Characteristics of Reconstituted Collagen Fibers from Chicken Keel Cartilage Depends on Salt Type for Removal of Proteoglycans
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the Raw Material
2.2. PG Extraction from Cartilage (Pretreatment of Cartilage)
2.3. Extraction of Pepsin-Solubilized Collagen—Reconstituted Fibers
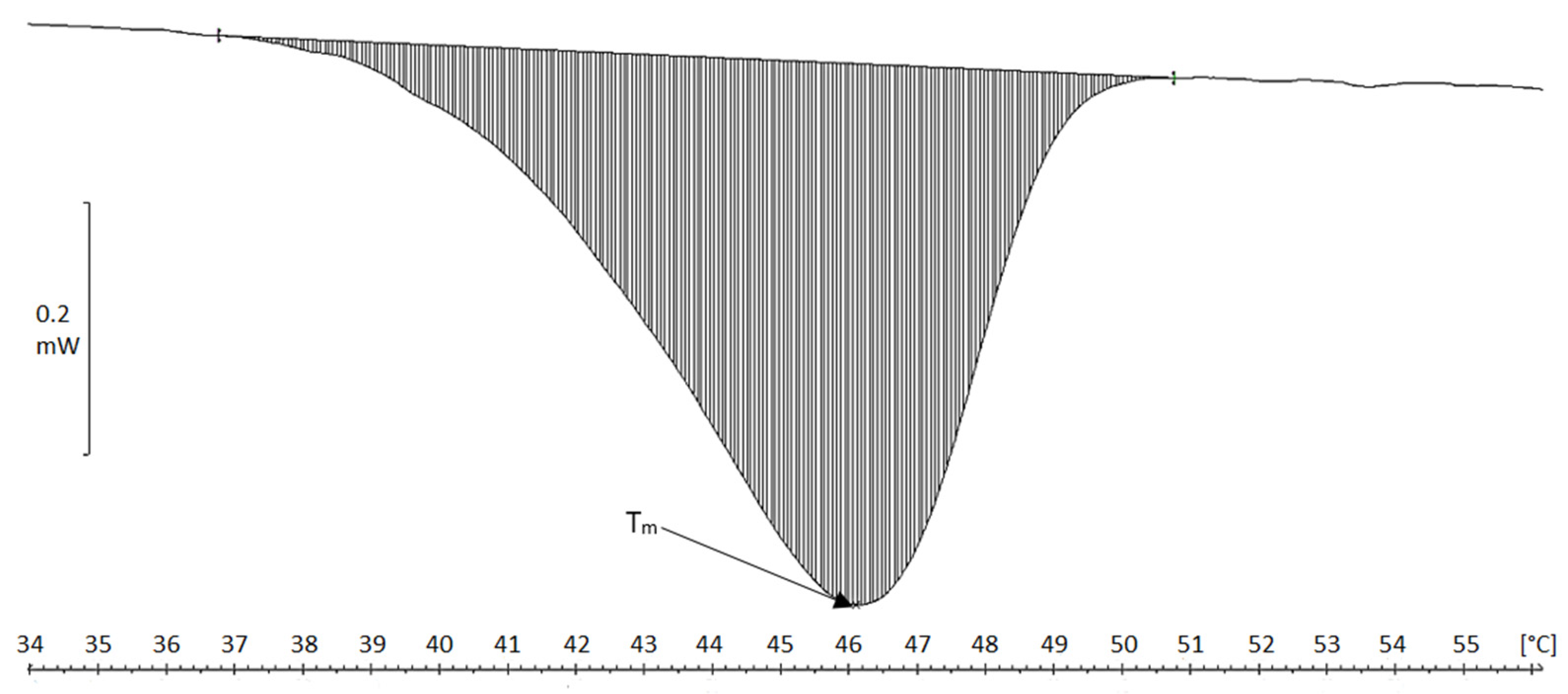
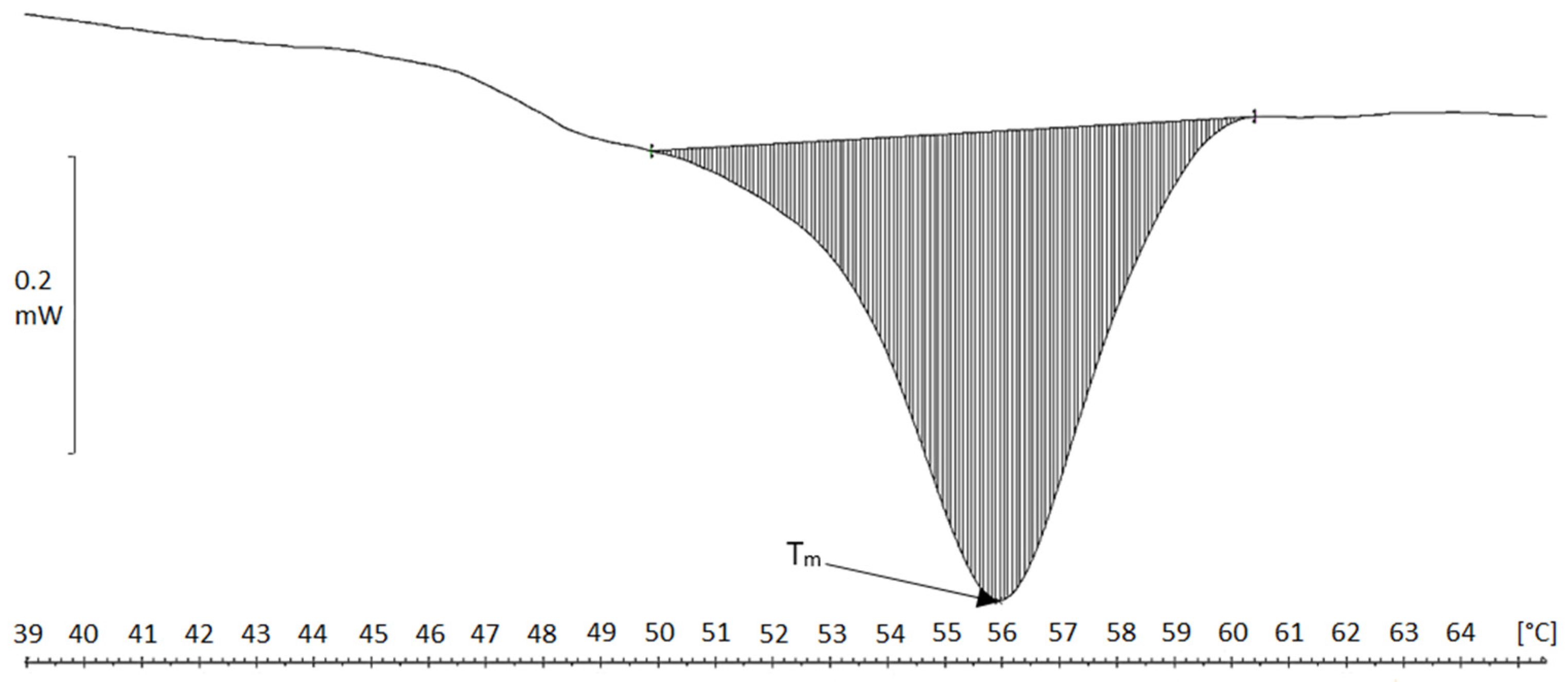
2.4. Differential Scanning Calorimetry (DSC)
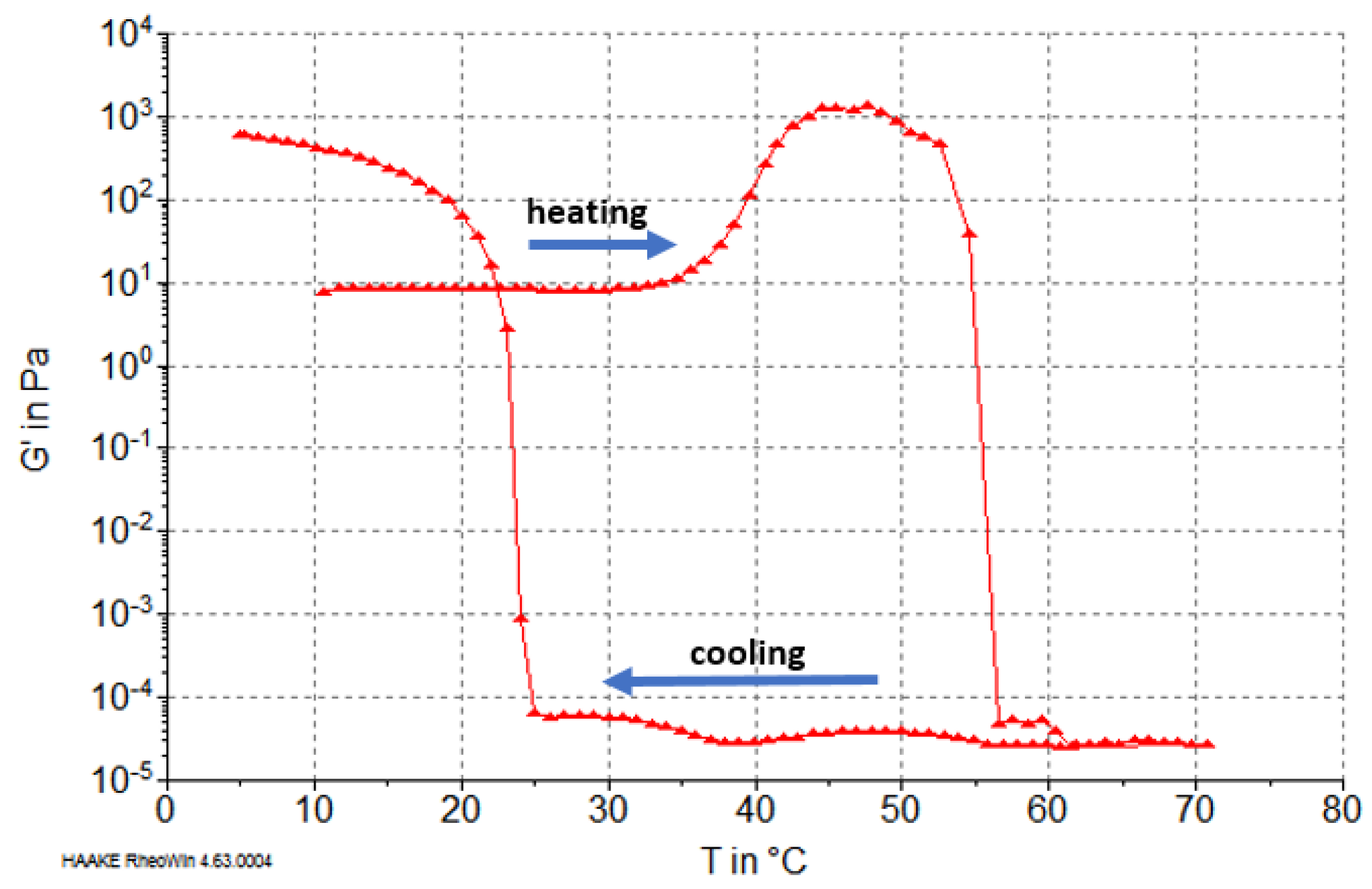
2.5. Rheological Properties of Reconstituted Collagen
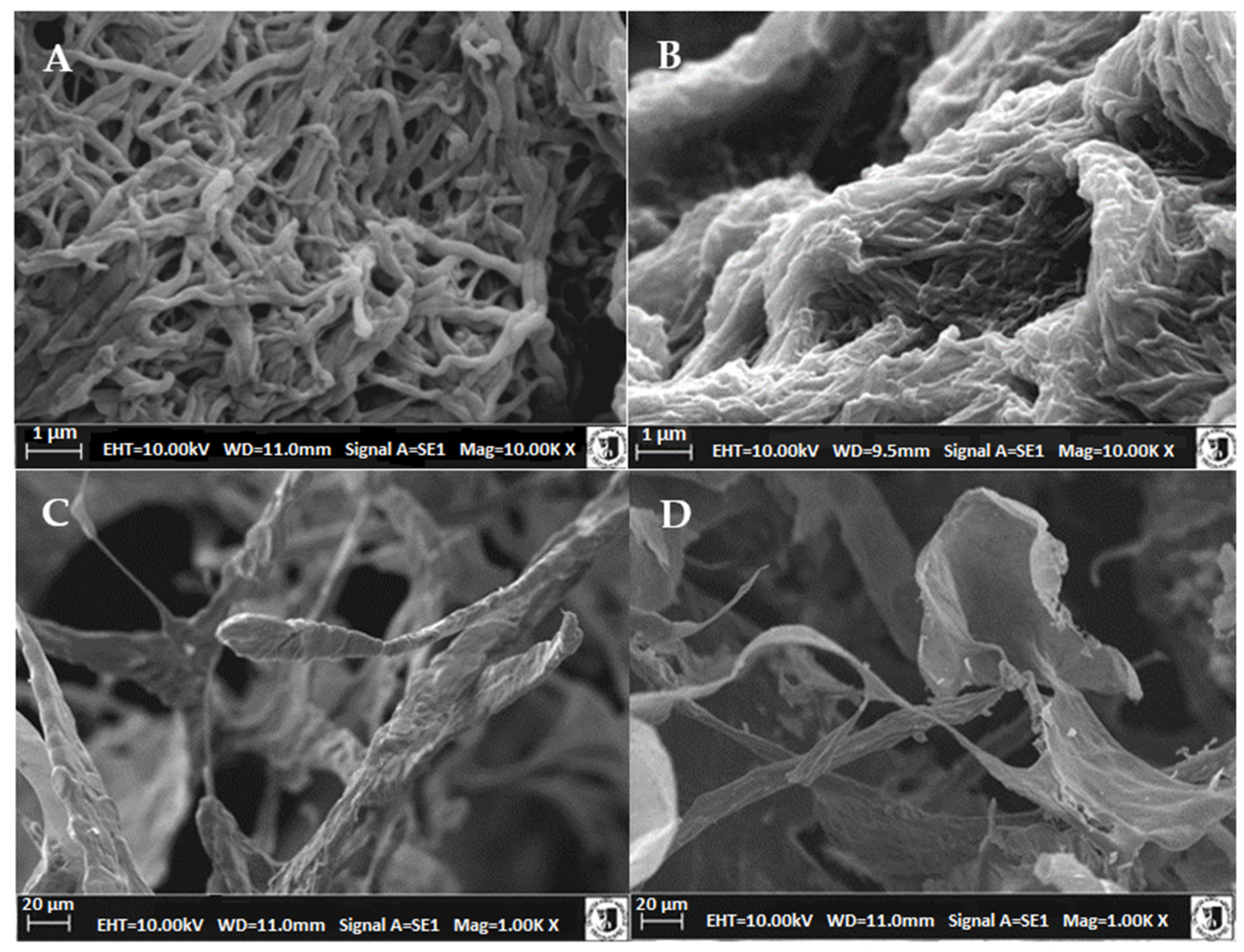
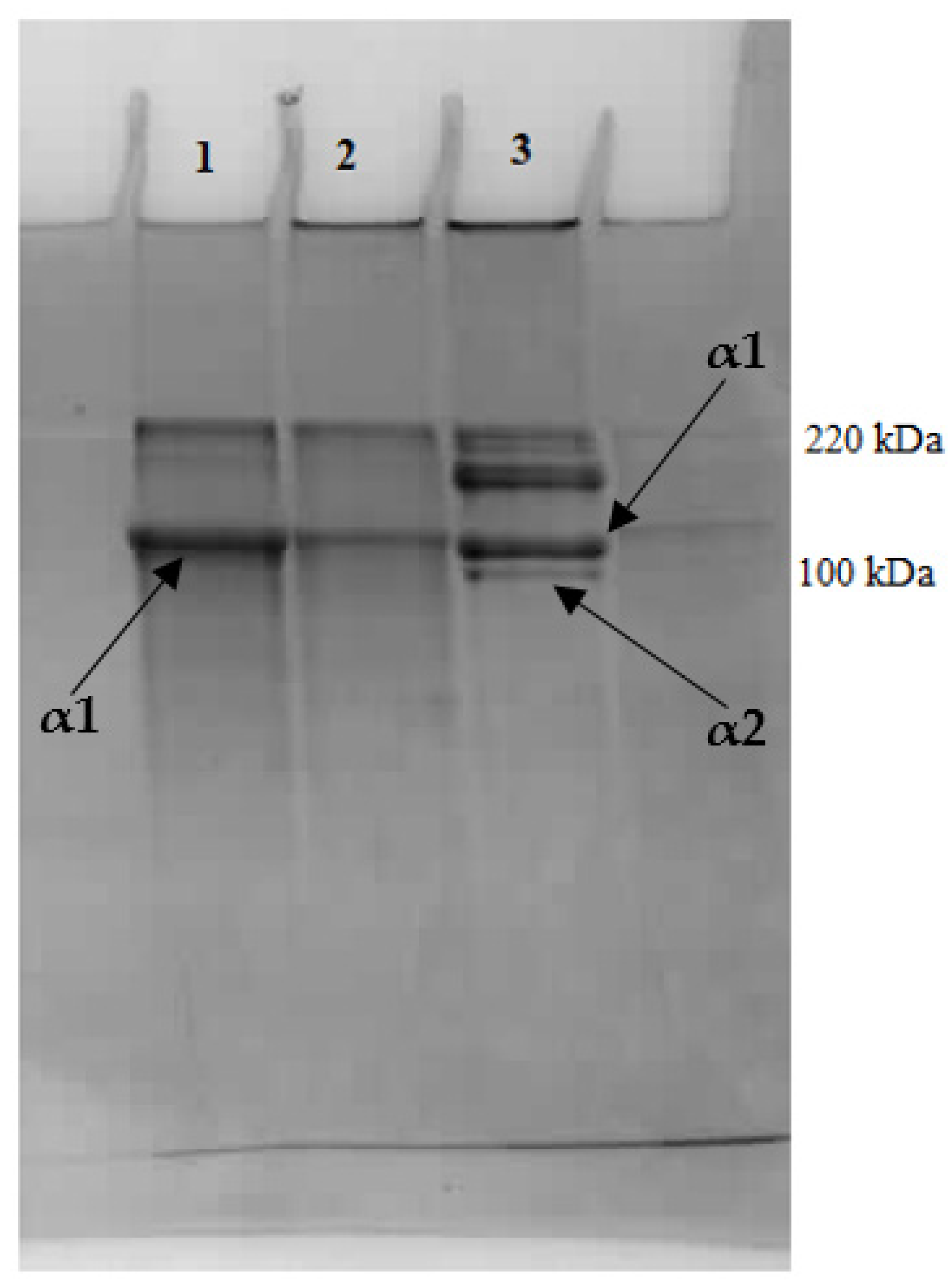
2.6. Microstructure of Atelocollagen Fibers (Type II Collagen)
3. Materials and Methods
3.1. Materials
3.2. Extraction of Collagen Type II
3.2.1. Pretreatment of the Raw Material (PG/GAG Extraction)
- (a)
- 0.2 M and 1.0 M NaCl solution in 0.05 M Tris-HCl buffer, pH 7.5, 24 h, 1:8 (w/v), 4 °C according to Cao and Xu [8],
- (b)
3.2.2. Extraction of Pepsin-Solubilized Collagen
3.3. Proximate Compositions of Cartilage
3.4. Quantitative Determination of Collagen (Hydroxyproline) and Proteoglycans or Glycosaminoglycans (Hyaluronic Acid and Chondroitin Sulfate) Content
3.5. Differential Scanning Calorimetry (DSC)
3.6. Rheological Properties of Reconstituted Collagen Fibers
3.7. Scanning Electron Microscopy (SEM)
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Vázquez, J.A.; Rodríguez–Amado, I.; Montemayor, M.I.; Fraguas, J.; del Pilar González, M.; Murado, M.A. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef]
- Lin, Y.C.; Tan, F.; Marra, K.G.; Jan, S.S.; Liu, D.C. Synthesis and characterization of collagen/hyaluronan/chitosan composite sponges for potential biomedical applications. Acta Biomater. 2009, 5, 2591–2600. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and characterization of collagen from the outer skin of squid (Doryteuthis singhalensis). Food Hydrocolloids 2015, 43, 708–716. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Balasubramanian, T. Isolation and characterization of thermostable collagen from the marine eel-fish (Evenchelys macrura). Process Biochem. 2013, 48, 1592–1602. [Google Scholar] [CrossRef]
- Chang, C.H.; Liu, H.C.; Lin, C.C.; Chou, C.H.; Lin, F.H. Gelatin–chondroitin–hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Biomaterials 2003, 24, 4853–4858. [Google Scholar] [CrossRef]
- Rigo, C.; Hartmann, D.J.; Bairati, A. Electrophoretic and immunochemical study of collagens from Sepia officinalis cartilage. Biochim. Biophys. Acta 2002, 1572, 77–84. [Google Scholar] [CrossRef]
- Cao, H.; Xu, S.-Y. Purification and characterization of type II collagen from chick sternal cartilage. Food Chem. 2008, 108, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Weis, M.A.; Kim, L.S.; Eyre, D.R. Type III collagen, a fibril network modifier in articular cartilage. J. Biol. Chem. 2010, 285, 18537–18544. [Google Scholar] [CrossRef]
- Gandhi, N.S.; Mancera, R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008, 72, 455–482. [Google Scholar] [CrossRef]
- Wilson, R.; Diseberg, A.F.; Gordon, L.; Zivkovic, S.; Tatarczuch, L.; Mackie, E.J.; Gorman, J.J.; Bateman, J.F. Comprehensive profiling of cartilage extracellular matrix formation and maturation using sequential extraction and label-free quantitative proteomics. Mol. Cell. Proteom. 2010, 9, 1296–1313. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.C.; You, S.J.; An, B.K.; Kang, C.W. Study on extraction of mucopolysaccharide-protein containing chondroitin sulfate from chicken keel cartilage. Asian Australas. J. Anim. Sci. 2006, 19, 601–604. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, C.; Jia, W.; Qin, X.; Cui, Z.; Mo, H.; Richel, A. Co-production of chondroitin sulfate and peptide from liquefied chicken sternal cartilage by hot-pressure. Carbohydr. Polym. 2019, 222, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.R.R.; Bezerra, T.K.A.; Queiroz, A.L.M.; Galvão, M.S.; Cavalcanti, M.T.; Pacheco, M.T.B.; Madruga, M.S. Collagen production from chicken keel bone using acid and enzymatic treatment at a temperature of 30 °C. Food Sci. Technol. 2020, 40, 491–497. [Google Scholar] [CrossRef]
- Garnjanagoonchorn, W.; Wongekalak, L.; Engkagul, A. Determination of chondroitin sulfate from different sources of cartilage. Chem. Eng. Process. 2007, 46, 465–471. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, L.; Sun, W.; Wang, Z.; Xu, J.; Ma, H. Isolation and characterization of collagen from the cartilage of Amur sturgeon (Acipenser schrenckii). Process Biochem. 2014, 49, 318–323. [Google Scholar] [CrossRef]
- Nakano, T.; Pietrasik, Z.; Ozimek, L.; Betti, M. Extraction, isolation and analysis of chondroitin sulfate from broiler chicken biomass. Process Biochem. 2012, 47, 1909–1918. [Google Scholar] [CrossRef]
- Silva, C.; Novoa-Carballal, R.; Reis, R.L.; Pashkuleva, I. Following the enzymatic digestion of chondroitin sulfate by a simple GPC analysis. Anal. Chim. Acta 2015, 885, 207–213. [Google Scholar] [CrossRef]
- Kang, A.H.; Piez, K.A.; Gross, J. Characterization of the α-chains of chick skin collagen and the nature of the amino-terminal cross-link region. Biochemistry 1969, 8, 3648–3655. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Isolation and characterization of collagen from the cartilages of brownbanded bamboo shark (Chiloscyllium punctatum) and blacktip shark (Carcharhinus limbatus). LWT Food Sci. Technol. 2010, 43, 792–800. [Google Scholar] [CrossRef]
- Matmaroh, K.; Benjakul, S.; Prodpran, T.; Encarnacion, A.B.; Kishimura, H. Characteristics of acid soluble collagen and pepsin soluble collagen from scale of spotted golden goatfish (Parupeneus heptacanthus). Food Chem. 2011, 129, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.K.; Liu, D.C. Effects of pepsin digestion at different temperatures and times on properties of telopeptide-poor collagen from bird feet. Food Chem. 2006, 94, 621–625. [Google Scholar] [CrossRef]
- Liu, D.; Liang, L.; Regenstein, J.M.; Zhou, P. Extraction and characterisation of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chem. 2012, 133, 1441–1448. [Google Scholar] [CrossRef]
- Yunoki, S.; Nagai, N.; Suzuki, T.; Munekata, M. Novel biomaterial from reinforced salmon collagen gel prepared by fibril formation and cross-linking. J. Biosci. Bioeng. 2004, 98, 40–47. [Google Scholar] [CrossRef]
- Zhang, X.; Ookawa, M.; Tan, Y.; Ura, K.; Adachi, S.; Takagi, Y. Biochemical characterisation and assessment of fibril-forming ability of collagens extracted from Bester sturgeon Huso huso x Acipenser ruthenus. Food Chem. 2014, 160, 305–312. [Google Scholar] [CrossRef]
- Wallace, D.G.; Condell, R.A.; Donovan, J.W.; Paivinen, A.; Rhee, W.M.; Wade, S.B. Multiple denaturational transitions in fibrillar collagen. Biopolymers 1986, 25, 1875–1893. [Google Scholar] [CrossRef]
- Pieper, J.S.; van der Kraan, P.M.; Hafmans, T.; Kamp, J.; Buma, P.; van Susante, J.L.C.; van den Berg, W.B.; Veerkamp, J.H.; van Kuppevelt, T.H. Crosslinked type II collagen matrices: Preparation, characterization, and potential for cartilage engineering. Biomaterials 2002, 23, 3183–3192. [Google Scholar] [CrossRef]
- Holmes, R.; Kirk, S.; Tronci, G.; Yang, X.; Wood, D. Influence of telopeptides on the structural and physical properties of polymeric and monomeric acid-soluble type I collagen. Mater. Sci. Eng. C 2017, 77, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Benjakul, S.; Maqsood, S.; Kishimura, H. Isolation and characterisation of collagen extracted from the skin of striped catfish (Pangasianodon hypophthalmus). Food Chem. 2011, 124, 97–105. [Google Scholar] [CrossRef]
- Forgacs, G.; Newman, S.A.; Hinner, B.; Maier, C.W.; Sackmann, E. Assembly of collagen matrices as a phase transition revealed by structural and rheologic studies. Biophys. J. Vol. 2003, 84, 1272–1280. [Google Scholar] [CrossRef]
- Yang, Y.; Leone, L.M.; Kaufman, L.J. Elastic moduli of collagen gels can be predicted from two-dimensional confocal microscopy. Biophys. J. 2009, 97, 2051–2060. [Google Scholar] [CrossRef]
- Latinovic, O.; Hough, L.A.; Ou-Yang, H.D. Structural and micromechanical characterization of type I collagen gels. J. Biomech. 2010, 43, 500–505. [Google Scholar] [CrossRef]
- Eysturskard, J.; Haug, I.J.; Elharfaoui, N.; Djabourov, M.; Draget, K.I. Structural and mechanical properties of fish gelatin as a function of extraction conditions. Food Hydrocoll. 2009, 23, 1702–1711. [Google Scholar] [CrossRef]
- Birk, D.E.; Silver, F.H. Collagen fibrillogenesis in vitro: Comparison of types I, II, and III. Arch. Biochem. Biophys. 1984, 235, 178–185. [Google Scholar] [CrossRef]
- Harris, R.; Reiber, A. Influence of saline and pH on collagen type I fibrillogenesis in vitro: Fibril polymorphism and colloidal gold labeling. Micron 2007, 38, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R.; Soliakov, A.; Lewis, R.J. In vitro fibrillogenesis of collagen type I in varying ionic and pH conditions. Micron 2013, 49, 60–68. [Google Scholar] [CrossRef]
- Douglas, T.; Heinemann, S.; Bierbaum, S.; Scharnweber, D.; Worch, H. Fibrillogenesis of collagen types I, II, and III with small leucine-rich proteoglycans decorin and biglycan. Biomacromolecules 2006, 7, 2388–2393. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.; Osatomi, K.; Yoshida, A.; Yamaguchi, A.; Tachibana, K.; Oda, T.; Hara, K. Characteristics of a self-assembled fibrillar gel prepared from red stingray collagen. Fisheries Sci. 2009, 75, 765–770. [Google Scholar] [CrossRef][Green Version]
- Stamov, D.R.; Müller, A.; Wegrowski, Y.; Brezillon, S.; Franz, C.M. Quantitative analysis of type I collagen fibril regulation by lumican and decorin using AFM. J. Struct. Biol. 2013, 183, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Paige, M.F.; Rainey, J.K.; Goh, M.C. A study of fibrous long spacing collagen ultrastructure and assembly by atomic force microscopy. Micron 2001, 32, 341–353. [Google Scholar] [CrossRef]
- Luo, X.M.; Fosmire, G.J.; Leach, R.M., Jr. Chicken keel cartilage as a source of chondroitin sulfate. Poultry Sci. 2002, 81, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Cliché, S.; Amiot, J.; Avezard, C.; Gariépy, C. Extraction and characterization of collagen with or without telopeptides from chicken skin. Poultry Sci. 2003, 82, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Farndale, W.R.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta 1986, 883, 173–177. [Google Scholar] [CrossRef]

| Parameters | Concentration [%] | |
|---|---|---|
| Raw Tissue ± SD | in Dry Matter | |
| dry matter | 13.10 ± 0.81 | 100.00 |
| protein | 8.50 ± 0.26 | 64.30 |
| ash | 0.90 ± 0.10 | 7.00 |
| fat | 0.09 ± 0.02 | 0.67 |
| hydroxyproline | 0.95 ± 0.13 | 7.25 |
| collagen | 7.19 ± 0.98 | 54.40 |
| uronic acid | 1.40 ± 0.34 | 10.70 |
| glycosaminoglycans | 3.80 ± 0.42 | 28.80 |
| Extraction Conditions | Yield [%] | |||
|---|---|---|---|---|
| Type of Salt Solution | Time [h] | Temperature [°C] | UA | GAG |
| 0.2/1.0 M NaCl | 24 | 4 | 97.3 | 98.5 |
| 3M MgCl2 | 24 | 23 | 85.6 | 83.8 |
| 48 | 97.9 | 98.0 | ||
| p-Value | 0.48 | 0.38 | ||
| Type of Material after Pretreatment | Yield of Collagen Recovery from Cartilage [%] | Recovery of Collagen in the Form of Fibers [%] | Amount of Freeze-Dried Collagen Fibers [mg/g Cartilage] |
|---|---|---|---|
| 0.2 /1.0 M NaCl | 95.1 | 95.2 a | 120 |
| 3 M MgCl2 | 97.3 | 70.8 b | 100 |
| p-Value | 0.33 | 0.02 | 0.29 |
| Material | Tm [°C] | ΔH [J/g Cartilage/Fibers] | ΔH [J/g Collagen] |
|---|---|---|---|
| Chicken keel cartilage | 64.5 | 0.5 | 7.1 |
| Cartilage residue after treatment in MgCl2 solution | 62.5 | 1.8 | 17.7 |
| Cartilage residue after treatment in NaCl solution | 65.0 | 2.1 | 23.3 |
| Collagen fibers MgCl2 (acidic) | 45.5 | 4.3 | 118 |
| Collagen fibers NaCl (acidic) | 46.1 | 4.3 | 96.4 |
| Collagen fibers MgCl2, neutral pH | 55.9 | 5.1 | 219 |
| Collagen fibers NaCl, neutral pH | 55.8 | 3.6 | 156 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pudło, A.; Juchniewicz, S.; Kopeć, W. Characteristics of Reconstituted Collagen Fibers from Chicken Keel Cartilage Depends on Salt Type for Removal of Proteoglycans. Molecules 2021, 26, 3538. https://doi.org/10.3390/molecules26123538
Pudło A, Juchniewicz S, Kopeć W. Characteristics of Reconstituted Collagen Fibers from Chicken Keel Cartilage Depends on Salt Type for Removal of Proteoglycans. Molecules. 2021; 26(12):3538. https://doi.org/10.3390/molecules26123538
Chicago/Turabian StylePudło, Anna, Szymon Juchniewicz, and Wiesław Kopeć. 2021. "Characteristics of Reconstituted Collagen Fibers from Chicken Keel Cartilage Depends on Salt Type for Removal of Proteoglycans" Molecules 26, no. 12: 3538. https://doi.org/10.3390/molecules26123538
APA StylePudło, A., Juchniewicz, S., & Kopeć, W. (2021). Characteristics of Reconstituted Collagen Fibers from Chicken Keel Cartilage Depends on Salt Type for Removal of Proteoglycans. Molecules, 26(12), 3538. https://doi.org/10.3390/molecules26123538






