Prenylated Flavonoid Glycosides with PCSK9 mRNA Expression Inhibitory Activity from the Aerial Parts of Epimedium koreanum
Abstract
1. Introduction
2. Results
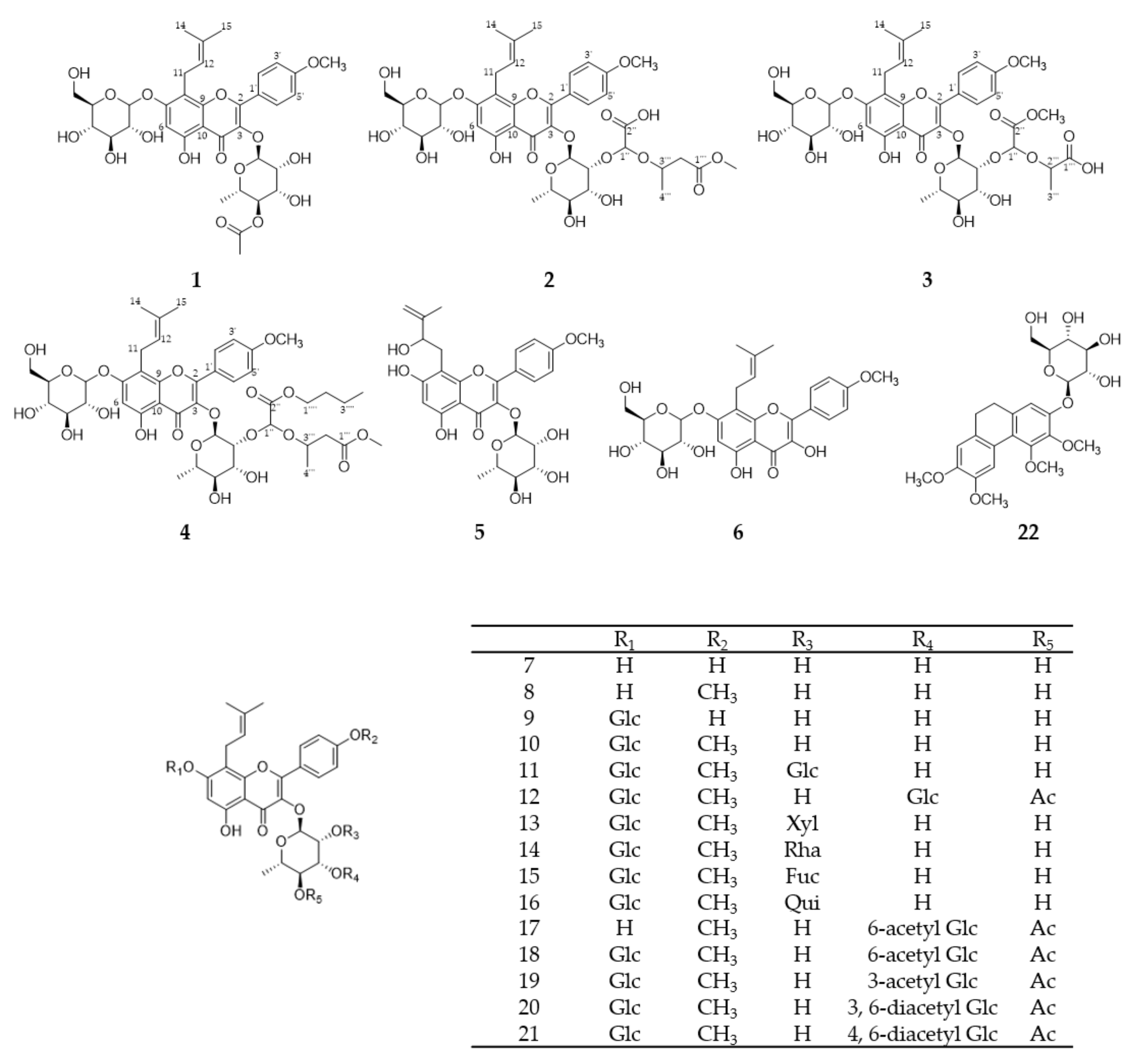
2.1. Isolation of Compounds from E. koreanum
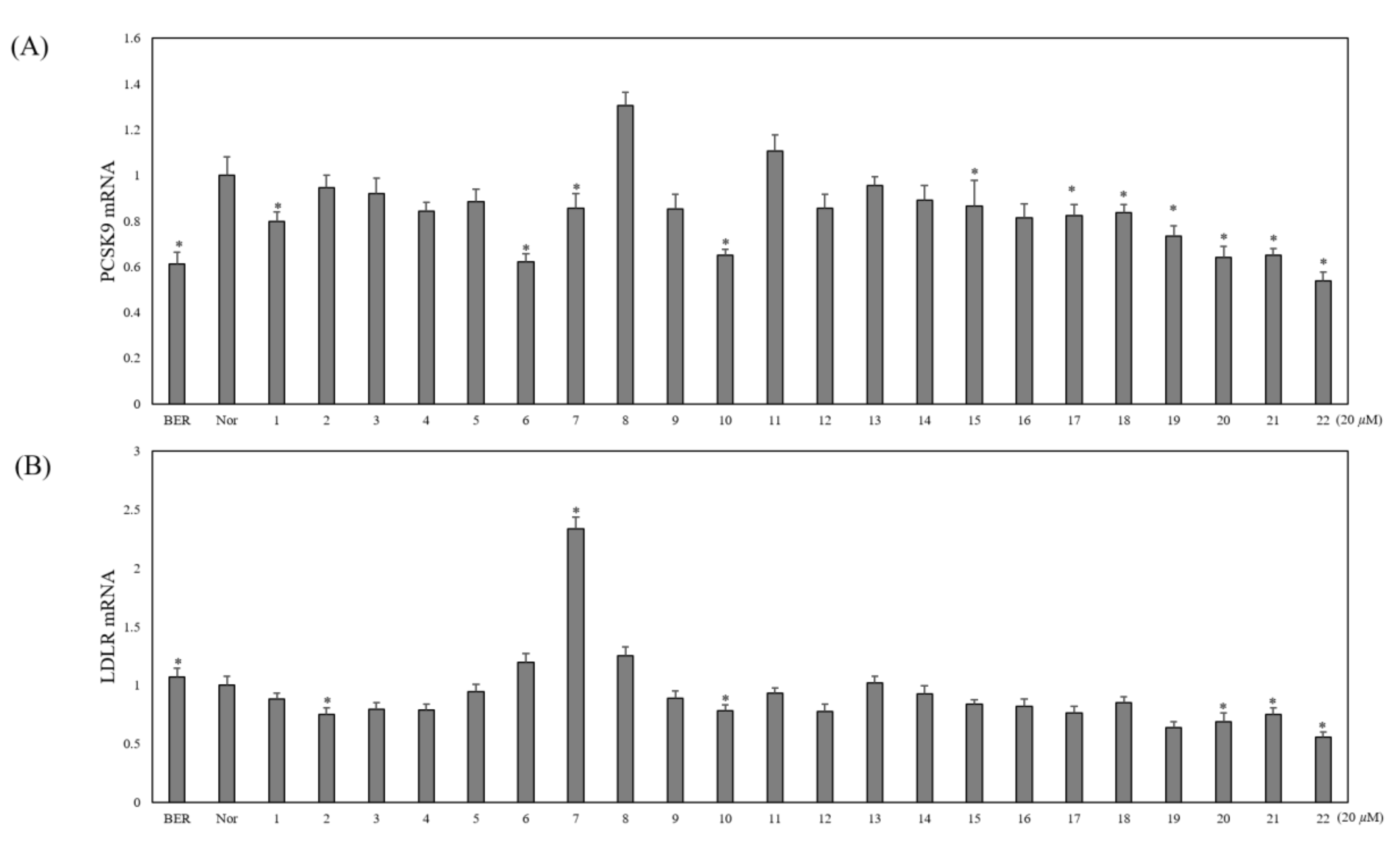
2.2. Bioactivity Evaluation
3. Discussion and Conclusions
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
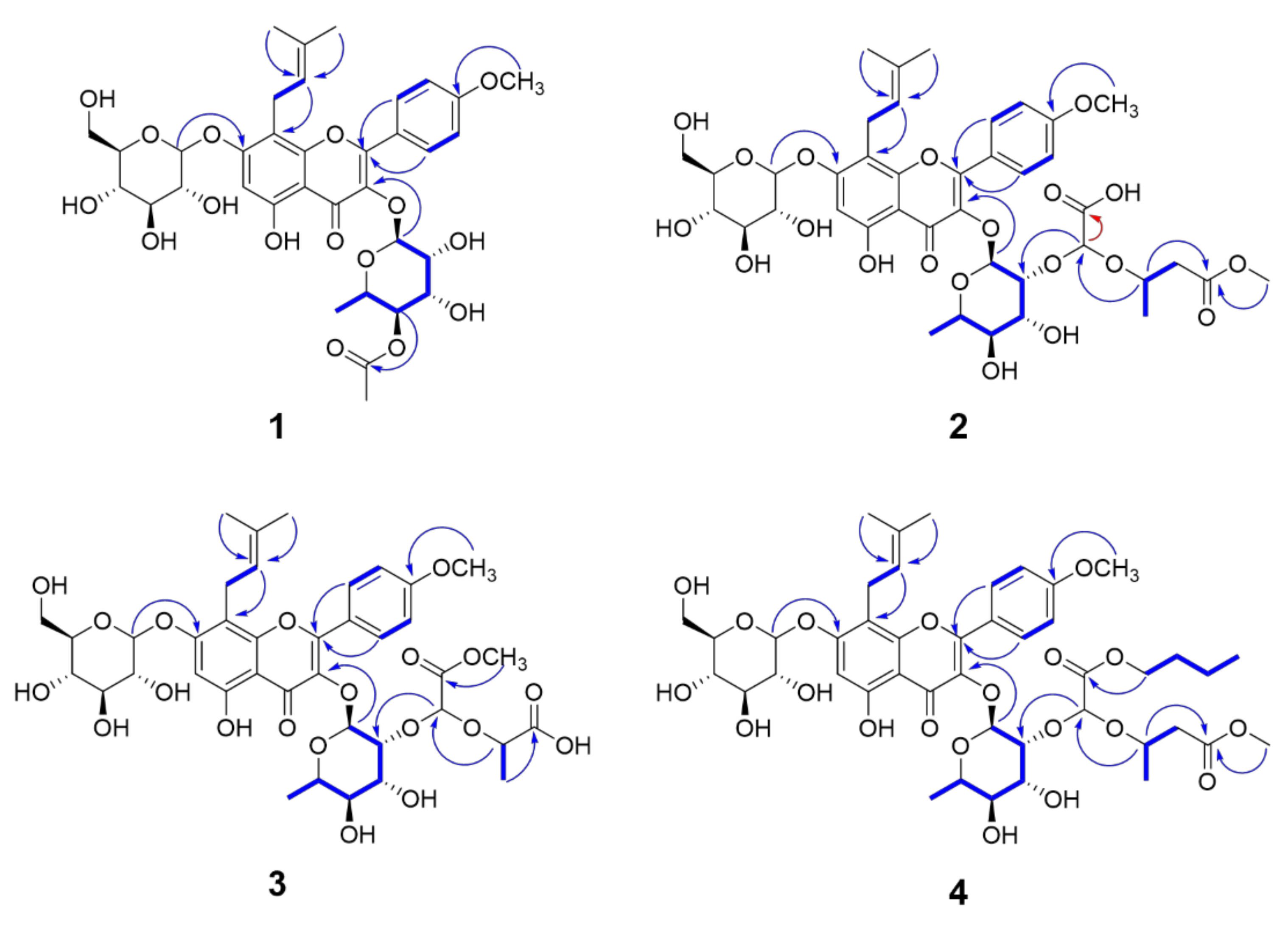
4.4. Characterization
4.5. Acid Hydrolysis
4.6. Cell Culture, Drugs and Chemicals
4.7. Quantitative Real-Time RT-PCR
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Perry, L.M. Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses; The MIT Press: Cambridge, MA, USA, 1980; p. 55. ISBN 0262160765. [Google Scholar]
- Tang, W.; Eisenbrand, G. Chinese Drugs of Plant. Origin: Chemistry, Pharmacology, and Use in Traditional and Modern Medicine; Springer: Berlin/Heidelberg, German, 1992; ISBN 3-642-73739-0. [Google Scholar]
- Ito, Y.; Hirayama, F.; Suto, K.; Sagara, K.; Toshida, T. Three flavonol glycosides from Epimedium koreanum. Phytochemistry 1988, 27, 911–913. [Google Scholar] [CrossRef]
- Kang, S.S.; Kang, Y.J.; Lee, M.W. Flavonoids from Epimedium Koreanum. J. Nat. Prod. 1991, 54, 542–546. [Google Scholar] [CrossRef]
- Li, W.; Xiao, P.; Tu, G.; Ma, L.; Zhang, R. Flavonol glycosides from Epimedium Koreanum. Phytochemistry 1995, 38, 263–265. [Google Scholar] [CrossRef]
- Su, X.; Li, W.; Ma, J.; Kim, Y. Chemical constituents from Epimedium Korenum Nakai and their chemotaxonomic significance. Nat. Prod. Res. 2018, 32, 2347–2351. [Google Scholar] [CrossRef]
- Lee, M.K.; Choi, Y.J.; Sung, S.H.; Shin, D.I.; Kim, J.W.; Kim, Y.C. Antihepatotoxic activity of icariin, a major constituent of Epimedium Koreanum. Planta Med. 1995, 61, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Makarova, M.N.; Pozharitskyay, O.N.; Shikov, A.N.; Tesakova, S.V.; Makarov, V.G.; Tikhonov, V.P. Effects of lipid-based suspenstion of Epimedium Koreanum Nakai extract on sexual behavior in rats. J. Ethnopharmacol. 2007, 114, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.J.; Sung, S.H.; Lee, H.S.; Jeon, M.H.; Kim, Y.C. Anti-hepatotoxic activity of icariside II, a constituent of Epimedium koreanum. Arch. Pharm. Res. 1995, 18, 289–292. [Google Scholar] [CrossRef]
- Meng, F.; Xiong, Z.; Jiang, Z.; Li, F. Osteoblastic proliferation stimulating activity of Epimedium koreanum Nakai extracts and its flavonol glycosides. Pharm. Biol. 2005, 43, 92–95. [Google Scholar] [CrossRef]
- Rhew, K.Y.; Han, Y. Immunoadjuvant activity of icarrin that induces Th1-type antibody in mice. Arch. Pharm. Res. 2012, 26, 796–806. [Google Scholar]
- Lin, X.; Li, W.; Xiao, P. Effects of icariside II from Epimedium koreanum on tumor cell lines in-vitro. J. Pharm. Pharmacol. 1999, 5, 701–703. [Google Scholar]
- Choi, H.J.; Eun, J.S.; Park, Y.R.; Kim, D.K.; Li, R.; Moon, W.S.; Park, J.M.; Kim, H.S.; Cho, N.P.; Cho, S.D.; et al. Ikarisoside A inhibits inducible nitric oxide synthase in lipopolysaccharide-stimulated RAW 264.7 cells via p38 kinase and nuclear factor-κB signaling pathways. Eur. J. Pharmacol. 2008, 601, 171–178. [Google Scholar] [CrossRef]
- Sabatine, M.S. PCSK9 inhibitors: Clinical evidence and implementation. Nat. Rev. Cardiol. 2019, 16, 155–165. [Google Scholar] [CrossRef]
- Dadu, R.T.; Ballantyne, C.M. Lipid lowering with PCSK9 inhibitors. Nat. Rev. Cardiol. 2014, 11, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Stoekenbroek, R.M.; Lambert, G.; Cariou, B.; Hovingh, G.K. Inhibiting PCSK9—biology beyond LDL control. Nat. Rev. Endocrinol. 2018, 15, 52–62. [Google Scholar] [CrossRef]
- Ahn, J.; Chae, H.S.; Pel, P.; Kim, Y.M.; Choi, Y.H.; Kim, J.; Chin, Y.W. Dilignans with a chromanol motif discovered by molecular networking from the stem barks of Magnolia obovata and their proprotein convertase subtilisin/kexin type 9 expression inhibitory activity. Biomolecules 2021, 11, 463. [Google Scholar] [CrossRef]
- Nhoek, P.; Chae, H.S.; Kim, Y.M.; Pel, P.; Huh, J.; Kim, H.W.; Choi, Y.H.; Lee, K.; Chin, Y.W. Sesquiterpenoids from the aerial parts of Salvia plebeia with inhibitory activities on proprotein convertase subtilisin/kexin type 9 expression. J. Nat. Prod. 2021, 84, 220–229. [Google Scholar] [CrossRef]
- Pel, P.; Chae, H.S.; Nhoek, P.; Kim, Y.M.; Khiev, P.; Kim, G.J.; Nam, J.W.; Choi, H.; Choi, Y.H.; Chin, Y.W. A stilbene dimer and flavonoids from the aerial parts of Chromolaena odorata with proprotein convertase subtilisin/kexin type 9 expression inhibitory activity. Bioorg. Chem. 2020, 99, 103869. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Kim, Y.M.; Chae, H.S.; Choi, Y.H.; Ahn, H.C.; Yoo, H.; Kang, M.; Kim, J.; Chin, Y.W. Prenylated flavonoids from the roots and rhizomes of Sophora tonkinensis and their effects on the expression of inflammatory mediators and proprotein convertase subtilisin/kexin type 9. J. Nat. Prod. 2019, 82, 309–317. [Google Scholar] [CrossRef]
- Li, H.; Zhou, C.; Chen, C.; Li, R.; Lee, K. Flavonoids isolated from heat-processed Epimedium koreanum and their anti-HIV-1 activities. Helv. Chim. Acta 2015, 98, 1177–1187. [Google Scholar] [CrossRef]
- Mei, Q.; Wang, C.; Zhao, Z.; Yuan, W.; Zhang, G. Synthesis of icariin from kaempferol through regioselective methylation and para-claisen-cope rearrangement. Beilstein J. Org. Chem. 2015, 11, 1220–1225. [Google Scholar] [CrossRef]
- Fukai, T.; Nomura, T. Seven prenylated flavonol glycosides from two Epimedium species. Phytochemistry 1998, 27, 259–266. [Google Scholar] [CrossRef]
- Liu, R.; Li, A.; Sun, A.; Cui, J.; Kong, L. Preparative isolation and purification of three flavonoids from the Chinese medicinal plant Epimedium koreanum Nakai by high-speed counter-current chromatography. J. Chromator. A 2005, 1064, 53–57. [Google Scholar] [CrossRef]
- Mizuno, M.; Kanie, Y.; Iinuma, M.; Tanaka, T.; Lang, F.A. Two flavonol glycosides, hexandrasides C and D, from the underground parts of Vancouveria hexandra. Phytochemistry 1991, 30, 2765–2768. [Google Scholar] [CrossRef]
- Kim, E.S.; Kim, M.K.; Kang, H.K.; Park, Y.I.; Dong, M.S.; Kim, D.H.; Chung, H.S. Flavonol glycosides with antioxidant activity from the aerial parts of Epimedium koreanum Nakai. Nat. Prod. Sci. 2008, 14, 233–238. [Google Scholar]
- Sun, P.; Chem, Y.; Shimizu, N.; Takeda, T. Studies on the constituents of Epimedium koreanum. III. Chem. Pharm. Bull. 1998, 46, 355–358. [Google Scholar] [CrossRef]
- Ueda, T.; Nakajima, K.; Chem, M.; Mihashi, H. Flavonoid glucoside and 5-lipoxygenase inhibitor containing the flavonoid as active ingredient. JP Patent: JP04159295A 2 June 1992. [Google Scholar]
- Ye, Z.; Wu, J.; Li, J.; Chang, X.; Tan, J.; Lv, W.; Zhu, H.; Sun, H.; Wnag, W.; Chem, Z.; et al. New prenylflavonol glycosides with xanthine oxidase inhibitory activity from the leaves of Cyclocarya paliurus. Bioorg. Chem. 2020, 101, 104018. [Google Scholar] [CrossRef]
- Sun, P.; Ye, W.; Zhao, J.; Pei, Y.; Wang, Z.; Chen, Y.; Ogihara, Y.; Takeda, T. Studies on the constituents of Epimedium Koreanum 1995, 43, 703–704. Chem. Pharm. Bull. 1995, 43, 703–704. [Google Scholar] [CrossRef]
- Li, J.; LI, H.; Liu, D.; Chen, X.; Chen, C.; Li, R. Three new acylated prenylflavonol glycosides from Epimedium Koreanum. Phytochem. Lett. 2016, 17, 206–212. [Google Scholar] [CrossRef]
- Li, W.; Pan, J.; Lü, M.; Zhang, R.; Xiao, P. A 9,10-dihydrophenanthrene derivate from Epimedium koreanum. Phytochemistry 1995, 39, 231–233. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899. [Google Scholar] [CrossRef]
- Taechalertpaisarn, J.; Zhao, B.; Liang, X.; Burgess, K. Small molecule inhibitors of the PCSK9·LDLR interaction. J. Am. Chem. Soc. 2018, 140, 3242–3249. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Luo, S.; Zhu, Z.; Xu, J. Small molecules as inhibitors of PCSK9: Current status and future challenges. Eur. J. Med. Chem. 2019, 162, 212–233. [Google Scholar] [CrossRef] [PubMed]
- Adorni, M.P.; Zimetti, F.; Lupo, M.G.; Ruscica, M.; Ferri, N. Naturally occurring PCSK9 inhibitors. Nutrients 2020, 12, 1440. [Google Scholar] [CrossRef]
- Chae, H.S.; Kim, H.J.; Ko, H.J.; Lee, C.H.; Choi, Y.H.; Chin, Y.W. Transcriptome analysis illuminates a hub role of SREBP2 in cholesterol metabolism by α-mangostin. ACS Omega 2020, 5, 31126–31136. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.S.; You, B.H.; Kim, D.Y.; Lee, H.; Ko, H.W.; Ko, H.J.; Choi, Y.H.; Choi, S.S.; Chin, Y.W. Sauchinone controls hepatic cholesterol homeostasis by the negative regulation of PCSK9 transcriptional network. Sci. Rep. 2018, 8, 6737. [Google Scholar] [CrossRef]
| Position | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 2 | 159.4 | 159.2 | 159.4 | 159.3 | ||||
| 3 | 135.9 | 136.8 | 136.6 | 136.9 | ||||
| 4 | 180.0 | 180.0 | 180.0 | 180.1 | ||||
| 5 | 161.0 | 161.0 | 160.9 | 161.1 | ||||
| 6 | 6.65, s | 99.4 | 6.65, s | 99.4 | 6.67, s | 99.9 | 6.67, s | 99.4 |
| 7 | 162.1 | 162.1 | 162.1 | 162.2 | ||||
| 8 | 110.5 | 110.6 | 110.7 | 110.6 | ||||
| 9 | 155.0 | 155.0 | 155.0 | 155.1 | ||||
| 10 | 107.5 | 107.5 | 107.5 | 107.5 | ||||
| 11 | 3.52, m, 3.57, m | 22.7 | 3.51, m, 3.57, m | 22.7 | 3.52, m, 3.58, m | 22.7 | 3.53, m, 3.57, m | 22.8 |
| 12 | 5.19, t (6.8) | 123.5 | 5.18, m | 123.6 | 5.20, m | 123.4 | 5.19, m | 123.6 |
| 13 | 132.6 | 132.7 | 132.8 | 132.7 | ||||
| 14 | 1.64, s | 25.9 | 1.64, s | 25.9 | 1.64, s | 25.9 | 1.64, s | 25.9 |
| 15 | 1.73, s | 18.3 | 1.72, s | 18.3 | 1.75, s | 18.3 | 1.72, s | 18.3 |
| 1′ | 123.9 | 123.8 | 123.7 | 123.9 | ||||
| 2′, 6′ | 7.85, d (8.8) | 131.9 | 7.86, d (8.8) | 131.9 | 7.90, d (8.4) | 131.9 | 7.89, d (8.8) | 132.0 |
| 3′, 5′ | 7.10, d (8.8) | 115.2 | 7.08, d (8.8) | 115.2 | 7.10, d (8.4) | 115.2 | 7.10, d (8.8) | 115.3 |
| 4′ | 163.6 | 163.5 | 163.6 | 163.6 | ||||
| 4’-OMe | 3.89, s | 56.1 | 3.89, s | 56.1 | 3.90, s | 56.1 | 3.89, s | 56.1 |
| Glucose | ||||||||
| 1 | 5.07, d (7.2) | 101.9 | 5.07, d (6.8) | 101.9 | 5.07, d (7.2) | 101.8 | 5.07, d (7.2) | 101.9 |
| 2 | 3.53, m | 74.9 | 3.53, m | 74.9 | 3.54, m | 74.9 | 3.53, m | 74.9 |
| 3 | 3.51, m | 78.2 | 3.51, m | 78.3 | 3.52, m | 78.3 | 3.51, m | 78.4 |
| 4 | 3.43, m | 71.1 | 3.43, m | 71.1 | 3.43, m | 71.1 | 3.42, m | 71.2 |
| 5 | 3.48, m | 78.3 | 3.48, m | 78.2 | 3.48, m | 78.2 | 3.49, m | 78.3 |
| 6 | 3.92, m 3.74, m | 62.4 | 3.92, m 3.74, m | 62.4 | 3.92, m 3.74, m | 62.3 | 3.92, m 3.74, m | 63.4 |
| Rhamnose | ||||||||
| 1 | 5.50, brs | 102.7 | 5.45, d (1.6) | 102.2 | 5.45, d (2.0) | 102.0 | 5.45, brs | 102.2 |
| 2 | 4.21, brs | 71.7 | 4.33, brs | 80.0 | 4.36, brs | 80.0 | 4.34, brs | 79.9 |
| 3 | 3.85, dd (9.8, 3.0) | 70.0 | 3.80, dd (9.2, 3.2) | 71.9 | 3.92, m | 72.2 | 3.80, dd (9.6, 3.2) | 71.9 |
| 4 | 4.82, t (10.0) | 74.9 | 3.38, m | 73.3 | 3.41, m | 71.7 | 3.39, m | 73.4 |
| 5 | 3.23, m | 69.6 | 3.33, m | 72.2 | 3.34, m | 72.9 | 3.29, m | 72.2 |
| 6 | 0.77, d (6.0) | 17.5 | 0.95, d (6.0) | 17.7 | 0.94, d (6.4) | 17.6 | 0.95, d (5.2) | 17.7 |
| 4-O-Ac | 172.3 | |||||||
| 2.01, s | 20.9 | |||||||
| Terminal | ||||||||
| 1″ | 5.21, s | 101.1 | 5.25, s | 100.4 | 5.21, s | 101.3 | ||
| 2″ | 170.0 | 169.4 | 169.7 | |||||
| 2″-OMe | 3.78, s | 52.9 | ||||||
| 1‴ | 173.1 | 174.4 | 173.1 | |||||
| 2‴ | 2.46, m 2.60, m | 42.5 | 4.53, q (6.8) | 73.2 | 2.46, m 2.62, m | 42.6 | ||
| 3‴ | 4.18, sextet (6.0) | 73.3 | 1.39, d (6.8) | 18.8 | 4.18, m | 73.4 | ||
| 4‴ | 1.21, d (6.4) | 21.6 | 1.21, d (6.0) | 21.7 | ||||
| 1‴-OMe | 3.65, s | 52.2 | 3.66, s | 52.2 | ||||
| 1⁗ | 4.16, m | 66.4 | ||||||
| 2⁗ | 1.66, m | 31.7 | ||||||
| 3⁗ | 1.42, m | 20.2 | ||||||
| 4⁗ | 0.95, t (7.2) | 14.1 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Kim, Y.-M.; Ahn, J.; Chae, H.-S.; Chin, Y.-W.; Kim, J. Prenylated Flavonoid Glycosides with PCSK9 mRNA Expression Inhibitory Activity from the Aerial Parts of Epimedium koreanum. Molecules 2021, 26, 3590. https://doi.org/10.3390/molecules26123590
Kim E, Kim Y-M, Ahn J, Chae H-S, Chin Y-W, Kim J. Prenylated Flavonoid Glycosides with PCSK9 mRNA Expression Inhibitory Activity from the Aerial Parts of Epimedium koreanum. Molecules. 2021; 26(12):3590. https://doi.org/10.3390/molecules26123590
Chicago/Turabian StyleKim, Eeray, Young-Mi Kim, Jongmin Ahn, Hee-Sung Chae, Young-Won Chin, and Jinwoong Kim. 2021. "Prenylated Flavonoid Glycosides with PCSK9 mRNA Expression Inhibitory Activity from the Aerial Parts of Epimedium koreanum" Molecules 26, no. 12: 3590. https://doi.org/10.3390/molecules26123590
APA StyleKim, E., Kim, Y.-M., Ahn, J., Chae, H.-S., Chin, Y.-W., & Kim, J. (2021). Prenylated Flavonoid Glycosides with PCSK9 mRNA Expression Inhibitory Activity from the Aerial Parts of Epimedium koreanum. Molecules, 26(12), 3590. https://doi.org/10.3390/molecules26123590







