Abstract
This paper aimed to investigate the potential antifungal influences of new alkaloids from Delphinium peregrinum L. var. eriocarpum Boiss. New Diterpenoid alkaloids Delcarpum (1), Hydrodavisine (4) and known alkaloids Peregrine (2), Delphitisine (3) were isolated by different chromatographic methods from the aerial parts of D. Peregrinum eriocarpum Boiss, which grows in Syria. The structures of alkaloids were proposed based on 1D NMR spectroscopy 1H-NMR, 13C-NMR, DEPT-135, DEPT-90, 2D NMR spectroscopy DQF-COSY, HMQC, EI-Ms mass spectrum, and IR spectroscopic measurements. The antifungal activity of the isolated alkaloids was evaluated against different dermatophyte fungal isolates compared with fluconazole. In the case of Peregrine (2) the minimum inhibitory concentrations(MICs) recorded 128–256, 32–64, and 32 for Epidermophyton floccosum, Microsporum canis, and Trichophyton rubrum, respectively, compared to 32–64, 16, and 32 μg/mL in the case of fluconazole, respectively. The MICs recorded on application of the four alkaloids mixture were 64, 32, and 16 in the case of E. floccosum, M. canis, and T. rubrum, respectively, which were significantly lower than that measured for each of the individual alkaloid and were compatible for fluconazole. In conclusion, MICs of the tested alkaloids showed a variable potential effect on the investigated fungal isolates. Peregrine (2) was the most effective alkaloid, however, the application of the mixture of alkaloids induced significant synergistic activity that was more pronounced than the application of individual ones.
1. Introduction
The genus Delphinium has been identified in many herbal species in Syria [1]. Delphinium is a medical herb used for the treatment of epilepsy, neurotoxic, and asthma [2,3]. The Delphinium alkaloids are currently under investigation in search for new analgesic, antiinflammatory drugs, muscular relaxation, anticonvulsant, pyramis lesion, and cardiac effects [3,4,5]. Moreover, a certain number of natural diterpenoid alkaloids have been reported to possess antiproliferative activities against various human cancer cell lines, indicating their great potential as new drugs for treating the corresponding cancers [6,7]. Due to their diterpenoid alkaloids components, Delphinium species have been considered as insecticidal potential [8]. Delphinium species have been used in folk medicine as a parasiticide and for the treatment of itches, skin eruptions. Delphinium was also used in the manufacture of dyes that are used to combat lice [3,9]. The plant D. peregrinum L. var. eriocarpum Boiss, which grows in Syria, has never been studied before. The first closest plant to it, called D. peregrinum var. elongatum Boiss, has been collected from Spain. C19-diterpenoid alkaloids (bicoloridine, dihydrogadesine, nudicaulidine, 13-acetylhetisinone, peregrine, peregrine alcohol, pergilone, delphiperegrine, and 14-O-acetylperegrine), norditerpenoid alkaloids (dehydrobicoloridine, bicoloridine alcohol, and peregrinine) and C20-diterpenoid alkaloids (hetisinone, hetisine, and atisinium chloride) have been isolated from this plant [10,11]. The second closest plant, called D. peregrinum has been collected from Turkey. C19-diterpenoid alkaloids (peregrine alcohol, pergilone, nudicaulidine, bicoloridine, peregrine, delphiperegrine) have been isolated from this plant [12]. Based on the aforementioned therapeutic effects, Delphinium alkaloids are expected to have antifungal effects. According to the available information, the literature has not sufficiently highlighted the expected antifungal effects of the new alkaloids isolated from D. Peregrinum L. Var. Eriocarpum Boiss in this study. This paper aimed to investigate the potential antifungal influences of new alkaloids isolated by different chromatographic methods from D. peregrinum L. var. eriocarpum Boiss (Figure 1).
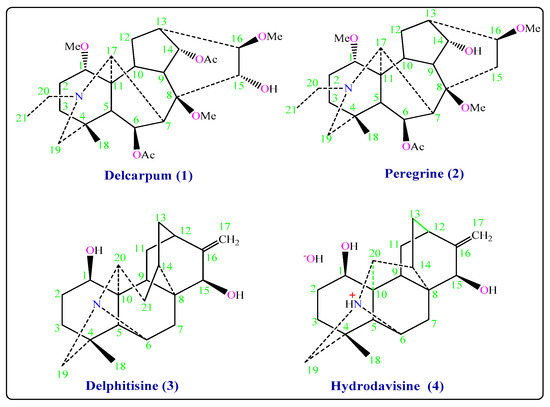
Figure 1.
Chemical structures of the isolated compounds (1–4).
2. Results and Discussion
2.1. Chemistry
The alkaloids were isolated by column chromatography (C.C) on silica gel of (8.02 g) using a Et2O and Et2O-CHCl3 then CHCl3 and CHCl3/MeOH/NH3 step gradient, followed by further C.C and flash chromatography (F.C), then by preparative TLC. Purification of diterpenoid alkaloids was assigned by TLC, GC, and HPLC.
New alkaloids were determined by mass and NMR data, showed characteristic signals of C19-diterpenoid alkaloids and C20-diterpenoid alkaloids in their NMR spectra and characteristic fragmentation of such compounds in their mass spectrum [13,14]. The NMR spectra of Delcarpum (1). C28H43NO8 gave signals at δH 1.14 (3H, t, J = 6.99 Hz), δC 11.9 (q) of an N-ethyl group, and a signal at δH 0.85 (3H, s), δC 25.2 (q) of methyl group, a signal at δH 3.32 and 3.45 (3H, s) of two methoxy groups, and one methoxy group at δH 3.17 (3H, m). The 13C-NMR spectrum (Table 1) of Delcarpum (1) gave signals at δC 48.5 (t) and δC 25.2 (q) of an angular methyl group, and one methoxy group at δC 48.6 (q) and at δC 20.2 (q), 20.6 (q), 170.0 (s), and 170.3 (s).

Table 1.
13C-NMR & HMQC NMR data assignments for Delcarpum (1), Peregrine (2), Delphitisine (3), and Hydrodavisine (4).
The 13C-NMR spectrum of Delcarpum (1) contained only three signals up field from 81 ppm at δC 32.8 (C-4), 47.6 (C-11), and 80.8 ppm (C-8) indicating the compound possessing is an aconitine-type C-19 diterpenoid alkaloids with a tertiary methoxy group at C-8 at δC 48.6 (q) [4].
The other two methoxy groups were situated at C-1α and C-16β to account for the one-proton signals at δH 3.22 (m, H-1β) and 3.11 (m, H-16α), which, in turn, gave one-bond connectivity with the methine carbon resonant at δC 82.3 (d) and 91.4 (d) ppm, respectively, in the HMQC spectrum (Table 1). Two acetate groups were situated at C-6β and C-14α to account for the one-proton signals at δH 5.29 (d, J = 7.53, H-6α) and 4.67 (t, J = 4.71 H-14β), which are connected with C-6, C-14, which showed a signal δC 71.4 (d) and 75.1 (d) ppm, respectively, in the HMQC spectrum (Table 1).
The methine carbon resonances at δC 74.1 (d) ppm suggested the presence of the secondary hydroxyl group at δH 3.52 (br s, OH) connected with C-15α in the molecule [10,11], and one proton of both protons connected with carbon C-12 gave a signal at δH 2.50 (dd, J1 = 3.17, J2 = 12.6 Hz, H-12) in which C-12 gave a signal at δC 28.2 (t) ppm, while one of the adjacent proton connected with C-13 gave a signal at δH 2.32 (t, J = 5.54 Hz, H-13) in which C-13 gave a signal at δC 36.4 (d) ppm, and two methylene protons showed a signal at δH 2.08 (d, J = 5.78 Hz, H-19) and δH 2.86 (d, J = 11.93 Hz, H-19) which are connected with C-19 that gave a signal at δC 56.8 (t) ppm. The other 1H- and 13C-NMR signals (Table 1) were in agreement with the proposed structure, and assignments were made by comparison with spectra of Peregrine (2) and 1H-COSY and HMQC data (Table 1). After connecting structure parts (A), (B), (C), and (D) we obtained Delcarpum (1) (Figure 2 and Figure S1a–g) [15].
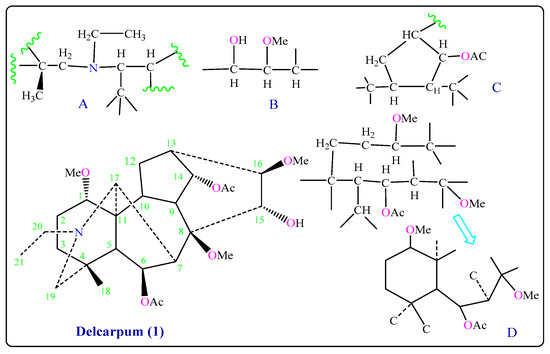
Figure 2.
Partial structures of Delcarpum (1).
The IR spectrum of compound (1) (amorphous) (IR νmax) showed absorbance at 3683–3435 cm−1, indicating the presence of OH group and the presence of a strong band at 1725 cm−1 indicating carbonyl group (C=O).
The mass spectrum (EIMS) of Delcarpum (1) has a peak at m/z 521, it represents the molecular ion M+ which is corresponding to its molecular weight with formula C28H43NO8, the spectral region adjacent to the M-peak may be used to reveal substituent and functional groups, such as the base peak appears at m/z 491, which indicated the presence of methoxy group, which converted into formyl H2C=O, CH3O (M-31), ethyl (M-29), which are corresponding to the following peaks of m/z 490(3), 492(24), respectively. The mass spectrum also gave the fragments of m/z -CH2OCH3 45, CH3-C=O 43
Peregrine (2), a colorless crystalline solid, gave a molecular ion at m/z 463 (M+, 3) in its EIMS, accounting for a molecular formula of C26H40NO6. The chemical structure of compound (2) was established according to its IR and NMR spectra (Table 1) [10,11,15].
The 1H-NMR spectrum of Delphitisine (3) exhibited characteristic signals at δH 1.10 (3H, s, CH3), 1.79 (2H, m, 2OH), 4.99 ppm (2H, d, C=CH2) ppm and tertiary amine with three carbon atoms (60.1, C-19; 2.50 d, & 2.69, d, H-19), (64.2, C-6; 4.23 m, H-6), and (64.4, C-20; 3.56 m, H-20). The 13C-NMR spectrum showed twenty-one signals, including one methyl, eight methylene, eight methine, and four quaternary carbons C-4, C-8, C-10, and C-16 that gave signals at δC 35.7, 44.1, 53.4, and 154.1 ppm, respectively (Table 1). The results of COSY and HMQC experiments (in CDCl3) and an inspection of literature values reported for C20-diterpenoid alkaloids clearly determined the existence of partial structures (A), (B), (C), and (D) (Figure 3 and Figure S2a–g) in Delphitisine (3). Its IR spectrum also showed absorption bands at 3633, 3452 (2 -OH), 3056 (=C-H), and 1605 (C=C) cm−1. Moreover, the mass spectrum (EIMS) of Delphitisine (3) has a peak at m/z 327 (54%) represents the molecular ion M+, which is corresponding to its molecular weight and a molecular formula of C21H29NO2. All that data can be satisfactorily supported by the structure of Delphitisine (3). The closest type is Hetisine-type, which is one of the most complex entries in the atisane-class [10,11,16,17].
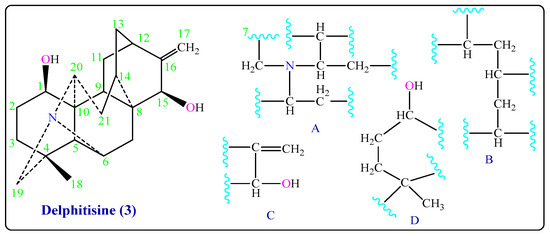
Figure 3.
Partial structures of Delphitisine (3).
The study spectrums of Hydrodavisine (4) gave signals at δH 5.01ppm (2H, d, J = 7.21 Hz), of an (-C=CH2), signals at δC 108.1, 154.2 ppm of quaternary carbon sp2, and IR in cm−1 at 1620 (C=C), 3019 (=C-H). The signal at δH (2H, 4, 89 (m) ppm) of an (-OH) group and in IR (3683–3620 & 3460) cm−1, with hydrogen bonding. A methyl group -CH3 was founded by δH 1.16 (3H, s) ppm and its δC 26.6 ppm (Table 1). The group (C3N+H) has been shown by signals at δC (C6: 63.6, C19: 59.4, C20: 66.3 ppm) and a hydrogen atom (1H, 3.74 ppm). 13C-NMR spectrum showed twenty signals, including one methyl, seven methylene, eight methine, and four quaternary carbons C-4, C-8, C-10, and C-16 that gave signals at δC 35.4, 36.3, 54.2, and 154.2 ppm, respectively (Table 1). After studied COSY and HMQC experiments (in CD3OD) it found the partial structures (A), (B), (C), and (D) (Figure 4 and Figure S3a–h), and after comparing the 13C-NMR of Hydrodavisine (4) with its of Delphitisine (3) and of C20-diterpenoid alkaloids [5,10,11]. We made sure that this compound has the same skeleton of Delphitisine (3), losing a secondary carbon atom. In addition to that, the mass spectrum of Hydrodavisine (4) (EIMS) the molecular weight M+ = 331 is corresponding to its molecular formula of [C20H29NO3], a peak at m/z 314 (96%) represents the molecular ion (M-OH)+. The peak at m/z 348 was for the fragment [M + OH]+, and the peak m/z 349 was for the fragment (M + H2O)+. The peak in 1H-NMR 4.89 ppm for OH hydrogen bonds with water molecules [15,18].
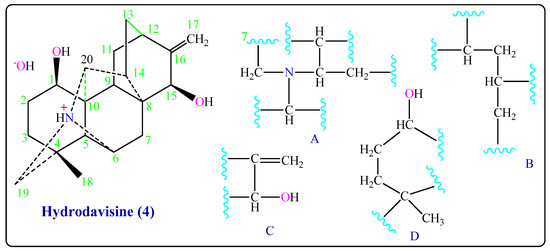
Figure 4.
Partial structures of Hydrodavisine (4).
2.2. Biology
Antifungal Activity
MIC of the tested alkaloids showed a different potential effect on the investigated fungal isolates. Peregrine was the most effective where the MIC recorded 128–256, 32–64, and 32 for Epidermophyton floccosum, Microsporum canis, and Trichophyton rubrum, respectively, compared to 32–64, 16, and 32 μg/mL in the case of fluconazole. Alkaloids extracted from the plant have been known to have important characteristics with biochemical, pharmacological, and medical effects in living organisms [19,20]. The investigators isolated three C19-diterpenoid alkaloids: Delbrunine, delbruline, and delbrusine, from Delphinium brunonianum and they indicated that these compounds have an antibacterial effect against Escherchia coli, Staphylococcus aureus, Pseudomonas aureginous, Salmonella flexinarie, and Bacillus subtilis. Sometimes, the use of individual bioactive compounds extracted from a particular plant does not induce the predicted inhibitory effects compared to its original synergistic combination with other associate candidates. The MIC and MFC of the mixture of tested alkaloids were significantly more pronounced than the application of individual ones. The MICs recorded on the application of the mixture of the four alkaloids were 64, 32, and 16 in the case of E. floccosum, M. canis, and T. rubrum, respectively, which was significantly lower than that measured for each of the individual alkaloids and were compatible for fluconazole as reference standard drug (Table 2). Hemaiswarya et al. [21] indicated that several plant extracts have shown synergistic activity against microorganisms. This review designates in detail the observed synergy and mechanism of action between natural products including flavonoids and essential oils and synthetic drugs in combating microbial infections. The mode of action of combination differs significantly from that of the same drugs acting individually, hence, isolating a single component may lose its therapeutic importance.

Table 2.
Range of minimum inhibitory (MIC) and minimum fungicidal concentrations (MFC) of synthesized alkaloid isolated from Delphinium peregrinum L. var. eriocarpum Boiss against different dermatophyte fungal isolates measured as µg/mL.
3. Materials and Methods
3.1. Chemistry
General. IR: CHCl3 for compounds (1, 2, and 3) and MeOH for compound (4) on FT-IR spectrum (Impact 410-Nicolet Madison, WI, USA). MS spectra were recorded on Varian GC/MS EI MS 70 eV. 3800 gas chromatograph directly interfaced with 2000 ion trap mass spectrometer (Varian, Walnut Creek, CA, USA). NMR spectra were recorded in CDCl3 for compounds (1, 2, and 3) and CD3OD for compound (4) on Bruker Avance 300 MHz (Bruker Biospin, Karlsruhe, Germany). Silica gel pH = 7, 230 mesh was used for column chromatography (C.C), pH = 7, 400 mesh was utilized for thin-layer chromatography (TLC) and silica gel 60 F254, TLC plates (Merck, Darmstadt Germany). All chemicals used in this study were obtained from Merck (Merck, Darmstadt, Germany).
Plant material: The investigated plant is related to the family Ranunculaceae, annual with herbaceous growth of 10–60 cm tall. The aerial parts (1 kg) were collected from several localities located at Umm Harten village between Homs and Tartous cities, Syria, during the flowering period (June to August) and authenticated in Botany Department, Faculty of Science, University of Damascus.
Extraction and isolation: Air-dried parts were extracted with methanolic NH3 (2%) at room temperature for a week. The resulting extract (208.5 g) was treated with 5% H2SO4. The acid was neutralized with 25% NaOH and extracted with Et2O then with CHCl3 to give crude alkaloid material (9.29 g). Column chromatography (C.C) on silica gel (8.02 g) using Et2O and Et2O/CHCl3 then CHCl3 and CHCl3/MeOH/NH3 step gradient, followed by further C.C flash chromatography (F.C) and preparative TLC.
Delcarpum (1): 67 mg resin were isolated by preparative TLC (Et2O:Me2CO 5:1), Rf = 0.212 in (MeOH:CH2Cl2 1:1), and TLC (C6H6:CH2Cl2:MeOH 1:6:3) Rf = 0.8, respectively. Peregrine (2): 56 mg crystalline, mp 123.5–125 °C from (hexane:EtOAc 1:1). Delphitisine (3): 31 mg resin was isolated by preparative TLC (MeOH:CH2Cl2 1:1) Rf = 0.515 and Hydrodavisine (4): 27 mg white crystals was isolated by preparative TLC (MeOH:CH2Cl2 1:1) recrystallization (CH2Cl2:MeOH 90:10) mp (dec) = 304–309 °C; Rf = 0.25 in (MeOH:CH2Cl2 1:1).
Purification of Delcarpum (1), Peregrine (2), Delphitisine (3) and Hydrodavisine (4) was assigned by TLC, GC, and HPLC. The structures were identified by 1D NMR spectroscopy 1H-NMR, 13C-NMR, DEPT-135, DEPT-90, 2D NMR spectroscopy DQF-COSY, HMQC, EI-Ms mass spectrum, and IR spectroscopy (Figure 1).
Delcarpum (1): Amorphous IR νmax CHCl3 cm−1: 3683, 3435, 3009, 2946, 1725, 1210, 1082, 784; 1H-NMR (300 MHz, CDCl3): δ 0.85 (3H, s), 1.14 (3H, t, J = 6.99 Hz), 2.08 (1H, d, J = 5.78 Hz), 2.32 (H, t, J = 5.54 Hz), 2.50 (H, dd, J1 = 3.17, J2 = 12.6 Hz), 2.86 (1H, d, J =11.93 Hz), 3.32 and 3.45 (3H each, s, 2 × OMe), 4.18 (H, d, J = 6.91 Hz), 4.67(H, t, J = 4.71 Hz), 3.52 (1H, br s, OH), 5.29 (H, d, J = 7.53 Hz); EIMS m/z (rel.int.) 521 (M+, 1), 492 (24), 491 (100), 490 (3), 431 (3), 401 (3), 400 (3), 98 (5), 91 (6), 79 (5), 77 (5), 58 (24), 56 (5), 45 (6), 44 (5), 43 (92), 42 (6), 41 (5). For 13C-NMR (300 MHz), see Table 1 and Table S1–S4.
Peregrine (2): Crystalline solid, mp = 123.5–125 °C from hexane-EtOAc; [α]D + 20° 9c 0.2). IR νmax KBr cm−1: 3633, 3438, 3001, 2946, 1733, 1230, 1081, 796; 1H-NMR (300 MHz, CDCl3): δ 0.85 (3H, s), 1.07 (3H, t, J = 7.12 Hz), 1.25 (1H,), 1.48 (1H, s), 1.57 (1H, -), 1.88 (1H, m), 2.01 (2H, -), 2.08 (7H, -), 2.25 (1H, dd, J = 5.31 Hz), 2.35 (1H, t, J = 5.67 Hz), 2.47 (2H, m), 2.61 (1H, d, J = 11.90 Hz), 2.74 (1H, d, J = 7.29 Hz), 3.05 (1H, t, J = 5.53 Hz), 3.11 (4H, -), 3.16 (1H, d, J = 2.07 Hz), 3.28 (3H, s), 3.39 (4H, -), 3.70 (1H, d, J = 6.48 Hz, OH), 4.02 (1H, q, J = 10.585 Hz), 5.25 (1H, d, J = 7.28 Hz); EIMS m/z (rel.int.) 463 (M+, 3), 434 (16), 433 (77), 432 (17), 403 (9), 402 (13), 401 (23), 400 (13), 373 (27), 372 (21), 341 (17), 340 (15), 123 (12), 96 (10), 91 (20), 79 (12), 77 (15), 71 (17), 65 (10), 58 (38), 56 (11), 55 (12), 45 (13), 43 (100), 42 (11), 41 (18). For 13C-NMR (300 MHz) see Table 1 and Table S1–S4. Peregrine (2) has been isolated before) [10,11,12].
Delphitisine (3): Amorphous IR νmax CHCl3 cm−1: 3633, 3438, 1605; 1H-NMR (300 MHz, CDCl3): δ 1.08 (3H, s), 1.10 (1H, m), 1.29 (3H, m), 1.77 (2H, m),1.79(6H, m) 1.94 (2H, m), 1.99 (1H, m), 2.07 (3H, m), 2.26 (1H, d, J = 2.29 Hz), 2.50 (1H, d, J = 12.45 Hz), 2.67 (1H, s), 2.69 (1H, d, J = 12.44 Hz), 3.56 (1H, m), 4.05 (1H, s), 4.23 (1H, m), 4.99 (1H, d, J = 7.60 Hz); EIMS m/z (rel.int.) 328 (M+ + 1, 13), 327 (M+, 54), 326 (100), 325 (13), 311 (20), 309 (75), 308 (32), 307 (14), 167 (19), 150 (24), 134 (20), 132 (24), 131 (24), 120 (32), 118 (41), 110 (22), 109 (25), 107 (38), 105 (21), 95 (29), 94 (30), 93 (21), 92 (81), 82 (25), 81 (26), 80 (61), 79 (27), 78 (73), 77 (16), 70 (19), 67 (38), 65 (10), 58 (38), 55 (43), 53 (38), 51 (38), 50 (12), 44 (32), 43 (35), 42 (26), 41 (71), 40 (15). For 13C-NMR (300 MHz), see Table 1 and Table S1–S4.
Hydrodavisine (4): Crystalline solid, mp(dec) = 304–309 °C from (MeOH:CH2Cl2); IR νmax MeOH cm−1: 3683, 3620, 3460, 3019, 2975, 2400, 1620,1521, 1423, 1213, 1046, 929, 759, 669; 1H-NMR (300 MHz, CD3OD): δ 1.16 (3H, s), 1.38 (3H, m), 1.70 (2H, m), 1.84 (4H, m), 1.90 (1H, m), 2.11 (2H, m), 2.21 (1H, m), 2.25 (1H, m), 2.86, (2H, q, J = 12.29 Hz), 3.10 (1H, s), 3.74 (+NH, m), 3.93 (1H, s), 3.99 (1H, s), 4.20 (1H, s), 4.89 (2OH + 2H2O, br s), 5.01 (2H, d, J = 7.21 Hz); EIMS m/z (rel.int.) 349(M+ + 18, 3), 348(M+ + 17, 5), 331 (M+, 3), 315 (M+ − O, 11), 314 (M+ − OH, 96), 313 (24), 312 (7), 311 (4), 299 (11), 298 (36), 297 (100), 296 (82), 295 (36), 294 (26), 293 (21), 292 (14), 291 (10), 277 (10), 276 (10), 252(8), 158 (10), 157 (11), 154 (10), 143 (10), 120 (13), 110 (10), 109 (12), 108 (12), 107 (11), 106 (11), 56 (7), 44 (10), 43 (7), 42 (12), 40 (6); In another MS: 331 (M+): [C20H28+NO2 + −OH], 349 (M +1 8): [C20H28+NO2 + −OH + H2O]. For 13C-NMR (300 MHz), see Table 1 and Table S1–S4.
3.2. Biology
Test organisms: Five different dermatophyte isolates from Epidermophyton floccosum, Microsporum canis, and Trichophyton rubrum were tested in this investigation. The test isolates were chosen from the identified stock specimens of the author and were isolated from patients with superficial clinical infections with different tinea infections admitted to dermatological and microbiological laboratories in Cairo, Egypt [22]. The inoculums were prepared from 10-day-old cultures. The isolates were maintained in Sabouraud dextrose agar (SDA) (Oxoid, Basingstoke, UK) with 20% glycerol, preserved at −80 °C, and cultured on potato dextrose agar (PDA) (Oxoid) plates incubated at 30 °C before investigation.
Antifungal activity: Assay of minimum inhibitory concentration (MIC): The experimental design was achieved according to CLSI M38-A protocols for filamentous fungi using the broth microdilution method following the Clinical and Laboratory Standards Institute (CLSI) M38-A2 guidelines [23]. Briefly, a fungal suspension was scraped from the margin of actively growing mycelium grown at 30 °C on potato dextrose agar (PDA). One percent solution Tween 80 in distilled water was added to the scraped mycelium, and the suspension was diluted 1:50 (v/v) in RPMI-1640 culture medium (Sigma, Darmstadt, Germany) in presence of a buffer, where the suspension contained about 5.0 × 104 CFU/mL. The compounds were prepared in RPMI medium supplemented with MOPS. Serial dilutions 1:2 were performed on the microplates and evaluated at bifold concentrations from 1 to 1024 μg/mL of each of the tested compounds or their mixture. Untreated controls were then tested, and 100 μL of the initially prepared inoculum was added to the microplates. The antifungal effect was observed after seven days of incubation at 30 °C by optical observation of turbidity. MIC was the lowest concentration of compound capable of inhibiting observed fungal growth in the wells by 100%. Fluconazole was used as an antifungal reference drug. All experiments were performed in triplicate.
Minimum fungicidal concentration (MFC): After recording the MIC value for each sample, 10 μL from clear wells, the last tube showing growth, were subcultured on Sabouraud dextrose agar medium (OXOID LTD, CM0041). The dishes were incubated at 30 °C for 7 days. A control without drugs was performed. MFC was defined as the lowest concentration of the compound at which growth was <3 CFU. All experiments were performed in triplicate.
4. Conclusions
Four alkaloids were isolated from the aerial parts of D. Peregrinum eriocarpum Boiss, two of them are from C19-diterpenoid alkaloids: Delcarpum (1) and Peregrine (2). The other two alkaloids are from C20-diterpenoid alkaloids: Delphitisine (3) and Hydrodavisine (4). Delcarpum (1) and Hydrodavisine (4) alkaloids are isolated for the first time. Concerning the antifungal effect, Peregrine (2) is the most effective in comparison with other alkaloids. In addition, the mixture of tested alkaloids is significantly inhibitory and is more pronounced than the application of individual ones.
Supplementary Materials
The following are available online, Figure S1a–g. Spectral characterization of Delcarpum (1), Figure S2a–g. Spectral characterization of Delphitisine (3), Figure S3a–h. Spectral characterization of Hydrodavisine (4), Table S1. 1H, 1H COSY (Correlation Spectroscopy Homonuclear) and HMQC (Heteronuclear Multiple Quantum Coherence) NMR data for Delcarpum (1), Table S2. 1H, 1H COSY (Correlation Spectroscopy Homonuclear) and HMQC (Heteronuclear Multiple Quantum Coherence) NMR data for Peregrine (2), Table S3. 1H, 1H COSY (Correlation Spectroscopy Homonuclear) and HMQC (Heteronuclear Multiple Quantum Coherence) NMR data. for Delphitisine (3) and Table S4. 1H, 1H COSY (Correlation Spectroscopy Homonuclear) and HMQC (Heteronuclear Multiple Quantum Coherence) NMR data for Hydrodavisine (4).
Author Contributions
Conceptualization, S.M.G.; Data curation, M.A., S.A. and S.A.O.; Formal analysis, M.A., S.A., S.M.G. and S.A.O.; Investigation, M.A., Y.A.M.S., S.A. and S.A.O.; Methodology, M.A.; Project administration, M.A.; Writing—original draft, M.A. and S.M.G.; Writing—review & editing, Y.A.M.S., S.A. and S.A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported and funded by Albaath University, Homs, Syria (Approval Number 37-99).
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
No potential conflict of interest was reported by the authors.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Post, G.E. Flore of Syria. Palestine and Sinai; American Press: Reston, VA, USA, 1933; Volume 2, pp. 21–25. [Google Scholar]
- Raza, M.; Shaheen, F.; Choudhary, M.; Sombati, S.; Rafiq, A.; Suria, A.; Rahman, A.-U.; DeLorenzo, R.J. Anticonvulsant activities of ethanolic extract and aqueous fraction isolated from Delphinium denudatum. J. Ethnopharmacol. 2001, 78, 73–78. [Google Scholar] [CrossRef]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer: New York, NY, USA, 2007. [Google Scholar]
- Zhang, Z.-T.; Jian, X.-X.; Ding, J.-Y.; Deng, H.-Y.; Chao, R.-B.; Chen, Q.-H.; Chen, D.-L.; Wang, F.-P. Further Studies on Structure-Cardiac Activity Relationships of Diterpenoid Alkaloids. Nat. Prod. Commun. 2015, 10, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-H.; Jiang, Z.-B.; Guo, Q.-L.; Shi, J.-G. A minor arcutine-type C 20-diterpenoid alkaloid iminium constituent of “fu zi. ” Chin. Chem. Lett. 2017, 28, 588–592. [Google Scholar] [CrossRef]
- Liang, X.; Gao, Y.; Luan, S. Two decades of advances in diterpenoid alkaloids with cytotoxicity activities. RSC Adv. 2018, 8, 23937–23946. [Google Scholar] [CrossRef]
- Yin, T.; Cai, L.; Ding, Z. An overview of the chemical constituents from the genus Delphinium reported in the last four decades. RSC Adv. 2020, 10, 13669–13686. [Google Scholar] [CrossRef]
- Ulubelen, A.; Meriçli, A.H.; Meriçli, F.; Kilinçer, N.; Ferizli, A.G.; Emekci, M.; Pelletier, S.W. Insect repellent activity of diterpenoid alkaloids. Phytotherapy Res. 2001, 15, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.-U.; Nasreen, A.; Akhtar, F.; Shekhani, M.S.; Clardy, J.; Parvez, M.; Choudhary, M.I. Antifungal Diterpenoid Alkaloids fromDelphinium denudatum. J. Nat. Prod. 1997, 60, 472–474. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, G.; Gavín, J.A.; Acosta, R.D.; Morales, J.A. Diterpenoid Alkaloids from Delphinium peregrinum. The Structure of Peregrine. Heterocycles 1988, 27, 1–5. [Google Scholar] [CrossRef]
- De La Fuente, G.; Ruiz-Mesía, L. Norditerpenoid alkaloids from Delphinium peregrinum var. elongatum. Phytochemistry 1995, 39, 1459–1465. [Google Scholar] [CrossRef]
- Ulubelen, A.; Meriçli, A.H.; Meriçli, F.; Ilarslan, R. Diterpene alkaloids from delphinuim peregrinum. J. Phytochem. 1992, 31, 1019–1022. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Chen, D.-L.; Chen, Q.-H.; Wang, F.-P. C20-Diterpenoid Alkaloids fromDelphiniumtrifoliolatum. J. Nat. Prod. 2005, 68, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Advancement in Research on Delphinium, sp. (Renunculaceae): A Review. Int. J. Green Herb. Chem. 2018, 7, 251–259. [Google Scholar] [CrossRef]
- Ulubelen, A.; Desai, H.K.; Srivastava, S.K.; Hart, B.P.; Park, J.-C.; Joshi, B.S.; Pelletier, S.W.; Meriçli, A.H.; Meriçli, F.; Ilarslan, R. Diterpenoid Alkaloids fromDelphinium davisii. J. Nat. Prod. 1996, 59, 360–366. [Google Scholar] [CrossRef]
- Alhilal, S.; Alhilal, M. Isolation and Structural Elucidation of a New Alkaloid from Delphinium peregrinum L. var. eriocarpum Boiss. Basic Appl. Sci. J. King Faisal Univ. 2020, 21, 54–59. [Google Scholar] [CrossRef]
- Wang, F.-P.; Liang, X.-T. C20-diterpenoid alkaloids. Alkaloids Chem. Biol. 2002, 59, 1–280. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.-H.; Zhang, J.-F.; Gao, F.; Huang, S.; Zhou, X.-L. Diterpenoid Alkaloids from Delphinium anthriscifolium var. majus. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Deng, W.; Sung, W.L. Three new C19-diterpenoid alkaloids, delbrunine, delbruline and desbrusine from Delphinium brunonianum royle. Heterocycles 1986, 24, 873–876. [Google Scholar]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Elmegeed, A.S.M.A.; Ouf, S.; Moussa, T.A.; Eltahlawi, S. Dermatophytes and other associated fungi in patients attending to some hospitals in Egypt. Braz. J. Microbiol. 2015, 46, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).