith, A.; Dimitrakis, G.; Obermayer, D.; Kappe, C.O. Understanding microwave heating effects in single mode type cavities—theory and experiment. Phys. Chem. Chem. Phys. 2010, 12, 4750–4758. [Google Scholar] [CrossRef]
- Cushing, B.L.; Kolesnichenko, V.; O’Connor, C.J. Recent Advances in the Liquid-Phase Syntheses of Inorganic Nanoparticles. Chem. Rev. 2004, 104, 3893–3946. [Google Scholar] [CrossRef]
- Leong, G.J.; Schulze, M.C.; Strand, M.B.; Maloney, D.; Frisco, S.L.; Dinh, H.N.; Pivovar, B.; Richards, R.M. Shape-directed platinum nanoparticle synthesis: Nanoscale design of novel catalysts: A review of shape-directed platinum nanoparticle synthesis. Appl. Organomet. Chem. 2014, 28, 1–17. [Google Scholar] [CrossRef]
- Casavola, M.; Buonsanti, R.; Caputo, G.; Cozzoli, P.D. Colloidal Strategies for Preparing Oxide-Based Hybrid Nanocrystals. Eur. J. Inorg. Chem. 2008, 2008, 837–854. [Google Scholar] [CrossRef]
- Pinna, N.; Niederberger, M. Surfactant-Free Nonaqueous Synthesis of Metal Oxide Nanostructures. Angew. Chem. Int. Ed. 2008, 47, 5292–5304. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Pellegrino, T.; Manna, L. Synthesis, properties and perspectives of hybrid nanocrystal structures. Chem. Soc. Rev. 2006, 35, 1195–1208. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. Ed. 2008, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, M.; Pinna, N. Metal Oxide Nanoparticles in Organic Solvents; Engineering Materials and Processes; Springer: London, UK, 2009; ISBN 978-1-84882-670-0. [Google Scholar]
- Bönnemann, H.; Khelashvili, G.; Bönnemann, H. Efficient fuel cell catalysts emerging from organometallic chemistry. Appl. Organomet. Chem. 2010, 24, 257–268. [Google Scholar] [CrossRef]
- Mazumder, V.; Sun, S. Oleylamine-Mediated Synthesis of Pd Nanoparticles for Catalytic Formic Acid Oxidation. J. Am. Chem. Soc. 2009, 131, 4588–4589. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Yang, H. Designer platinum nanoparticles: Control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today 2009, 4, 143–164. [Google Scholar] [CrossRef]
- Yu, W.; Tu, A.W.; Liu, H. Synthesis of Nanoscale Platinum Colloids by Microwave Dielectric Heating. Langmuir 1999, 15, 6–9. [Google Scholar] [CrossRef]
- Carpenter, M.K.; Moylan, T.E.; Kukreja, R.S.; Atwan, M.H.; Tessema, M.M. Solvothermal Synthesis of Platinum Alloy Nanoparticles for Oxygen Reduction Electrocatalysis. J. Am. Chem. Soc. 2012, 134, 8535–8542. [Google Scholar] [CrossRef]
- Long, N.V.; Chien, N.D.; Hayakawa, T.; Hirata, H.; Lakshminarayana, G.; Nogami, M.; Gandham, L. The synthesis and characterization of platinum nanoparticles: A method of controlling the size and morphology. Nanotechnol. 2009, 21, 035605. [Google Scholar] [CrossRef]
- Kang, Y.; Li, M.; Cai, Y.; Cargnello, M.; Diaz, R.E.; Gordon, T.R.; Wieder, N.L.; Adzic, R.R.; Gorte, R.J.; Stach, E.A.; et al. Heterogeneous Catalysts Need Not Be so “Heterogeneous”: Monodisperse Pt Nanocrystals by Combining Shape-Controlled Synthesis and Purification by Colloidal Recrystallization. J. Am. Chem. Soc. 2013, 135, 2741–2747. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Daimon, H.; Lee, Y.; Kim, A.J.; Sun, S. Synthesis of Monodisperse Pt Nanocubes and Their Enhanced Catalysis for Oxygen Reduction. J. Am. Chem. Soc. 2007, 129, 6974–6975. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.M.; Ortiz, D.; De La Osa, R.A.; González, F.; Brown, A.S.; Losurdo, M.; Everitt, H.O.; Moreno, F. UV Plasmonic Behavior of Various Metal Nanoparticles in the Near- and Far-Field Regimes: Geometry and Substrate Effects. J. Phys. Chem. C 2013, 117, 19606–19615. [Google Scholar] [CrossRef]
- Wang, C.; Daimon, H.; Onodera, T.; Koda, T.; Sun, S. A General Approach to the Size- and Shape-Controlled Synthesis of Platinum Nanoparticles and Their Catalytic Reduction of Oxygen. Angew. Chem. Int. Ed. 2008, 47, 3588–3591. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lim, B.; Lee, E.P.; Xia, Y. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 2009, 4, 81–95. [Google Scholar] [CrossRef]
- Dahal, N.; García, S.; Zhou, J.; Humphrey, S.M. Beneficial Effects of Microwave-Assisted Heating versus Conventional Heating in Noble Metal Nanoparticle Synthesis. ACS Nano 2012, 6, 9433–9446. [Google Scholar] [CrossRef]
- Kou, J.; Bennett-Stamper, C.; Varma, R.S. Green Synthesis of Noble Nanometals (Au, Pt, Pd) Using Glycerol under Microwave Irradiation Conditions. ACS Sustain. Chem. Eng. 2013, 1, 810–816. [Google Scholar] [CrossRef]
- Komarneni, S.; Li, D.; Newalkar, B.; Katsuki, A.H.; Bhalla, A.S. Microwave—Polyol Process for Pt and Ag Nanoparticles. Langmuir 2002, 18, 5959–5962. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J.; Deng, K.; Gui, A.L.; Tang, Y. Preparation of Tractable Platinum, Rhodium, and Ruthenium Nanoclusters with Small Particle Size in Organic Media. Chem. Mater. 2000, 12, 1622–1627. [Google Scholar] [CrossRef]
- Cao, Y.; Jiang, L.; Guo, H.; Zheng, Q. Nano-layered K4Nb6O17 as an efficient photocatalyst for methyl orange degradation: Influence of solution pH and surface-dispersed gold nanoparticles. J. Mol. Catal. A Chem. 2014, 383-384, 209–216. [Google Scholar] [CrossRef]
- Maeda, K.; Eguchi, M.; Youngblood, W.J.; Mallouk, T. Niobium Oxide Nanoscrolls as Building Blocks for Dye-Sensitized Hydrogen Production from Water under Visible Light Irradiation. Chem. Mater. 2008, 20, 6770–6778. [Google Scholar] [CrossRef]
- Sarahan, M.C.; Carroll, E.C.; Allen, M.; Larsen, D.S.; Browning, N.D.; Osterloh, F.E. K4Nb6O17-derived photocatalysts for hydrogen evolution from water: Nanoscrolls versus nanosheets. J. Solid State Chem. 2008, 181, 1678–1683. [Google Scholar] [CrossRef]
- Domen, K.; Yoshimura, J.; Sekine, T.; Tanaka, A.; Onishi, T. A novel series of photocatalysts with an ion-exchangeable layered structure of niobate. Catal. Lett. 1990, 4, 339–343. [Google Scholar] [CrossRef]
- Maeda, K.; Sahara, G.; Eguchi, M.; Ishitani, O. Hybrids of a Ruthenium (II) Polypyridyl Complex and a Metal Oxide Nanosheet for Dye-Sensitized Hydrogen Evolution with Visible Light: Effects of the Energy Structure on Photocatalytic Activity. ACS Catal. 2015, 5, 1700–1707. [Google Scholar] [CrossRef]
- Osterloh, F. Inorganic Materials as Catalysts for Photochemical Splitting of Water. Chem. Mater. 2008, 20, 35–54. [Google Scholar] [CrossRef]
- Sarac, F.E.; Yilmaz, C.; Acar, F.Y.; Unal, U. CdTe quantum dot sensitized hexaniobate nanoscrolls and their photoelectrochemical properties. RSC Adv. 2012, 2, 10182–10184. [Google Scholar] [CrossRef]
- Maeda, K.; Eguchi, M.; Lee, S.-H.A.; Youngblood, W.J.; Hata, H.; Mallouk, T.E. Photocatalytic Hydrogen Evolution from Hexaniobate Nanoscrolls and Calcium Niobate Nanosheets Sensitized by Ruthenium (II) Bipyridyl Complexes. J. Phys. Chem. C 2009, 113, 7962–7969. [Google Scholar] [CrossRef]
- Adireddy, S.; Carbo, C.E.; Yao, Y.; Vargas, J.M.; Spinu, L.; Wiley, J.B. High-Yield Solvothermal Synthesis of Magnetic Peapod Nanocomposites via the Capture of Preformed Nanoparticles in Scrolled Nanosheets. Chem. Mater. 2013, 25, 3902–3909. [Google Scholar] [CrossRef]
- Rostamzadeh, T.; Adireddy, S.; Wiley, J.B. Formation of Scrolled Silver Vanadate Nanopeapods by Both Capture and Insertion Strategies. Chem. Mater. 2015, 27, 3694–3699. [Google Scholar] [CrossRef]
- Yao, Y.; Chaubey, G.S.; Wiley, J.B. Fabrication of Nanopeapods: Scrolling of Niobate Nanosheets for Magnetic Nanoparticle Chain Encapsulation. J. Am. Chem. Soc. 2011, 134, 2450–2452. [Google Scholar] [CrossRef] [PubMed]
- Adireddy, S.; Carbo, C.E.; Rostamzadeh, T.; Vargas, J.M.; Spinu, L.; Wiley, J.B. Peapod-Type Nanocomposites through the In Situ Growth of Gold Nanoparticles within Preformed Hexaniobate Nanoscrolls. Angew. Chem. Int. Ed. 2014, 53, 4614–4617. [Google Scholar] [CrossRef] [PubMed]
- Adireddy, S.; Rostamzadeh, T.; Carbo, C.E.; Wiley, J.B. Particle Placement and Sheet Topological Control in the Fabrication of Ag–Hexaniobate Nanocomposites. Langmuir 2014, 31, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Rostamzadeh, T.; Adireddy, S.; Davis-Wheeler Chin, C.; Abdallah, M.H.; Wiley, J.B. Formation of Mixed-Metal Ceria Nanopeapod Composites within Scrolled Hexaniobate Nanosheets. ChemNanoMat 2019, 5, 1373–1380. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsu, Y.K.; Popescu, R.; Gerthsen, D.; Lin, Y.G.; Feldmann, C. Au@Nb@HxK1-xNbO3 nanopeapods with near-infrared active plasmonic hot-electron injection for water splitting. Nat. Commun. 2018, 9, 232. [Google Scholar] [CrossRef]
- Liu, L.; Lee, W.; Scholz, R.; Pippel, E.; Gösele, U. Tailor-Made Inorganic Nanopeapods: Structural Design of Linear Noble Metal Nanoparticle Chains. Angew. Chem. Int. Ed. 2008, 47, 7004–7008. [Google Scholar] [CrossRef]
- Byoun, W.; Yoo, H. Peapod Assemblies of Au and Au/Pt Nanoparticles Encapsulated within Hollow Silica Nanotubes. ChemistrySelect 2017, 2, 2414–2419. [Google Scholar] [CrossRef]
- Takai, A.; Sakamoto, Y.; Terasaki, O.; Yamauchi, Y.; Kuroda, K. Platinum Nanopeapods: Spatial Control of Mesopore Arrangements by Utilizing a Physically Confined Space. Chem. A Eur. J. 2013, 19, 11564–11567. [Google Scholar] [CrossRef]


| Usage/Reaction | Benefits of MW Heating |
|---|---|
| General operation | Risk reduction (health and safety) [2] |
| Automation [9] Scalability (experimental→batch) [10] | |
| Experimental parameters | Mild reaction conditions [1] Reduced reaction times [4] |
| Precise temperature control [11] In-situ temperature and pressure monitoring [12] Lack of thermal gradients [8] | |
| Colloidal catalyst NPs | Smaller size distribution [13] |
| Spontaneous nucleation event [14] | |
| Homogeneous nucleation and growth [14] | |
| Prevention of agglomeration [7] | |
| Catalyst nanocomposites | Intricate nanocomposite production [15] Monodispersed products [16] Elucidation of formation mechanisms [6] |
| Repeatability [17] Uniform heat distribution improves product yields [18] |
| LOW: tan δ < 0.1 | MEDIUM: 0.1 ≤ tan δ ≤ 0.5 | HIGH: tan δ > 0.5 | |||
|---|---|---|---|---|---|
| Solvent | tan δ | Solvent | tan δ | Solvent | tan δ |
| Acetonitrile | 0.062 | DMF | 0.161 | 1,2-Ethanediol | 1.350 |
| THF | 0.047 | 1,2-Dichloroethane | 0.127 | Ethanol | 0.941 |
| Toluene | 0.040 | Water | 0.123 | DMSO | 0.659 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, C.D.-W.; Treadwell, L.J.; Wiley, J.B. Microwave Synthetic Routes for Shape-Controlled Catalyst Nanoparticles and Nanocomposites. Molecules 2021, 26, 3647. https://doi.org/10.3390/molecules26123647
Chin CD-W, Treadwell LJ, Wiley JB. Microwave Synthetic Routes for Shape-Controlled Catalyst Nanoparticles and Nanocomposites. Molecules. 2021; 26(12):3647. https://doi.org/10.3390/molecules26123647
Chicago/Turabian StyleChin, Clare Davis-Wheeler, LaRico J. Treadwell, and John B. Wiley. 2021. "Microwave Synthetic Routes for Shape-Controlled Catalyst Nanoparticles and Nanocomposites" Molecules 26, no. 12: 3647. https://doi.org/10.3390/molecules26123647
APA StyleChin, C. D.-W., Treadwell, L. J., & Wiley, J. B. (2021). Microwave Synthetic Routes for Shape-Controlled Catalyst Nanoparticles and Nanocomposites. Molecules, 26(12), 3647. https://doi.org/10.3390/molecules26123647